Drought Stress and High Temperature Affect the Antioxidant Metabolism of Cotton (Gossypium hirsutum L.) Anthers and Reduce Pollen Fertility
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Data Collection
2.2.1. Midday Leaf Water Potential (ΨMD)
2.2.2. Pollen Viability
2.2.3. Anther Peroxide Content
2.2.4. Peroxides Labeling
2.2.5. Anther AsA and GSH Contents
2.2.6. Gene Expression
2.2.7. Data Analysis
3. Results
3.1. Midday Leaf Water Potential (ΨMD)
3.2. Pollen Viability and Pollen Germination Rate
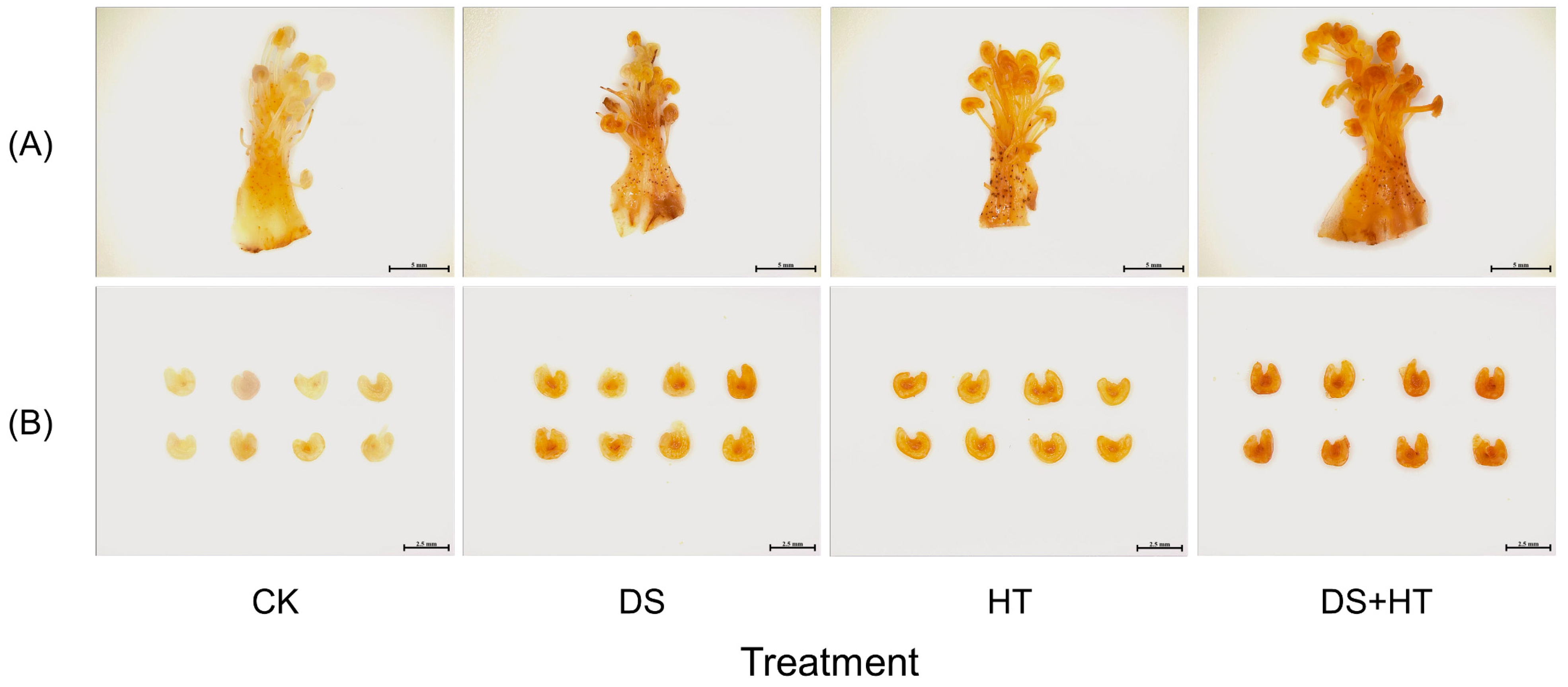
3.3. Peroxides Labeling
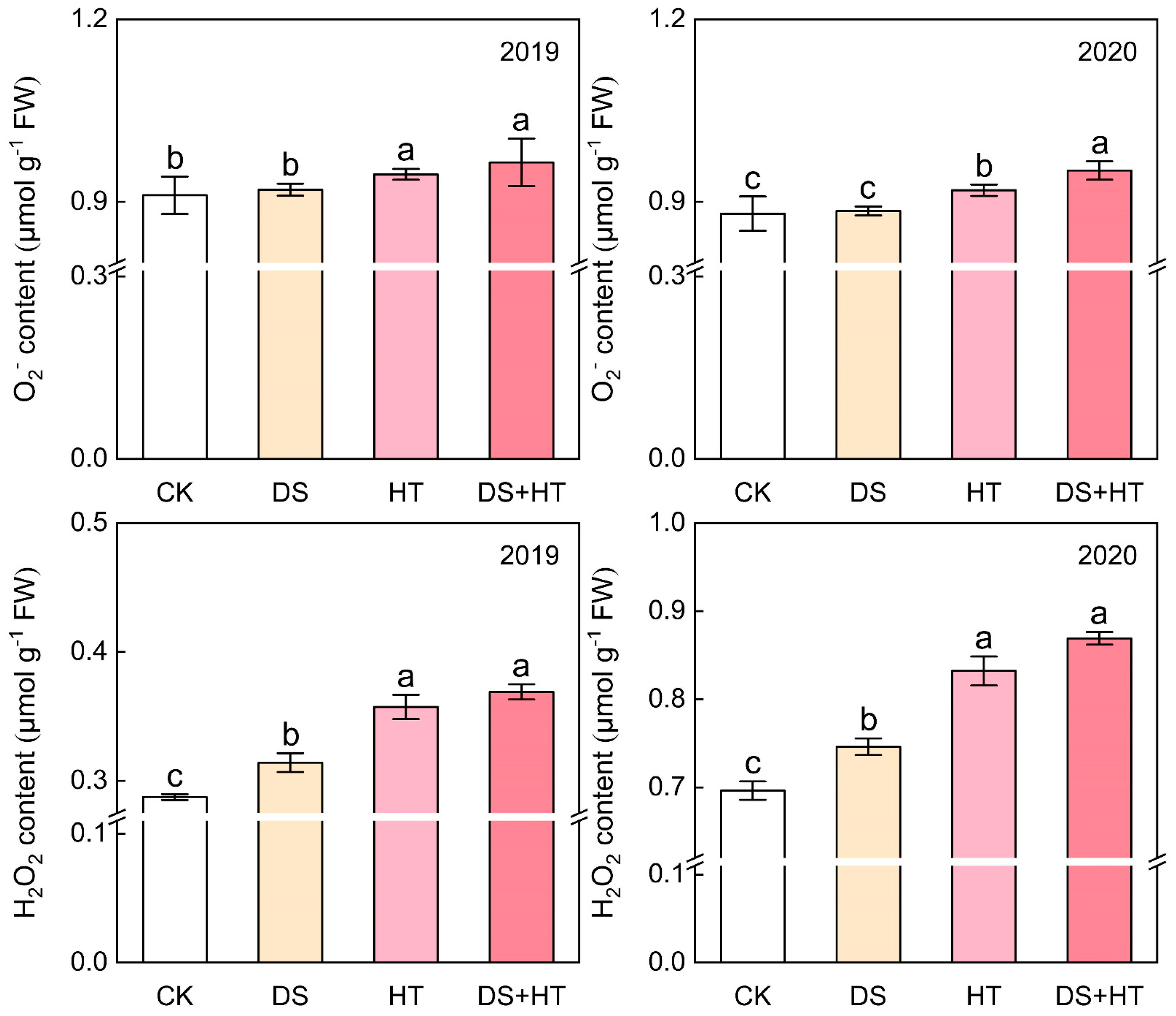
3.4. Anther ROS Content
3.5. Anther MDA Content
3.6. Expression of genes associated with antioxidant enzymes in cotton anthers
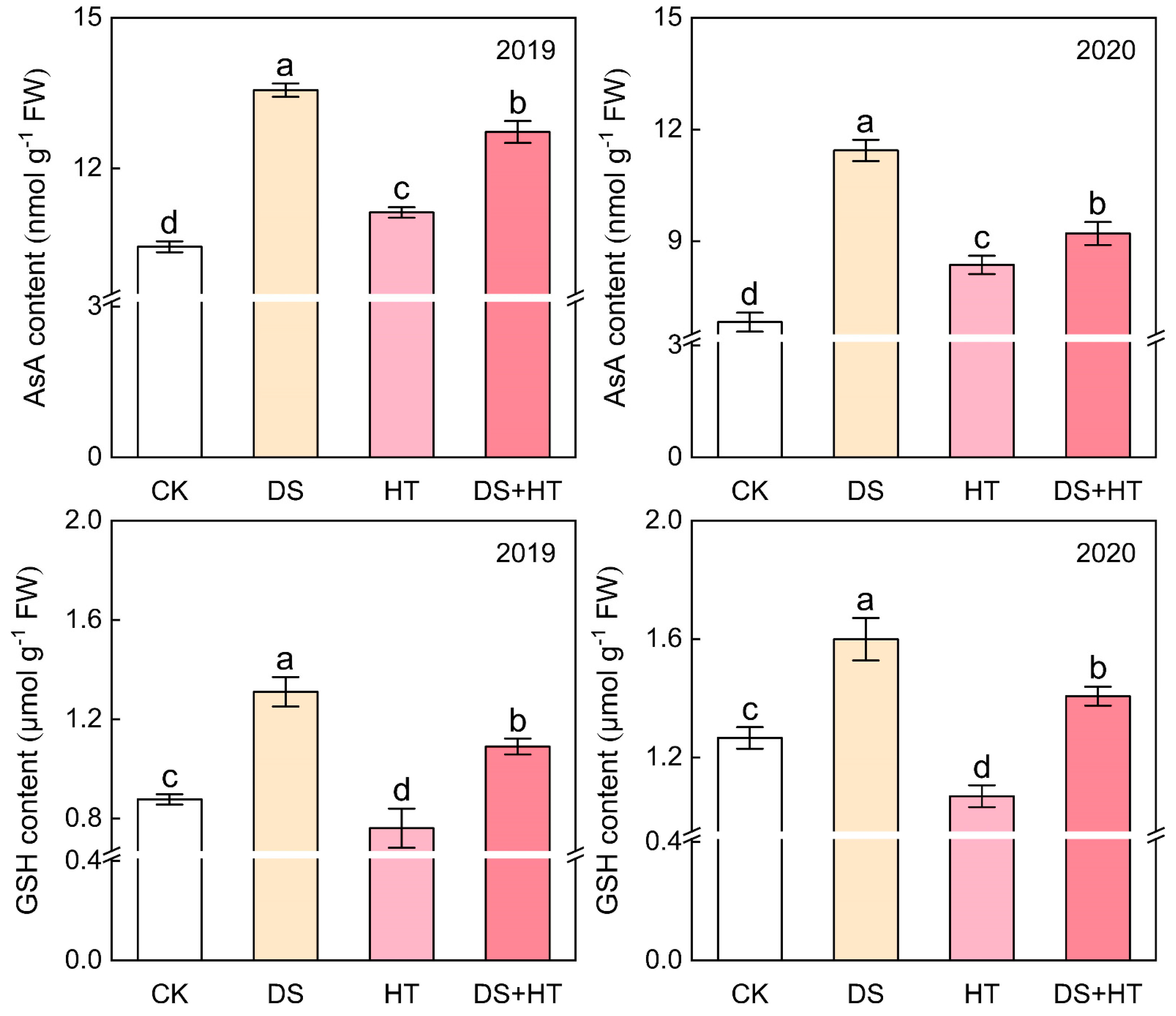
3.7. Anther AsA and GSH Contents
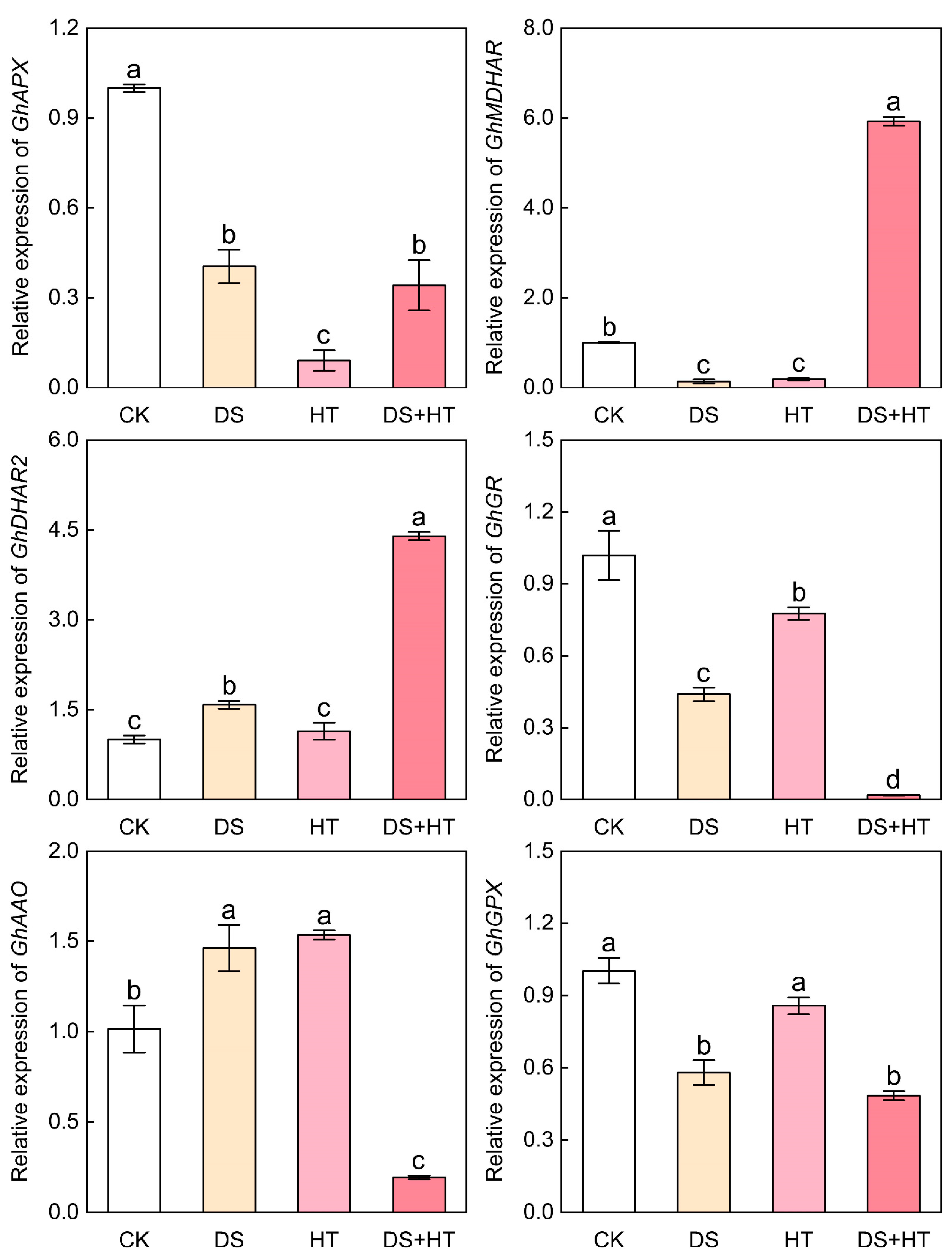
3.8. Expression of Genes Associated with AsA–GSH Cycle in Cotton Anthers
3.9. Correlation between Pollen Viability and ROS Content
3.10. Principal Component Analysis of Antioxidant Metabolism in Cotton Anthers
4. Discussion
4.1. Effects of Drought Stress and High Temperature on ROS Content and Its Relationship with Pollen Fertility
4.2. Effects of Drought Stress and High Temperature on Antioxidant Enzyme System in Anthers
4.3. Effects of Drought Stress and High Temperature on AsA–GSH Cycle in Anthers
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Akasofu, S.I. On the Present Halting of Global Warming. Climate 2013, 1, 4–11. [Google Scholar] [CrossRef]
- Solomon, S.; Qin, D.; Manning, M.; Chen, Z.; Marquis, M.; Averyt, K.B.; Tignor, M.; Miller, H. The Physical Science Basis: Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Comput. Geom. 2007, 996, 95–123. [Google Scholar]
- Peng, S.; Huang, J.; Sheehy, J.E.; Laza, R.C.; Visperas, R.M.; Zhong, X.; Centeno, G.S.; Khush, G.S.; Cassman, K.G. Rice Yields Decline with Higher Night Temperature from Global Warming. Proc. Natl. Acad. Sci. USA 2004, 101, 9971–9975. [Google Scholar] [CrossRef] [PubMed]
