Abstract
The Australian tomato Solanum orbiculatum ssp. orbiculatum is an edible bush tomato endemic to the more arid areas of Western Australia, South Australia, and the Northern Territory. Breeding system data indicate that the plants are potentially self-compatible but are unable to carry out spontaneous autogamy or agamospermy. The flower is protogynous, as the stigma become receptive to pollen germination while still in bud condition and the anthers do not release pollen immediately after anthesis. This arrangement is a simple and common way to avoid too much self-pollination, favours cross pollination, and would allow forced bud pollination for hybrid development. The floral structure and morphology of this species can also encourage cross pollination, as the stigma is mostly exserted above the anther’s tips. In an attempt to examine the hypothesis of a positive correlation between pollen grain size and style length, we found a statistically significant difference between the pollen size of the long-styled and short-styled flowers. Pollen in vitro germination and viability tests have been optimised to facilitate effective breeding work on this species. A modified Brewbake and Kwack (BK) medium supplemented with 20% sucrose and 2.5% PEG 4000 has been found to be the most efficient media components for the in vitro germination of viable pollen grains. Alternatively, Alexander’s and acetocarmine (1%) stains have shown the highest positive correlation with the in vitro pollen germination test and, therefore, can be used as quick tests for checking pollen viability. Moreover, pollen grains stored for three months under 4 °C and dry conditions can be used efficiently to effect fertilisation in breeding programs, as it can maintain more than 50% of the original viability. This study will contribute to understanding the evolution and systematic relationships of species and for founding effective conservation programs. Furthermore, understanding the reproductive biology of this species is also of interest because of its potential for tomato breeding.
1. Introduction
Many of the native species commonly known as bush tomatoes, such as Solanum orbiculatum, S. centrale, S. diversiflorum, S. phlomoides, S. chippendalei, S. aviculare, and S. laciniatum, were used as a food source by Indigenous Australians, and several species are in commercial production or evaluation as ‘bush tucker’ [1]. Solanum species are also required for mine site restoration in Australia, particularly because of the resurgence of mining activity in the arid zone, where the genus most commonly occurs. The species required for restoration include S. orbiculatum and S. diversiflorum, as both species are common and widespread components of the pre-mind vegetation [2,3].
The wild relatives of tomato that originated in South America have been exploited mostly as sources of resistance to major diseases. In Australia, there are over 60 Solanum species, which are endemic and are widely distributed in the most arid regions, including, but not limited to, Central Australia [4]. Many of these Solanum species, and particularly the edible ones, have adaptations to extremes of environment and can be considered as valuable sources of genes for tolerance to many abiotic stresses, such as drought conditions and high temperature [5].
A total of 19 diclinous Solanum species native to Australia have been studied in the field and laboratory to clarify the nature and evolution of andromonoecy, androdioecy, and dioecy in the genus Solanum [6]. These species are separable into two groups, based on sex expression, as discerned from floral morphology. The species in Group I are ten andromonoecious species, including the edible species S. chippendalei, S. diversiflorum, and S. phlomoides. Typically, these species bear inflorescences with a single, large basal hermaphroditic flower and 12–60 distal, smaller staminate flowers. The species in Group II are nine cryptically dioecious species, with morphologically hermaphrodite flowers producing pollen without pores (inaperturate)—thus rendering the flowers (and the plants that bear them) functionally female. This study has described the reproductive biology of both of these groups, confirmed andromonoecy for Group I and functional dioecy for Group II, and has discussed the evolution of andromonoecy and dioecy both in Solanum and in general. This study has also suggested that the andromonoecious condition was derived from hermaphroditic-flowered ancestors in part by hemisterilisation of flowers but largely by the addition of staminate flowers.
Solanum cowiei is a new cryptically dioecious species of bush tomato from the Northern Territory, Australia [7]. It has been found that the new species is cryptically dioecious via the production of inaperturate pollen grains in morphologically hermaphrodite flowers. Remarkable variations in the vegetative and reproductive characteristics within many Australian Solanum spp. have been observed, but not appropriately reported. This study is concerned with investigating the reproductive biology of S. orbiculatum, as one of the most important edible bush tomatoes besides S. centrale, and the diversity within its reproductive system, as knowing this information could be the initial building block for producing improved elite lines or F1 hybrids of this Australian endemic wild species, as well as improving the domesticated tomatoes for abiotic stresses.
To date, no studies have investigated the reproductive system of this endemic species. Here, we address questions about its breeding system, pollen biology, pollination, and cross-compatibility with the domesticated Solanum lycopersicum and other wild species native to Australia and other geographical regions in the world. This will accumulate the important information needed for improving the agronomic characteristics of this species so that it can be considered for commercial production. More importantly, we aim for the exploitation of the genes of this species in the genetic improvement of the commercial tomato varieties for resistance to pests and diseases, in addition to tolerance to various abiotic stresses, and perhaps quality characteristics.
2. Materials and Methods
2.1. Plant Material
Seeds of Solanum orbiculatum have been collected from wild populations found in natural habitats in Wiluna, Western Australia, during the summer of 2013 and stored until we started the experiment in 2016. More than 200 seeds have been surface-sterilised by immersion in 50% bleach (4.5% sodium hypochlorite), with 0.1% Tween-20, for 30 min, followed by 3× rinsing with sterilised deionised water, before being placed in plastic Petri dishes (90 mm) lined with Whatman® filter paper moistened with 50 ppm GA3 to enhance germination. All Petri dishes have been incubated at constant 26 ± 0.5 °C in a Contherm Digital Series incubator (Contherm Scientific Limited, Lower Hutt, New Zealand) and have been monitored and scored for germination on a daily basis [3]. Germinated seeds with emerged radicles have been carefully shifted into nursery trays and placed in a microclimate room at a temperature regime of 26/20 °C (day/night temperature). Seedlings of S. orbiculatum (≈5.0 cm long) have been transplanted into cocopeat bags, supplied by Galuko Pty Ltd. (Vaucluse, Australia), fertigated as needed, and were grown to maturity in the experimental facilities of the Plant Breeding Institute (PBI), University of Sydney, Camden, NSW (Latitude: −34.016467, Longitude: 150.670687, Sea level: 87 m).
Other species of tomato have been obtained from different sources to enable interspecific compatibility tests. These include the following: (a) Solanum centrale, collected from Central Australia; (b) Wild species of Solanum, which originated in Peru, obtained from the Tomato Genetics Resources Center (TGRC), University of California, Davis, which are S. pimpinellifolium (Accession No. LA0373) and S. Pennellii (Accession No. LA0716); and (c) S. lycopersicum, obtained from The World Vegetable Center (AVRDC), Taiwan. The same procedure of seed disinfection and germination outlined above for S. orbiculatum has been used for S. centrale, while the seeds of all other species have been sown directly in nursery trays without any treatment.
