Climate Change and an Agronomic Journey from the Past to the Present for the Future: A Past Reference Investigation and Current Experiment (PRICE) Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Descriptions of the PRICE
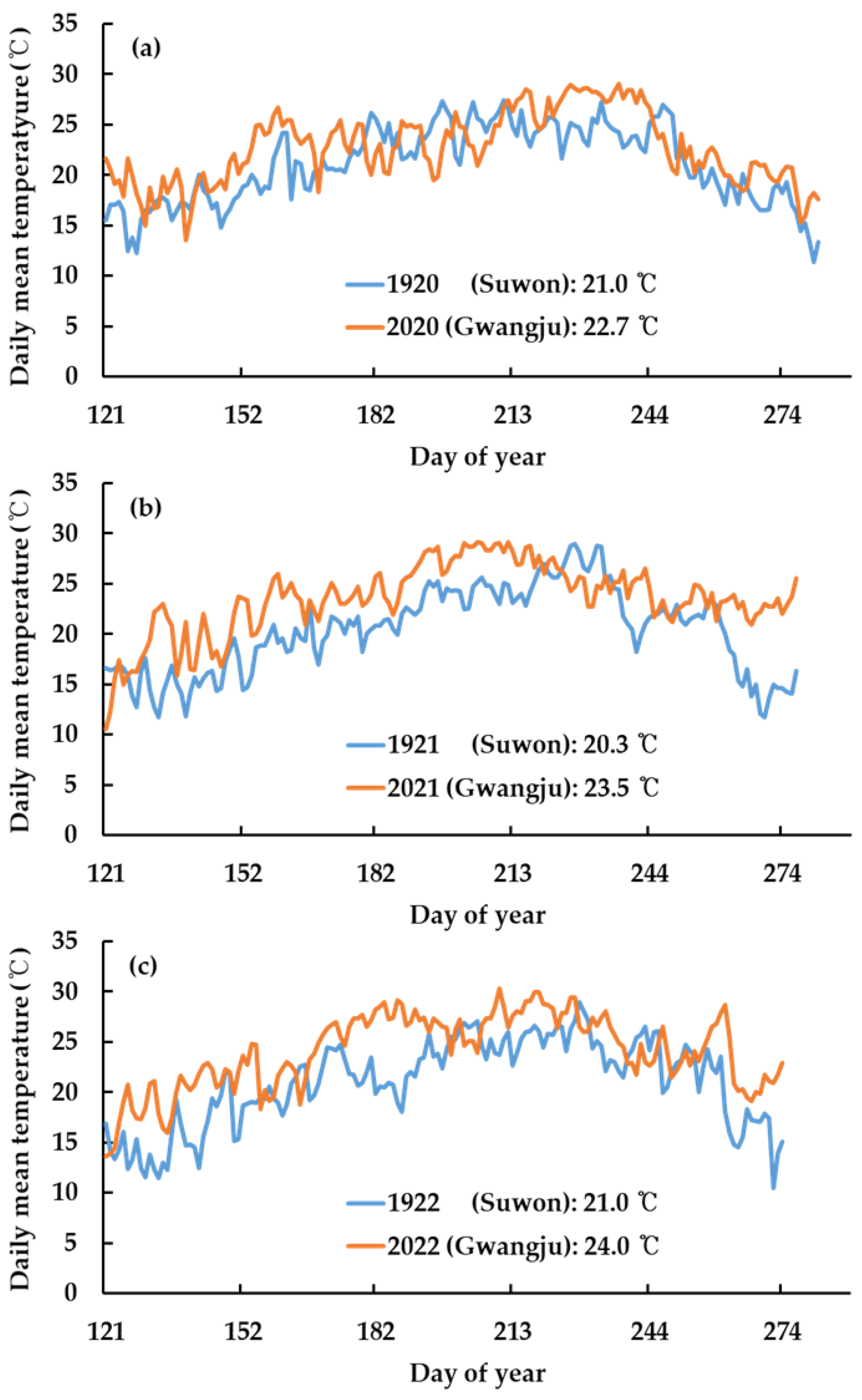
2.2. Atmospheric [CO2] and Temperature in the PRICE
2.3. Agronomic Practices in the PRICE
2.4. Measurements of Crop Parameters and Yield
2.5. Experimental Design and Statistical Analysis
3. Results
3.1. Plant Height and Tiller Number
3.2. Flowering Phenology and Shoot Biomass
3.3. Grain Yield and Harvest Index
4. Discussion
4.1. Changes in Rice Phenotypic Traits over the Last 100 Years
4.2. Changes in Rice Shoot Biomass, Grain Yield, and HI over the Last 100 Years
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IPCC. Climate Change 2021: The Physical Science Basis. In Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Masson-Delmotte, V., Zhai, P., Pirani, A., Connors, S.L., Péan, C., Berger, S., Caud, N., Chen, Y., Goldfarb, L., Gomis, M.I., et al., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2021. [Google Scholar]
- KMA. Korean Climate Change Assessment Report 2020: Summary for Policymakers. In The Physical Science Basis; Kim, N.W., Lee, J.H., Cho, G.H., Kim, S.H., Eds.; Korean Meteorology Administration: Seoul, Republic of Korea, 2020; ISBN 978-89-654715-7-9. [Google Scholar]
- NIMS. Report of Global Atmosphere Watch 2022: The Greenhouse Gases. In Atmospheric CO2 Concentrations; Lee, S.R., Lee, S.J., Kim, J., Eds.; National Institute of Meteorological Sciences: Jeju-do, Republic of Korea, 2023; pp. 6–13. ISBN 11-1360000-000991-10. [Google Scholar]
- Neftel, A.; Moor, E.; Oeschger, H.; Stauffer, B. Evidence from polar ice cores for the increase in atmospheric CO2 in the past two centuries. Nature 1985, 315, 45–47. [Google Scholar] [CrossRef]
- Etheridge, D.M.; Steele, L.P.; Langenfelds, R.L.; Francey, R.J.; Barnola, J.-M.; Morgan, V.I. Historical CO2 records from the Law Dome DE08, DE08-2, and DSS ice cores. In Trends a Compendium of Data on Global Change; Carbon Dioxide Information Analysis Centre, Oak Ridge National Laboratory, US Department of Energy: Oak Ridge, TN, USA, 1998. [Google Scholar]
- Kimball, B.A. Carbon dioxide and agricultural yield: An assemblage and analysis of 430 prior observations. Agron. J. 1983, 75, 779–788. [Google Scholar] [CrossRef]
- Allen, L.H.; Boote, K.J.; Jones, J.W.; Jones, P.H.; Valle, R.R.; Acock, B.; Rogers, H.H.; Dahlman, R.C. Response of vegetation to rising carbon dioxide: Photosynthesis, biomass, and yield of soybean. Glob. Biogeochem. Cycles 1987, 1, 1–14. [Google Scholar] [CrossRef]
- Allen, L.H. Free-air CO2 enrichment field experiments: An historical overview. Crit. Rev. Plant Sci. 1992, 11, 121–134. [Google Scholar] [CrossRef]