- Zinn, K.E.; Tunc-Ozdemir, M.; Harper, J.F. Temperature Stress and Plant Sexual Reproduction: Uncovering the Weakest Links. J. Exp. Bot. 2010, 61, 1959–1968. [Google Scholar] [CrossRef]
- Giorgi, F.; Lionello, P. Climate Change Projections for the Mediterranean Region. Glob. Planet. Chang. 2008, 63, 90–104. [Google Scholar] [CrossRef]
- Guo, C.; Ge, X.; Ma, H. The Rice OsDIL Gene Plays a Role in Drought Tolerance at Vegetative and Reproductive Stages. Plant Mol. Biol. 2013, 82, 239–253. [Google Scholar] [CrossRef] [PubMed]
- Pettigrew, W.T. Moisture Deficit Effects on Cotton Lint Yield, Yield Components, and Boll Distribution. Agron. J. 2004, 96, 377–383. [Google Scholar] [CrossRef]
- Wang, R.; Ji, S.; Zhang, P.; Meng, Y.; Zhou, Z. Drought Effects on Cotton Yield and Fiber Quality on Different Fruiting Branches. Crop Sci. 2016, 56, 1265–1276. [Google Scholar] [CrossRef]
- Gao, M.; Xu, B.; Wang, Y.; Zhou, Z.; Hu, W. Quantifying Individual and Interactive Effects of Elevated Temperature and Drought Stress on Cotton Yield and Fibre Quality. J. Agron. Crop Sci. 2021, 207, 422–436. [Google Scholar] [CrossRef]
- Xu, B.; Hu, W.; Gao, M.; Zhao, W.; Wang, Y.; Zhou, Z. Effects of Elevated Air Temperature Coupling with Soil Drought on Carbohydrate Metabolism and Oil Synthesis during Cottonseed Development. Physiol. Plant. 2022, 174, e13643. [Google Scholar] [CrossRef]
- Liu, Z.; Zhu, Y.; Hu, C.; Wei, G.; Yang, L.; Zhang, G. Effects of NaCl Stress on the Growth, Antioxidant Enzyme Activities and Reactive Oxygen Metabolism of Grafted Eggplant. J. Appl. Ecol. 2007, 18, 537–541. [Google Scholar]
- Liu, Z.; Shi, X.; Yan, P.; Duan, Y.; Geng, X.; Ye, J.; Li, S.; Yang, X.; Zhang, G.; Jia, Y.; et al. Tapetal Programmed Cell Death, Antioxidant Response and Oxidative Stress in Wheat Anthers Associated with D2-type Cytoplasmic Male-Sterility. Sci. Agric. Sin. 2017, 50, 4071–4086. [Google Scholar]
