Comparative Study between Silvopastoral and Agroforest Systems on Soil Quality in a Disturbed Native Forest of South-Central Chile
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Description and Experimental Design
2.2. Soil Sampling and Characterization
2.3. SQI Estimation
2.4. Statistical Analysis
3. Results and Discussion
3.1. Soil Characterization
3.1.1. Physical Properties as SINDs
3.1.2. Chemical Properties as SINDs
3.1.3. Microbiological Properties as SINDs
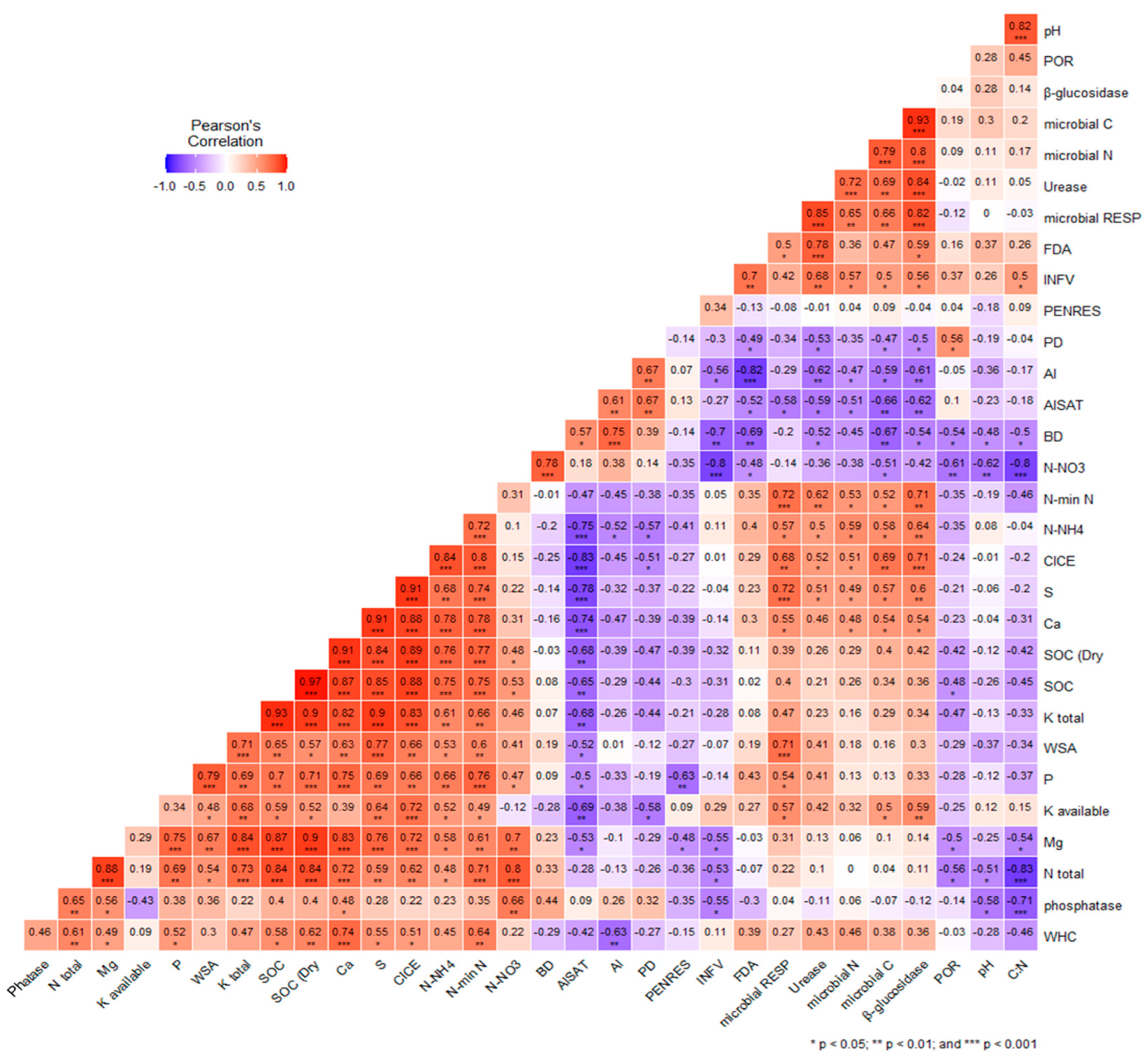
3.2. Interactions among Soil Quality Indicators (SINDs)
3.3. Carbon Sequestration
3.4. Determination of SQI
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Abbreviation | Description | Abbreviation | Description |
| AFS | agroforestry system | Mg2+ | magnesium |
| SPS | silvopastoral systems | Na+ | sodium |
| AGROFRST | agroforest | S | sulphur |
| RA | Ranchillo Alto | AlEXCH | exchangeable Al |
| Pg | petagrams | %AlSAT | % of Al saturation |
| C | carbon | ECEC | effective cation exchange capacity |
| CO2eq | carbon equivalent | pH | soil reactivity |
| SOM | soil organic matter | PD | particle density |
| SOC | soil organic carbon | BD | bulk density |
| CSEQ | carbon sequestration | POR | total porosity (%) |
| SQ | soil quality | WSA | % of water stable aggregates |
| SQCHE | chemical soil quality | INFV | infiltration velocity |
| SQPHY | physical soil quality | WHC | water holding capacity |
| SQBIOL | biological soil quality | PENR | penetration resistance |
| SQI | soil quality index | MBIOMSS | microbial biomass |
| SIND | soil quality indicator | MSOC | microbial biomass C |
| N | nitrogen | MN | microbial N |
| C/N | carbon–nitrogen ratio | MRESP | microbial respiration |
| NH4+ | ammonium | NMIN | N mineralization |
| NO3- | nitrate | β-GLU | β-glucosidase activity |
| P | phosphorous | ||
| URS | urease activity | ||
| K+ | potassium | PHOSP | phosphatase activity |
| Ca2+ | calcium | FDA | fluorescein diacetate hydrolysis |
Appendix A. Subindices for Each Soil Quality Indicator (SIND) Considered for the Soil Quality Index (SQI) Estimation
| PHYSICAL SIND | Level | Interpretation | Subindex | Source |
|---|---|---|---|---|
| PD (g cm−3) | <2 | Desirable | 1 | [64] |
| >2 | Without effect | 0 | ||
| BD (g cm−3) | <1.10 | Optimum | 2 | [77] |
| 1.10−1.47 | Desirable | 1 | ||
| >1.47 | Low | 0 | ||
| POR (%) | <5 | Critical | −5 | [79] |
| 5–10 | Restrictive | 0 | ||
| 10–25 | Acceptable | 1 | ||
| 25–40 | Desirable | 2 | ||
| >40 | Optimum | 5 | ||
| WHC (%) | >60 | Optimum | 10 | [80] |
| 51–60 | Acceptable | 5 | ||
| 41–50 | Low | 0 | ||
| <40 | Critical | −10 | ||
| INFVk (cm day−1) | <8.64 | Undesirable | −5 | [81] |
| 8.64–20 | Acceptable | 0 | ||
| 20–43.2 | Optimum | 5 | ||
| PENR (psi) | >300 | Undesirable | 0 | [82,83] |
| 200–300 | Acceptable | 1 | ||
| 100–200 | Optimum | 2 | ||
| WSA (%) | <50 | Undesirable | 0 | [82] |
| 50–70 | Medium | 1 | ||
| 70–90 | High | 2 | ||
| >90 | Optimum | 3 |
Appendix B
| CHEMICAL SIND | Level | Interpretation | Sub-Index | Source |
|---|---|---|---|---|
| pH | <3.0 | Super critical | −1 | [84] |
| 3.01–4.0 | Critical | 0 | ||
| 4.01–5.5 | Limiting | 1 | ||
| 5.51–6.8 | Desirable | 2 | ||
| 6.81–7.2 | Optimum | 2 | ||
| 7.21–7.5 | Acceptable | 1 | ||
| 7.51–8.5 | Limiting | 1 | ||