For controlled pollination studies, some plants have been kept inside the microclimate room, while all of the other plants, including more than 100 S. orbiculatum plants, have been shifted into a greenhouse with a hydroponic growing system for further assessment of their vegetative growth and reproductive structures.
2.2. Floral Structure Study
The morphological features of 20 fresh flowers were analysed under a binocular stereomicroscope (Zeiss Stemi 2000-C with KL1500 LCD light source, Oberkochen, Germany). A digital calliper has been used to measure the length of one petal from each flower and its broadest width, as well as the length of anthers and styles. The sequential growth events were recorded from the day that a new floral bud appeared and have been monitored throughout all developmental stages by physical examination.
2.3. Pollen Collection and Storage Conditions
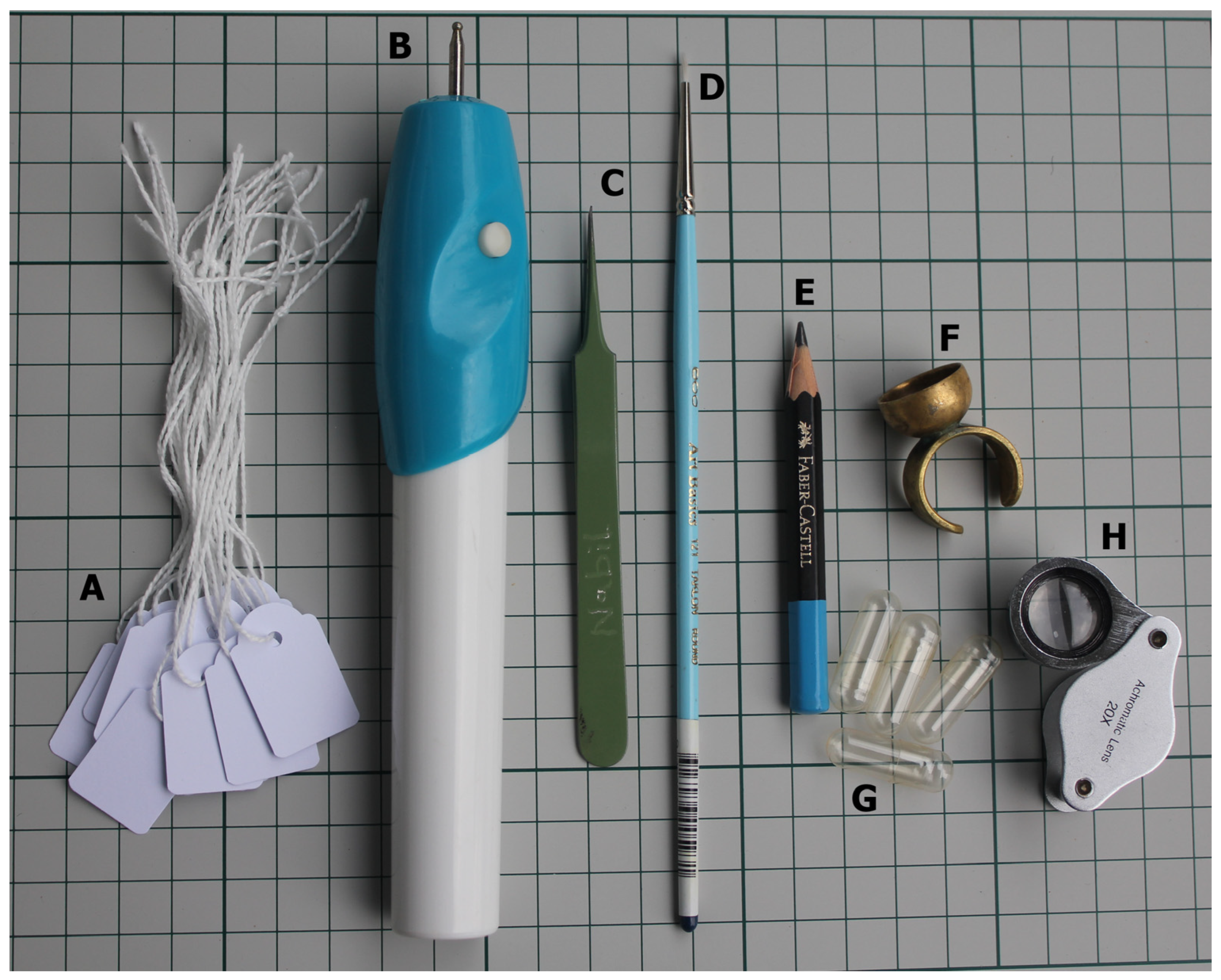
The collection of pollen grains in a viable condition is the primary requirement for any experimental study on pollen. Anthers can be vibrated at anthesis using a hand-held battery-operated engraver and can be collected in a brass ring (Figure 1) or a gelatine capsule before being used to (1) pollinate attached or detached flowers for in vivo and semi-in-vivo compatibility studies and fruit setting, and (2) can be dusted over the germination medium for in vitro germination tests, or onto glass slides for stainability tests.
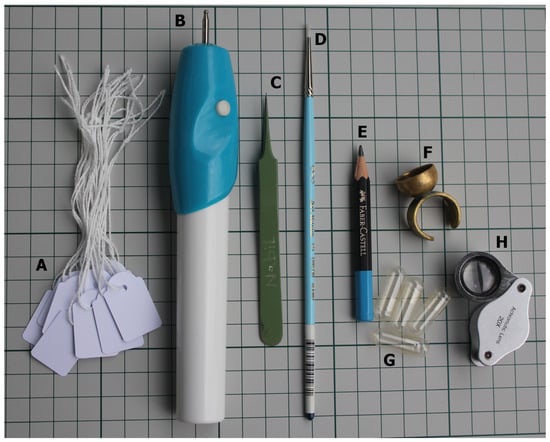
Figure 1.
Tools used to aid in hand-, self-, and cross-pollination. (A) Paper tags. (B) Battery-operated engraver used to vibrate pollen out of anthers. (C) Fine forceps used to emasculate flowers. (D) Small brush used to apply pollen to stigmata. (E) Pencil. (F) Brass ring used as pollen collector. (G) Gelatine capsules. (H) 20× magnification lens.