- Horie, T.; Nakagawa, H.; Nakano, J.; Hamotani, K.; Kim, H.Y. Temperature gradient chambers for research on global environment change. III. A system designed for rice in Kyoto, Japan. Plant Cell Environ. 1995, 18, 1064–1069. [Google Scholar] [CrossRef]
- Moya, T.B.; Ziska, L.H.; Weldon, C.; Quilang, J.E.P.; Jones, P. Microclimate in open-top chambers: Implications for predicting climate change effects on rice production. Trans. ASABE 1997, 40, 739–747. [Google Scholar] [CrossRef]
- Leadley, P.W.; Niklaus, P.; Stocker, R.; Körner, C. Screen-aided CO2 control (SACC): A middle ground between FACE and open-top chambers. Acta Oecol. 1997, 18, 207–219. [Google Scholar] [CrossRef]
- Okada, M.; Lieffering, H.; Nakamura, H.; Yoshimoto, M.; Kim, H.Y.; Kobayashi, K. Free-air CO2 enrichment (FACE) using pure CO2 injection: System description. New Phytol. 2001, 150, 251–260. [Google Scholar] [CrossRef]
- Kimball, B.A.; Conley, M.M.; Wang, S.; Lin, X.; Luo, C.; Morgan, J.; Smith, D. Infrared heater arrays for warming ecosystem field plots. Glob. Chang. Biol. 2008, 14, 309–320. [Google Scholar] [CrossRef]
- Cai, C.; Yin, X.; He, S.; Jiang, W.; Si, C.; Struik, P.C.; Luo, W.; Li, G.; Xie, Y.; Xiong, Y.; et al. Responses of wheat and rice to factorial combinations of ambient and elevated CO2 and temperature in FACE experiments. Glob. Chang. Biol. 2016, 22, 856–874. [Google Scholar] [CrossRef]
- Baker, J.T.; Allen, L.H.; Boote, K.J. Growth and yield response of rice to carbon dioxide concentration. J. Agri. Sci. 1990, 115, 313–320. [Google Scholar] [CrossRef]
- Baker, J.T.; Allen, L.H.; Boote, K.J. Temperature effects on rice at elevated CO2 concentration. J. Exp. Bot. 1992, 43, 959–964. [Google Scholar] [CrossRef]
- Sakai, H.; Yagi, K.; Kobayashi, K.; Kawashima, S. Rice carbon balance under elevated CO2. New Phytol. 2001, 150, 241–249. [Google Scholar] [CrossRef]
- Cheng, W.; Sakai, H.; Yagi, K.; Hasegawa, T. Interactions of elevated [CO2] and night temperature on rice growth and yield. Agri. For. Met. 2009, 149, 51–58. [Google Scholar] [CrossRef]
- Allen, L.H.; Kimball, B.A.; Bunce, J.A.; Yoshimoto, M.; Harazono, Y.; Baker, J.T.; Boote, K.J.; White, J.W. Fluctuations of CO2 in Free-Air CO2 Enrichment (FACE) depress plant photosynthesis, growth, and yield. Agric. For. Meteorol. 2020, 284, 107899. [Google Scholar] [CrossRef]
- Kim, H.-Y.; Horie, T.; Nakagawa, H.; Wada, K. Effects of elevated CO2 concentration and high temperature on growth and yield. 1. The effect on development, dry matter production and some growth characteristics. Jpn. J. Crop Sci. 1996, 65, 634–643. [Google Scholar] [CrossRef]
- Kim, H.-Y.; Horie, T.; Nakagawa, H.; Wada, K. Effects of elevated CO2 concentration and high temperature on growth and yield. 2. The effect on yield and its components of Ahihikari rice. Jpn. J. Crop Sci. 1996, 65, 644–651. [Google Scholar] [CrossRef]