| >8.5 | Critical | 0 | ||
| SOC (%) | >15 | Excellent | 20 | [80] |
| 5–15 | High | 10 | ||
| 3–5 | Moderate | 1 | ||
| <2 | Low | −10 | ||
| N (%) | >0.5 | Desirable | 2 | [84] |
| 0.1–0.5 | Adequate | 1 | ||
| <0.1 | Insufficient | 0 | ||
| NO3− (mg kg−1) | <10 | Critical | −5 | [80] |
| 10–20.1 | Insufficient | 0 | ||
| 20.1–40 | Adequate | 5 | ||
| >40 | Desirable | 10 | ||
| NH4+ (mg kg−1) | <25 | Critical | −5 | [80] |
| 25–50 | Insufficient | 0 | ||
| 51–75 | Adequate | 5 | ||
| >75 | Desirable | 10 | ||
| C:N ratio | 1–10 | Adequate | 2 | [84] |
| 10–20 | Moderate | 1 | ||
| >20 | Insufficient | 0 | ||
| P (mg kg−1) | >15 | Adequate | 10 | [62] |
| 5–15 | Moderate | 1 | ||
| <5 | Insufficient | −5 | ||
| K (mg kg−1) | >500 | Adequate | 2 | [84] |
| 100–500 | Moderate | 1 | ||
| <100 | Insufficient | 0 | ||
| S (mg kg−1) | >100 | Insufficient | 0 | [84] |
| 1–100 | Adequate | 1 | ||
| <1 | Insufficient | 0 | ||
| Ca (mg kg−1) | >1000 | Desirable | 2 | [84] |
| 101–1000 | Adequate | 1 | ||
| 10–100 | Insufficient | 0 | ||
| <10 | Critical | −1 | ||
| Mg (mg kg−1) | >500 | Adequate | 2 | [84] |
| 50–500 | Moderate | 1 | ||
| <50 | Insufficient | 0 | ||
| ECEC (cmol kg−1) | >6.27 | Adequate | 10 | [75,85] |
| 1.65–6.27 | Moderate | 5 | ||
| <1.65 | Insufficient | 0 | ||
| Exchangeable % Na | <15 | Critical | −10 | [84] |
| ≤15 | Acceptable | 1 | ||
| AlEXCH (cmol kg−1) | <0.1 | Adequate | 0 | [85] |
| 0.11–0.51 | Moderate | −1 | ||
| 0.51–0.81 | Undesirable | −2 | ||
| >0.81 | Critical | −3 | ||
| Sat Al (%) | 1.1–3.1 | Adequate | 0 | [85] |
| 3.2–6.1 | Moderate | −1 | ||
| 6.2–12 | High | −2 | ||
| >12 | Critical | −5 |
Appendix C
| BIOLOGICAL SIND | Level | Interpretation | Subindex | Source |
|---|---|---|---|---|
| MN (µg N g dw−1) | >4067 | Desirable | 2 | [86] |
| <4067 | Undesiderable | 1 | ||
| MSOC (µg C g d w−1) | >28,608 | Adequate | 3 | [87] |
| 2814–28,608 | Moderate | 2 | ||
| <2814 | Low | 1 | ||
| MRESP (mg CO2 g dw−1) | <0.3 | Critical | 1 | [88] |
| 0.3–0.5 | Restrictive | 3 | ||
| 0.5–0.65 | Limited | 5 | ||
| 0.65–0.85 | Desirable | 8 | ||
| >0.85 | Optimum | 10 | ||
| N mineralization N-min N (µg N kg dw−1) | <9 | Critical | 1 | [89] |
| 9–13 | Restrictive | 3 | ||
| 13–17 | Limited | 5 | ||
| 17–21 | Desirable | 8 | ||
| >21 | Optimum | 10 | ||
| β-GLU (µg PNF g dw−1 h−1) | <14,304 | Undesirable | 0 | [88] |
| 14,304–28,608 | Acceptable | 5 | ||
| >28,608 | Optimum | 10 | ||
| URS (µg N-NH4 g dw−1 h−1) | <28 | Undesirable | 0 | [89,90] |
| 28–560 | Acceptable | 1 | ||
| >560 | Optimum | 2 | ||
| PHOSP (µg PNF g dw−1 h−1) | <60 | Undesirable | 0 | [91] |
| 60–170 | Medium | 1 | ||
| >170 | Optimum | 3 | ||
| FDA (µg F g dw−1) | >66 | Optimum | 3 | [60] |
| 50–66 | Adecuate | 2 | ||
| 33-50 | Average | 1 | ||
| <33 | Limited | 0 |
References
- Poore, J.; Nemecek, T. Reducing food’s environmental impacts through producers and consumers. Science 2018, 360, 987. [Google Scholar] [CrossRef] [PubMed]
- Mbow, H.O.P.; Reisinger, A.; Canadell, J.; O’Brien, P. Food Security in Climate Change and Land in: Special Report on Climate Change and Land; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2019; p. 32. Available online: https://www.ipcc.ch/site/assets/uploads/2018/07/sr2_background_report_final.pdf (accessed on 18 January 2023).