For the pollen storage experiment, 100 mg of pollen was collected after anthesis. The pollen was divided into four samples and stored for 0, 30, 60, and 90 days in individual gelatine capsules to avoid the effects of successive openings for sampling on pollen viability. The capsules were placed in an air-tight plastic container with silica gel to create a low-humidity environment and were stored in a refrigerator at 4 °C.
In the controlled pollination experiment, the pollen grains of Solanum orbiculatum were collected 24 h after flower anthesis, as the pollen grains could not be released from the anther sacs immediately after anthesis.
2.4. Pollen Culture in Artificial Media
In vitro pollen germination provides a direct and reliable assessment of viability but does not necessarily produce scores closely correlated with fertilising capacity in all instances [8]. The reliability of the in vitro germination test depends critically on the ability of the investigator to formulate a suitable medium for obtaining consistent germination and tube growth, a requirement that has been met for S. orbiculatum in this study.
- A.
- Media composition: Culture media with different components were screened for optimal pollen germination. The components of the pollen germination medium (PGM) were optimised using BK basal medium [9] enriched with different concentrations of sucrose and polyethylene glycol 4000 (PEG). The experiment has been carried out using 6 different sucrose concentrations (0, 5, 10, 15, 20, and 25% w/v) and 3 different concentrations of PEG (0, 2.5, and 5% w/v). The basic BK medium contains 100 mg/L H3BO3, 300 mg/L Ca (NO3)2·4H2O), 200 mg/L MgSO4·7H2O, and 100 mg/L KNO3.
- B.
- Slide preparation: Pollen was collected from the flowers of a single plant the morning after anthesis and distributed on glass slides with one drop of medium placed onto each slide. The slides were inverted and maintained on Petri plates lined with moist filter paper (RH > 95%) for 2 h at 22.5 °C.
- C.
- Scoring of pollen cultures: The responses of cultured pollen grains are assessed as a percentage for pollen germination. A pollen grain is considered germinated when the length of its tube is equal to or more than the diameter of the pollen grain. The pollen grains were scored for germination using arbitrarily selected microscope fields in a completely randomised design, with a factorial treatment structure. Each treatment contained 3 replicates, and 3 fields of view were assessed in each replicate. Pollen grains in at least three arbitrarily selected microscopic fields of each slide were observed after 2 h incubation at 22.5 °C. It was convenient to move the preparation under the microscope in consecutive rows and score one or two arbitrary fields in each row. This would eliminate the probability of scoring the same group of pollen grains again. For each field, the total number of pollen grains and the number of germinated grains were recorded.
- D.
- Pollen hydration: The pollen grains were hydrated by incubation at 22.5 °C inside the brass ring over moist filter paper in a covered container for 15 min.
2.5. Pollen Histo-Chemical Tests
- A.
- Cotton blue-lactophenol (0.5%): Hydrated fresh and mature pollen grains were dusted onto a drop of stain on a slide, a cover glass was placed on the smear, and the preparation was warmed for 15 min. The pollen grains were then examined under the high power of a microscope and classified as follows: (1) deeply stained (dark blue, uniform intensity), (2) moderately stained (light blue), and (3) unstained (colourless, shrivelled, often without cytoplasm).
- B.
- Acetocarmine (1%): Pollen grains were dispersed in a drop of stain on a microscope slide, covered with a coverslip, and examined under a light microscope to be scored for stainability. Red-stained pollen grains were considered viable, while light and unstained pollen grains were considered non-viable.
- C.
- I-KI (1% and 2%) solution: The same procedure was carried out as that for acetocarmine. Darkly stained pollen grains were considered viable, whereas light-brown-coloured and unstained pollen grains were considered non-viable.
- D.
- Fluorochromatic reaction test (FCR): The test media were prepared using the rapid semi-empirical method described by Heslop-Harrison et al. [10]. Fluorescein diacetate was made up as a stock solution in acetone at 2 mg/mL. Immediately before use, dilutions of 1% were prepared in 25% sucrose solution. The pollen samples were dispersed in a drop of this medium on a microscope slide, and the fluorochromatic reaction was allowed to proceed for 10 min at room temperature under a covered Petri dish lined with moistened filter paper before viewing with fluorescence microscopy using a Leica DMIL compound microscope. The viable pollen grains fluoresced bright yellow-green under ultraviolet light.
- E.
- Alexander’s stain: Alexander’s stain [11] has been used as a differential stain to differentiate between aborted and non-aborted pollen grains. Pollen grains have been incubated on a slide in a drop of the stain and incubated for at least one hour in the dark at 22.5 °C before viewing under the compound microscope, as mentioned above.
2.6. Controlled Pollination for Compatibility Studies
For experimental controlled pollination, we used the following four treatments: (1) bagging intact flowers, to test for spontaneous self-pollination; (2) manual self-pollination, to test for self-compatibility; (3) manual cross-pollination and emasculation, to test for interspecific compatibility; and (4) emasculation of 10 pre-anthetic flowers, to check for possible apomixes. The treatments were performed on 10 randomly selected plants of S. orbiculatum and 3 plants of each of the other species. On each plant, seven self-pollinations and four crosses were performed, and two flowers served as the controls (no pollination). To avoid any contamination by cross-pollination, the self-pollinated flowers were only allowed to self-pollinate within gelatine capsules. The open-pollinated flowers were scored from the same plants to ensure that bagging the flowers had no deleterious effect on the seed set. On the other hand, pollinations were carried out on excised pistils implanted in 1% agar in Petri dishes, as described by Lundqvist [12]. The behaviour of the pollen tubes was assessed after staining the pistils in 0.05% aniline blue in 0.1 M phosphate buffer (pH 7) for 20 min at room temperature. The stigmas were viewed with a Leica DMIL compound microscope using fluorescence optics with short-wavelength ultraviolet light and photographed with a Nikon Coolpix Photo camera (Nikon Corporation, Tokyo, Japan) mounted on the same microscope. The callose deposits in the pollen tubes fluoresced, providing an indication of the distance that the tubes had grown, if any [13].
The fruit setting for some self-pollination and cross-pollination has been counted on each plant to calculate fruit setting percentages (number of fruits/total number of pollinated flowers).
2.7. Data Collection and Analysis
Data were statistically analysed, and the treatment means were separated according to the least significant difference (LSD) at a p = 0.05. The differences in the size of the pollen and the length of the styles were tested by t-test. All analyses were performed using SPSS statistics (version 19.0, Chicago, IL, USA).