- Kim, H.-Y.; Lim, S.-S.; Kwak, J.-H.; Lee, D.-S.; Lee, S.-M.; Ro, H.-M.; Choi, W.-J. Dry matter and nitrogen accumulation and partitioning in rice (Oryza sativa L.) exposed to experimental warming with elevated CO2. Plant Soil 2011, 342, 59–71. [Google Scholar] [CrossRef]
- Choi, W.J.; Lee, M.S.; Choi, J.E.; Yoon, S.; Kim, H.-Y. How do weather extremes affect rice productivity in a changing climate? An answer to episodic lack of sunshine. Glob. Chang. Boil. 2013, 19, 1300–1310. [Google Scholar] [CrossRef]
- Zaher, M.A.B.; Kumagai, E.; Yabiku, T.; Nakajima, M.; Matsunami, T.; Matsuyama, N.; Thinh, N.C.; Hasegawa, T.; Kawasaki, M. Effects of elevated atmospheric CO2 concentration on growth and photosynthesis in eddo at two different air temperatures. Plant Prod. Sci. 2021, 24, 363–373. [Google Scholar] [CrossRef]
- Oh-e, I.; Saitoh, K.; Kuroda, T. Effects of high temperature on growth, yield and dry-matter production of rice grown in the paddy field. Plant Prod. Sci. 2007, 10, 412–422. [Google Scholar] [CrossRef]
- Ziska, L.H.; Namuco, O.; Moya, T.; Quilang, J. Growth and yield response of field-grown tropical rice to increasing carbon dioxide and air temperature. Agron. J. 1997, 89, 45–53. [Google Scholar] [CrossRef]
- Chaturvedi, A.K.; Bahuguna, R.N.; Shah, D.; Pal, M.; Jagadish, S.V.K. High temperature stress during flowering and grain filling offsets beneficial impact of elevated CO2 on assimilate partitioning and sink strength in rice. Sci. Rep. 2017, 7, 8277. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Li, Y.; Yu, Z.; Wang, G.; Liu, X.; Liu, J.; Liu, J.; Zhang, X.; Yin, K.; Jin, J. Co-elevation of atmospheric [CO2] and temperature alters photosynthetic capacity and instantaneous water use efficiency in rice cultivars in a cold-temperature region. Front. Plant Sci. 2022, 13, 1037720. [Google Scholar] [CrossRef]
- Kim, H.-Y.; Lieffering, M.; Miura, S.; Kobayashi, K.; Okada, M. Growth and nitrogen uptake of CO2-enriched rice under field conditions. New Phytol. 2001, 150, 223–229. [Google Scholar] [CrossRef]
- Kim, H.-Y.; Lieffering, M.; Kobayashi, K.; Okada, M.; Miura, S. Seasonal changes in the effects of elevated CO2 on rice atthree levels of nitrogen supply: A free air CO2 enrichment(FACE) experiment. Glob. Chang. Biol. 2003, 9, 826–837. [Google Scholar] [CrossRef]
- Kim, H.-Y.; Lieffering, M.; Kobayashi, K.; Okada, M.; Mitchell, M.W.; Gumpertz, M. Effects of free-air CO2 enrichment and nitrogen supply on the yield of temperate paddy rice crops. Field Crops Res. 2003, 83, 261–270. [Google Scholar] [CrossRef]
- Long, S.P.; Ainsworth, E.A.; Leakey, A.D.B.; Nösberger, J.; Ort, D.R. Food for thought: Lower-than-expected crop yield stimulation with rising CO2 concentrations. Science 2006, 312, 1918–1921. [Google Scholar] [CrossRef]
- Yang, L.; Huang, J.; Yang, H.; Zhu, J.; Liu, H.; Dong, G.; Liu, G.; Han, Y.; Wang, Y. The impact of free-air CO2 enrichment (FACE) and N supply on yield formation of rice crops with large panicle. Field Crops Res. 2006, 98, 141–150. [Google Scholar] [CrossRef]