- Kelliher, T.; Walbot, V. Hypoxia Triggers Meiotic Fate Acquisition in Maize. Science 2012, 337, 345–348. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Jiang, Y.; Ma, J.; Gao, Z.; Chen, G. Metabolism of Reactive Oxygen Species in Anthers and Leaves of Cytoplasmic Male-sterile Rice. Chin. Agric. Sci. Bull. 2016, 32, 6–12. [Google Scholar]
- Mittler, R. Oxidative Stress, Antioxidants and Stress Tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef] [PubMed]
- García-Limones, C.; Hervás, A.; Navas-Cortés, J.A.; Jiménez-Dı́az, R.M.; Tena, M.J.P.; Pathology, M.P. Induction of an Antioxidant Enzyme System and Other Oxidative Stress Markers Associated with Compatible and Incompatible Interactions between Chickpea (Cicer arietinum L.) and Fusarium oxysporum f. sp. ciceris. Physiol. Mol. Plant Pathol. 2002, 61, 325–337. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.; Zhang, J. Advances in the Research on the AsA–GSH Cycle in Horticultural Crops. Front. Agric. China 2010, 4, 84–90. [Google Scholar] [CrossRef]
- Foyer, C.H.; Noctor, G. Oxidant and Antioxidant Signalling in Plants: A Re-evaluation of the Concept of Oxidative Stress in a Physiological Context. Plant Cell Environ. 2005, 28, 1056–1071. [Google Scholar] [CrossRef]
- Eltayeb, A.E.; Kawano, N.; Badawi, G.H.; Kaminaka, H.; Sanekata, T.; Morishima, I.; Shibahara, T.; Inanaga, S.; Tanaka, K. Enhanced Tolerance to Ozone and Drought Stresses in Transgenic Tobacco Overexpressing Dehydroascorbate Reductase in Cytosol. Physiol. Plant. 2006, 127, 57–65. [Google Scholar] [CrossRef]
- Navari-Izzo, F.; Pinzino, C.; Quartacci, M.F.; Sgherri, C.L.M.; Izzo, R. Intracellular Membranes: Kinetics of Superoxide Production and Changes in Thylakoids of Resurrection Plants upon Dehydration and Rehydration. Proc. R. Soc. Edinb. Sect. B Biol. 1994, 102, 187–191. [Google Scholar] [CrossRef]
