Abstract
A high pod abscission rate in soybean plants results in a significant decrease in the yield per plant. Under dense planting conditions, dense tolerant soybean cultivars had a relatively low rate of pod abscission, thereby facilitating higher yield. In this experiment, two planting densities were used to analyze the differentially expressed genes and metabolites between the abscised and non-abscised flowers of two soybean cultivars on the basis of transcriptomic and metabolomic techniques. The flower abscission rate of LD32 was significantly lower than that of SND28. Both cultivars were enriched in the photosynthesis, sugar, and starch metabolism; MAPK signaling; and phenylalanine metabolism pathways at different planting densities. However, under dense planting, the trend of differential gene changes in the density-tolerant CV LD32 was opposite to that of the conventional CV SND28. The results of the joint analysis indicated that the co-regulation of cytokinin dehydrogenase 6 (CKX6) and cis-zeatin riboside monophosphate (CZRM) in the zeatin biosynthesis pathway of LD32 under dense planting conditions was the main factor for the relatively low rate of pod abscission under dense planting conditions.
1. Introduction
Pod abscission occurs frequently during the development of soybean plants (Glycine max (L.) Merr.), resulting in a reduced yield [1,2,3]. Factors such as planting density, temperature, light intensity, daylength, air humidity, and water and fertilizer management in the field contribute to pod abscission in soybean [4,5]. Planting density is an important cultivation practice that affects crop yield and represents an important factor for regulating the crop’s population structure and yield [6,7]. Dense cultivation allows the soybean population to maximize the use of light and nutrients, thus increasing yield. However, increasing the planting density causes stronger competition among soybean plants, leading to an increase in the rate of pod abscission. Thus, if not under control, dense cultivation indirectly leads to reduced yield rather than the anticipated increase [8,9]. Reasonably close planting of soybeans and coordination of the relationship between planting density and flower or pod abscission have become important issues for improving the yield of soybean. Nevertheless, density-tolerant soybean varieties have a lower rate of flower and pod abscission, and have a better pods per plant quantity under dense planting conditions than that seen in conventional varieties [10]. Thus, tolerance of density is a beneficial characteristic for achieving high yields. However, the effect of dense planting on the differences in flower and pod abscission among different density-tolerant soybean varieties remains unclear.
With the development of biotechnology, a number of genomic technologies, including gene localization, genome-wide association studies, marker development, and molecular breeding, have been widely implemented [11]. For example, transcriptional metabolism-based association analysis has become an important tool for research on crop-related mechanisms. Both hormone metabolic regulation and the related gene expression levels affect the abscission of plant organs; among such plant hormones, cytokinin is involved in regulating plants’ growth and development processes, such as floral meristem development, abiotic and biotic stress responses, and formation and maintenance of floral organs [12].
Cytokinin, which is present in more than a dozen interconvertible forms in most higher plants, is degraded only by specific cytokinin dehydrogenases (CKX) to complete the metabolic process [13,14]. Members of the CKX gene family, which encodes CKX enzymes, are the only enzymes that have been clearly shown to catalyze the metabolism of specific cytokinins and play important roles in the developmental process of the cell cycle in plants [15]. They play an important role in the regulation of organ abscission in crops [16,17].
In soybean, altering the expression of cytokinin-related genes (CKX and IPT) led to changes in the cytokinin content of flowers, which in turn affected pod abscission in soybean [18,19,20]. The concentrations of cytokinin during different growth stages and in different plant parts of soybean regulate the abscission and senescence of the floral pod apparatus [21], and changes in the concentration of cytokinin in soybean flowers directly affects the abscission of floral organs [22]. A decrease in the concentration of cytokinin in roots during the growth and development of pods in soybeans has been demonstrated to cause the abscission of floral organs [23]. This impact on abscission also affected the soybean yield. Griffiths et al. [24] showed that cytokinin affected yield by inducing the abscission of floral organs in soybean, and that during pod formation, cytokinin regulated the abscission of soybean pods, which in turn affected the yield traits.
Most studies have attempted to analyze different genes/metabolites to study the density tolerance of maize [25,26]. This experiment applied this technology to study the metabolism of abscised and non-abscised flowers in soybean. In this study, two soybean cultivars with significantly different flower abscission rates—namely, the density-tolerant CV LD32 and the conventional CV SND28—were used as the research materials. Transcriptome and metabolome sequencing techniques were then performed to compare and analyze the differences in the genes and differentially expressed metabolites (DEMs) of abscised and non-abscised flowers of the two soybean varieties under different planting densities. Subsequently, the effects of planting density on flower abscission in soybean were discussed.
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
In 2021, the soybean cultivars Liaodou32 (LD32) and Shennogndou28 (SND28), which were used as the research materials, were grown under two different planting densities (150,000 plants ha−1 and 225,000 plants ha−1) for the field trial (Table S1). The experiment was located at the Agronomy Experimental Base of Shenyang Agricultural University in Shenyang, Liaoning Province (41°48′11.75″ N, 123°25′31.18″ E, Shenyang, Liaoning Province). The local conventional planting density is 150,000 plants ha−1. The experiment was conducted using a randomized group design with three replicates, and each plot was 20 m2. The fields were managed according to local soybean management practices (the soil in the area contained 42.3 g/kg organic matter, 0.201 g/kg total nitrogen, 39.5 mg/kg fast-acting phosphorus, and 226 mg/kg fast-acting potassium, and it had a pH value of 6.30).
2.2. Investigation and Statistics of Flowers Based on Their Flowering Stages
Flowers were observed at R1 (flowering stage), R2 (prime bloom period), and R3 (podding stage). An area of 1 m2 was randomly selected in the experimental plots and screen pockets were laid on the ground both to record the number of abscised flowers and for timely collection of abscised flowers as backup samples. During the sampling period from 8:00 a.m. to 8:00 p.m. every day, the abscised flowers were immediately placed into liquid nitrogen for preservation, to be used in subsequent experiments. The total number of abscised flowers, the total number of opened flowers, and the rate of abscised flowers were subsequently recorded.
2.3. Yield and Yield Components
At maturity, uniformly growing plants of each of the two soybean cultivars were planted at different densities, and their agronomic traits, including plant height, stem thickness, height of the lowest pod, pods per plant, grains per plant, 100-grain weight, total dry matter, and the harvest index were measured. Soybean yield was also measured at a moisture content of 13.0%. To determine the physiological parameters, at least three replicates were performed.
2.4. RNA-Seq Analysis
The abscised flowers from plants at the R1, R2, and R3 stages and non-abscised flowers from plants at the later R3 stage were taken as samples. The abscised and non- abscised flowers were collected from both treatment and control samples, snap-frozen in liquid nitrogen for 30 min, removed, and then placed in a refrigerator at −80 °C. After all samples had been collected, they were sent to Lianchuan Biotech (Hangzhou, China) for sequencing and analysis. Differences in the genes between the non-abscised and abscised flowers under different planting densities were investigated using RNA-Seq. The experiment was conducted on eight sample groups, numbered 13–20, and the representative meanings of each number are noted (Table 1). Total RNA was extracted using TRIzol reagent (Thermo Fisher, Santa Clara, CA, USA) following the manufacturer’s instructions. High-quality RNA samples with RIN numbers greater than 7.0 were used to construct the sequencing libraries. After the total extraction of RNA, two rounds of purification of the total RNA (5 µg) were performed using Oligo (dT) Dynabeads (Thermo Fisher, CA, USA). The cDNA libraries constructed from the pooled RNA of the samples were sequenced using the Illumina Novaseq 6000 sequencing platform. The transcriptome was sequenced using the Illumina paired-end RNA-seq method. Reads from all samples were mapped to the soybean reference genome using the HISAT2 software package (version hisat2-2.0.4). A portion of the reads was initially removed on the basis of the quality of the information accompanying each read, and the remaining reads were mapped to the reference genome. The transcriptomes of all samples were then merged using software (version: gffcompare-0.9.8) to reconstruct a comprehensive transcriptome. After the final transcriptome was generated, StringTie and BallGown were used to estimate the expression levels of all transcripts and the abundance of the mRNAs by calculating the fragments per kilobase of exons per million mapped fragments (FPKM). The false discovery rate (FDR) was set to <0.05, and a fold change of ≥2 (|log2FC| ≥ 1) and q < 0.05 (q value is the corrected p value) were used as the criteria for screening differentially expressed genes (DEGs) [27]. The DEGs were then analyzed for their GO function and enrichment using the Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways [28].

Table 1.
Numeric codes of the transcriptome samples and the corresponding physiological significance.
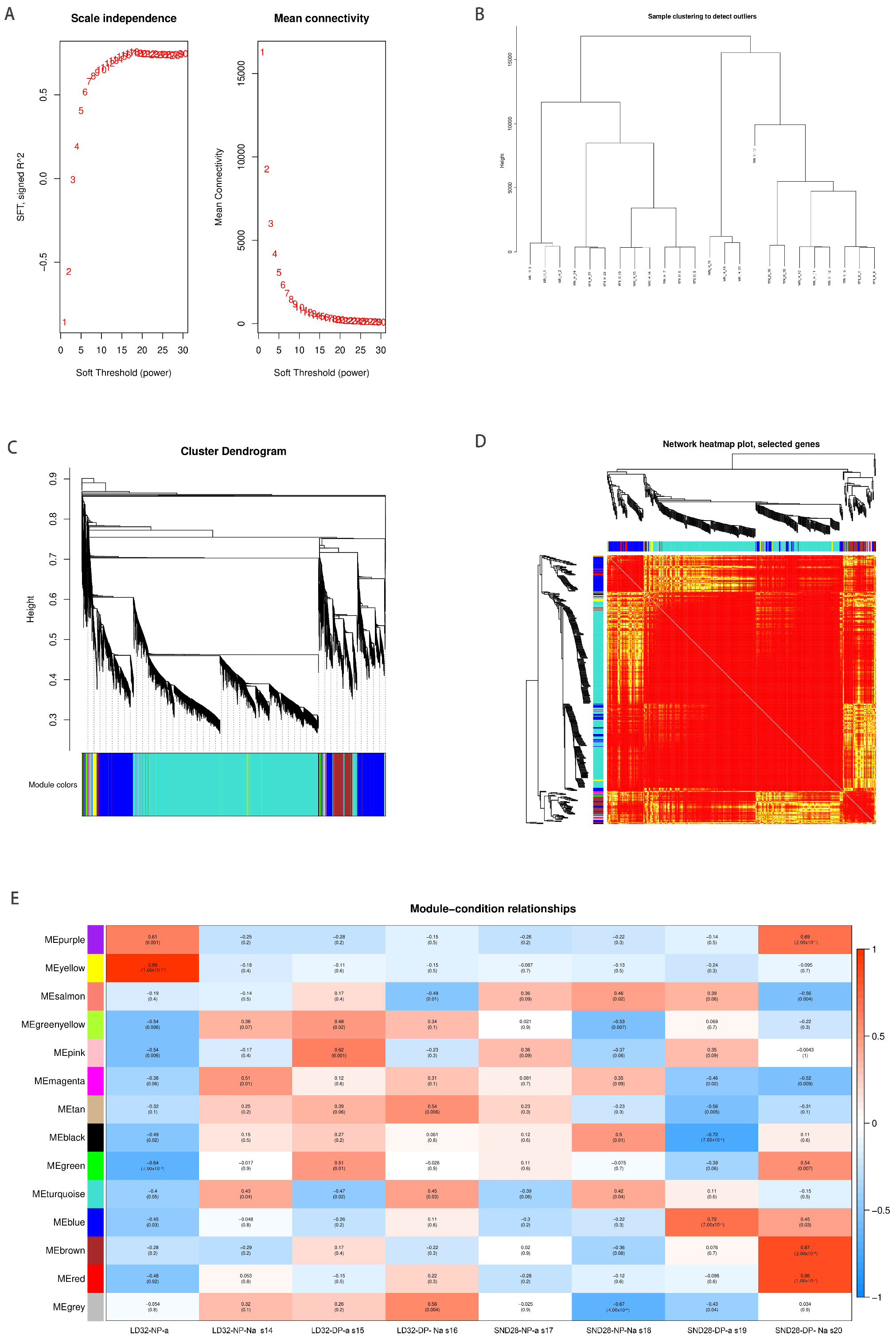
Co-expression analysis was performed using the weighted gene correlation network analysis (WGCNA) package. Genes with FPKM < 1 in the samples were filtered. Hierarchical clustering of the samples was conducted on the basis of the Euclidean distance computed from the gene expression data. The network topology analysis ensured a scale-free topology network with a defined soft-thresholding power of 1. On the basis of the dynamic tree cutting algorithm, we identified the total number of modules (excluding gray modules) with the parameters minModuleSize = 30 and mergeCutHeight = 0.25. For each module, the co-expression relationships among the module’s genes were analyzed and visualized by Cytoscape (Java, 3.8.2).
2.5. Quantitative Real-Time PCR (qRT-PCR) Analysis
The relative gene expression levels of the DEGs were verified using qRT-PCR. Fluorescent quantification primer sequences of the DEGs were designed (Table S2), and β-actin was selected as the internal reference gene for a control. RNA was extracted from the samples by reagents provided by OMEGA Bio-Tek. The All-in-One First-Strand cDNA Synthesis Kit was used to reverse-transcribe the RNA into cDNA, which was used as a template for qRT-PCR validation using an Mx3000p real-time fluorescence quantitative analyzer (Agilent, Santa Clara, CA, USA). The results for the target genes were quantified using the 2−ΔΔCt method.