- Rosenzweig, C.; Mbow, C.; Barioni, L.G.; Benton, T.G.; Herrero, M.; Krishnapillai, M.; Liwenga, E.T.; Pradhan, P.; Rivera-Ferre, M.G.; Sapkota, T.; et al. Climate change responses benefit from a global food system approach. Nat. Food 2020, 1, 94–97. [Google Scholar] [CrossRef] [PubMed]
- Crippa, M.; Solazzo, E.; Guizzardi, D.; Monforti-Ferrario, F.; Tubiello, F.N.; Leip, A. Food systems are responsible for a third of global anthropogenic GHG emissions. Nat. Food 2021, 2, 198–209. [Google Scholar] [CrossRef] [PubMed]
- Giraldo, C.; Escobar, F.; Chará, J.D.; Calle, Z. The adoption of silvopastoral systems promotes the recovery of ecological processes regulated by dung beetles in the Colombian Andes: Ecological processes regulated by dung beetles. Insect Conserv. Divers. 2011, 4, 115–122. [Google Scholar] [CrossRef]
- Gerber, P.J.; Hristov, A.N.; Henderson, B.; Makkar, H.; Oh, J.; Lee, C.; Meinen, R.; Montes, F.; Ott, T.; Firkins, J.; et al. Technical options for the mitigation of direct methane and nitrous oxide emissions from livestock: A review. Animal 2013, 7, 220–234. [Google Scholar] [CrossRef]
- FAO. Livestock’s Long Shadow: Environmental Issues and Options; Steinfeld, H., Ed.; Food and Agriculture Organization of the United Nations: Rome, Italy, 2006; p. 390. [Google Scholar]
- O’Mara, F.P. The role of grasslands in food security and climate change. Ann. Bot. 2012, 110, 1263–1270. [Google Scholar] [CrossRef]
- Sarabia, L.; Solorio, F.J.; Ramírez, L.; Ayala, A.; Aguilar, C.; Ku, J.; Almeida, C.; Cassador, R.; Alves, B.J.; Boddey, R.M. Improving the Nitrogen Cycling in Livestock Systems through Silvopastoral Systems. In Nutrient Dynamics for Sustainable Crop Production; Meena, R.S., Ed.; Springer Singapore: Singapore, 2020; pp. 189–213. [Google Scholar] [CrossRef]
- Li, B.V.; Jiang, B. Responses of forest structure, functions, and biodiversity to livestock disturbances: A global meta-analysis. Glob. Chang. Biol. 2021, 27, 4745–4757. [Google Scholar] [CrossRef]
- Sollins, P.; Homann, P.; Caldwell, B.A. Stabilization and destabilization of soil organic matter: Mechanisms and controls. Geoderma 1996, 74, 65–105. [Google Scholar] [CrossRef]
- Amerongen-Madison, J.; Bulmer, C.; Trofymow, T.; Prescott, C.; Wallace, B.; Philpott, T.; Dymond, C.; Fredeen, A. Soil Carbon in Forest Ecosystems: Pools and Processes. Forest Carbon Initiative. 2021. Available online: https://a100.gov.bc.ca/pub/eirs/finishDownloadDocument.do;jsessionid=8F62A9315428CC8F653587E211134F7F?subdocumentId=21641 (accessed on 18 January 2023).
- Blanco-Canqui, H.; Lal, R. Principles of Soil Conservation and Management; Springer: Berlin/Heidelberg, Germany, 2008; p. 617. [Google Scholar]
- Juhos, K.; Madarász, B.; Kotroczó, Z.; Béni, Á.; Makádi, M.; Fekete, I. Carbon sequestration of forest soils is reflected by changes in physicochemical soil indicators—A comprehensive discussion of a long-term experiment on a detritus manipulation. Geoderma 2021, 385, 114918. [Google Scholar] [CrossRef]
- Lorenz, K.; Lal, R. Soil organic carbon sequestration in agroforestry systems. A review. Agron. Sustain. Dev. 2014, 34, 443–454. [Google Scholar] [CrossRef]
- Nair PK, R. Carbon sequestration studies in agroforestry systems: A reality-check. Agrofor. Syst. 2012, 86, 243–253. [Google Scholar] [CrossRef]
- Ortiz, J.; Neira, P.; Panichini, M.; Curaqueo, G.; Stolpe, N.B.; Zagal, E.; Dube, F.; Gupta, S.R. Silvopastoral Systems on Degraded Lands for Soil Carbon Sequestration and Climate Change Mitigation. In Agroforestry for Sustainable Intensification of Agriculture in Asia and Africa; Dagar, J.C., Gupta, S.R., Sileshi, G.W., Eds.; Sustainability Sciences in Asia and Africa; Springer Nature Singapore: Singapore, 2023; pp. 207–242. Available online: https://link.springer.com/10.1007/978-981-19-4602-8_7 (accessed on 18 January 2023).