- Tambussi, E.A.; Bartoli, C.G.; Beltrano, J.; Guiamet, J.J.; Araus, J.L. Oxidative Damage to Thylakoid Proteins in Water-stressed Leaves of Wheat (Triticum aestivum). Physiol. Plant. 2000, 108, 398–404. [Google Scholar] [CrossRef]
- Moller, I.M.; Jensen, P.E.; Hansson, A. Oxidative Modifications to Cellular Components in Plants. Annu. Rev. Plant Biol. 2007, 58, 459–481. [Google Scholar] [CrossRef]
- Rong, Z.; Zhang, X.; Yang, S.; Xu, Z.; Li, J.; Huang, G.; Zhao, J.; Gong, M. Involvement of Antioxidant Defense System in Enhancement of Drought Resistance in Tobacco (Nicotiana tabacum L.) Plants Through Circular Drought-hardening. Plant Physiol. J. 2012, 48, 705–713. [Google Scholar]
- Fu, G.; Song, J.; Xiong, J.; Li, Y.; Chen, H.; Le, M.; Tao, L. Changes of Oxidative Stress and Soluble Sugar in Anthers Involve in Rice Pollen Abortion under Drought Stress. Agric. Sci. China 2011, 10, 1016–1025. [Google Scholar] [CrossRef]
- Selote, D.S.; Khanna-Chopra, R. Drought-induced Spikelet Sterility is Associated with an Inefficient Antioxidant Defence in Rice Panicles. Physiol. Plant. 2004, 121, 462–471. [Google Scholar] [CrossRef]
- Ma, Y.; Ma, F.; Ma, X.; Li, M.; Wang, Y.; Han, M.; Shu, H. Effects of Drought Stress on Ascorbic Acid Contents and Activities of Related Metabolic Enzymes in Apple Leaves. J. Northwest A F Univ. (Nat. Sci. Ed.) 2008, 36, 150–154+160. [Google Scholar]
- Liu, X.; Huang, B. Heat Stress Injury in Relation to Membrane Lipid Peroxidation in Creeping Bentgrass. Crop Sci. 2000, 40, 503–510. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, S.; Xiao, L.; Tang, W.; Xiao, Y.; Chen, L. Effect of High Temperature Stress on Physiological Characteristics of Anther, Pollen and Stigma of Rice During Heading-flowering Stage. Chin. J. Rice Sci. 2014, 28, 155–166. [Google Scholar]