3. Results and Discussion
3.1. Floral Structure and Morphological Variation
The premating barriers include a variety of floral morphological characters correlated with a diversity of mating systems. The floral structures of Solanum orbiculatum ssp. orbiculatum have been studied using detailed physical examination in order to evaluate the magnitude of the morphological intra-sub-specific variations. The studied flowers showed broad variation in petal colour (wide range of purple colour shades and patterns) and shape (Figure 2).

Figure 2.
Morphological diversity of floral structures of S. orbiculatum ssp. Orbiculatum, showing variation in shape, colour, and number of petals and anthers. (a,d–j,l,m) Flowers with 5 petals and 5 anthers. (b,c) Flowers with 4 petals and 4 anthers and lower length/width ratio. (k) A flower with six petals and 6 anthers. (n) A flower with 3 petals and 3 anthers. Lines of small grids are 10 mm apart.
The anthers and petals within each flower are always of a similar number, with a range from three (Figure 2n) to six (Figure 2k). However, most of the plants have flowers with five petals and five anthers, similar to the domesticated tomato S. lycopersicum. The size of the flowers showed large variation, as indicated through the petals’ length and width in Table 1. The recorded petal length was from about 10 mm to 20 mm, and the petal width was 5.3 mm to 9.8 mm, with a 2:1 ratio (length:width). However, some of the flowers had a lower length:width ratio, such as those in Figure 2c,g, making the flower more condensed and probably more suitable for landing big insects that have more vibrating powers. This characteristic, as well as the whole flower size and brighter colour, can have a major impact on attracting insects and, therefore, can affect mating.

Table 1.
Floral structure of 20 flowers collected randomly from 100 plants of Solanum orbiculatum growing hydroponically in coco-peat media inside a greenhouse at PBI/Cobbitty.
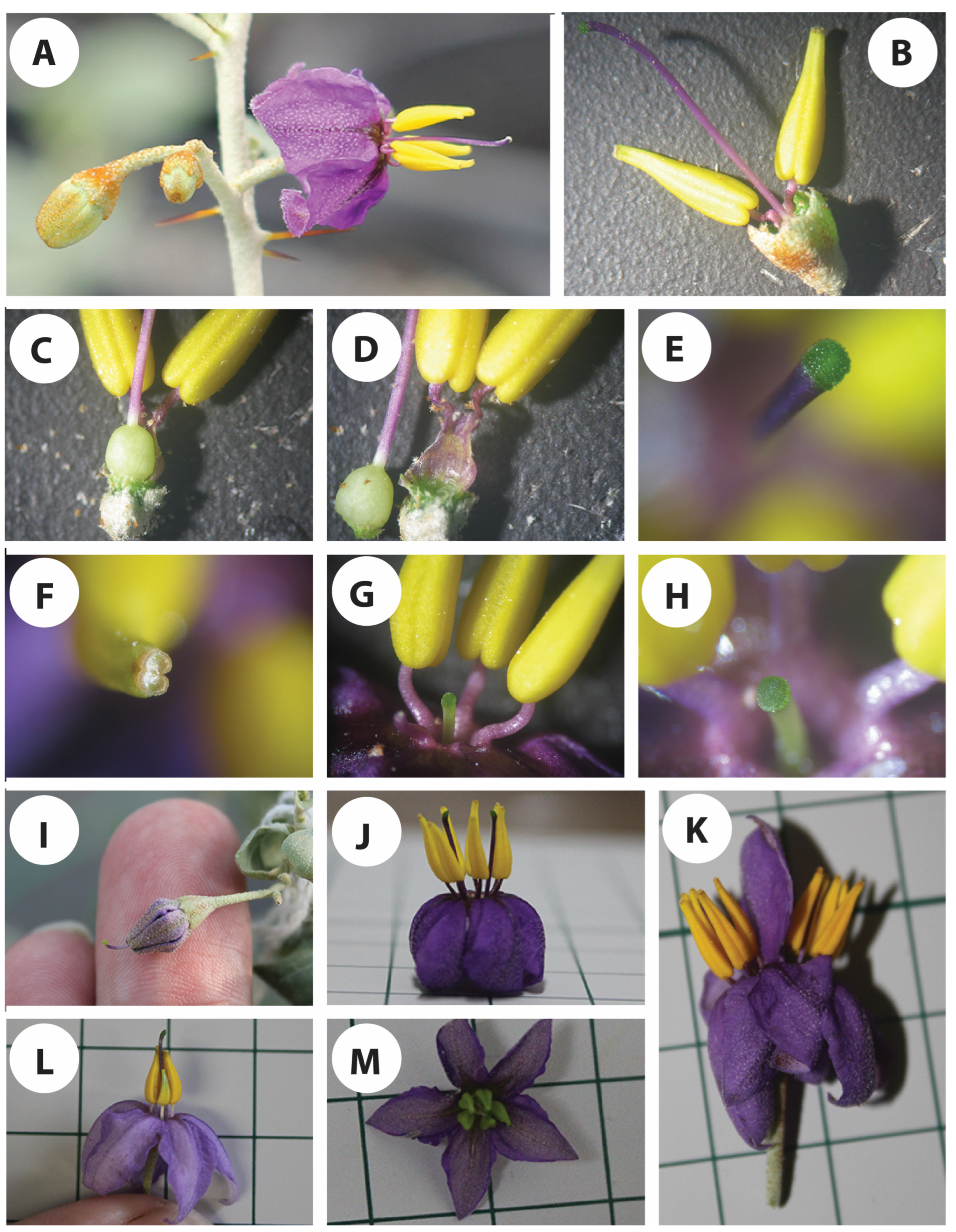
In normal cases, the stigmata are always exserted or protruding above the anthers at the time of anthesis (Figure 3A), a case that can mostly encourage cross-pollination. However, short-styled flowers (Figure 3G,H) have also been observed in all of the plants kept under the microclimate conditions, although it was in a low percentage (<5%). There was a whole range of style lengths, which can raise doubts about their effective sexual identity. Some of the manually pollinated, short-styled flowers were able to set fruits, however, more testing is needed to verify if there is any correlation between the style length and the fertility of the gynoecium. On the other hand, the anthers on those short-styled flowers were found to accommodate fertile pollen grains with no significant difference compared to the flowers with exserted stigmata. In a few cases, stigma exsertion has been observed in the floral buds before anthesis (Figure 3I). This has been observed in the plants growing inside the greenhouse under a low temperature when the plants were first shifted there, a case that can highly encourage cross-pollination, as the stigma are fully receptive to foreign pollen. The large variation in the style length could be related to the environmental conditions, particularly temperatures, and needs more investigation under different controlled constant and alternating temperatures.