2.6. Metabolomic Assay
The samples were subjected to metabolite extraction using the protein precipitation method using an organic reagent, and quality control samples (QC) were prepared simultaneously. The samples were subjected to untargeted metabolomic analysis using a Q Exactive high-resolution mass spectrometer (Thermo Fisher Scientific, Waltham, MA, USA) with LC-MS/MS technology to acquire data in both the positive ion (pos) and negative ion (neg) modes to explore the metabolomic composition and biological functions within the samples. Raw data from the mass spectrometry were converted to readable mzXML data using ProteoWizard’s MSConvert software(3.0.230xx). Peak extraction was performed using XCMS software (3.22.0), and the quality of the peak extraction was controlled. The extracted material was annotated with the total number of ions using CAMERA and then identified at the first level using MetaX software (1.4.17). Information from the primary mass spectrometry examination was used for identification, and information from the secondary mass spectrometry examination was matched with an in-house standard database. Candidate identification substances were used for the annotation of the metabolites, using HMDB, KEGG, and other databases to explain the physicochemical properties and biological functions of the metabolites. Quantitative screening of the DEMs was performed using MetaX software [29]. Depending on the contribution to the degree of variance of the PLS-DA model (VIP) of the first two principal components of the multivariate analysis, the partial least squares method-discriminant analysis (PLS-DA) model, and the univariate analysis; the fold change and t-test (Student’s t-test) results were used to screen for DEMs. The screening criteria for DEMs between groups were based on an extensive targeted metabolomic analysis, and DEMs were defined as follows: a ratio of ≥2 or a ratio ≤ ½, a q of value < 0.05 (or p < 0.05), and a VIP of ≥1 [30]. Finally, Pearson’s correlation analysis was used to integrate the metabolomic and transcriptomic analyses.
2.7. Statistical Analysis
The data were analyzed by one-way analysis of variance (ANOVA) and Duncan’s multiple range test (p < 0.05) using SPSS 22.0 Statistics (SPSS Inc., Chicago, IL, USA).
3. Results
3.1. Effects of Planting Density on the Flower Number and Flower Abscission Rate in Soybean
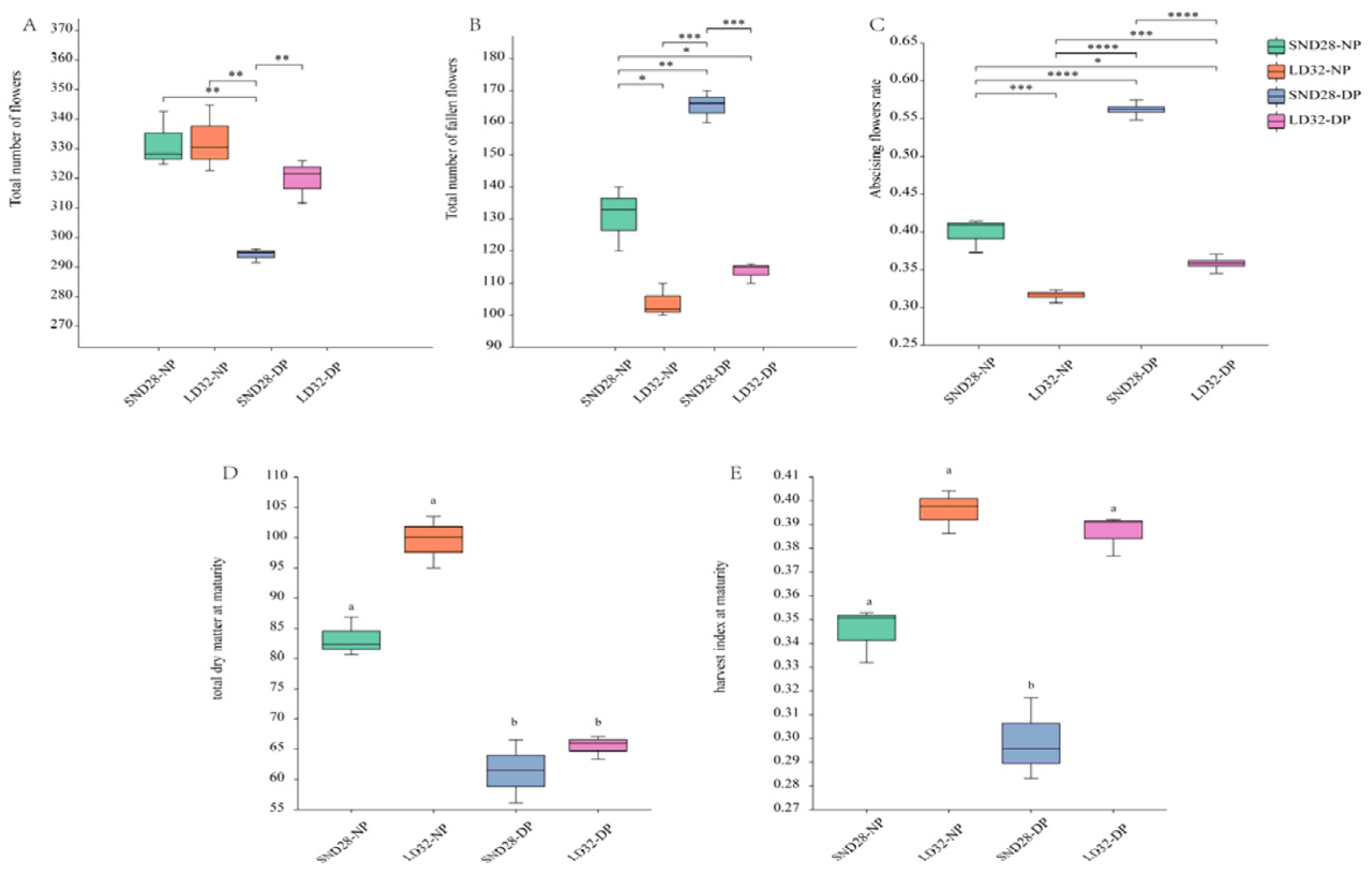
Significant differences were not observed between the total number of opened flowers in LD32 and SND28 plants under normal planting densities. However, significant differences were observed in the number of abscised flowers and in the rate of abscission. Under dense planting, the total number of open flowers of the two soybean cultivars showed a decreasing trend, while the total number of abscised flowers and abscission rate showed an increasing trend. Moreover, the total number of fallen flowers and shedding rate of SND28 were higher than those of LD32 (Figure 1A–C). Increased planting density had a significant impact on the total dry matter mass at maturity of both LD32 and SND28, resulting in a decrease compared with the normal planting density. Moreover, LD32 exhibited a greater total dry matter mass than SND28 when grown under dense planting (Figure 1D). The harvest index also indicated that increased planting density led to a decrease in the harvest index for both varieties, with SND28 experiencing a significant decrease compared with LD32 (Figure 1E). These findings suggest that LD32 demonstrated better tolerance than SND28, as shown by the abscission rate, total dry matter mass at maturity, and harvest index under high density.
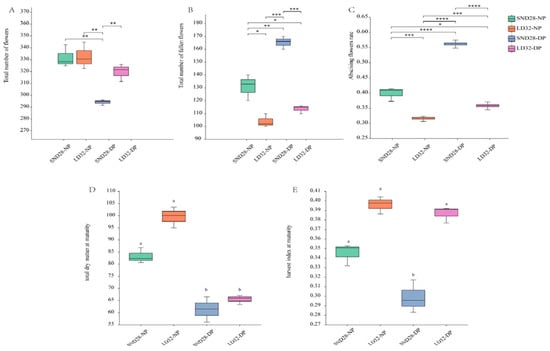
Figure 1.
Effect of planting density on the number of flowers, the flower abscission rate, total dry matter mass, and harvest index of different soybean cultivars at the flowering stage. (A) The effect of planting density on the total number of flowers. (B) The effect of planting density on the total number of fallen flowers. (C) The effect of planting density on the flower abscission rate. (D) The effect of planting density on the total dry matter mass. (E) The effect of planting density on the harvest index. Different lowercase letters and * indicate p < 0.05. **, ***, **** indicate p < 0.01.
3.2. Transcriptome Differences and Enrichment Analysis
The number of abscised flowers and the abscission rates in SND28 and LD32 were significantly different under the two planting densities. To investigate the mechanism underlying these differences, we conducted a comparative study of the transcriptome of the abscised and non-abscised flowers of the two cultivars under different densities.
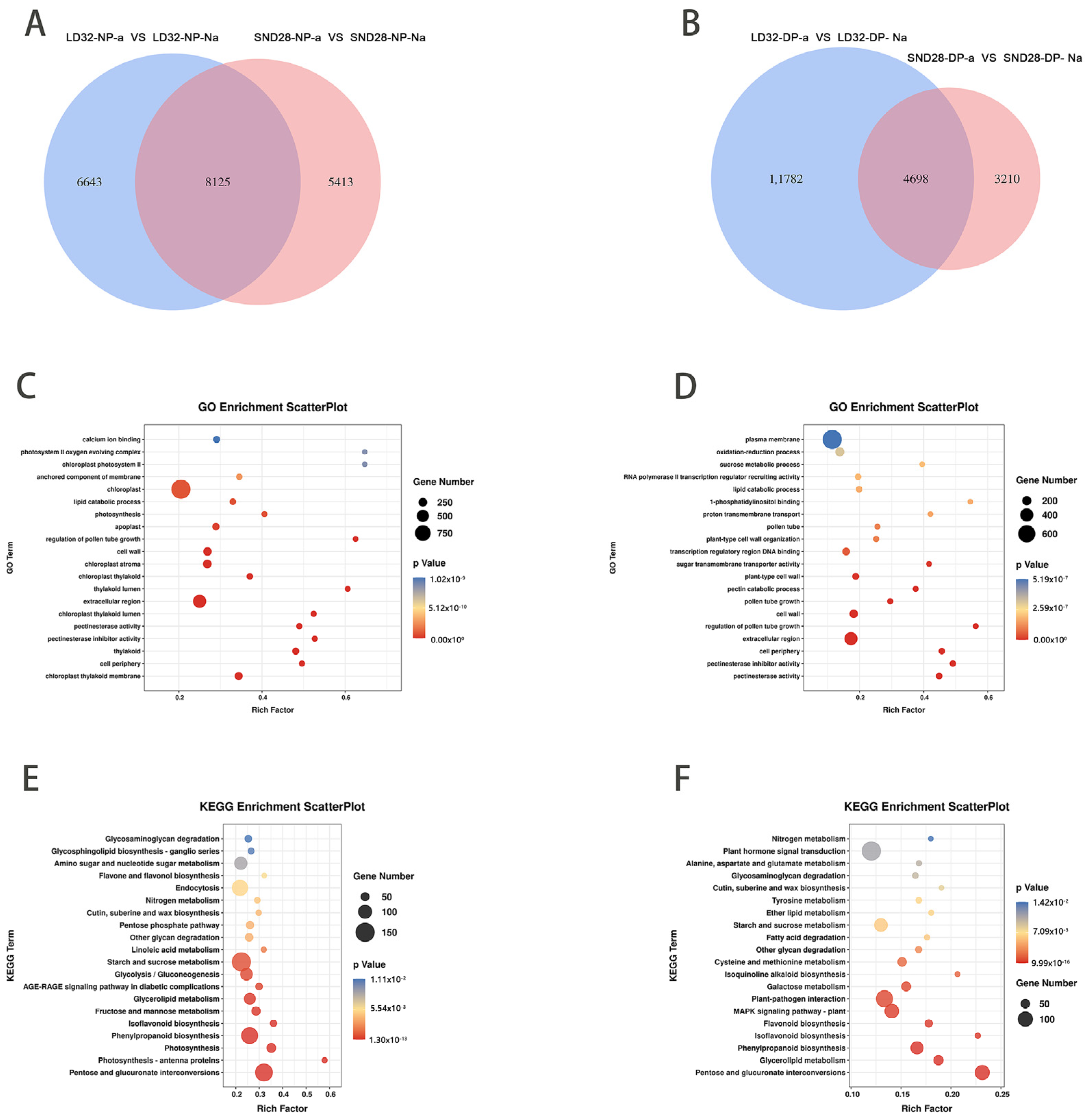
The results showed that 15,110 differentially expressed genes (DEGs) were detected between the abscised and non-abscised flowers of LD32 (LD32-NP-A vs. LD32-NP-NA), and 13,395 DEGs were detected between the abscised and non-abscised flowers of SND28 (SND28-NP-A vs. SND28-NP-NA) under normal planting densities. We then detected 15,187 DEGs between the non-abscised flowers of SND28 (SND28-NP-NA vs. SND28-DP-NA) under the two different cropping densities, 12,276 DEGs between the abscised flowers of SND 28 (SND28-NP-A vs. SND28-DP-A) under the two different cropping densities, 2359 DEGs between the abscised flowers of LD32 (LD32-NP-A vs. LD32-DP-A) under the two different cropping densities, and 6824 DEGs between the non-abscised flowers of LD32 (LD32-NP-NA vs. LD32-DP-NA) under the two different cropping densities. An analysis of the DEGs and enriched pathways of abscised and non-abscised flowers at different planting densities revealed (Figure 2A) 8125 DEGs between the abscised flowers and non-abscised flowers of SND28 (SND28-NP-A vs. SND28-NP-NA) and LD32 (LD32-NP-A vs. LD32-NP-NA) under the normal planting density, and 461 pathways that were enriched in various GO categories (Figure 2C), which mainly included sucrose transport and photosynthesis pathways. These DEGs were subjected to a KEGG analysis. The findings showed that (Figure 2E) 31 pathways were enriched in these genes, which were mainly photosynthesis, phenylpropanoid biosynthesis, photosynthesis, starch metabolism, sucrose metabolism, MAPK signaling, and zeatin biosynthesis pathways.
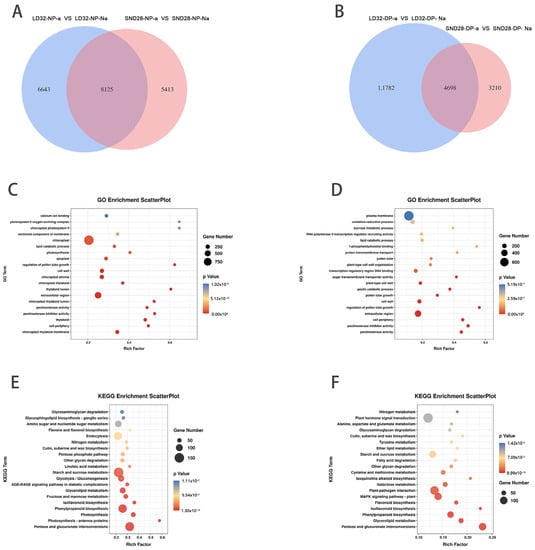
Figure 2.