- Ahammed, G.J.; Xu, W.; Liu, A.; Chen, S. Endogenous Melatonin Deficiency Aggravates High Temperature-induced Oxidative Stress in Solanum lycopersicum L. Environ. Exp. Bot. 2019, 161, 303–311. [Google Scholar] [CrossRef]
- Nico, D.S.; Danny, G. The Impact of Environmental Stress on Male Reproductive Development in Plants: Biological Processes and Molecular Mechanisms. Plant Cell Environ. 2014, 37, 1–18. [Google Scholar]
- Djanaguiraman, M.; Prasad, P.V.V.; Al-Khatib, K. Ethylene Perception Inhibitor 1-MCP Decreases Oxidative Damage of Leaves Through Enhanced Antioxidant Defense Mechanisms in Soybean Plants Grown under High Temperature Stress. Environ. Exp. Bot. 2011, 71, 215–223. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhou, L.; Liu, J.; Du, X.; Asad, M.; Huang, F.; Pan, G.; Cheng, F. Relationship of ROS Accumulation and Superoxide Dismutase Isozymes in Developing Anther with Floret Fertility of Rice under Heat Stress. Plant Physiol. Biochem. 2017, 122, 90–101. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; He, X.; Chen, W.; Li, J.; Zhang, J. Effects of Heat Acclimation on High-temperature Stress Resistance and Heattolerance Mechanism of Festuca arundinacea and Lolium perenne. Acta Ecol. Sin. 2008, 28, 162–171. [Google Scholar]
- Zou, M.; Yuan, L.; Zhu, S.; Liu, S.; Ge, J.; Wang, C. Response of Osmotic Adjustment and Ascorbate-glutathione Cycle to Heat Stress in a Heat-sensitive and a Heat-tolerant Genotype of wucai (Brassica campestris L.). Sci. Hortic. 2016, 211, 87–94. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, J. Effect of High-temperature Stress on the Activity of Key Enzymes in the AsA–GSH Cycle in ‘Yali’ Pears. Front. Agric. China 2010, 4, 463–467. [Google Scholar] [CrossRef]
- Loka, D.A.; Oosterhuis, D.M.; Baxevanos, D.; Noulas, C.; Hu, W. Single and Combined Effects of Heat and Water Stress and Recovery on Cotton (Gossypium hirsutum L.) Leaf Physiology and Sucrose Metabolism. Plant Physiol. Biochem. 2020, 148, 166–179. [Google Scholar] [CrossRef]