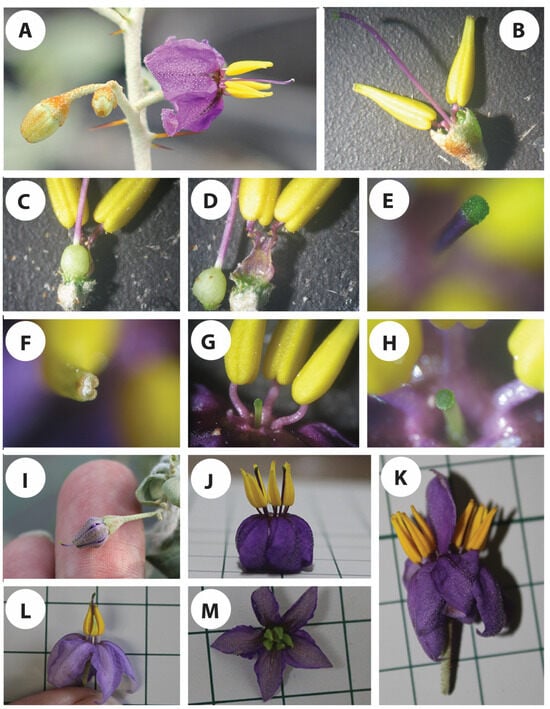
Figure 3.
Floral structure of S. orbiculatum. (A) Three-flowered cyme inflorescence, with flowers at different developmental stages. (B–D) Androecium and gynoecium. (E) Close-up view of the stigma. (F) Distal end of the anther showing pore. (G,H) Short-styled flower, showing a close-up view in (H). (I) Flower at pre-anthesis stage showing stigma exsertion. (J) A flower with 2 gynoecia. (K) Two fused flowers sharing one pedicel. (L,M) Flowers at anthesis showing underdeveloped anther in (L) and immature anthers in (M).
We have also observed differences among the different flowers in terms of the angle at which the anthers spread around the gynoecium. In some of the flowers, the anthers are very tight to the style (Figure 2h,m), whereas others are very loose and have a wider angle to the style. This characteristic can have a significant effect on the chances of self-pollination.
The flower pedicel length can affect the erectness of the flower stem. The long pedicels observed in some plants can cause the flower to bend and, therefore, may have an influence on the access to the flower for insects and may augment the chances of self-pollen to reach the stigma.
More investigation is needed in order to find the differences in the pedicel length among flowers of the same plant, as well as flowers on different plants. This characteristic is genetically controlled, however, environmental conditions such as temperature and light intensity may have a significant effect on its gene expression. The plants grown in the greenhouse under fluctuating temperatures have shown more variation in pedicel lengths and require a detailed investigation and data recording as the plants grow through the summer season.
3.2. Relationship between Pollen Size and Style Length
A correlation between pollen grain size and style length was proposed in 1867 and recently by Torres [14] and Aguilar et al. [15] based on pollen grain provisioning, i.e., pollen grains of different sizes contain sufficient nutrients to grow through respective styles of different lengths. However, Darwin rejected this hypothesis because he observed species with a single size of pollen grain but variable style lengths. As mentioned above, in the Solanum orbiculatum floral structure, we found flowers with different style lengths on the same plant and, therefore, it has become important to investigate whether there is a relationship between the pollen size and style length within the subspecies.
In this study, we found a significant difference between the means of the area of the pollen grains taken from long-styled and short-styled flowers (Table 2) and a significant difference between the style lengths of two groups of flowers (flowers with exserted stigmata and inserted stigmata), as shown in Table 3. However, more investigation is needed in order to find a correlation between the style length and the pollen size or volume using a wide range of flowers with different style lengths, a study that could not be carried out here due to the limited number of short-styled flowers.

Table 2.
Means of pollen area (µm2) from long-styled and short-styled flowers of S. orbiculatum.

Table 3.
Means of length of styles (µm) from long-styled and short-styled flowers of S. orbiculatum.
More investigation could also be carried out to find out whether pollen from other species of Solanum can have a different success rate in pollinating long-styled and short-styled flowers of S. orbiculatum, and vice versa, in order to establish if this can act as a reproductive barrier among our collection of Solanum species.
3.3. Optimising the In Vitro Pollen Germination Medium
The sucrose and PEG 4000 concentrations significantly affected the germination of the pollen grains of Solanum orbiculatum ssp. orbiculatum (Table 4). The best response was obtained on a medium containing 20% sucrose and 2.5% PEG. However, no significant differences were found between medium 11 (20% sucrose, 2.5% PEG) and both media 9 (15% sucrose, 5% PEG) and 12 (20% sucrose, 5% PEG). The lowest germination rate was found under the concentration with 30% sucrose and 5% PEG, which was due to a possible osmotic imbalance that caused pollen tube bursting and, therefore, reduced the germination percentage. A higher concentration of PEG (5%) was effective in increasing pollen germination when a less-than-optimal concentration of sucrose was used (<20%), underscoring the importance of sucrose as an osmoticum, as well as its role as a source of energy. The pollen germination values were low in the media with either low or high concentrations of sucrose, indicating that sucrose plays an important role as an osomoticum, as well as being a source of energy for germinating pollen grains.

Table 4.
In vitro pollen germination of Solanum orbiculatum using Brewbaker and Kwack basic media enriched with 18 combinations of sucrose and PEG at different concentrations.