Results of the transcription analysis of different groups. (A) Comparison of the number of DEGs in abscised and non-abscised flowers of SND28 (SND28-NP-A vs. SND28-NP-NA), and abscised and non-abscised flowers of LD32 (LD32-NP-A vs. LD32-NP-NA) at a normal planting density. (B) Comparison of the number of DEGs in abscised and non-abscised flowers of SND28 (SND28-DP-A vs. SND28-DP-NA), and abscised and non-abscised flowers of LD32 (LD32-DP-A vs. LD32-DP-NA) under dense planting. (C) GO enrichment bubble plots of DEGs in abscised and non-abscised flowers of SND28 (SND28-NP-A vs. SND28-NP-NA), and abscised and non-abscised flowers of LD32 (LD32-NP-A vs. LD32-NP-NA). (D) GO enrichment bubble plots of DEGs in abscised and non-abscised flowers of SND28 (SND28-DP-A vs. SND28-DP-NA) and LD32 abscised and non-abscised flowers (LD32-DP-A vs. LD32-DP-NA) under dense planting conditions. (E) KEGG enrichment bubble plots of DEGs in abscised and non-abscised flowers of SND28 (SND28-NP-A vs. SND28-NP-NA) and LD32 (LD32-NP-A vs. LD32-NP-NA). (F) KEGG enrichment bubble plots of DEGs in abscised and non-abscised flowers of SND28 (SND28-DP-A vs. SND28-DP-NA) and LD32 (LD32-DP-A vs. S16) under dense planting.
In total, 4698 DEGs were identified in the abscised versus non-abscised flowers of SND28 (SND28-DP-A vs. SND28-DP-NA) and LD32 (LD32-DP-A vs. LD32-DP-NA) under high-density planting (Figure 2B). The common DEGs were subjected to GO enrichment analysis, and 357 pathways were found to be enriched, including sucrose metabolic processes, sucrose transport, and other GO pathways (Figure 2D). These DEGs were subjected to KEGG analysis (Figure 2F), and 28 pathways were found to be enriched in these genes. They included metabolic pathways such as phenylpropanoid biosynthesis, MAPK signaling, starch and sucrose metabolism, and phytohormone pathways. The results showed that both abscised and non-abscised flowers were affected by the accumulation and metabolism of sugars under different planting densities, while the MAPK signaling pathway was equally involved in the process of flower abscission. Different planting densities affected the metabolic regulation of the photosynthetic pathway.
3.3. Pathways Associated with Flower Pod Abscission and Related Differential Genes
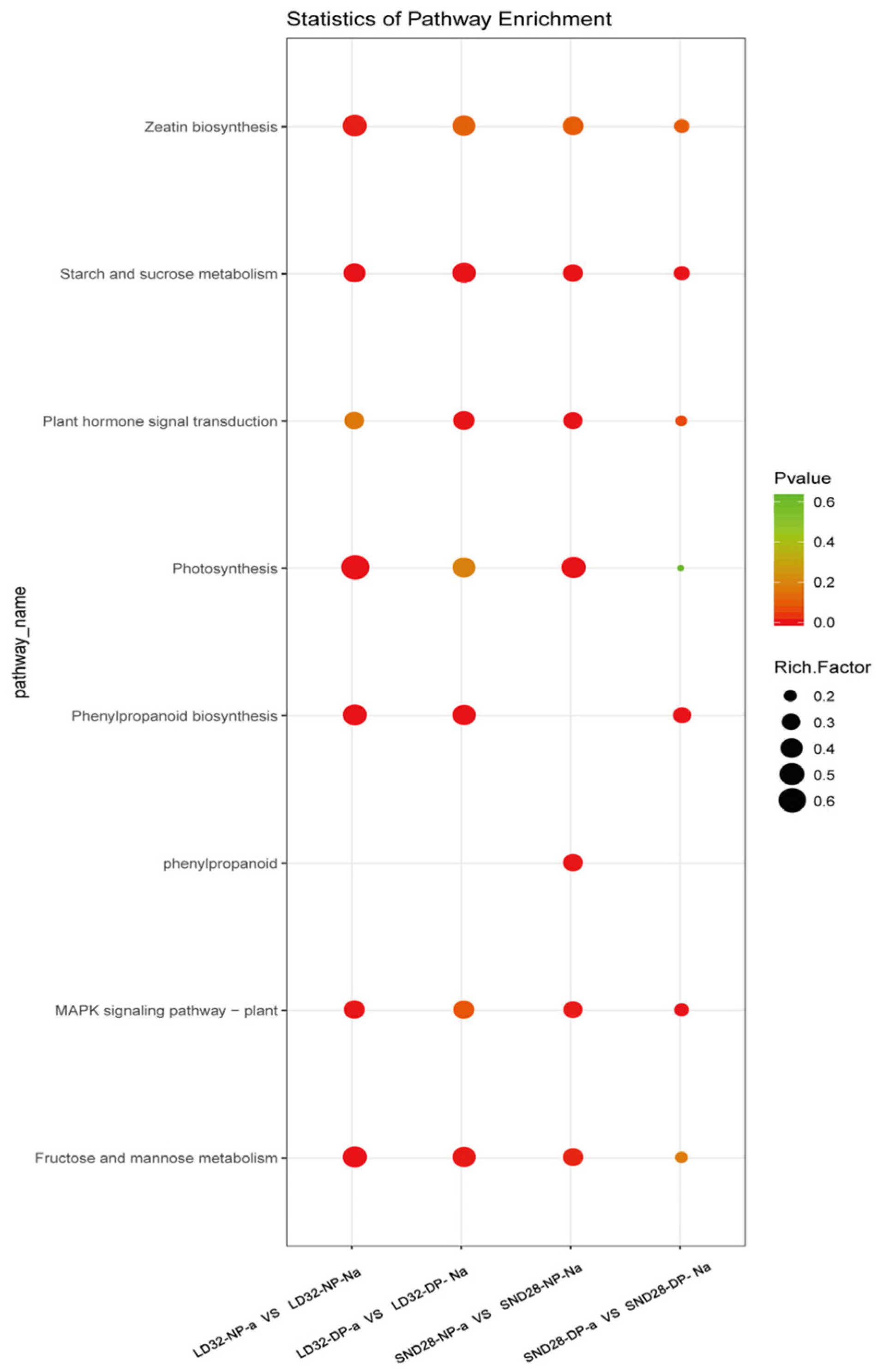
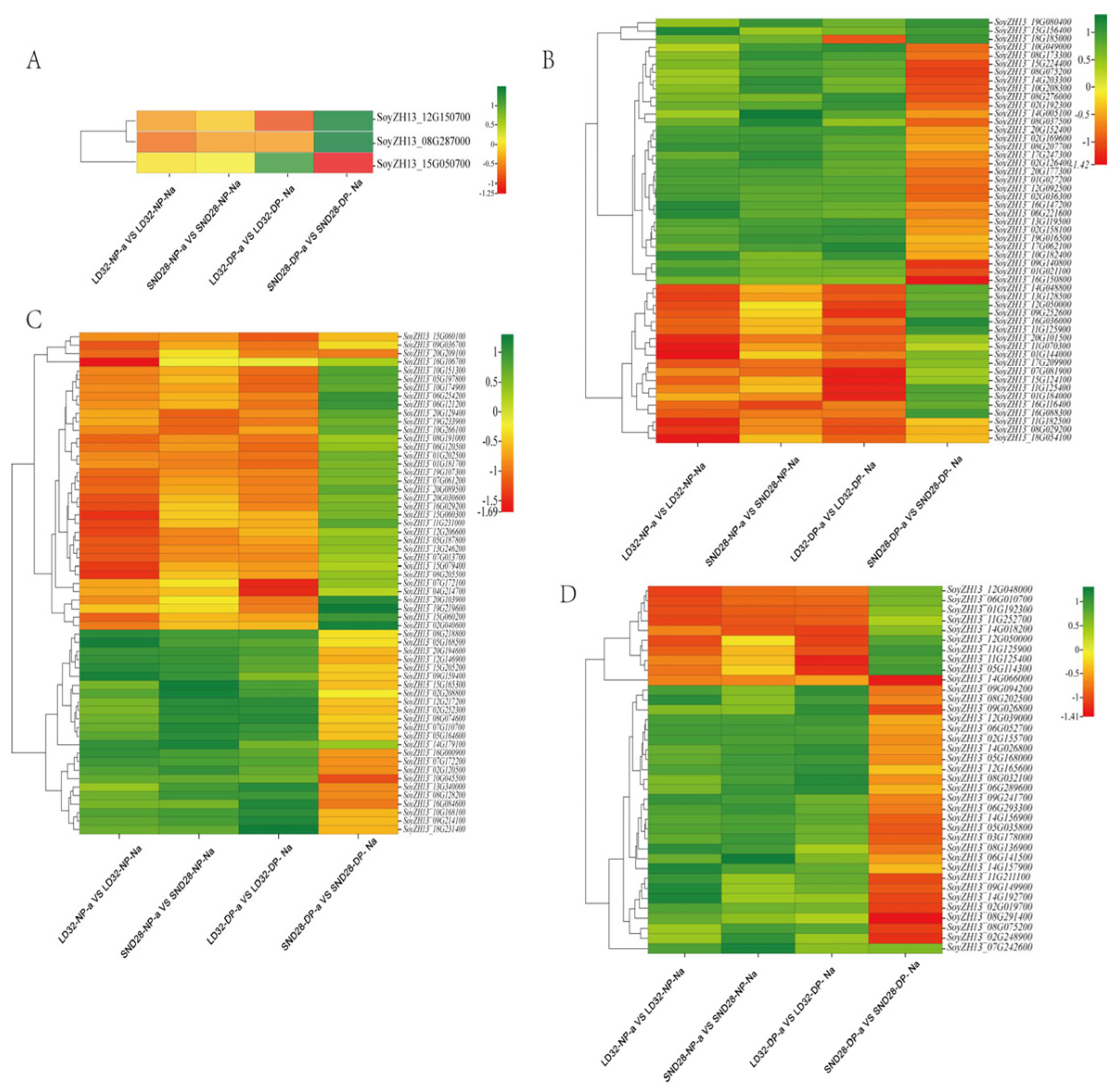
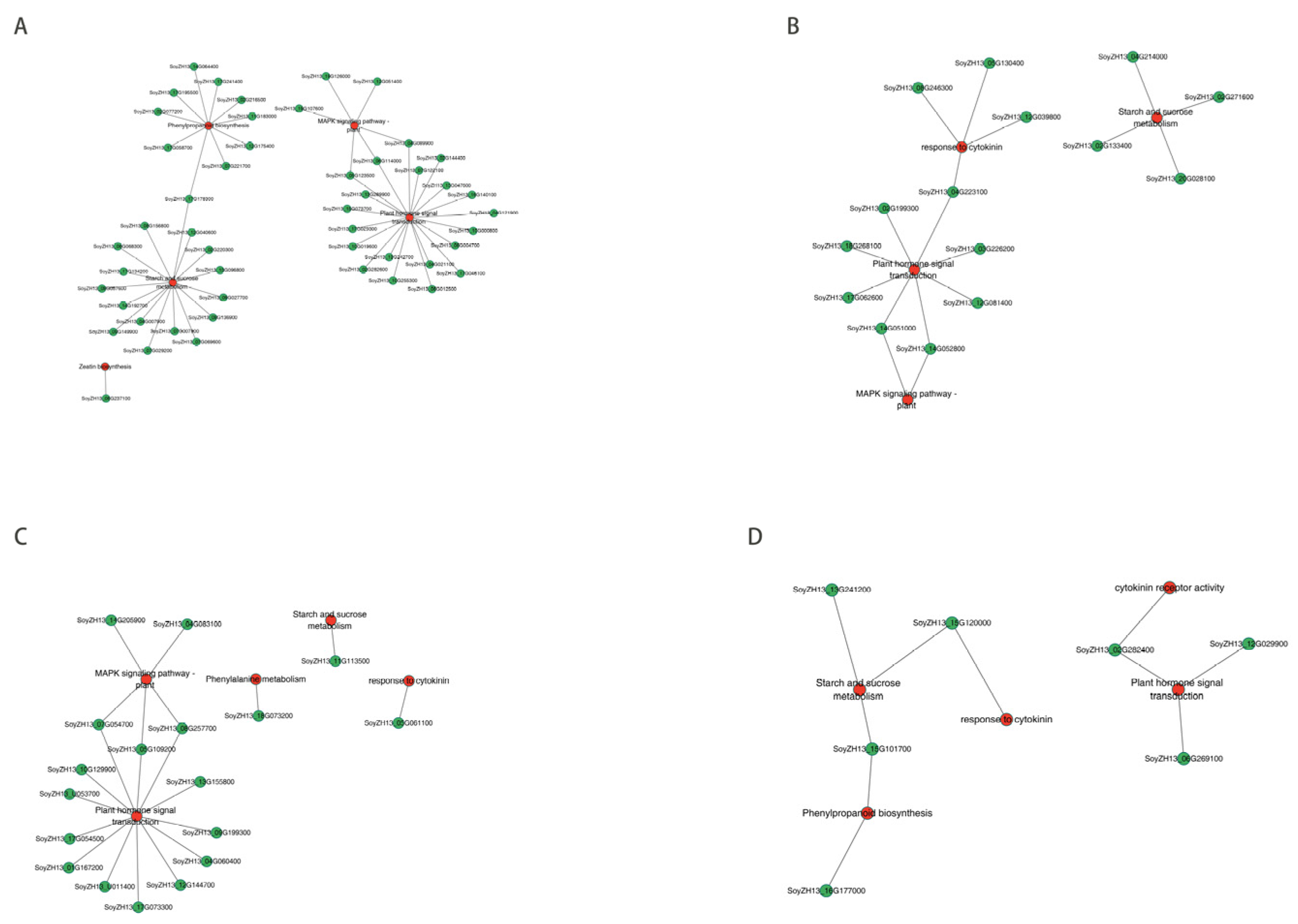
A comparative analysis of the transcriptome data from abscised and non-abscised flowers under different densities showed that the following pathways were significantly enriched in the DEGs (Figure 3): photosynthesis, sugar, and starch metabolism; MAPK signaling; and phenylalanine metabolism. Among them, three photosynthesis-related genes (Figure 4A), 51 phenylalanine metabolism-related genes (Figure 4B), 59 MAPK signaling pathway-related genes (Figure 4C), and 37 sugar and starch metabolism-related genes were identified (Figure 4D). Among the pathways enriched in terms of expression, the DEGs that were co-enriched showed the same trend of expression in all three comparison groups, i.e., LD32-NP-A vs. LD32-NP-NA, LD32-DP-A vs. LD32-DP-NA, and SND28-NP-A vs. SND28-NP-NA, and the opposite trend was observed in the SND28-DP-A vs. SND28-DP-NA comparison. These results suggested that the conventional CV SND28 exhibited a significant increase in the flower abscission rate under dense planting, which was influenced by the expression of DEGs in these pathways.