- Yang, L.; Huang, J.; Yang, H.; Dong, G.; Liu, G.; Zhu, J.; Wang, Y. Seasonal changes in the effects of free-air CO2 enrichment (FACE) on dry matter production and distribution of rice (Oryza sativa L.). Field Crops Res. 2006, 98, 12–19. [Google Scholar] [CrossRef]
- Liu, H.; Yang, L.; Wang, Y.; Huang, J.; Zhu, J.; Yunxia, W.; Dong, G.; Liu, G. Yield formation of CO2-enriched hybrid cultivar Shanyou 63 under fully open-air field conditions. Field Crops Res. 2008, 108, 93–100. [Google Scholar] [CrossRef]
- Yang, L.; Liu, H.; Wang, Y.; Zhu, J.; Hung, J.; Liu, G.; Dong, G.; Wang, Y. Yield formation of CO2-enriched inter-subspecific hybrid rice cultivar Liangyoupeijiu under fully open-air field condition in a warm sub-tropical climate. Agric. Ecosyst. Environ. 2009, 129, 193–200. [Google Scholar] [CrossRef]
- Hasegawa, T.; Sakai, H.; Tokida, T.; Nakamura, H.; Zhu, C.; Usui, Y.; Yoshimoto, M.; Fukuoka, M.; Wakatsuki, H.; Katayanagi, N.; et al. Rice cultivar responses to elevated CO2 at two free-air CO2 enrichment (FACE) sites in Japan. Funct. Plant Biol. 2013, 40, 148–159. [Google Scholar] [CrossRef]
- Zhu, C.; Xu, X.; Wang, D.; Zhu, J.; Liu, G. An indica rice genotype showed a similar yield enhancement to that of hybrid rice under free air carbon dioxide enrichment. Sci. Rep. 2015, 5, 12719. [Google Scholar] [CrossRef]
- Sakai, H.; Tokida, T.; Usui, Y.; Nakamura, H.; Hasegawa, T. Yield responses to elevated CO2 concentration among Japanese rice cultivars released since 1882. Plant Prod. Sci. 2019, 22, 352–366. [Google Scholar] [CrossRef]
- Hu, S.; Wang, Y.; Yang, L. Response of rice yield traits to elevated atmospheric CO2 concentration and its interaction with cultivar, nitrogen application rate and temperature: A meta-analysis of 20 years FACE studies. Sci. Total Environ. 2021, 764, 142797. [Google Scholar] [CrossRef]
- Hu, S.; Chen, W.; Tong, K.; Wang, Y.; Jing, L.; Wang, Y.; Yang, L. Response of rice growth and leaf physiology to elevated CO2 concentrations: A meta-analysis of 20-year FACE studies. Sci. Total Environ. 2022, 807, 151017. [Google Scholar] [CrossRef] [PubMed]
- Ainsworth, E.A.; Long, S.P. 30 years of free-air carbon dioxide enrichment (FACE): What have we learned about future crop productivity and its potential for adaptation? Glob. Chang. Biol. 2021, 27, 27–49. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yang, T.; Chen, J.; Bell, S.M.; Wu, S.; Jiang, Y.; Sun, Y.; Zeng, Y.; Zeng, Y.; Pan, X.; et al. Effects of free-air temperature increase on grain yield and greenhouse gas emissions in a double rice cropping system. Field Crops Res. 2022, 281, 108489. [Google Scholar] [CrossRef]
- Wang, W.; Cai, C.; Lam, S.K.; Liu, G.; Zhu, J. Elevated CO2 cannot compensate for japonica grain yield losses under increasing air temperature because of the decrease in spikelet density. Eur. J. Agron. 2018, 99, 21–29. [Google Scholar] [CrossRef]