3.4. Pollen Stainability Test
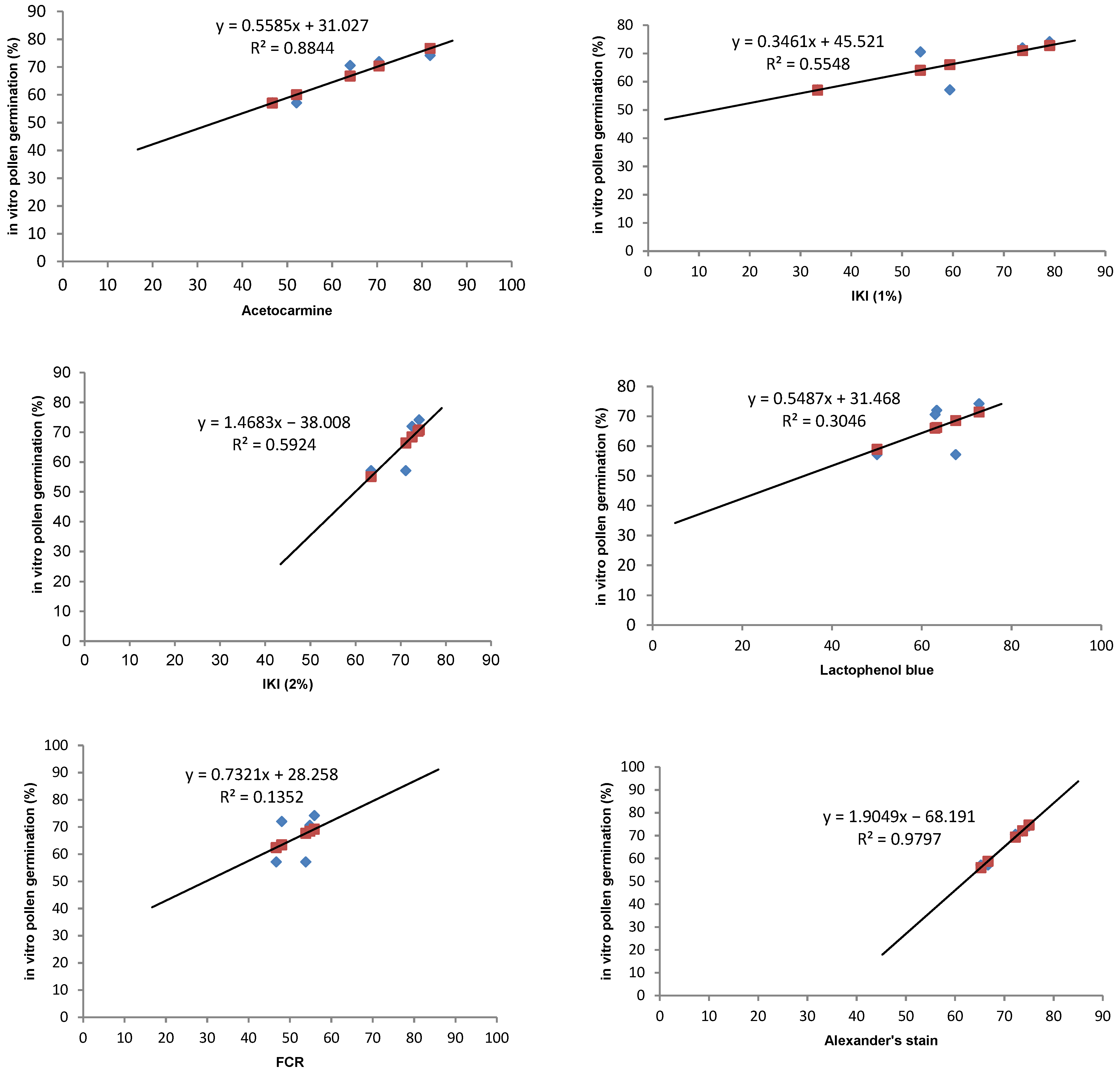
In any crop, there is a need to optimise stainability tests to check the viability of pollen grains. Stainability tests are quick tests and are accurate if a high correlation can be established with the in vitro germination test. Five different types of stains have been tried to test the viability of pollen grains in Solanum orbiculatum ssp. orbiculatum (Table 5). Acetocarmine and Alexander’s stains were found to have the highest correlation (88.4 and 98%, respectively) (Table 5, Figure 4). Alexander’s stain can be efficiently used to test the viability within a short period of time, and the scoring is easier than other methods because the aborted non-viable pollen grains appear green, while the viable pollen grains appear dark red (Figure 5F). It is possible to predict the in vitro germination result using the regression equation shown in Figure 4.

Table 5.
Correlation between the in vitro pollen germination test and various stainability tests.
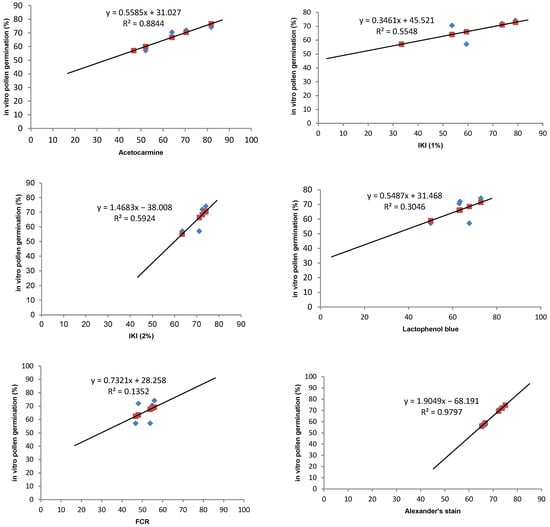
Figure 4.
Regression lines and correlation coefficients between 5 staining methods (acetocarmine, IKI, lactophenol blue, FCR, and Alexander’s stain) and the in vitro pollen germination of the bush tomato S. orbiculatum ssp. orbiculatum. Blue points represent in vitro germination data and red points represent predicted data.