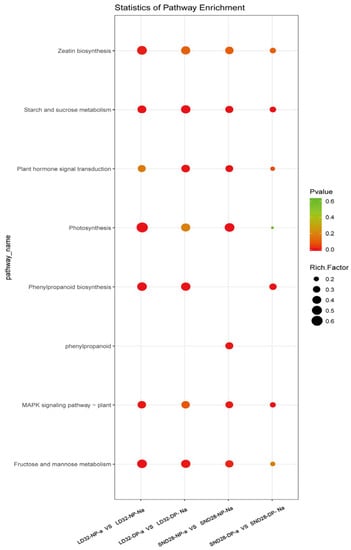
Figure 3.
Pathways associated with flower and pod abscission, and the related DEGs.
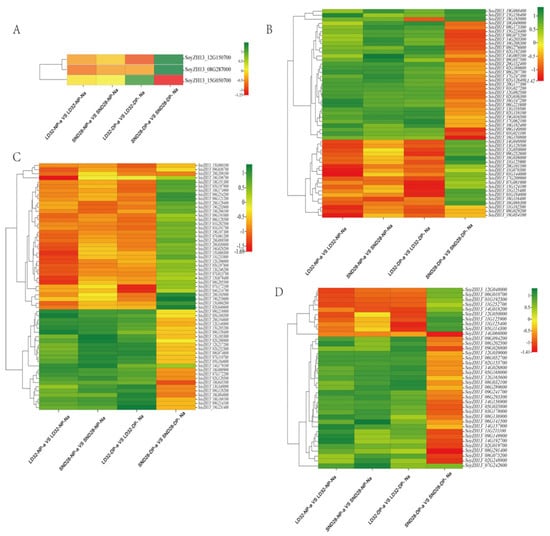
Figure 4.
Heat map analysis of common genes expressed in the common enrichment pathways in abscised and non-abscised flowers of LD32 and SND28 under different densities. (A) Heat map of the expression of three genes in the photosynthetic pathway. (B) Heat map of the expression of 51 genes in the phenylalanine metabolic pathway. (C) Heat map of the expression of 59 genes in the MAPK metabolic pathway. (D) Heat map of the expression of 37 genes in the sugar and starch metabolic pathways.
3.4. WGCNA Analysis Based on Differentially Expressed Genes
The samples were further subjected to a weighted correlation network analysis to identify the gene clusters or modules associated with the response to flower abscission in soybean under different cropping densities (Figure 5A–D). In total, 38,531 DEGs were selected across all comparison groups, and all the genes were clustered into 26 modules (Figure 5E). The maximum number of genes in the turquoise module was 24,215, and the minimum number of genes in the salmon-orange module was 42. Except for the invalid MEgrey module, the number of genes in the other modules ranged from 54 (the Metan module) to 8647 (the Meblue module). The KEGG enrichment analysis of the four modules showed that the starch and sucrose metabolism, plant hormone signal transduction, and MAPK signaling pathways, which were key components of the Meblue module, were associated with LD32-NP-A, LD32-NP-NA, LD32-DP-A, and LD32-DP-NA (Figure 6). Moreover, the MAPK signaling pathway and the zeatin biosynthesis pathways were enriched. Among the DEGs, the expression of SoyZH13_04G223100 and SoyZH13_05G130400 was downregulated in abscised and non-abscised flowers of LD32 under normal planting densities, while the expression of SoyZH13_05G130400 and SoyZH13_05G061100 was downregulated in the abscised flowers of LD32 under higher planting densities. This indicated that the responses of abscised and non-abscised flowers of CV LD32 under different planting densities were regulated by multiple metabolic pathways and DEGs.
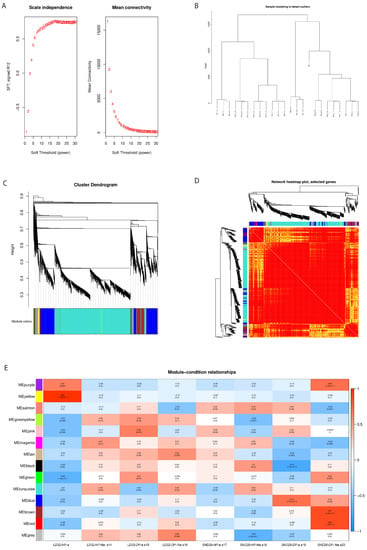
Figure 5.
WGCNA analysis. (A) Determination of the soft thresholds, where the abscissa represents the soft threshold. Left: the ordinate corresponds to the index of the scale-free network model; right: the average degree of linkage of each soft threshold. (B) Clustering dendrogram of the samples and the corresponding tissue information. The gene cluster tree is based on the Euclidean distance. (C) Gene cluster dendrograms and detection of the modules. (D) Gene expression heat map. (E) Heat map of the modules and the samples’ correlations.
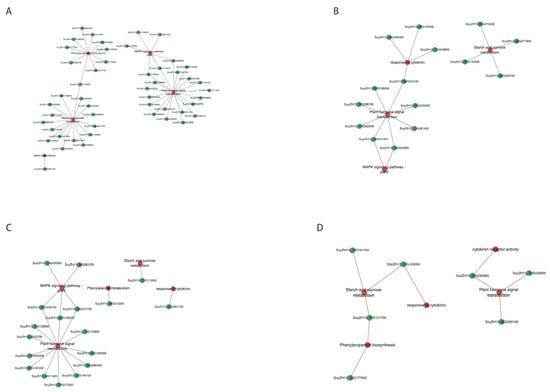
Figure 6.
WGCNA of the enriched gene co-expression network map. (A) Gene co-expression network of LD32-NP-A; (B) gene co-expression network of LD32-NP-NA; (C) gene co-expression network of LD32-DP-A; (D) gene co-expression network of LD32-DP-NA.
3.5. Metabolome Analysis
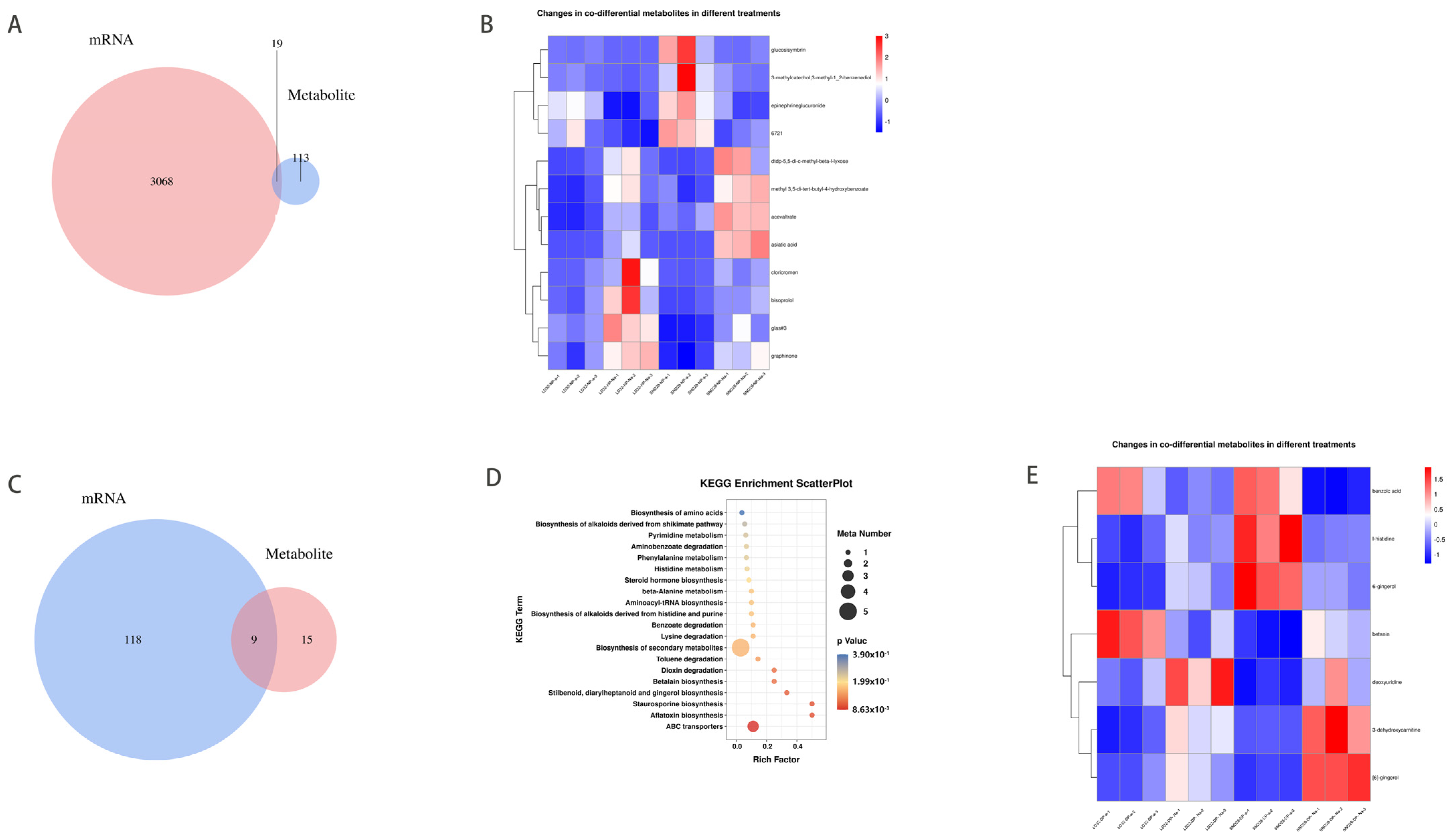
Under normal planting densities, 132 differentially expressed metabolites (DEMs) were detected in the abscised and non-abscised flowers of LD32 (LD32-NP-A vs. LD32-NP-NA), and 3087 DEMs were detected in the abscised and non-abscised flowers of SND28 (SND28-NP-A vs. SND28-NP-NA). Under the two different planting densities, 140 and 441 DEMs were detected in the abscised (LD32-NP-A vs. LD32-DP-A) and non-abscised flowers of LD32 (LD32-NP-NA vs. LD32-DP-NA), respectively, and 3161 and 1739 DEMs were detected in the abscised (SND28-NP-A vs. SND28-DP-A) and non-abscised flowers of SND28 (SND28-NP-NA vs. SND28-DP-NA), respectively. DEM and enrichment analyses were performed for the abscised and non-abscised flowers under different planting densities. Under a normal planting density, 19 DEMs were found to be common in the comparison of abscised and non-abscised flowers of SND28 (SND28-NP-A vs. SND28-NP-NA), and of abscised and non-abscised flowers of LD32 (LD32-NP-A vs. LD32-NP-NA) (Figure 7A). However, these 19 DEMs were not enriched in the relevant metabolic pathways, and their specific functions are unknown. The list of these 19 DEMs can be found in Supplemental Table S3. Additionally, we compared 12 metabolites with metabolic significance among the 19 common DEMs (Figure 7B). Under dense planting, 95 DEMs (Figure 7C) were common to the abscised and non-abscised flowers of SND28 (SND28-DP-A vs. SND28-DP-NA) and LD32 (LD32-DP-A vs. LD32-DP-NA), and these DEMs were enriched together with ABC transporters, aflatoxin biosynthesis, and staurosporine biosynthesis, as well as 25 other metabolic pathways (Figure 7D). The list of these 95 DEMs can be found in Supplemental Table S4. Additionally, we screened eight DEMs involved in the metabolic pathway from 51 metabolites for a comparison (Figure 7E). Therefore, these DEMs and enrichment pathways were involved in the regulation of flowering in soybean.
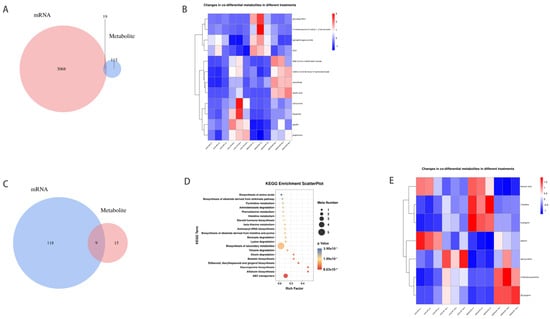
Figure 7.