- Somarriba, E.; Beer, J.; Alegre-Orihuela, J.; Andrade, H.J.; Cerda, R.; DeClerck, F.; Detlefsen, G.; Escalante, M.; Giraldo, L.A.; Ibrahim, M.; et al. Mainstreaming Agroforestry in Latin America. In Agroforestry—The Future of Global Land Use; Nair, P.K.R., Garrity, D., Eds.; Springer: Dordrecht, The Netherlands, 2012; Volume 9, pp. 429–453. [Google Scholar] [CrossRef]
- Alonso, J. Silvopastoral systems and their contribution to the environment. Cuba. J. Agric. Sci. 2011, 45, 9. [Google Scholar]
- Udawatta, R.P.; Jose, S. Carbon Sequestration Potential of Agroforestry Practices in Temperate North America. In Carbon Sequestration Potential of Agroforestry Systems; Kumar, B.M., Nair, P.K.R., Eds.; Springer: Dordrecht, The Netherlands, 2011; Volume 8, pp. 17–42. [Google Scholar] [CrossRef]
- Feliciano, D.; Ledo, A.; Hillier, J.; Nayak, D.R. Which agroforestry options give the greatest soil and above ground carbon benefits in different world regions? Agric. Ecosyst. Environ. 2018, 254, 117–129. [Google Scholar] [CrossRef]
- Karlen, D.L.; Mausbach, M.J.; Doran, J.W.; Cline, R.G.; Harris, R.F.; Schuman, G.E. Soil Quality: A Concept, Definition, and Framework for Evaluation (A Guest Editorial). Soil Sci. Soc. Am. J. 1997, 61, 4–10. [Google Scholar] [CrossRef]
- Arshad, M.A.; Martin, S. Identifying critical limits for soil quality indicators in agro-ecosystems. Agric. Ecosyst. Environ. 2002, 88, 153–160. [Google Scholar] [CrossRef]
- Doran, J.W.; Jones, A.J. (Eds.) Methods for Assessing Soil Quality; Soil Science Society of America: Madison, WI, USA, 1997. [Google Scholar] [CrossRef]
- Muñoz-Rojas, M. Soil quality indicators: Critical tools in ecosystem restoration. Curr. Opin. Environ. Sci. Health 2018, 5, 47–52. [Google Scholar] [CrossRef]
- Tale, K.S.; Ingole, S. A review on role of physico-chemical properties in soil quality. Chem. Sci. Rev. Lett. 2015, 4, 57–66. [Google Scholar]
- Li, J.; Nie, M.; Powell, J.R.; Bissett, A.; Pendall, E. Soil physico-chemical properties are critical for predicting carbon storage and nutrient availability across Australia. Environ. Res. Lett. 2020, 15, 094088. [Google Scholar] [CrossRef]
- Karlen, D.L.; Andrews, S.S.; Doran, J.W. Soil quality: Current concepts and applications. In Advances in Agronomy; Elsevier: Amsterdam, The Netherlands, 2001; Volume 74, pp. 1–40. [Google Scholar] [CrossRef]
- Drobnik, T.; Greiner, L.; Keller, A.; Grêt-Regamey, A. Soil quality indicators–From soil functions to ecosystem services. Ecol. Indic. 2018, 94, 151–169. [Google Scholar] [CrossRef]
- Flores, J.P.; Martínez, E.; Espinosa, M.; Ahumada, I.; Avendaño, P.; Henríquez, G.; Torres, P. Determinación de la erosión actual y potencial de los suelos de Chile: Región de La Araucanía, 2010. Síntesis de Resultados (Pub. CIREN N° 149). Available online: https://bibliotecadigital.ciren.cl/handle/20.500.13082/2133 (accessed on 15 January 2023).
- Casanova, M.; Salazar, O.; Seguel, O.; Luzio, W. Human-Induced Soil Degradation in Chile. In The Soils of Chile; Casanova, M., Salazar, O., Seguel, O., Luzio, W., Eds.; Springer: Dordrecht, The Netherlands, 2013; pp. 121–158. [Google Scholar] [CrossRef]
- Lara, A.; Solari, M.E.; Prieto MD, R.; Peña, M.P. Reconstrucción de la cobertura de la vegetación y uso del suelo hacia 1550 y sus cambios a 2007 en la ecorregión de los bosques valdivianos lluviosos de Chile (35°—43°30′ S). Bosque (Valdivia) 2012, 33, 3–4. [Google Scholar] [CrossRef]
- Schmidt, A.; Alonso, V.; Schmidt, H. Manejo Silvopastoril de los Bosques de Ñirre en la XII Región de Magallanes; Fondo de Investigación del Bosque Nativo: Santiago, Chile, 2013; p. 63. Available online: https://investigacion.conaf.cl/archivos/repositorio_documento/2018/10/Informe-final-029-2013_final_observaciones.pdf (accessed on 18 January 2023).