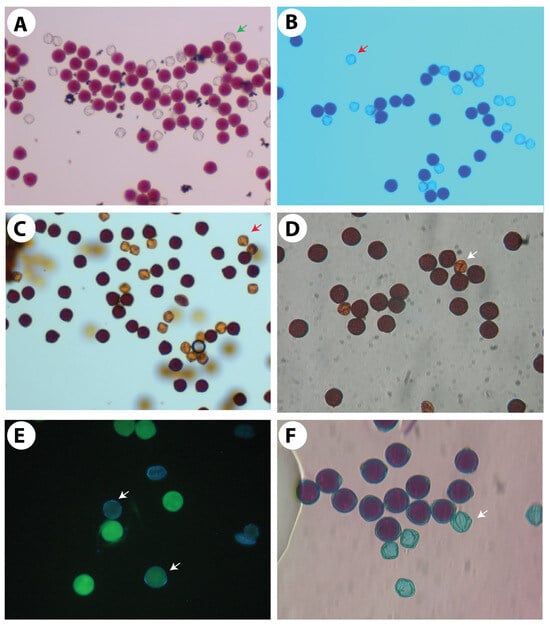
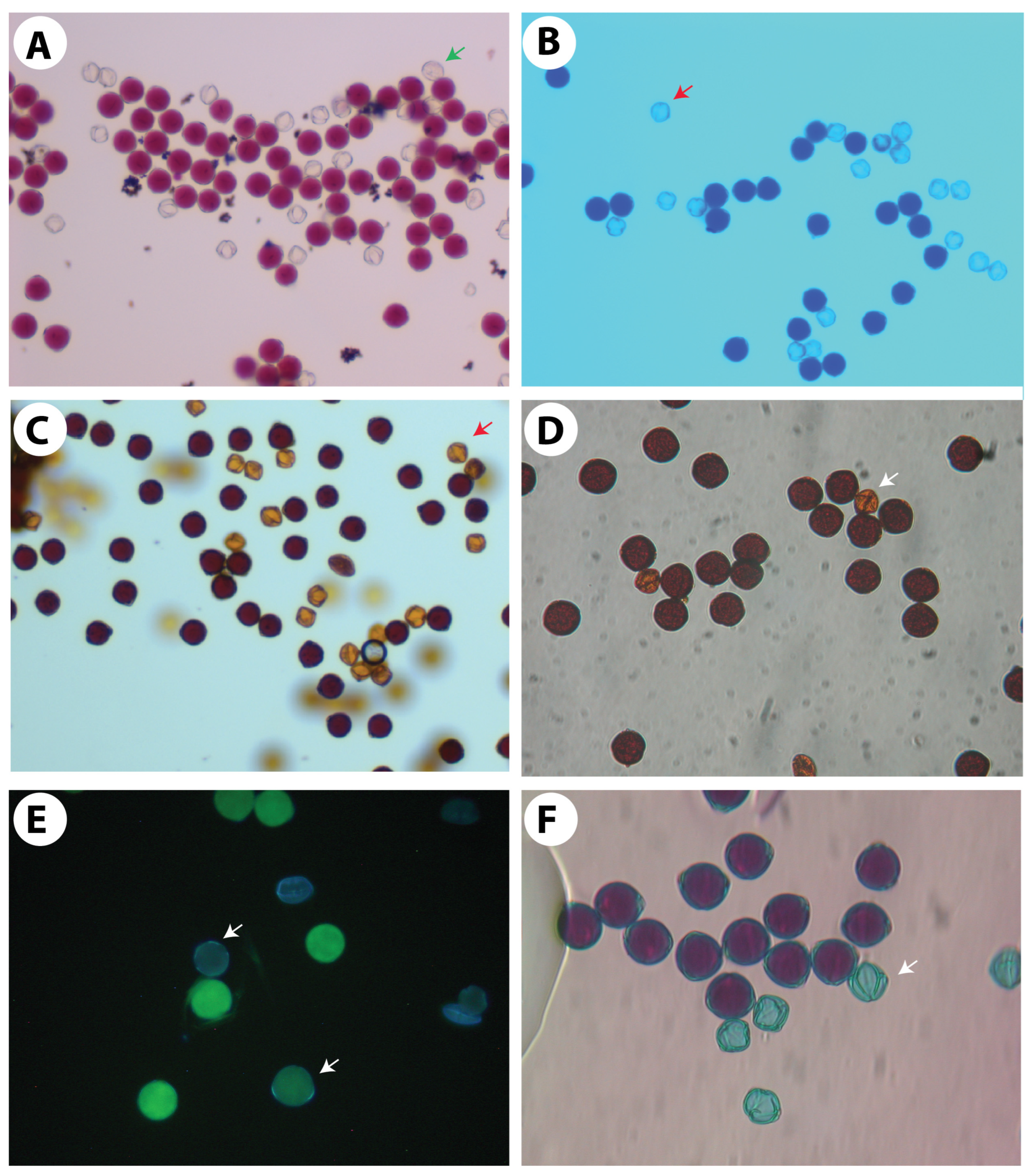
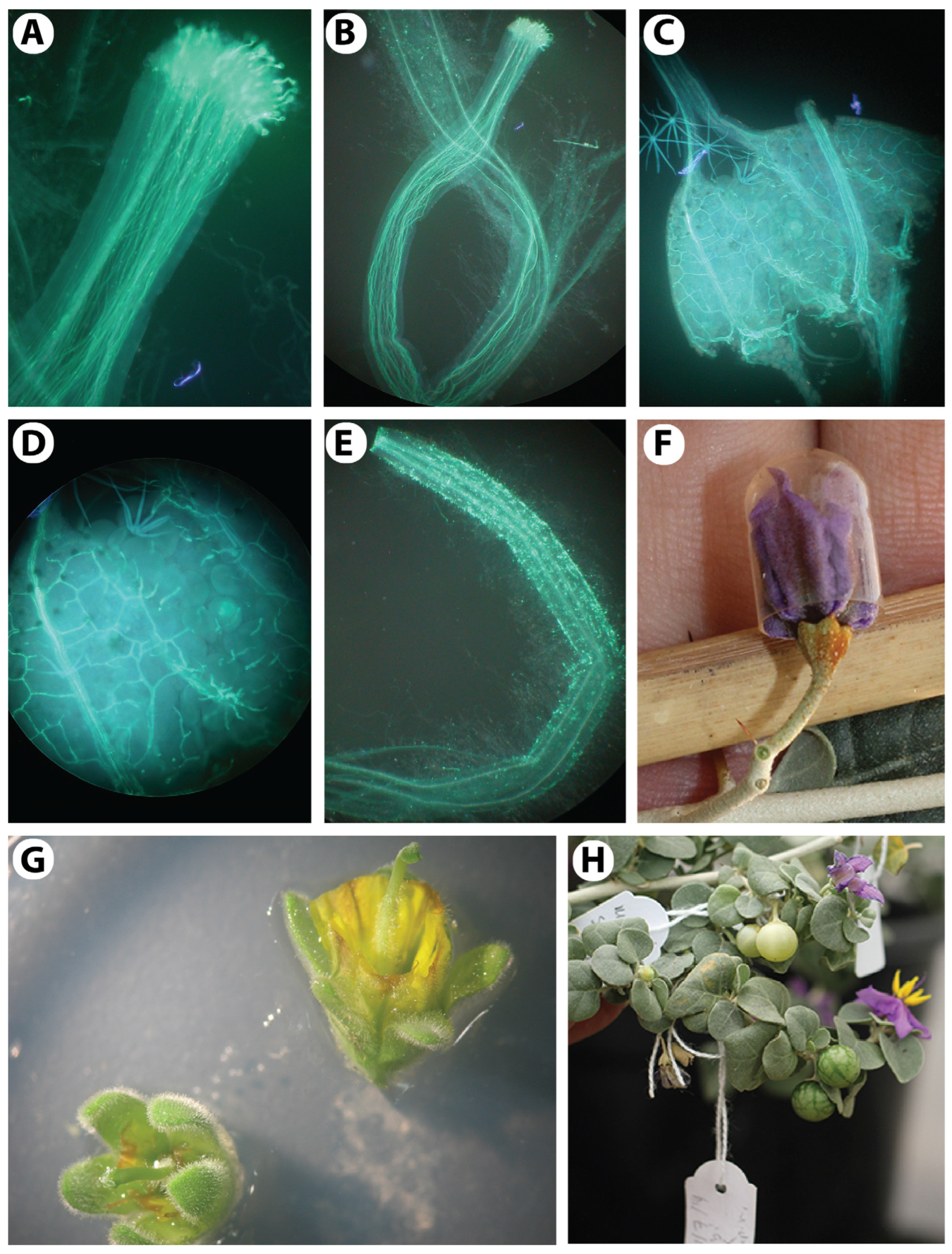
Figure 5.