Heat map of the number of common DEMs and DEGs in different groups. (A) Comparison of the number of DEMs in abscised and non-abscised flowers of SND28 (SND28−NP−A vs.SND28−NP−NA), and abscised and non-abscised flowers of LD32 (LD32−NP−A vs. LD32−NP−NA) at a normal planting density. (B) Comparison of common DEMs in abscised and non-abscised flowers of SND28 (SND28−NP−A vs. SND28−NP−NA), and abscised and non-abscised flowers of LD32 (LD32−NP−A vs. LD32−NP−NA) at a normal planting density in the heat map. (C) Comparison of the differential amounts of DEMs in the abscised and non-abscised flowers of SND28 (SND28−DP−A vs. SND28−DP−NA), and the abscised and non-abscised flowers of LD32 (LD32−DP−A vs. LD32−DP−NA) under dense planting. (D) Comparison of the differential amounts of metabolites in the abscised and non-abscised flowers of SND28 (SND28−DP−A vs. SND28−DP−NA), and the abscised flowers of LD32 (LD32−DP−A vs. LD32−DP−NA) under dense planting. KEGG enrichment bubble plots of DEMs in abscised and non-abscised flowers (LD32−DP−A vs. LD32−DP−NA). (E) Heat map of common DEMs in abscised and non-abscised flowers (SND28−DP−A vs. SND28−DP−NA) of SND28 under dense planting versus abscised and non-abscised flowers (LD32−DP−A vs. LD32−DP−NA) of LD32 under dense planting.
3.6. Integrative Analysis of DEGs and Differential Metabolites
3.6.1. Integrative Analysis of DEGs and Differential Metabolites under Different Planting Density Conditions
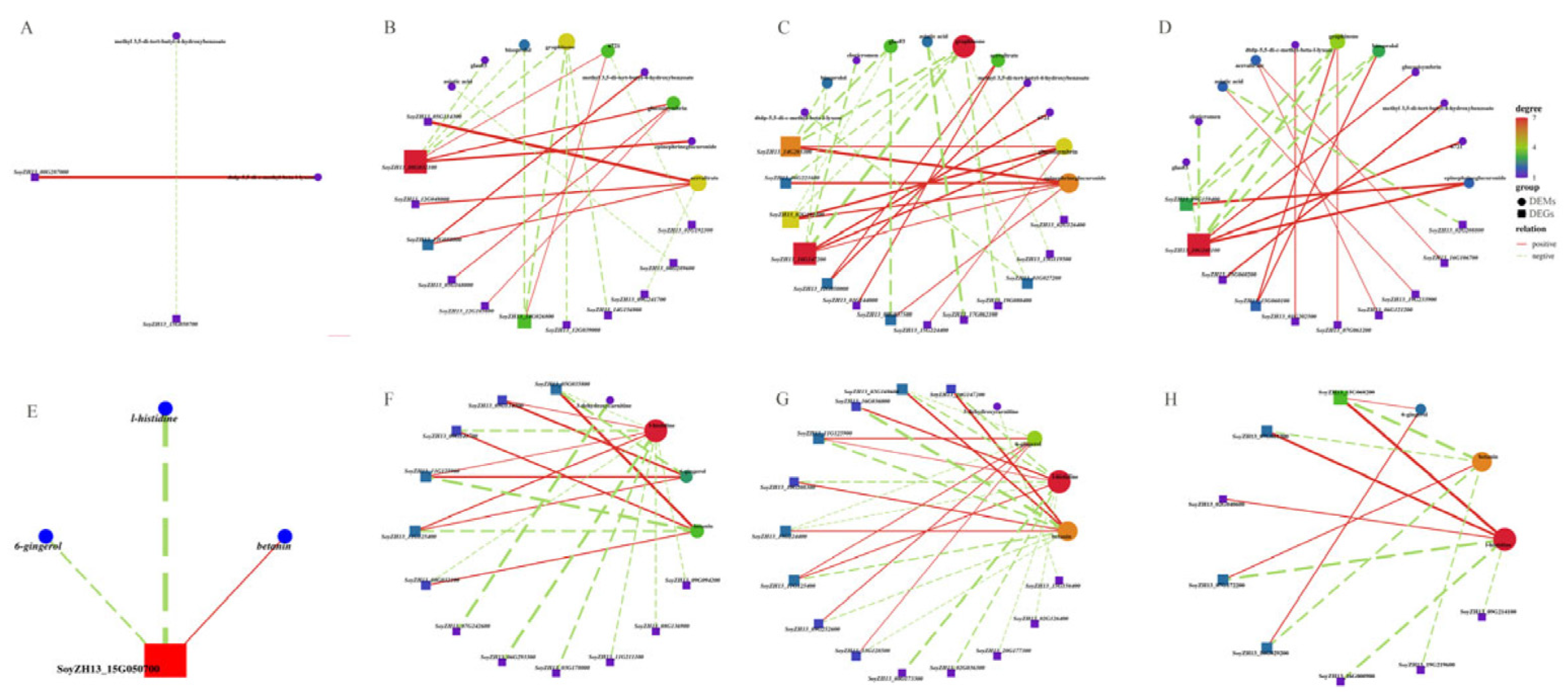
Further investigations with correlation network plots (|r| >0.90) were performed for the common DEGs and DEMs in the abscised and non-abscised flowers of SND28 (SND28-NP-A vs. SND28-NP-NA) and LD32 (LD32-NP-A vs. LD32-NP-NA) under normal planting densities, and those of SND28 (SND28-DP-A vs. SND28-DP-NA) and LD32 (LD32-DP-A vs. LD32-DP-NA) under dense planting. The correlation analysis showed that the DEGs in the photosynthesis pathways (Figure 8A,E), sugar and starch metabolic pathways (Figure 8B,F), the phenylalanine pathways (Figure 8C,G), and the MAPK signaling pathways (Figure 8D,H) play direct or indirect regulatory roles in the metabolism of key DEMs in LD32 and SND28 under different planting densities.
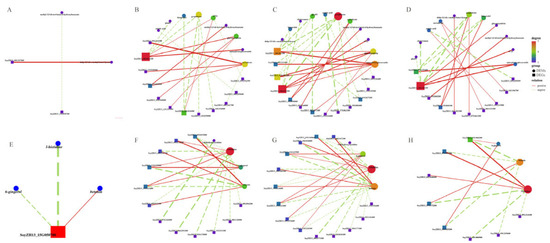
Figure 8.
Network diagram of the correlations of common DEGs and metabolites in different groups. (A) Correlation network diagram of common DEGs and DEMs in the photosynthetic pathway of the abscised and non-abscised flowers of SND28 (SND28-NP-A vs. SND28-NP-NA) and LD32 (LD32-NP-A vs. LD32-NP-NA) under normal planting densities. (B) Correlation network diagram of common DEGs and DEMs in the sugar and starch metabolism pathways of the abscised and non-abscised flowers of SND28 (SND28-NP-A vs. SND28-NP-NA) and LD32 (LD32-NP-A vs. LD32-NP-NA) under normal planting densities. (C) Correlation network diagram of common DEGs and DEMs in the phenylalanine pathways of the abscised and non-abscised flowers of SND28 (SND28-NP-A vs. SND28-NP-NA) and LD32 (LD32-NP-A vs. LD32-NP-NA) under normal planting densities. (D) Correlation network diagram of common DEGs and DEMs in the MAPK signaling pathways of the abscised and non-abscised flowers of SND28 (SND28-NP-A vs. SND28-NP-NA) and LD32 (LD32-NP-A vs. LD32-NP-NA) under normal planting densities. (E) Correlation network diagram of common DEGs and DEMs in the photosynthetic pathway of the abscised and non-abscised flowers of SND28 (SND28-DP-A vs. SND28-DP-NA) and LD32 (LD32-DP-A vs. LD32-DP-NA) under dense planting. (F) Correlation network diagram of common DEGs and DEMs in the sugar and starch metabolism pathways of the abscised and non-abscised flowers of SND28 (SND28-DP-A vs. SND28-DP-NA) and LD32 (LD32-DP-A vs. LD32-DP-NA) under dense planting. (G) Correlation network diagram of common DEGs and DEMs in the phenylalanine pathways of the abscised and non-abscised flowers of SND28 (SND28-DP-A vs. SND28-DP-NA) and LD32 (LD32-DP-A vs. LD32-DP-NA) under dense planting. (H) Correlation network diagram of common DEGs and DEMs in the MAPK signaling pathways of the abscised and non-abscised flowers of SND28 (SND28-DP-A vs. SND28-DP-NA) and LD32 (LD32-DP-A vs. LD32-DP-NA) under dense planting.
3.6.2. Integrative Analysis of DEGs and DEMs of Non-Abscised Flowers of the Two Varieties under Different Planting Densities
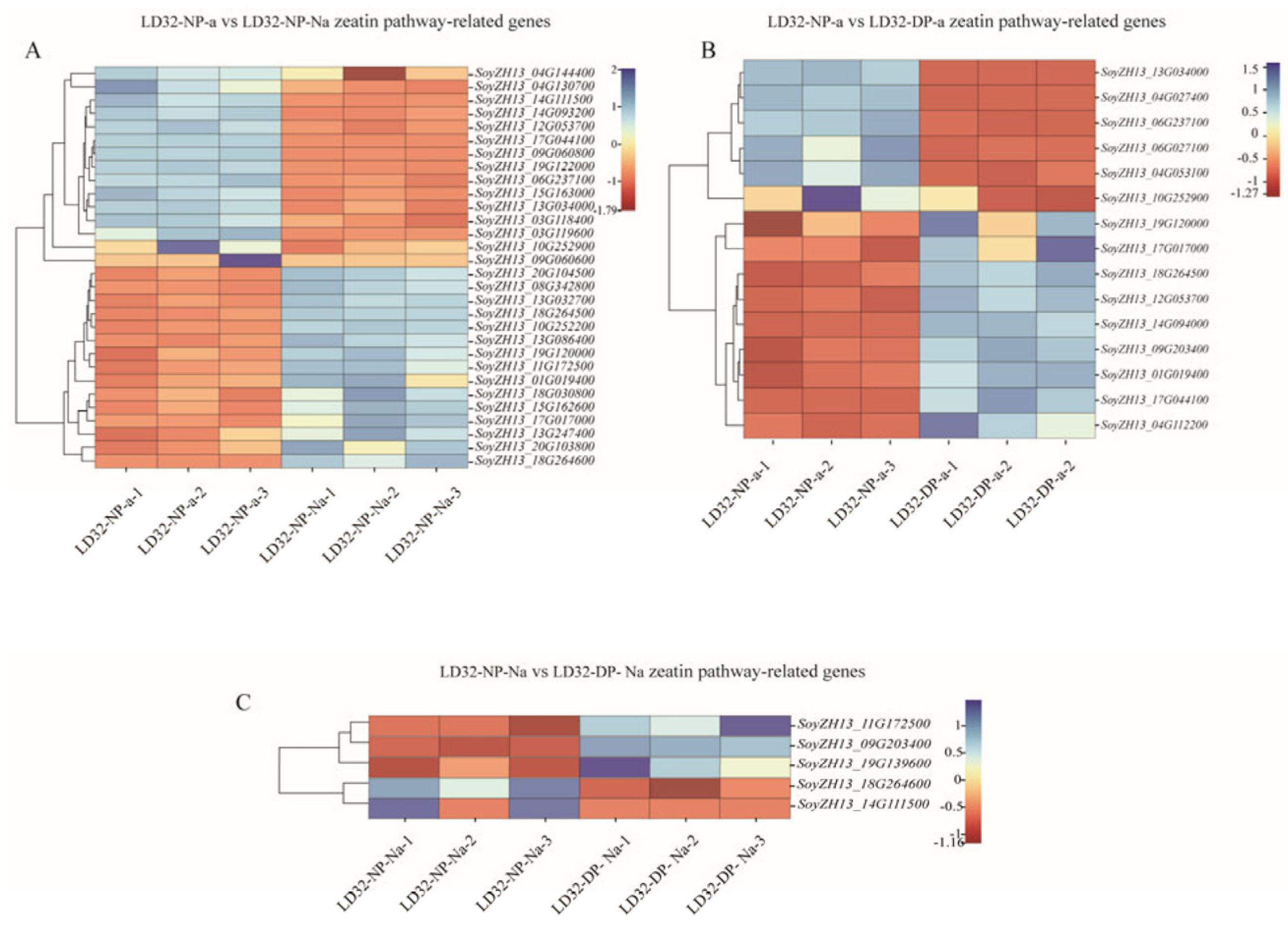
Interestingly, LD32 was significantly enriched in the zeatin biosynthesis pathway in the comparisons (LD32-NP-A vs. LD32-NP-NA and LD32-NP-A vs. LD32-DP-A) compared with SND28. Under normal planting densities, 30 DEGs between abscised flowers and non-abscised flowers of LD32 (LD32-NP-A vs. LD32-NP-NA) (Figure 9A) were observed in the zeatin biosynthesis pathway, including 15 upregulated and 15 downregulated genes. Moreover, 10 of the 30 DEGs of the cytokinin hydroxylase family were differentially expressed in the zeatin biosynthesis pathway and four were upregulated. In addition, abscised flowers of LD32 had 15 DEGs (Figure 9B) in the zeatin biosynthesis pathway under the two planting densities (LD32-NP-A vs. LD32-DP-A); six were up-regulated and nine were downregulated, while 8 of the 15 DEGs in the cytokinin hydroxylase family were downregulated. The zeatin biosynthesis pathway likely plays a key role in the development of floral organs in LD32 plants under different planting densities.
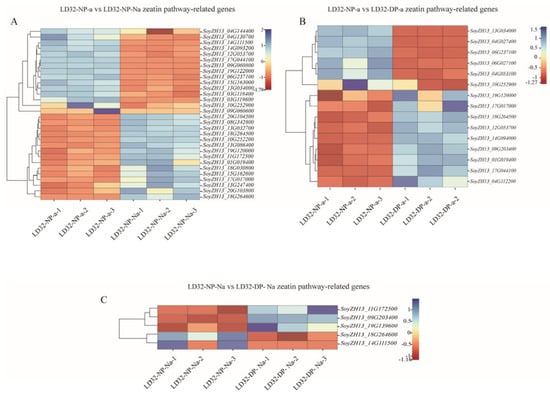
Figure 9.
Heat map of DEGs of LD32 enriched in the zeatin pathway. (A) Heat map analysis of DEGs between LD32−NP−A and LD32−NP−NA in the zeatin pathway. (B) Heat map of DEGs between LD32−NP−A and LD32−DP−A in the zeatin pathway. (C) Heat map of DEGs between LD32−NP−NA and LD32−DP−NA in the zeatin pathway.