- Wang, W.; Cai, C.; He, J.; Gu, J.; Zhu, G.; Zhang, W.; Zhu, J.; Liu, G. Yield, dry matter distribution and photosynthetic characteristics of rice under elevated CO2 and increased temperature conditions. Field Crops Res. 2020, 248, 107605. [Google Scholar] [CrossRef]
- Yuan, M.; Cai, C.; Wang, X.; Li, G.; Wu, G.; Wang, J.; Geng, W.; Liu, G.; Zhu, J.; Sun, X. Warm air temperatures increase photosynthetic acclimation to elevated CO2 concentrations in rice under field conditions. Field Crops Res. 2021, 262, 108036. [Google Scholar] [CrossRef]
- Ziska, L.H.; Bunce, J.A. Predicting the impact of changing CO2 on crop yields: Some thoughts on food. New Phytol. 2007, 175, 607–618. [Google Scholar] [CrossRef] [PubMed]
- Tubiello, F.N.; Amthor, J.S.; Boote, K.J.; Donatelli, M.; Easterling, W.; Fischer, G.; Gifford, R.M.; Howden, M.; Reilly, J.; Rosenzweig, C. Crop response to elevated CO2 and world food supply. A comment on “Food for Thought…” by Long et al., Science 312, 1918–1921 2006. Eur. J. Agron. 2007, 26, 215–223. [Google Scholar] [CrossRef]
- Ainsworth, E.A. Rice production in a changing climate: A meta-analysis of responses to elevated carbon dioxide and elevated ozone concentration. Glob. Chang. Biol. 2008, 14, 1642–1650. [Google Scholar] [CrossRef]
- Aronson, E.L.; McNulty, S.G. Appropriate experimental ecosystem warming methods by ecosystem, objective, and practicality. Agric. For. Meteorol. 2009, 149, 1791–1799. [Google Scholar] [CrossRef]
- Amthor, J.S.; Hanson, P.J.; Norby, R.J.; Wullschleger, S.D. A comment on ‘‘Appropriate experimental ecosystem warming methods by ecosystem, objective, and practicality’’ by Aronson and McNulty. Agric. For. Meteorol. 2010, 150, 497–498. [Google Scholar] [CrossRef]
- Aronson, E.L.; McNulty, S.G. Reply to comment on ‘‘Appropriate experimental ecosystem warming methods by ecosystem, objective, and practicality’’ by Aronson and McNulty. Agric. For. Meteorol. 2010, 150, 499–500. [Google Scholar] [CrossRef]
- Kimball, B.A. Comment on the comment by Amthor et al. on “Appropriate experimental ecosystem warming methods” by Aronson and McNulty. Agric. For. Meteorol. 2011, 151, 420–424. [Google Scholar] [CrossRef]
- Wang, J.; Wang, C.; Chen, N.; Xiong, Z.; Wolfe, D.; Zou, J. Response of rice production to elevated [CO2] and its interaction with rising temperature or nitrogen supply: A meta-analysis. Clim. Chang. 2015, 130, 529–543. [Google Scholar] [CrossRef]
- Allen, L.H.; Boote, K.J.; Jones, J.W.; Jones, P.H.; Pickering, N.B.; Baker, J.T.; Vu1, J.C.V.; Gesch, R.W.; Thomas, J.M.G.; Prasad, P.V.V. Sunlit, controlled-environment chambers are essential for comparing plant responses to various climates. Agron. J. 2020, 112, 4531–4549. [Google Scholar] [CrossRef]