- Burke, J.J.; Mahan, J.R.; Hatfield, J.L. Crop-specific Thermal Kinetic Windows in Relation to Wheat and Cotton Biomass Production. Agron. J. 1988, 80, 553–556. [Google Scholar] [CrossRef]
- Zuo, D.; Ming, J.; Liu, C.; Wang, L. Advance in Technique of Plant Pollen Viability. J. Anhui Agric. Sci. 2007, 35, 4742–4745. [Google Scholar]
- Liu, X.; Wang, X.; Wang, X.; Gao, J.; Luo, N.; Meng, Q.; Wang, P. Dissecting the Critical Stage in the Response of Maize Kernel Set to Individual and Combined Drought and Heat Stress around Flowering. Environ. Exp. Bot. 2020, 179, 104213. [Google Scholar] [CrossRef]
- Hu, W.; Lv, X.; Yang, J.; Chen, B.; Zhao, W.; Meng, Y.; Wang, Y.; Zhou, Z.; Oosterhuis, D.M. Effects of Potassium Deficiency on Antioxidant Metabolism Related to Leaf Senescence in Cotton (Gossypium hirsutum L.). Field Crops Res. 2016, 191, 139–149. [Google Scholar] [CrossRef]
- Kampfenkel, K.; Vanmontagu, M.; Inzé, D. Extraction and Determination of Ascorbate and Dehydroascorbate from Plant Tissue. Anal. Biochem. 1995, 225, 165–167. [Google Scholar] [CrossRef]
- Barrow, J.R. Comparisons among Pollen Viability Measurement Methods in Cotton. Crop Sci. 1983, 23, 734–736. [Google Scholar] [CrossRef]
- Jiang, P.; Zhang, X.; Zhu, Y.; Zhu, W.; Xie, H.; Wang, X. Metabolism of Reactive Oxygen Species in Cotton Cytoplasmic Male Sterility and Its Restoration. Plant Cell Rep. 2007, 26, 1627–1634. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhou, L.; Liu, J.; Cao, Z.; Du, X.; Huang, F.; Pan, G.; Cheng, F. Involvement of CAT in the Detoxification of HT-induced ROS Burst in Rice Anther and Its Relation to Pollen Fertility. Plant Cell Rep. 2018, 37, 741–757. [Google Scholar] [CrossRef]
- Nguyen, G.N.; Hailstones, D.L.; Wilkes, M.; Sutton, B.G. Drought-induced Oxidative Conditions in Rice Anthers Leading to a Programmed Cell Death and Pollen Abortion. J. Agron. Crop Sci. 2010, 195, 157–164. [Google Scholar] [CrossRef]