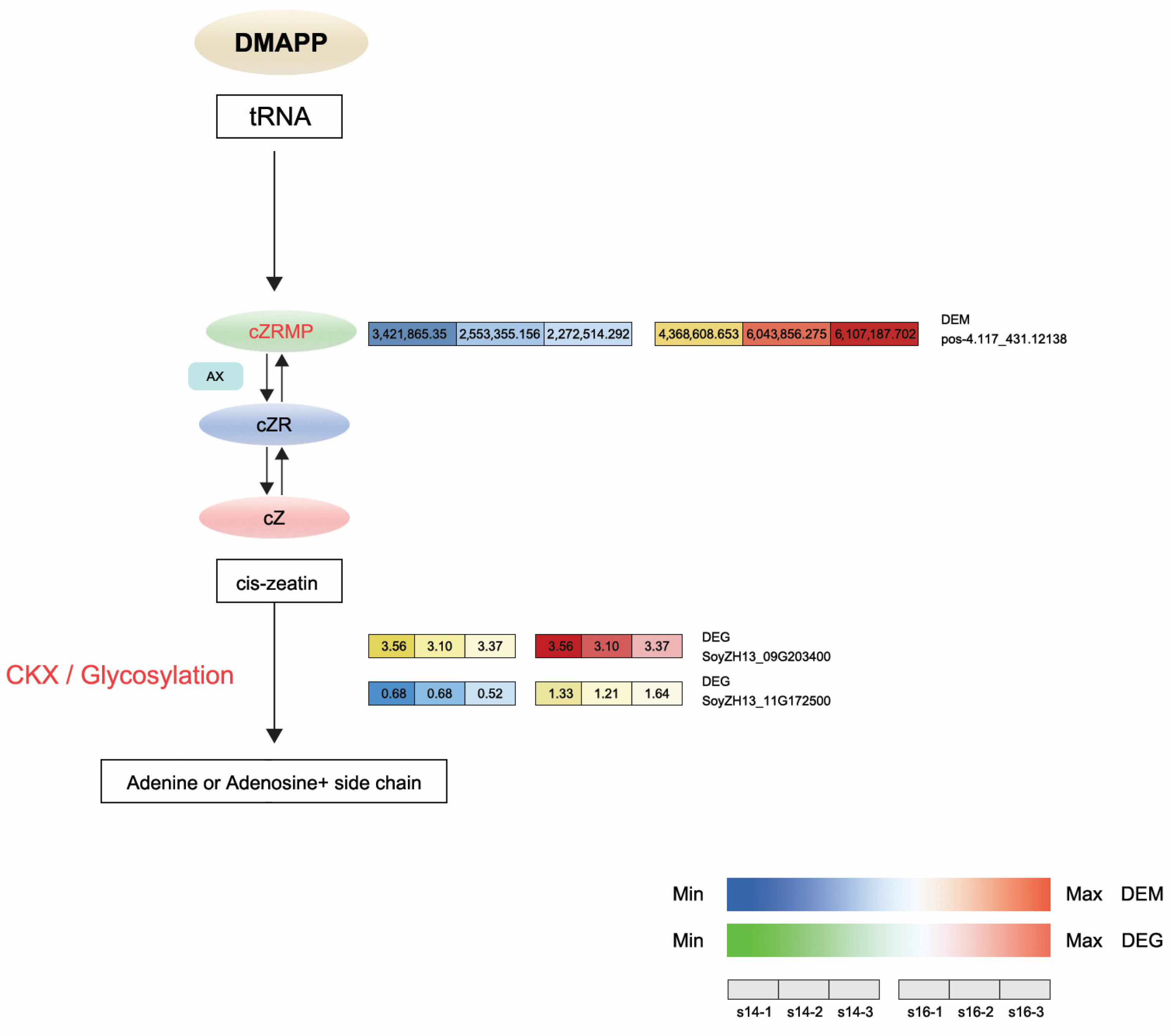
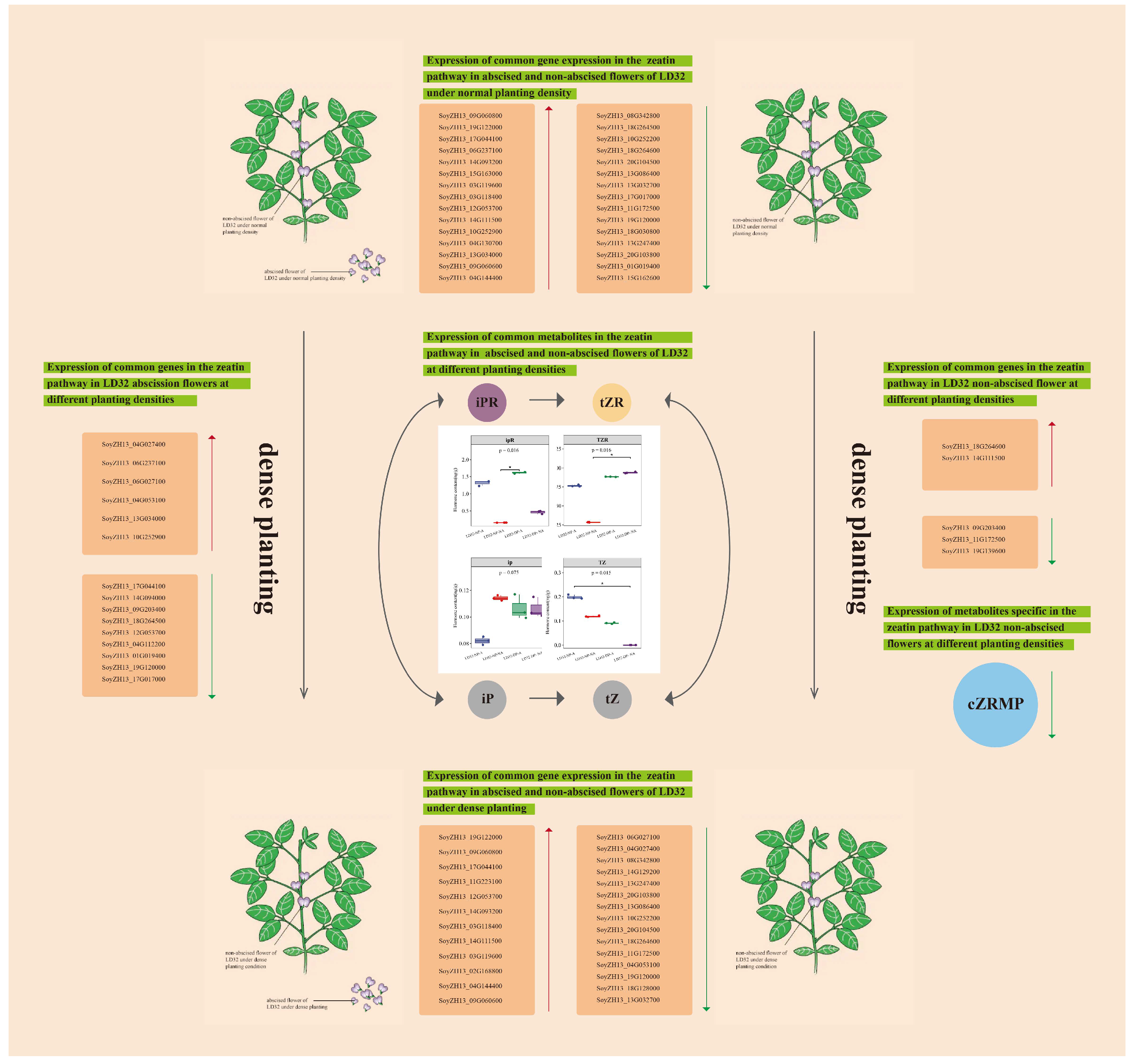
In the DEM analysis, the levels of cis-zeatin riboside monophosphate (CZRM) detected in LD32 non-abscised flowers under dense planting were significantly higher than in non-abscised flowers under normal conditions. In the zeatin biosynthesis pathway, cis-zeatin riboside monophosphate (CZRM) induces the phosphorylation of the riboside cZR through the enzyme adenosine kinase (AK). Furthermore, CZRM was converted to cis-zeatin (cZ) by the enzymes cytokinin phosphoribosyl hydrolase (LOG) and cytokinin oxidase/dehydrogenase (CKX), leading to the breakdown of the free base cZ into adenine or adenosine. The differential gene cytokinin dehydrogenase 6 and the differential metabolite CZRM were downregulated in non-abscised flowers of LD32 under both normal densities and dense planting (LD32-NP-NA vs. LD32-DP-NA). This indicated that the levels of CKX6 (SoyZH13_09G203400 and SoyZH13_11G172500) (Figure 9C) and CZRM were higher in normal flowers of LD32 under dense planting than under the normal planting density. The content in non-abscised flowers of LD32 was higher than that in abscised flowers of LD32 under normal planting densities. Changes in the number of non-abscised flowers of LD32 under dense planting and the lack of significant differences in the number of non-abscised flowers of LD32 under the normal planting density were hypothesized to be related to the co-regulation of CKX6 and CZRM in the zeatin biosynthesis pathway (Figure 10).
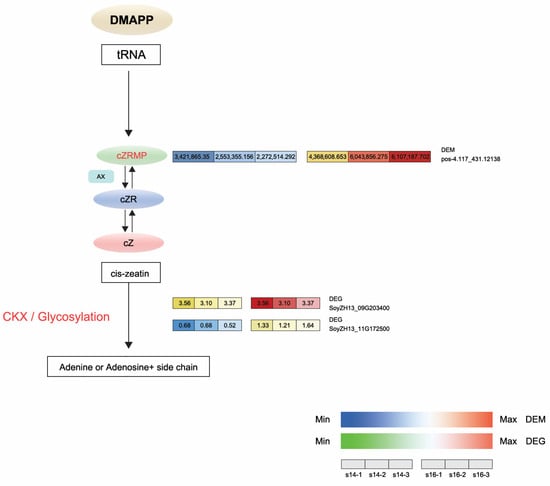
Figure 10.
Expression profiles of DEGs and DEMs associated with the biosynthesis and metabolism of zeatin in the comparison between LD32-NP-NA and LD32-DP-NA. cZ, cis-zeatin; DMAPP, dimethylallyl pyrophosphate; AK, adenosine kinase causing the phosphorylation of riboside cZR; CKX, cytokinin oxidase/dehydrogenase leading to the free base of cZ to adenine or adenosine catabolism.
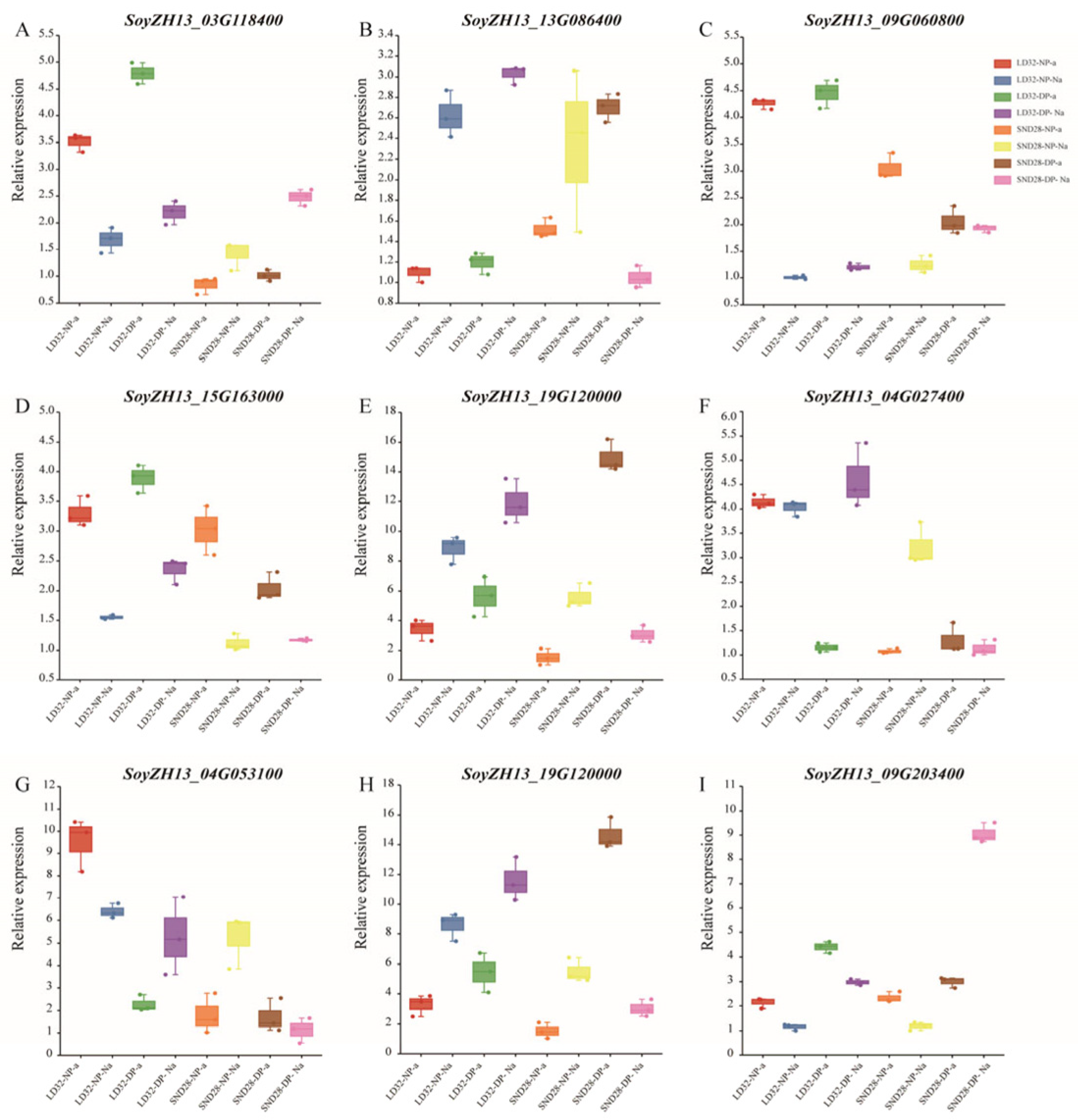
3.7. Validation of DEGs by qRT-PCR Analysis
The expression levels of some DEGs were assessed using qRT-PCR to verify the reliability of the RNA-Seq data (Figure 11). We found that DEGs in each comparison showed the same trend in the RNA-Seq and qRT-PCR results, indicating that the RNA-Seq data were reliable.