- Neira, P.; Henríquez-Castillo, C.; Ortiz, J.; Stolpe, N.; Dube, F. Do different densities of tree cover affect pasture biomass and soil microbial communities? Agrofor. Syst. 2021, 95, 1465–1478. [Google Scholar] [CrossRef]
- Sotomayor, A.; Schmidt, H.; Salinas, J.; Schmidt, A.; Sánchez-Jardón, L.; Alonso, M.; Moya, I.; Teuber, O. Silvopastoral Systems in the Aysén and Magallanes Regions of the Chilean Patagonia. In Silvopastoral Systems in Southern South America; Peri, P.L., Dube, F., Varella, A., Eds.; Springer International Publishing: Berlin/Heidelberg, Germany, 2016; Volume 11, pp. 213–230. [Google Scholar] [CrossRef]
- Ortiz, J.; Dube, F.; Neira, P.; Panichini, M.; Stolpe, N.B.; Zagal, E.; Martínez-Hernández, P.A. Soil Quality Changes within a (Nothofagus obliqua) Forest Under Silvopastoral Management in the Andes Mountain Range, South Central Chile. Sustainability 2020, 12, 6815. [Google Scholar] [CrossRef]
- Dube, F.; Thevathasan, N.V.; Zagal, E.; Gordon, A.M.; Stolpe, N.B.; Espinosa, M. Carbon Sequestration Potential of Silvopastoral and Other Land Use Systems in the Chilean Patagonia. In Carbon Sequestration Potential of Agroforestry Systems; Kumar, B.M., Nair, P.K.R., Eds.; Springer: Dordrecht, The Netherlands, 2011; Volume 8, pp. 101–127. [Google Scholar] [CrossRef]
- Dube, F.; Sotomayor, A.; Loewe, V.; Müller-Using, B.; Stolpe, N.; Zagal, E.; Doussoulin, M. Silvopastoral Systems in Temperate Zones of Chile. In Silvopastoral Systems in Southern South America; Peri, P.L., Dube, F., Varella, A., Eds.; Springer International Publishing: Berlin/Heidelberg, Germany, 2016; Volume 11, pp. 183–211. [Google Scholar] [CrossRef]
- Dube, F.; Stolpe, N.B.; Zagal, E.; Figueroa, C.R.; Concha, C.; Neira, P.; Carrasco, C.; Schwenke, J.M.; Schwenke, V.; Müller-Using, B. Novel agroforestry systems in temperate Chile. In Temperate Agroforestry Systems, 2nd ed.; Gordon, A.M., Newman, S.M., Coleman, B.R.W., Eds.; CABI: Wallingford, UK, 2018; pp. 237–251. [Google Scholar] [CrossRef]
- Leal, F.; Aburto, F.; Aguilera, N.; Echeverría, C.; Gatica-Saavedra, P. Forest degradation modifies litter production, quality, and decomposition dynamics in Southern temperate forests. Front. Soil Sci. 2023, 3, 1111694. [Google Scholar] [CrossRef]
- United States Department of Agriculture (USDA). Soil Survey Staff: Keys to Soil Taxonomy; USDA: Washington, DC, USA, 2014.
- Stolpe, N. Descripciones de los Principales Suelos de la VIII Región de Chile; Department of Soils and Natural Resources, Faculty of Agronomy, Universidad de Concepción: Chillán, Chile, 2006. [Google Scholar]
- Besoain, E.; Sepúlveda, G. Minerales secundarios. In Suelos Volcánicos de Chile; Tosso, J., Ed.; INIA: Santiago, Chile, 1985; pp. 153–214. [Google Scholar]
- CIREN (Centro de Información de Recursos Naturales). Descripciones de suelos, materiales y símbolos. In Estudio Agrológico VIII Región; CIREN: Santiago, Chile, 1999; Volume 1, p. 121. [Google Scholar]
- Blake, G.R.; Hartge, K.H. Particle Density 1. In Methods of Soil Analysis: Part 1—Physical and Mineralogical Methods, 2nd ed.; Klute, A., Ed.; American Society of Agronomy, Soil Science Society of America Inc.: Madison, WI, USA, 1986; pp. 377–382. [Google Scholar] [CrossRef]
- Kemper, W.D.; Rosenau, R.C. Aggregate Stability and Size Distribution. In SSSA Book Series; Klute, A., Ed.; Soil Science Society of America, American Society of Agronomy: Madison, WI, USA, 2018; pp. 425–442. [Google Scholar] [CrossRef]
- Zhang, R. Determination of Soil Sorptivity and Hydraulic Conductivity from the Disk Infiltrometer. Soil Sci. Soc. Am. J. 1997, 61, 1024–1030. [Google Scholar] [CrossRef]
- Zagal, E.; Longeri, L.; Vidal, I.; Hoffman, G.; González, R. Influencia de la adición de nitrógeno y fosforo sobre la descomposición de paja de trigo en un suelo derivado de cenizas volcánicas. Agric. Técnica 2003, 63, 403–415. [Google Scholar] [CrossRef]
- Sadzawka, A.; Carrasco, M.A.; Grez, R.; Mora, M.L.; Flores, H.; Neaman, A. Métodos de Análisis Recomendados para los Suelos Chilenos; Comisión de Normalización y Acreditación, Sociedad Chilena de la Ciencia del Suelo: Santiago, Chile, 2006; p. 113. [Google Scholar]
- Wright, A.F.; Bailey, J.S. Organic carbon, total carbon, and total nitrogen determinations in soils of variable calcium carbonate contents using a Leco CN-2000 dry combustion analyzer. Commun. Soil Sci. Plant Anal. 2001, 32, 3243–3258. [Google Scholar] [CrossRef]
- Anderson JP, E.; Domsch, K.H. A physiological method for the quantitative measurement of microbial biomass in soils. Soil Biol. Biochem. 1978, 10, 215–221. [Google Scholar] [CrossRef]
- Jenkinson, D.S.; Brookes, P.C.; Powlson, D.S. Measuring soil microbial biomass. Soil Biol. Biochem. 2004, 36, 5–7. [Google Scholar] [CrossRef]
- Horwath, W.R.; Paul, E.A. Microbial Biomass. In SSSA Book Series; Weaver, R.W., Angle, S., Bottomley, P., Bezdicek, D., Smith, S., Tabatabai, A., Wollum, A., Eds.; Soil Science Society of America: Madison, WI, USA, 2018; pp. 753–773. [Google Scholar] [CrossRef]
- Linn, D.M.; Doran, J.W. Effect of Water-Filled Pore Space on Carbon Dioxide and Nitrous Oxide Production in Tilled and Nontilled Soils. Soil Sci. Soc. Am. J. 1984, 48, 1267–1272. [Google Scholar] [CrossRef]