- Yan, Z.; Liu, D.; Jia, X.; Yang, Q.; Chen, Y.; Dong, P.; Wang, Q. Maize Tassel Development, Physiological Traits and Yield under Heat and Drought Stress during Flowering Stage. Sci. Agric. Sin. 2021, 54, 3592–3608. [Google Scholar]
- Liu, M.; Guo, L.; Yue, Y.; Wu, J.; Fan, X.; Xiao, G.; Teng, K. Physiological and Antioxidant Enzyme Gene Expression Differences Between Female and Male Buchloe dactyloides Plants under Drought Stress. Acta Prataculturae Sin. 2023, 32, 93–103. [Google Scholar]
- Gupta, M.; Cuypers, A.; Vangronsveld, J.; Clijsters, H. Copper Affects the Enzymes of the Ascorbate-glutathione Cycle and Its Related Metabolites in the Roots of Phaseolus vulgaris. Physiol. Plant. 1999, 106, 262–267. [Google Scholar] [CrossRef]
- Gill, S.S.; Anjum, N.A.; Hasanuzzaman, M.; Gill, R.; Trivedi, D.K.; Ahmad, I.; Pereira, E.; Tuteja, N. Glutathione and Glutathione Reductase: A Boon in Disguise for Plant Abiotic Stress Defense Operations. Plant Physiol. Biochem. 2013, 70, 204–212. [Google Scholar] [CrossRef]
- Xu, S.; Li, J.; Zhang, X.; Wei, H.; Cui, L. Effects of Heat Acclimation Pretreatment on Changes of Membrane Lipid Peroxidation, Antioxidant Metabolites, and Ultrastructure of Chloroplasts in Two Cool-season Turfgrass Species under Heat Stress. Environ. Exp. Bot. 2006, 56, 274–285. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, J.; Zhang, J. Effect of Exogenous Substance Pretreatment on Antioxidative Ability of Pear Fruits under High Temperature and Excessive Sunlight Stress. Acta Hortic. 2015, 1094, 389–394. [Google Scholar] [CrossRef]
- Noctor, G.; Foyer, C.H. Ascorbate and Glutathione: Keeping Active Oxygen under Control. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1998, 49, 249–279. [Google Scholar] [CrossRef] [PubMed]