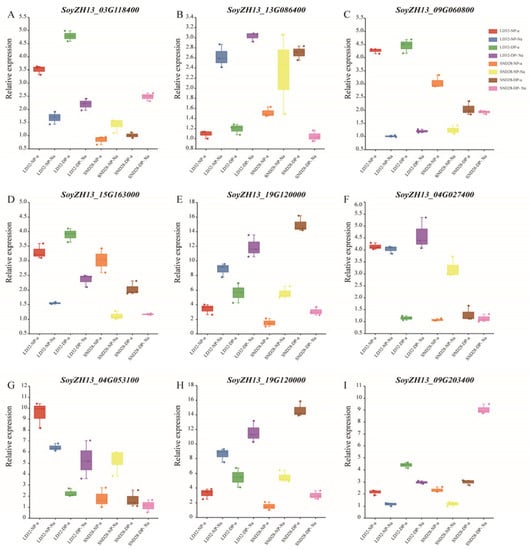
Figure 11.
Validation of DEGs in different comparisons by qRT-PCR. (A) SoyZH13_03G118400 expression levels in different comparisons. (B) SoyZH13_13G086400 expression levels in different comparisons. (C) SoyZH13_09G060800 expression levels in different comparisons. (D) SoyZH13_15G163000 expression levels in different comparisons. (E) SoyZH13_19G120000 expression levels in different comparisons. (F) SoyZH13_04G027400 expression levels in different comparisons. (G) SoyZH13_04G053100 expression levels in different comparisons. (H) SoyZH13_19G120000 expression levels in different comparisons. (I) SoyZH13_09G203400 expression levels in different comparisons.
3.8. HPLC-MS/MS Quantification of LD32 Floral Organ Hormones
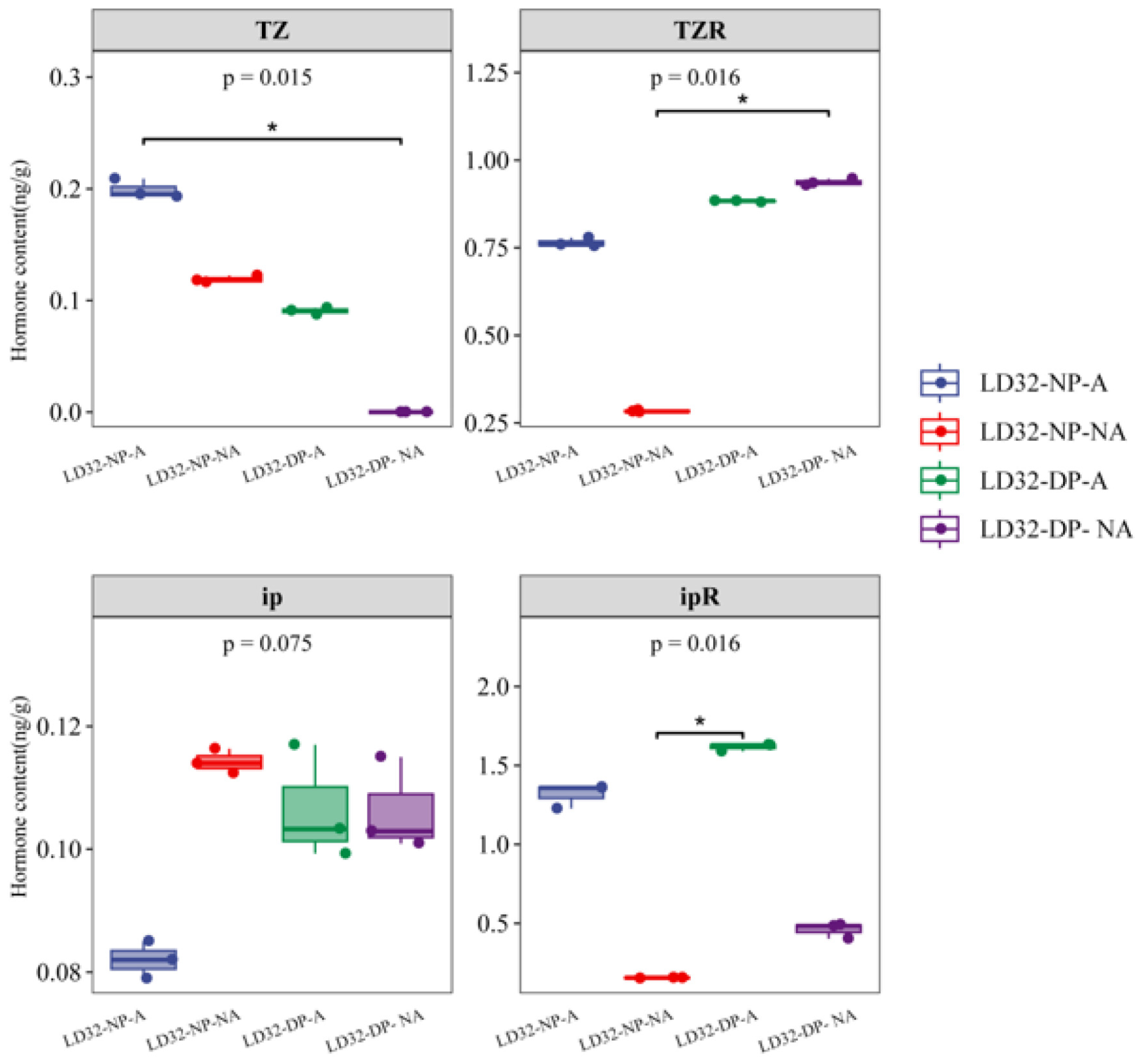
To further verify why LD32 did not experience a significant reduction in flower abscission under dense planting conditions, the expression levels of different hormones in the abscised and non-abscised flowers of LD32 under different planting densities were determined by UPLC-Orbitrap-MS (UPLC, Vanquish; MS, QE) (Figure 12). Abscised flowers of LD32 under the normal planting density had the highest levels of trans-zeatin, and the N6-(delta2-isopentenyl) adenine level was higher than that in non-abscised flowers under the normal planting density, abscised flowers under dense planting, and non-abscised flowers under the normal planting density. The increased content of these hormones is responsible for the non-significant reduction in flower abscission in LD32 under dense planting. Combined with the pre-transcriptome data, the change in the hormone content appeared to be related to the downregulated expression of Cytokinin dehydrogenase 6 (CKX6) in both abscised and non-abscised flowers of LD32.
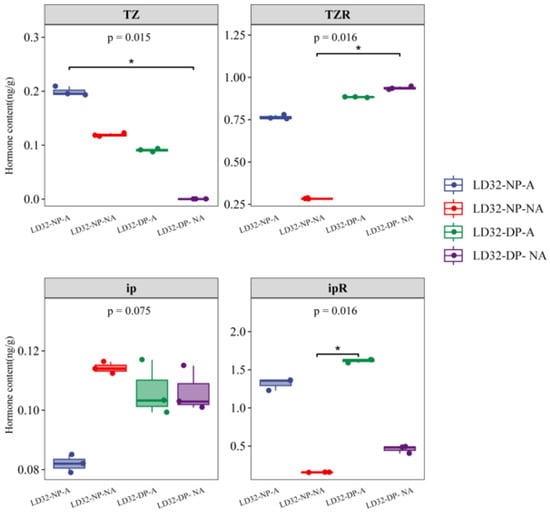
Figure 12.
Determination of the hormone content in abscised and non-abscised flowers of LD32 under different planting densities. * indicate p < 0.05.
4. Discussion
The abscission/non-abscission of flowers strongly depends on the availability of carbohydrates (source), hormones, photosynthesis, and other factors for their development and growth. Denser planting conditions would cause some change in the availability and supply. The effect of density on the agronomic traits and yield (Table S5) of the two soybean varieties showed that at crop maturity, the aboveground dry matter mass and harvest index of SND28 decreased significantly under high-density planting, while the harvest index of LD32 did not change significantly. Under the normal planting density, there was no significant difference in the yield per plant between the two cultivars, but under dense planting, the yield per plant decreased significantly less in LD32 than in SND28. These results indicated that the performance of SND28 was more erratic under high-density planting, whereas LD32 is a density-tolerant variety that can be used for the selection and breeding of densely planted high-yielding soybean varieties.
Increasing the planting density affects the crop’s photosynthetic utilization, while inhibiting photosynthesis increases the abscission of flowers and fruit [31]. The changes in the expression of DEGs in the photosynthetic pathway observed in this study may be an important reason for the increased flower abscission in the conventional variety SND28 under dense planting. The DEGs enriched in the photosynthetic pathways included SoyZH13_15G050700, SoyZH13_08G287000, and SoyZH13_12G150700, which are all regulatory genes in the chloroplasts associated with repair or other functions [32]. Sugar acts as a signal for the transition from nutritional to reproductive growth [33]. Changes in DEGs in the sugar and starch metabolic pathways in this experiment may also serve as a signal that affects the abscission of soybean flowers. The DEGs SoyZH13_09G149900, SoyZH13_11G211100, and SoyZH13_14G192700 were enriched in the sugar and starch metabolic pathways belonging to the sucrose synthase family. Sucrose synthase (SuSy, SS) is a key enzyme that regulates sucrose metabolism [34], and can regulate sucrose catabolism while affecting the shedding of various reproductive organs of the crop [35]. Thus, the differential expression of genes related to sugar and starch metabolism pathways caused changes in sugar and starch in flowers that led to changes in this metabolic pathway, thereby causing flower abscission in soybean. Further in-depth studies on the roles of photosynthesis, and sugar and starch metabolism in soybean flower abscission are necessary. LD32 did not show significant changes in the number of abscised and non-abscised flowers, or in the abscission rates under different planting densities. Therefore, WGCNA was used to mine the modules related to the sample’s characteristics, identify relevant candidate genes on the basis of the enrichment pathways, and indirectly mine for genes that regulated abscission only in LD32. Four modules (MEyellow, MEturquoise, MEmagenta, MEtan, and MEpink) were associated with phytohormone signaling (SoyZH13_04G223100, SoyZH13_05G13040, SoyZH13_05G130400, and SoyZH13_05G061100). These genes include the two-component response regulator ARR9 isoform B (Glycine soja), serine/threonine-protein kinase CTR1, sucrose synthase, and the abscisic acid receptor PYL4. It has been shown that IDA, MAPK, and abscisic acid are involved in organ abscission through the regulation of cell wall hydrolases in delaminated zones [36,37]. Genes encoding sucrose synthase, MAPK, and the abscisic acid receptor played key roles in regulating LD32 flower abscission.
Cytokinin plays critical roles in plants’ growth and development, including cell division and differentiation, senescence, leaf abscission, and responses to biotic and abiotic stresses [38]. Cytokinin oxidase/dehydrogenase (CKX) catalyzes the irreversible degradation of the cytokinin isopentenyl adenine, zeatin, and its riboside in a single enzymatic step through oxidative side chain cleavage, and it is the only enzyme that has been clearly shown to catalyze the catabolism of specific cytokinins [39]. Metabolomic analysis showed that dense planting led to a reduction in CZRM. Directed hormone assays revealed that LD32 showed higher levels of trans-zeatin and N6-(delta2-isopentenyl) adenine in the abscised flowers under the normal planting density than in the non-abscised flowers under the normal planting density, abscised flowers under dense planting, and non-abscised flowers under dense planting. Transcriptome analysis also revealed that CKX family genes (cytokinin oxidase/dehydrogenase) were differentially altered in abscised and non-abscised flowers of LD32 under different planting densities; moreover, the content of cytokinin dehydrogenase 6 (CKX6) was higher in abscised flowers of LD32 than in non-abscised flowers under the normal planting density. The expression of CKX6 increased in both the abscised and non-abscised flowers of LD32 under dense planting, indicating that CKX6 was upregulated in non-abscised flowers and that lower cytokinin concentrations may be more favorable for the development of non-abscised flowers in LD32 under dense planting. Changes in the activity of CKX altered the tissue concentrations of cytokinin, as suggested by Motoyuki Ashikari et al. [40], who showed that the expression of OsCKX2 in meristematic tissues of inflorescences regulated cytokinin levels and thus controlled flower numbers. Changes in the content of CZRM, trans-zeatin, N6-(delta2-isopentenyl) adenine, and the degradation gene CKX were all associated with a decrease in cytokinin. Bartrina et al. [35] showed that CKX regulated cytokinin levels in SAM and directly controlled the number of reproductive organs and grains.
5. Conclusions
In this study, two soybean cultivars, LD32 (a density-tolerant variety) and SND28 (a conventional cultivar), were compared using transcriptomic and metabolomic analyses. According to the results, the conventional CV SND28 exhibited a significant increase in the flower abscission rate, while the tolerant CV LD32 did not show a similar trend under dense planting conditions, which was probably due to the influence of differentially expressed genes in the photosynthesis, sugar, and starch metabolism, MAPK signaling, and phenylalanine metabolism pathways. Furthermore, the results of the joint analysis showed that CKX6 (cytokinin dehydrogenase 6) and CZRM (cis-zeatin riboside monophosphate) were enriched in the zeatin biosynthesis pathway in normal flowers of LD32 under dense planting, and showed downregulated expressions compared with the control. This suggested that the simultaneous expression of DEGs and DEMs resulted in the more stable performance of non-abscised flowers of LD32 under dense planting, and they were involved in regulating the flowering mechanism in soybean under dense planting. In line with the results above, we proposed a hypothetical model to illustrate the regulation pattern of non-abscised and abscised flowers of LD32 under different planting densities (Figure 13). Under different planting densities, abscised and non-abscised flowers of LD32 shared common DEMs (iPR, tZR, iP, and tZ) in the zeatin pathway, but differed in the content of the common metabolites as influenced by the expression of different DEGs. In addition, CZRMP, which was unique to LD32-NP-NA and LD32-DP-NA, may be related to the expression of CZRMP by downregulation with CKX6 (SoyZH13_09G203400, SoyZH13_11G172500, and SoyZH13_19G139600). This suggested that the expression of related DEGs and DEMs in the zeatin synthesis pathway may be the main factor influencing both the insignificant reduction in the flowering rate and the more stable performance of LD32 under high density. However, the specific regulatory mechanism remains to be studied further.