- Alef, K. Nitrogen mineralization in soils. In Methods in Applied Soil Microbiology and Biochemistry; Alef, K., Nannipieri, P., Eds.; Academic Press: London, UK, 1995; pp. 234–245. [Google Scholar]
- Tabatabai, M.A. Soil Enzymes. In SSSA Book Series; Weaver, R.W., Angle, S., Bottomley, P., Bezdicek, D., Smith, S., Tabatabai, A., Wollum, A., Eds.; Soil Science Society of America: Madison, WI, USA, 1994; pp. 775–833. [Google Scholar] [CrossRef]
- Kandeler, E.; Poll, C.; Frankenberger Jr, W.T.; Tabatabai, M.A. Nitrogen cycle enzymes. In Methods in Soil Enzymology; Dick, R.P., Ed.; Soil Science Society of America Book Series; Soil Science Society of America: Madison, WI, USA, 2011; Volume 9, pp. 211–244. [Google Scholar]
- Tabatabai, M.A.; Bremner, J.M. Use of p-nitrophenyl phosphate for assay of soil phosphatase activity. Soil Biol. Biochem. 1969, 1, 301–307. [Google Scholar] [CrossRef]
- Bello, D.; García-Carballal, S.; Muiño, F.; Salgado, J.; Trasar-Cepeda, C. A pH-adjusted method to measure urease activity in soils spiked with tetrafluoroborate 1-butyl-3-methylimidazolium [BMIM][BF4]. In Proceedings of the 20th International Electronic Conference on Synthetic Organic Chemistry, Santiago de Compostela, Online, 1–30 November 2016. [Google Scholar] [CrossRef]
- Alef, K. Estimation of the hydrolysis of fluorescein diacetate. In Methods in Applied Soil Microbiology and Biochemistry; Alef, K., Nannipieri, P., Eds.; Academic Press: London, UK, 1995; pp. 232–233. [Google Scholar]
- Green, V.S.; Stott, D.E.; Diack, M. Assay for fluorescein diacetate hydrolytic activity: Optimization for soil samples. Soil Biol. Biochem. 2006, 38, 693–701. [Google Scholar] [CrossRef]
- Amacher, M.C.; O’Neil, K.P.; Perry, C.H. Soil Vital Signs: A New Soil Quality Index (SQI) for Assessing Forest Soil Health; RMRS-RP-65; U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station: Washington, DC, USA, 2007; p. RMRS-RP-65. [CrossRef]
- Dube, F.; University of Concepción, Chillán, Región del Bio Bio, Chile. Personal communication, 2019.
- Nanzyo, M. Unique properties of volcanic ash soils. Glob. Environ. Res.-Eng. Ed. 2002, 6, 99–112. [Google Scholar]
- Panichini, M.; INIA Carillanca, Temuco, Región de La Araucanía, Chile. Personal communication, 2020.
- Nissen, J.; Quiroz, C.; Seguel, O.; Roberto Mac Donald, H.; Sch, A.E. Variación del potencial mátrico durante el movimiento de agua en andisoles. Agro Sur 2005, 33, 36–47. [Google Scholar] [CrossRef]
- Zadzawka, A.; Carrasco, A. Quimica de los Suelos Volcanicos; Biblioteca Digital INIA: Santiago, Chile, 1985; Available online: https://biblioteca.inia.cl/handle/123456789/26667 (accessed on 18 January 2023).
- Qi, K.; Pang, X.; Yang, B.; Bao, W. Soil carbon, nitrogen and phosphorus ecological stoichiometry shifts with tree species in subalpine plantations. PeerJ 2020, 8, e9702. [Google Scholar] [CrossRef] [PubMed]
- Schroth, A.W.; Bostick, B.C.; Graham, M.; Kaste, J.M.; Mitchell, M.J.; Friedland, A.J. Sulfur species behavior in soil organic matter during decomposition. J. Geophys. Res. Biogeosci. 2007, 112, G04011. [Google Scholar] [CrossRef]
- Jones, C.A. Estimation of percent aluminum saturation from soil chemical data. Commun. Soil Sci. Plant Anal. 1984, 15, 327–335. [Google Scholar] [CrossRef]
- Alfaro, M.; Dube, F.; Zagal, E. The Influence of Overmature, Degraded Nothofagus Forests with Strong Anthropic Disturbance on the Quality of an Andisol and its Gradual Recovery with Silvopasture in Southwestern South America. In Agroforestry for Degraded Landscapes; Dagar, J.C., Gupta, S.R., Teketay, D., Eds.; Springer: Singapore, 2020; pp. 67–85. [Google Scholar] [CrossRef]
- Decker KL, M.; Boerner RE, J. Elevation and vegetation influences on soil properties in Chilean Nothofagus forests. Rev. Chil. Hist. Nat. 2003, 76, 371–381. [Google Scholar] [CrossRef]
- Schnürer, J.; Rosswall, T. Fluorescein diacetate hydrolysis as a measure of total microbial activity in soil and litter. Appl. Environ. Microbiol. 1982, 43, 1256–1261. [Google Scholar] [CrossRef]
- Reyes, F.; Lillo, A.; Ojeda, N.; Reyes, M.; Alvear, M. Efecto de la exposición y la toposecuencia sobre actividades biológicas del suelo en bosque relicto del centro-sur de Chile. Bosque (Valdivia) 2011, 32, 255–265. [Google Scholar] [CrossRef]
- Muñoz, C.; Torres, P.; Alvear, M.; Zagal, E. Physical protection of C and greenhouse gas emissions provided by soil macroaggregates from a Chilean cultivated volcanic soil. Acta Agric. Scand. Sect. B—Soil Plant Sci. 2012, 62, 739–748. [Google Scholar] [CrossRef]
- Casanova, M.; Salazar, O.; Seguel, O.; Luzio, W. (Eds.) Main Features of Chilean Soils. In The Soils of Chile; Springer: Dordrecht, The Netherlands, 2013; pp. 25–97. [Google Scholar] [CrossRef]
- Prosser, J.A.; Speir, T.W.; Stott, D.E. Soil Oxidoreductases and FDA Hydrolysis. In SSSA Book Series; Dick, R.P., Ed.; American Society of Agronomy, Crop Science Society of America, and Soil Science Society of America: Madison, WI, USA, 2015; pp. 103–124. [Google Scholar] [CrossRef]
- Ortiz, J.; University of Concepción, Chillán, Región del Bio Bio, Chile. Unpublished work. 2021.