- Shila, S.K.; Murali, A.V.M.; Sibasis, S.; Malireddy, K.R.; Arulandu, A. Biochemical and Structural Characterization of a Robust and Thermostable Ascorbate Recycling Monodehydroascorbate Reductase (MDHAR) from Stress Adapted Pearl Millet. Biochem. Biophys. Res. Commun. 2023, 662, 135–141. [Google Scholar]
- Krishna, D.B.; Ali, K.W.; Nellootil, S.S.; Kannapiran, P.; Murali, A.V.M.; Seetharami, R.E.; Balasubramaniam, D.; Anmol, C.; Malireddy, K.R.; Arulandu, A. Plant Dehydroascorbate Reductase Moonlights as Membrane Integrated Ion Channel. Arch. Biochem. Biophys. 2023, 741, 109603. [Google Scholar]
- Gaspard, S.; Monzani, E.; Casella, L.; Gullotti, M.; Maritano, S.; Marchesini, A. Inhibition of Ascorbate Oxidase by Phenolic Compounds. Enzymatic and Spectroscopic Studies. Biochemistry 1997, 36, 4852–4859. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Cheng, M.; Cao, N.; Li, Y.; Wang, S.; Zhou, Z.; Hu, W. Drought Stress and High Temperature Affect the Antioxidant Metabolism of Cotton (Gossypium hirsutum L.) Anthers and Reduce Pollen Fertility. Agronomy 2023, 13, 2550. https://doi.org/10.3390/agronomy13102550
Zhang J, Cheng M, Cao N, Li Y, Wang S, Zhou Z, Hu W. Drought Stress and High Temperature Affect the Antioxidant Metabolism of Cotton (Gossypium hirsutum L.) Anthers and Reduce Pollen Fertility. Agronomy. 2023; 13(10):2550. https://doi.org/10.3390/agronomy13102550
Chicago/Turabian StyleZhang, Jipeng, Mengdie Cheng, Nan Cao, Yongjun Li, Shanshan Wang, Zhiguo Zhou, and Wei Hu. 2023. "Drought Stress and High Temperature Affect the Antioxidant Metabolism of Cotton (Gossypium hirsutum L.) Anthers and Reduce Pollen Fertility" Agronomy 13, no. 10: 2550. https://doi.org/10.3390/agronomy13102550
APA StyleZhang, J., Cheng, M., Cao, N., Li, Y., Wang, S., Zhou, Z., & Hu, W. (2023). Drought Stress and High Temperature Affect the Antioxidant Metabolism of Cotton (Gossypium hirsutum L.) Anthers and Reduce Pollen Fertility. Agronomy, 13(10), 2550. https://doi.org/10.3390/agronomy13102550