- Omae, I. Effect of various nitrogen fertilizers on rice. Bull. Model Farm 1926, 6, 491–512. [Google Scholar]
- Dusenge, M.E.; Duarte, A.G.; Way, D.A. Plant carbon metabolism and climate change: Elevated CO2 and temperature impacts on photosynthesis, photorespiration and respiration. New Phytol. 2019, 221, 32–49. [Google Scholar] [CrossRef] [PubMed]
- Misu, H.; Shirosida, T. Nitrogen application study on rice (Tamanishiki and Nakate Ginbouzu). Bull. Agric. Expt. Station 1938, 10, 470–475. [Google Scholar]
- Experimental and Research Report. In Agricultural Chemistry, Soil Survey, and Soil Fertility in 1972; Part 1; RDA: Suwon, Republic of Korea, 1973; p. 32.
- NAIST (National Institute of Agricultural Science and Technology). Taxonomical Classification of Korean Soil; RDA: Suwon, Republic of Korea, 2000. [Google Scholar]
- Rice in Chosen. In Chosen Product; Part 1 (4); Chosen Shokusan Bank: Seoul, Republic of Korea, 1928; pp. 33–36.
- Lee, H.S.; Hwang, W.H.; Jeong, J.H.; Yang, S.Y.; Lim, Y.H.; Choi, M.G.; Jeong, N.J.; Lee, C.G.; Choi, K.J. Studies on the temperature response and critical day-length affecting the heading date of major cultivating rice varieties in recent Korean paddy field. Korean J. Crop Sci. 2019, 64, 323–336. [Google Scholar] [CrossRef]
- Long, S.P.; Elizabith, A.A.; Rogers, A.; Ort, D.R. Rising atmospheric carbon dioxide: Plants FACE the future. Annu. Rev. Plant Biol. 2004, 55, 591–628. [Google Scholar] [CrossRef]
- Kimball, B.A. Crop responses to elevated CO2 and interactions with H2O, N, and temperature. Curr. Opin. Plant Biol. 2016, 31, 36–43. [Google Scholar] [CrossRef]
- Chen, J.; Liu, Y.; Zhou, W.; Zhang, J.; Pan, T. Effects of climate change and crop management on changes in rice phenology in China from 1981 to 2010. J. Sci. Food Agric. 2021, 101, 6311–6319. [Google Scholar] [CrossRef]
- Zhang, T.; Huang, Y.; Yang, X. Climate warming over the past three decades has shortened rice growth duration in China and cultivar shifts have further accelerated process for late rice. Glob. Chang. Biol. 2013, 19, 563–570. [Google Scholar] [CrossRef]
- Wang, H.; Ghosh, A.; Linquist, B.A.; Hijmans, R.J. Satellite-based observations reveal effects of weather variation on rice phenology. Remote Sens. 2020, 12, 1522. [Google Scholar] [CrossRef]
- Horie, T. Global warming and rice production in Asia: Modeling, impact prediction and adaptation. Proc. Jpn. Acad. Ser. 2019, 95, 211–245. [Google Scholar] [CrossRef]
- Bishop, K.A.; Leakey, A.D.B.; Ainsworth, E.A. How seasonal temperature or water inputs affect the relative response of C3 crops to elevated [CO2]: A global analysis of open top chamber and free air CO2 enrichment studies. Food Energy Secur. 2014, 3, 33–45. [Google Scholar] [CrossRef]
- Peng, S.; Huang, J.; Sheehy, J.E.; Cassman, K.G. Rice yields decline with higher night temperature from global warming. Proc. Natl. Acad. Sci. USA 2004, 101, 9971–9975. [Google Scholar] [CrossRef] [PubMed]