- Arshad, M.A.C.; Lowery, B.; Grossman, B. Physical Tests for Monitoring Soil Quality; Doran, E.J.W., Jones, A.J., Eds.; SSSA Special Publications (Soil Science Society of America): Madison, WI, USA, 2015; pp. 123–141. [Google Scholar] [CrossRef]
- Pagliai, M.; Vignozzi, N. The soil pore system as an indicator of soil quality. Adv. Geoecol. 2002, 35, 69–80. [Google Scholar]
- Vidal, I. Fertirrigación Cultivos y Frutales; Publications of Department of Soils and Natural Resources, Faculty of Agronomy, Universidad de Concepción: Chillan, Chile, 2007; Volume 1, p. 118. [Google Scholar]
- Reynolds, W.; Yang, X.; Drury, C.; Zhang, T.; Tan, C. Effects of selected conditioners and tillage on the physical quality of a clay loam soil. Can. J. Soil Sci. 2003, 83, 381–393. [Google Scholar] [CrossRef]
- Lal, R.; Elliot, W. Erodibility and Erosivity. In Soil Erosion Research Methods, 2nd ed.; Soil and Water Conservation Society (U.S.), Ed.; St. Lucie Press: Boca Raton, FL, USA, 1994; p. 340. [Google Scholar] [CrossRef]
- Carter, M.R. Soil Quality for Sustainable Land Management: Organic Matter and Aggregation Interactions that Maintain Soil Functions. Agron. J. 2002, 94, 38–47. [Google Scholar] [CrossRef]
- Villarroel, R.B. Diagnóstico de la Fertilidad del Suelo; Instituto de Investigaciones Agropecuarias–Centro Regional de Investigación Remehue Serie Acta: Osorno, Chile, 2000; p. 71. [Google Scholar]
- Villaroel, R. Análisis de Suelo, Metodología e Interpretación; INIA: Osorno, Chile, 1989; p. 79. [Google Scholar]
- Xu, X.; Thornton, P.E.; Post, W.M. A global analysis of soil microbial biomass carbon, nitrogen and phosphorus in terrestrial ecosystems: Global soil microbial biomass C, N and P. Glob. Ecol. Biogeogr. 2013, 22, 737–749. [Google Scholar] [CrossRef]
- Moebius-Clune, B.N.; Moebius-Clune, D.J.; Gugino, B.K.; Idowu, O.J.; Schindelbeck, R.R.; Ristow, A.J.; van Es, H.M.; Thies, J.E.; Shayler, H.A.; McBride, M.B.; et al. Comprehensive Assessment of Soil Health: The Cornell Framework Manual; Cornell University: Ithaca, NY, USA, 2016; Edition 3.1; Available online: https://www.css.cornell.edu/extension/soil-health/manual.pdf (accessed on 18 January 2023).
- Stott, D.E.; Andrews, S.S.; Liebig, M.A.; Wienhold, B.J.; Karlen, D.L. Evaluation of β-Glucosidase Activity as a Soil Quality Indicator for the Soil Management Assessment Framework. Soil Sci. Soc. Am. J. 2010, 74, 107–119. [Google Scholar] [CrossRef]
- Nannipieri, P.; Kandeler, E.; Ruggiero, P. Enzyme Activities and Microbiological and Biochemical Processes in Soil. In Enzymes in the Environment; Burns, R., Dick, R., Eds.; CRC Press: Boca Raton, FL, USA, 2002; Volume 84, pp. 1–34. [Google Scholar] [CrossRef]
- Maulood, P.; Darwesh, D. Soil Quality Index Models for Assessing WalnutOrchards in Northern Erbil Province, Iraq. Pol. J. Environ. Stud. 2020, 29, 1275–1285. [Google Scholar] [CrossRef] [PubMed]


| System | ||||
|---|---|---|---|---|
| SIND | SPS 0–5 | SPS 5–20 | AGROFRST 0–5 | AGROFRST 5–20 |
| A INFVk * | 19.41 ± 1.2 × 10−5 | 19.41 ± 1.2 × 10−5 | 13.08 ± 1.3 × 10−5 | 13.08 ± 1.3 × 10−5 |
| WHC ** | 70.83 ± 1.67 a | 65.97 ± 1.86 a | 74.44 ± 0.78 a | 65.97 ± 2.75 a |
| BD *** | 0.57 ± 0.01 a | 0.61 ± 0.01 a | 0.65 ± 0.02 b | 0.73 ± 0.02 b |
| PD *** | 1.91 ± 0.01 a | 2.09 ± 0.01 a | 2.0 ± 0.01 a | 2.16 ± 0.02 a |
| B POR (%) ** | 70.21 ± 0.79 a | 70.83 ± 0.87 a | 67.09 ± 0.71 a | 66.21 ± 0.66 a |
| WSA ** | 49.20 ± 0.05 a | 48.65 ± 0.02 a | 49.74 ± 0.43 a | 48.82 ± 0.20 a |
| PENRES **** | 100–200 ± 0.00 a | 100–200 ± 0.00 a | 100–200 ± 0.00 a | 100–200 ± 0.00 a |
| System | ||||
|---|---|---|---|---|
| SIND | SPS 0–5 | SPS 5–20 | AGROFRST 0–5 | AGROFRST 5–20 |
| •pH | 6.03 ± 0.07 a | 6.06 ± 0.06 a | 5.62 ± 0.17 a | 5.51 ± 0.04 a |
| SOC * | 13.94 ± 0.02 a | 10.65 ± 0.16 b | 14.63 ± 0.03 a | 11.95 ± 0.15 c |
| C:N | 12.73 ± 0.25 a | 13.48 ± 1.00 a | 10.47 ± 0.15 a | 10.90 ± 0.11 a |