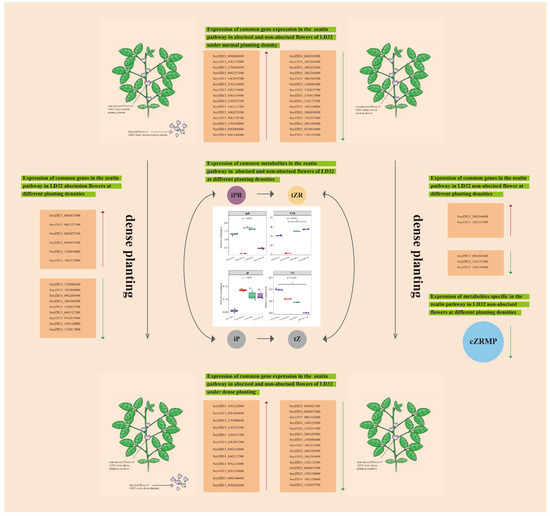
Figure 13.
The proposed model affecting the difference in non-abscised and abscised flower of LD32 under different planting densities. Red arrows represent upregulation, green arrows represent downregulation, and the black arrow represents promotion. * indicate p < 0.05.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agronomy13102561/s1, Table S1: Determination of planting density; Table S2: Fluorescence quantitative primer sequence; Table S3: Detailed information on 19 common DEMs in SND28 and LD32 under normal planting density; Table S4: Detailed information on 95 common DEMs in SND28 and LD32 Under dense planting; Table S5: The effect of density on the agronomic traits and yield.
Author Contributions
N.W. investigation, data curation, validation, and writing—original draft; D.H. investigation and formal analysis; H.S. investigation; X.Y. writing—review and editing; F.X. conceptualization, writing—review, editing, and funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Key R&D Program of China (2021YFD1201102-01).
Data Availability Statement
All sequencing reads are available in NCBI SRA: PRJNA1009706. Metabolomics data have been deposited to the EMBL-EBI MetaboLights database (DOI: 10.1093/nar/gkz1019, PMID:31691833) with the identifier MTBLS8587.
Acknowledgments
We are grateful to the National Key Research and Development Program project for supporting our high-yield and high-quality specialty soybean resources and material preparation. We thank the Soybean Research Institute of Shenyang Agricultural University for providing us with soybean germplasm resources.
Conflicts of Interest
The authors declare that they have no competing interests.
References
- Dybing, C.D.; Ghiasi, H.; Paech, C. Biochemical Characterization of Soybean Ovary Growth from Anthesis to Abscission of Aborting Ovaries. Plant Physiol. 1986, 81, 1069–1074. [Google Scholar] [CrossRef] [PubMed]
- Heitholt, J.J.; Egli, D.B.; Leggett, J.E.; Mackown, C.T. Role of Assimilate and Carbon-14 Photosynthate Partitioning in Soybean Reproductive Abortion. Crop Sci. 1986, 26, 999–1004. [Google Scholar] [CrossRef]
- Schaik, P.H.V.; Probst, A.H. Effects of Some Environmental Factors on Flower Production and Reproductive Efficiency in Soybeans. Agron. J. 1958, 50, 810–816. [Google Scholar] [CrossRef]
- Cui, W.; Song, Q.; Zuo, B.; Han, Q.; Jia, Z. Effects of Gibberellin (GA4+7) in Grain Filling, Hormonal Behavior, and Antioxidants in High-Density Maize (Zea mays L.). Plants 2020, 9, 978. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Liu, X.; Wang, C.; Jin, J.; Herbert, S.J.; Hashemi, M. Responses of soybean yield and yield components to light enrichment and planting density. Int. J. Plant Prod. 2010, 4, 1–10. [Google Scholar]
- Board, J.E.; Harville, B.G. Soybean Yield Component Responses to a Light Interception Gradient during the Reproductive Period. Crop Sci. 1993, 33, 772–777. [Google Scholar] [CrossRef]
- Liu, B.; Wang, C.; Jin, J.; Liu, J.D.; Zhang, Q.Y.; Liu, X.B. Effects of Light Enrichment and Shade during Reproductive Stage on Yield and Yield Components in Soybean. Soy. Sci. 2008, 27, 764–772. [Google Scholar]
- Ghiasi, H.; Dybing, C.P.D. Free amino Acid content and metabolic activities of setting and aborting soybean ovaries. Plant Physiol. 1987, 85, 91–95. [Google Scholar] [CrossRef]
- Angotti, J.J. Effects of Planting Date and Maturity Group on Reproductive Growth and Disease Incidence of Soybeans in Southeast Missouri; Arkansas State University: Jonesboro, AR, USA, 2012. [Google Scholar]
- Zhang, J.; Zhou, T.; Jia, K. Formation and Space-time Distribution of Flowers and Pods for Super-high-yielding Soybeans. Soybean Sci. 2012, 5, 12. [Google Scholar]
- Aggarwal, S.K.; Singh, A.; Choudhary, M.; Kumar, A.; Rakshit, S.; Kumar, P.; Bohra, A.; Varshney, R.K. Pangenomics in Microbial and Crop Research: Progress, Applications, and Perspectives. Genes 2022, 13, 598. [Google Scholar] [CrossRef]
- Shoaib, M.; Yang, W.; Shan, Q.; Sajjad, M.; Zhang, A. Genome-wide identification and expression analysis of new cytokinin metabolic genes in bread wheat (Triticum aestivum L.). PeerJ 2019, 7, e6300. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.N.; Kambhampati, S.; Kisiala, A.; Seegobin, M.; Emery, R.J.N. The soybean (Glycine max L.) cytokinin oxidase/dehydrogenase multigene family; identification of natural variations for altered cytokinin content and seed yield. Plant Direct 2020, 5, e00308. [Google Scholar] [CrossRef]
- Liu, P.; Zhang, C.; Ma, J.-Q.; Zhang, L.-Y.; Yang, B.; Tang, X.-Y.; Huang, L.; Zhou, X.-T.; Lu, K.; Li, J.-N. Genome-Wide Identification and Expression Profiling of Cytokinin Oxidase/Dehydrogenase (CKX) Genes Reveal Likely Roles in Pod Development and Stress Responses in Oilseed Rape (Brassica napus L.). Genes 2018, 9, 168. [Google Scholar] [CrossRef]
- Kurakawa, T.; Ueda, N.; Maekawa, M.; Kobayashi, K.; Kojima, M.; Nagato, Y.; Sakakibara, H.; Kyozuka, J. Direct control of shoot meristem activity by a cytokinin-activating enzyme. Nature 2007, 445, 652–655. [Google Scholar] [CrossRef]
- Tamas, I.A.; Wallace, D.H.; Ludford, P.M.; Ozbun, J.L. Effect of Older Fruits on Abortion and Abscisic Acid Concentration of Younger Fruits in Phaseolus vulgaris L. Plant Physiol. 1979, 64, 620–622. [Google Scholar] [CrossRef] [PubMed]
- Kim, J. Behavior. Four shades of detachment: Regulation of floral organ abscission. Plant Signal. Behav. 2014, 9, e976154. [Google Scholar] [CrossRef] [PubMed]
- Atkins, C.A.; Pigeaire, A. Application of cytokinins to flowers to increase pod set in Lupinus angustifolius L. Aust. J. Agric. Res. 1993, 44, 1799–1819. [Google Scholar] [CrossRef]
- Pigeaire, A.; Atkins, C.A. Effect of cytokinin application to flowers on pod set in Lupinus angustifolius L. Aust. J. Agric. Res. 1992. [Google Scholar]
- Miyawaki, K.; Tarkowski, P.; Matsumoto-Kitano, M.; Kato, T.; Sato, S.; Tarkowska, D.; Tabata, S.; Sandberg, G.; Kakimoto, T. Roles of Arabidopsis ATP/ADP isopentenyltransferases and tRNA isopentenyltransferases in cytokinin biosynthesis. Proc. Natl. Acad. Sci. USA 2006, 103, 16598–16603. [Google Scholar] [CrossRef]
- Nonokawa, K.; Nakajima, T.; Nakamura, T.; Kokubun, M. Effect of Synthetic Cytokinin Application on Pod Setting of Individual Florets within Raceme in Soybean. Plant Prod. Sci. 2015, 15, 79–81. [Google Scholar] [CrossRef]
- Kemmerer, E.C.; Tucker, M.L. Comparative study of cellulases associated with adventitious root initiation, apical buds, and leaf, flower, and pod abscission zones in soybean. Plant Physiol. 1994, 104, 557–562. [Google Scholar] [CrossRef] [PubMed]
- Nonokawa, K.; Kokubun, M.; Nakajima, T.; Nakamura, T.; Yoshida, R. Roles of Auxin and Cytokinin in Soybean Pod Setting. Plant Prod. Sci. 2007, 10, 199–206. [Google Scholar] [CrossRef]
- Griffiths, C.A.; Sagar, R.; Geng, Y.; Primavesi, L.F.; Patel, M.K.; Passarelli, M.K.; Gilmore, I.S.; Steven, R.T.; Bunch, J.; Paul, M.J.; et al. Chemical intervention in plant sugar signalling increases yield and resilience. Nature 2016, 540, 574–578. [Google Scholar] [CrossRef] [PubMed]
- Hong, D.-F.; Ma, J.-F.; Ma, Y.; Wei, F.; Wei, X.-Y.; Wang, J.-M.; Zhang, X.-S. Study on the Property of Density Tolerance in Maize. J. Maize Sci. 2019. [Google Scholar] [CrossRef]
- Potts, S. Identification of QTL and Candidate Genes for Plant Density Tolerance in Maize. Doctoral Dissertation, University of Illinois at Urbana-Champaign, Urbana, IL, USA, 2014. [Google Scholar]
- Sahraeian, S.M.E.; Mohiyuddin, M.; Sebra, R.; Tilgner, H.; Afshar, P.T.; Au, K.F.; Bani Asadi, N.; Gerstein, M.B.; Wong, W.H.; Snyder, M.P.; et al. Gaining comprehensive biological insight into the transcriptome by performing a broad-spectrum RNA-seq analysis. Nat. Commun. 2017, 8, 59. [Google Scholar] [CrossRef]
- Yi, L.; Pimentel, H.; Bray, N.L.; Pachter, L. Gene-level differential analysis at transcript-level resolution. Genome Biol. 2018, 19, 53. [Google Scholar] [CrossRef]
- Want, E.J.; O’Maille, G.; Abagyan, R.; Siuzdak, G.; Smith, C.A. XCMS: Processing Mass Spectrometry Data for Metabolite Profiling Using Nonlinear Peak Alignment, Matching, and Identification. Anal. Chem. 2006, 78, 779–787. [Google Scholar] [CrossRef]
- Wen, B.; Mei, Z.; Zeng, C.; Liu, S. metaX: A flexible and comprehensive software for processing metabolomics data. BMC Bioinform. 2017, 18, 183. [Google Scholar] [CrossRef]
- Wingler, A.; Henriques, R. Sugars and the speed of life—Metabolic signals that determine plant growth, development and death. Physiol. Plant. 2022, 174, e13656. [Google Scholar] [CrossRef]
- Marc, G.; Maia, R.; Smith, H.M. The role of auxin and sugar signaling in dominance inhibition of inflorescence growth by fruit load. Plant Physiol. 2021, 187, 1189–1201. [Google Scholar] [CrossRef]
- Horacio, P.; Martínez-Noël, G. Sucrose signaling in plants: A world yet to be explored. Plant Signal. Behav. 2013, 8, e23316. [Google Scholar] [CrossRef]
- Santiago, J.; Brandt, B.; Wildhagen, M.; Hohmann, U.; Hothorn, L.A.; Butenko, M.A.; Hothorn, M. Mechanistic insight into a peptide hormone signaling complex mediating floral organ abscission. eLife 2016, 5, e15075. [Google Scholar] [CrossRef]
- Bartrina, I.; Otto, E.; Strnad, M.; Werner, T.; Schmulling, T. Cytokinin Regulates the Activity of Reproductive Meristems, Flower Organ Size, Ovule Formation, and Thus Seed Yield in Arabidopsis thaliana. Plant Cell 2011, 23, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.-Y.; Zong, X.-J.; Li, D.-Q. Mitogen-activated protein kinase cascade is involved in abscisic acid signal transduction in plant. Life Sci. 2010, 22, 736–742. [Google Scholar] [CrossRef]
- Manna, M.; Rengasamy, B.; Sinha, A.K. Revisiting the role of MAPK signalling pathway in plants and its manipulation for crop improvement. Plant Cell Environ. 2023, 46, 2277–2295. [Google Scholar] [CrossRef]
- Werner, T.; Motyka, V.; Laucou, V.; Smets, R.; Van Onckelen, H.; Schmülling, T. Cytokinin-Deficient Transgenic Arabidopsis Plants Show Multiple Developmental Alterations Indicating Opposite Functions of Cytokinins in the Regulation of Shoot and Root Meristem Activity. Plant Cell 2003, 15, 2532–2550. [Google Scholar] [CrossRef]
- Jameson, P.E.; Song, J. Cytokinin: A key driver of seed yield. J. Exp. Bot. 2016, 67, 593–606. [Google Scholar] [CrossRef]
- Ashikari, M.; Sakakibara, H.; Lin, S.; Yamamoto, T.; Takashi, T.; Nishimura, A.; Angeles, E.R.; Qian, Q.; Kitano, H.; Matsuoka, M. Cytokinin oxidase regulates rice grain production. Science 2005, 309, 741–745. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).