- Lewis, J.D.; Olszyk, D.; Tingey, D.T. Seasonal patterns of photosynthetic light response in Douglas-fir seedlings subjected to elevated atmospheric CO2 and temperature. Tree Physiol. 1999, 19, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Matsui, T.; Omasa, K.; Horie, T. High temperature-induced spikelet sterility of japonica rice at flowering in relation to air temperature, humidity and wind velocity conditions. Jpn. J. Crop. Sci. 1997, 66, 449–455. [Google Scholar] [CrossRef]
- Nakagawa, H.; Horie, T.; Matsui, T. Effects of climate change on rice production and adaptive technologies. In Rice Science: Innovations and Impacts for Livelihood; Mew, T.W., Brar, D.S., Peng, S., Dawe, D., Hardy, B., Eds.; IRRI: Los Banos, Philippines, 2003; pp. 635–658. [Google Scholar]
- Poorter, H.; Fiorani, F.; Pieruschka, R.; Wojciechowski, T.; van der Putten, W.H.; Kleyer, M.; Schurr, U.; Postma, J. Pampered inside, pestered outside? Differences and similarities between plants growing in controlled conditions and in the field. New Phytol. 2016, 212, 838–855. [Google Scholar] [CrossRef]








| Parameters | RE1920–1922 | CE2020–2022 |
|---|---|---|
| pH | 5.3 b | 6.3 |
| Total C (g kg−1) | 11.5 b | 13.0 |
| Total N (g kg−1) | 1.10 a | 1.04 |
| Available P (mg P2O5 kg−1) | 5.9 b | 1.9 |
| CEC (cmol kg−1) | 7.8 b | 12.8 |
| Series | Gangseo | Seogcheon |
| Texture | Coarse Loamy | Coarse Loamy |
| Agronomic Practices | Descriptions | |
|---|---|---|
| RE1920–1922 | CE2020–2022 | |
| Cultivar used | Tamanishiki (Oryza sativa L.) | Used the same cultivar as that in the RE1920–1922 |
| Seed selection | Used salt solution with a density of 1.13 | Conducted using the same methodology as that in the RE1920–1922 |
| Nursery bed preparation in paddy field | On 25–28 April, applied compost (N 0.6%) at 1.10 kg m−2 and soybean meal (N 6.78%) at 113.6 g m−2 | On 25–28 April, applied compost (N 1.5%) at 0.45 kg m−2 and soybean meal (N 8.06%) at 95.6 g m−2 |
| Sowing date | Unclear, but given that the duration of raising the seedlings at this time was around 40 days and that planting date ranged from 13 to 16 June, it was estimated to be approximately 5 May | On 5 May across 2020–2022 |
| Sowing density | 273 mL m−2 (presoaked and germinated seed) | Conducted using the same methodology as that in the RE1920–1922 |
| Raising of seedlings | In a traditional flooded nursery in a paddy field | Conducted using the same methodology as that in the RE1920–1922 |
| Additional fertilizer for raising seedlings | 3 weeks after sowing, applied N at 3.5 g m−2 in the form of (NH4)2SO4 and human manure at 1.6 L m−2 | 3 weeks after sowing, applied N at 3.5 g m−2 in the form of (NH4)2SO4, 1.5 g N m−2 added as a proxy of human manure, which we failed to procure due to coronavirus disease 2019 |