| N * | 1.10 ± 0.02 a | 0.80 ± 0.05 b | 1.40 ± 0.02 c | 1.10 ± 0.02 a |
| •P+ ** | 3.11 ± 0.05 a | 1.89 ± 0.13 a | 4.88 ± 1.12 a | 1.83 ± 0.17 a |
| NH4+ ** | 12.13 ± 0.53 a | 9.30 ± 0.37 b | 11.62 ± 0.64 ab | 9.59 ± 0.08 ab |
| •N-NO3− ** | 3.32 ± 0.34 ab | 2.46 ± 0.41 b | 28.70 ± 4.15 a | 22.69± 3.12 ab |
| K ** | 47.37 ± 1.57 ab | 30.72 ± 4.58 c | 51.33 ± 1.69 a | 37.58 ± 0.11 bc |
| •Ca2++ ** | 5.48 ± 2.29 a | 0.97 ± 0.22 a | 6.66 ± 0.59 a | 1.50 ± 0.32 a |
| Mg2+ ** | 0.20 ± 0.01 a | 0.08 ± 0.03 b | 0.36 ± 0.03 c | 0.15 ± 0.07 ab |
| S ** | 7.71 ± 0.44 ab | 2.10 ± 0.10 c | 7.80 ± 0.49 a | 2.87 ± 0.40 c |
| •ECEC *** | 9.22 ± 0.38 a | 1.77 ± 0.52 b | 7.68 ± 1.69 ab | 3.19 ± 0.30 ab |
| Na * | 1.61 × 10−6 ± 0.00 a | 1.61 × 10−6 ± 0.00 a | 1.38 × 10−6 ± 0.00 a | 1.38 × 10−6 ± 0.00 a |
| •AlEXCH *** | 0.09 ± 0.01 ab | 0.18 ± 0.01 a | 0.14 ± 0.03 b | 0.29 ± 0.15 ab |
| •AlSAT * | 0.86 ± 0.05 b | 4.68 ± 1.57 ab | 1.57 ± 0.21 ab | 6.45 ± 0.45 a |
| System | ||||
|---|---|---|---|---|
| SIND | SPS 0–5 | SPS 5–20 | AGROFRST 0–5 | AGROFRST 5–20 |
| •MSOC | 1889.50 ± 373.68 b | 315.23 ± 292.00 ab | 463.43 ± 109.34 ab | 69.6 ± 23.76 a |
| MN | 196.67 ± 72.74 a | 46.77 ± 19.16 a | 68.77 ± 6.88 a | 10.30 ± 1.06 a |
| MRESP | 0.18 ± 0.01 a | 0.13 ± 0.01 a | 0.14 ± 0.03 a | 0.13 ± 0.01 a |
| •β-GLU | 2.43 ± 0.23 b | 1.15 ± 0.32 ab | 1.34 ± 0.60 ab | 0.94 ± 0.26 a |
| URS | 1247.55 ± 1.33 a | 994.69 ± 2.20 a | 1063.50 ± 2.68 a | 650.07 ± 0.12 a |
| PHOSP | 713.62 ± 0.09 a | 699.71 ± 0.07 a | 759.52 ± 0.02 a | 740.05 ± 0.04 a |
| FDA | 55.85 ± 0.51 a | 56.74 ± 1.15 a | 54.64 ± 4.71 a | 39.93 ± 3.48 a |
| NMIN | 19.36 ± 1.93 ab | 1.78 ± 2.13 a | 18.21 ± 4.86 ab | 8.41 ± 1.49 ab |
| System | ||
|---|---|---|
| SPS 0–20 | AGROFRST 0–20 | |
| Previous SOC (%) | 8.5 * | 9.2 ** |
| SOC2019 | 11.5 | 12.6 |
| Previous SOC stock (Mg ha−1) | 61.09 * | 79.92 ** |
| SOC stock 2019 (Mg ha−1) | 83.02 | 107.44 |
| Theoretical annual CSEQ | 5.48 | 5.50 |
| Systems | ||||
|---|---|---|---|---|
| SPS 0–5 | SPS 5–20 | AGROFRST 0–5 | AGROFRST 5–20 | |
| (5) Physical SQI | 64.26 | 64.26 | 64.26 | 64.26 |
| (6) Chemical SQI | 9.09 | 1.52 | 28.79 | 16.67 |
| (7) Microbiological SQI | 41.86 | 25.58 | 41.86 | 23.26 |
| (8) Global SQI | 38.41 | 30.46 | 44.98 | 34.74 |
| (9) % SQI | 37.9 | 44.8 | 31.0 | 37.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ortiz, J.; Dube, F.; Neira, P.; Hernández Valera, R.R.; de Souza Campos, P.M.; Panichini, M.; Pérez-San Martín, A.; Stolpe, N.B.; Zagal, E.; Curaqueo, G. Comparative Study between Silvopastoral and Agroforest Systems on Soil Quality in a Disturbed Native Forest of South-Central Chile. Agronomy 2023, 13, 2683. https://doi.org/10.3390/agronomy13112683
Ortiz J, Dube F, Neira P, Hernández Valera RR, de Souza Campos PM, Panichini M, Pérez-San Martín A, Stolpe NB, Zagal E, Curaqueo G. Comparative Study between Silvopastoral and Agroforest Systems on Soil Quality in a Disturbed Native Forest of South-Central Chile. Agronomy. 2023; 13(11):2683. https://doi.org/10.3390/agronomy13112683
Chicago/Turabian StyleOrtiz, Juan, Francis Dube, Pablo Neira, Rafael R. Hernández Valera, Pedro M. de Souza Campos, Marcelo Panichini, Andrés Pérez-San Martín, Neal B. Stolpe, Erick Zagal, and Gustavo Curaqueo. 2023. "Comparative Study between Silvopastoral and Agroforest Systems on Soil Quality in a Disturbed Native Forest of South-Central Chile" Agronomy 13, no. 11: 2683. https://doi.org/10.3390/agronomy13112683
APA StyleOrtiz, J., Dube, F., Neira, P., Hernández Valera, R. R., de Souza Campos, P. M., Panichini, M., Pérez-San Martín, A., Stolpe, N. B., Zagal, E., & Curaqueo, G. (2023). Comparative Study between Silvopastoral and Agroforest Systems on Soil Quality in a Disturbed Native Forest of South-Central Chile. Agronomy, 13(11), 2683. https://doi.org/10.3390/agronomy13112683