| Soil preparation | Soils from paddy field under non-fertilized conditions cropped for several years | Soils from paddy fields under non-fertilized conditions cropped for 3 years |
| Pot preparation and preconditioning | In 1/2000 a Wagner’s pots, filled with gravel up to 3.0 cm from the bottom, and then filled with soil (11.2 kg pot−1) mixed with limestone powder (10 g pot−1) in late May. These pots were kept flooded for 2 weeks | Conducted using the same methodology as that in the RE1920–1922 |
| Fertilizing | 3 days before transplanting, all fertilizers (N:P:K = 0.75:0.75:0.75 g pot−1) were well mixed with flooded soil in pots Total amount of fertilizers applied only as a basal dressing | Conducted using the same methodology as that in the RE1920–1922 |
| Transplanting date and density | On 16 June 1920–1921 and on 13 June 1922; 5 seedlings hill−1 (1 hill pot−1) | Conducted using the same methodology as that in the RE1920–1922 |
| Irrigation and weeding | Carefully managed both the water and the weeds | Kept flooded up to 5 days before harvest and weeding frequently |
| Fertilizers | Sources | RE1920–1922 | CE2020–2022 | ||
|---|---|---|---|---|---|
| Content (%) | Application (g N–P–K pot−1) | Content (%) | Application (g N–P–K pot−1) | ||
| N | Ammonium sulfate | 20.77 | 0.75 | 22.43 | 0.75 |
| Chile saltpeter | 15.24 | 0.75 | 15.98 | 0.75 | |
| Calcium cyanamide | 17.54 | 0.75 | 24.73 | 0.75 | |
| Rice bran | 2.19 | 0.75 | 2.93 | 0.75 | |
| Bone meal | 4.14 | 0.75 | 2.79 | 0.75 | |
| Soybean meal | 6.78 | 0.75 | 8.06 | 0.75 | |
| Perilla seed meal | 5.10 | 0.75 | 4.92 | 0.75 | |
| Cottonseed meal | 6.32 | 0.75 | 8.03 | 0.75 | |
| Blood meal | 11.94 | 0.75 | 16.14 | 0.75 | |
| P | Calcium superphosphate | − | 0.75 | 16.0 | 0.75 |
| K | Potassium sulfate | − | 0.75 | 48.0 | 0.75 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Min, H.; Lee, H.-S.; Lee, C.-K.; Choi, W.-J.; Ha, B.-K.; Lee, H.; Shin, S.-H.; An, K.-N.; Kim, D.-K.; Kwon, O.-D.; et al. Climate Change and an Agronomic Journey from the Past to the Present for the Future: A Past Reference Investigation and Current Experiment (PRICE) Study. Agronomy 2023, 13, 2692. https://doi.org/10.3390/agronomy13112692
Min H, Lee H-S, Lee C-K, Choi W-J, Ha B-K, Lee H, Shin S-H, An K-N, Kim D-K, Kwon O-D, et al. Climate Change and an Agronomic Journey from the Past to the Present for the Future: A Past Reference Investigation and Current Experiment (PRICE) Study. Agronomy. 2023; 13(11):2692. https://doi.org/10.3390/agronomy13112692
Chicago/Turabian StyleMin, Hyunkyeong, Hyeon-Seok Lee, Chun-Kuen Lee, Woo-Jung Choi, Bo-Keun Ha, Hyeongju Lee, Seo-Ho Shin, Kyu-Nam An, Dong-Kwan Kim, Oh-Do Kwon, and et al. 2023. "Climate Change and an Agronomic Journey from the Past to the Present for the Future: A Past Reference Investigation and Current Experiment (PRICE) Study" Agronomy 13, no. 11: 2692. https://doi.org/10.3390/agronomy13112692