Pollen histochemical viability tests. (A,B) Staining with the nuclear dyes acetocarmine in (A) and cotton blue in lactophenol in (B). (C,D) For starch staining, I + KI was used as 1% solution in (C) and 2% solution in (D). (E) Fluorochromatic reaction test (FCR) to test the integrity of the plasmalemma of the vegetative cell using fluorescein diacetate. (F) Differential staining of aborted and non-aborted pollen using Alexander’s staining solution. Arrows in all pictures indicate non-viable pollen grains.
3.5. Pollen Storage and Viability
In a trial to find out the possibility to store Solanum orbiculatum pollen grains under low temperatures and humidity, we found that the pollen grains can maintain their viability without any significant drop for the first month after storage at 4 °C (Table 6). However, there was a significant decrease in viability (39.31%) after two months of storage under the same conditions, and the pollen viability remained significantly unchanged one month later (35.05%). More investigation is needed to check if the viability can be maintained at that level if we store the pollen grains for several months more.

Table 6.
Means of in vitro pollen germination (%) of S. orbiculatum after storage durations (months) at 4 °C.
It would also be beneficial if the pollen could be stored under lower temperatures (−80 °C). This would probably allow us to store it for years, which could facilitate breeding programs and the easy transport of the genetic material between plant breeders.
3.6. Timing of Pollen Viability and Stigma Receptivity
The pollen grain viability of Solanum orbiculatum ssp. orbiculatum was tested for 3 days after anthesis. No major changes in the pollen viability were detected during the first 2 days, however, the pollen grains harvested on the 3rd day have minimum viability (<20%) and no pollen could be harvested after the 3rd day, as the anthers wither and the distal pore becomes blocked.
On the other hand, the stigma receptivity was tested up to 5 days before anthesis and 3 days after anthesis. We found, through in vivo and semi-in-vivo stigma receptivity tests, that the stigma became receptive when the floral bud was still unopened (4 days before anthesis) and continued to remain receptive for 3 days after anthesis, or up to the end of the anther dehiscence stage, indicating the initial female phase, followed by a bisexual phase only.
The temporal separation of sexual tracks shown above shows the adaptation of Solanum orbiculatum ssp. orbiculatum towards allogamy. This type of separation in timing between male and female organs is called protogyny.
3.7. Sequence of Floral Development
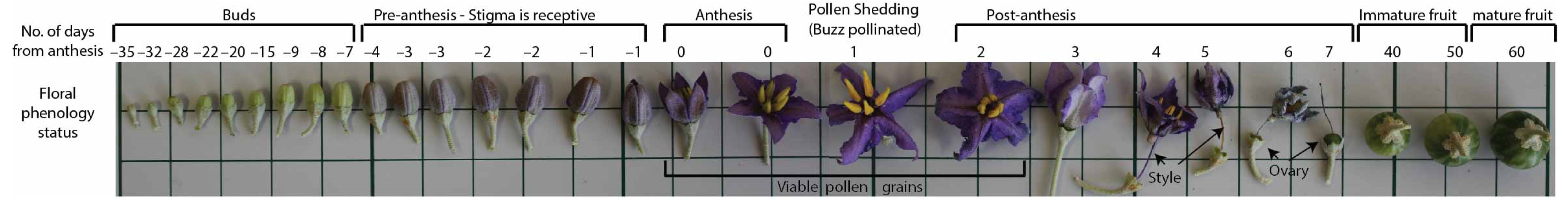
The floral development has been traced with time. At the early stages of bud development, the development progression is very slow, requiring about 1 month to reach the pre-anthesis stage (Figure 6). The petal colour starts to change to purple about 4 days before anthesis. The stigma receptivity tests have shown that the stigma is fully receptive during this pre-anthesis stage and maintains its receptivity 2 days after anthesis.

Figure 6.
Morphological floral developmental sequence of S. orbiculatum ssp. orbiculatum, as observed under controlled condition.
The petals start to curve backward one day after anthesis and then start to curve upward on the second day after anthesis, as the fertilised ovary starts growing and pushing the withered petals away from it. The drying petals eventually become disconnected on the third day, and the whole corolla slides down on the style on the fourth day and becomes stuck on the wider portion of the stigma, facing down, until it falls on the sixth or seventh day, leaving the style connected to the growing ovary. The fruits keep growing in size until reaching full maturity about 2 months after fertilisation. The fruits ripen on the plants and stay attached for a longer period of time compared to domesticated tomatoes.
3.8. Developing an Efficient Pollination Technique
Relying on all of the results above, an efficient pollination technique has been developed using the tool set shown in Figure 1. The anthers are buzzed using a battery-operated engraver (Figure 7B). The collected fresh pollen grains (Figure 7C) can be used to self-pollinate the flower without emasculation or to cross-pollinate the flowers after emasculation. Since the pollen grains cannot be easily freed from the anther through the terminal or distal pores (Figure 3F), there is no risk of contamination with its own pollen if we emasculate the flower during the first few hours after anthesis (Figure 7E). However, emasculation and cross-pollination can also be performed up to four days before anthesis, as the stigma is fully receptive during that time (Figure 7D–F). The stigma can be gently dipped into the pollen (Figure 7G), and the flower can then be tagged using a paper tag with all of the information written on it, with a pencil, including the name of the pollen parent, the date of pollination, and the name of the pollinating person (Figure 7H,I).

Figure 7.
Controlled pollination of Solanum orbiculatum. (A) Harvesting pollen grains from flowers on the morning after anthesis. (B) A hand-held battery-operated engraver is used to vibrate the anthers to release pollen grains through the opening at the distal end of each anther. (C) The amount of harvested pollen grains from 2 to 3 flowers. (D) Mature flower a few hours before full anthesis, ready for emasculation and pollination, as the stigma is fully receptive. (E) Emasculation after anthesis and before pollen shed. (F) Emasculated flower showing a pistil with a receptive stigma. (G) Pollination by gently dipping the stigma into the collected pollen grains. (H) Tagging the pollinated flower. (I) Image of Solanum orbiculatum showing controlled self- and cross-pollinated flowers.
3.9. Self- and Cross-Compatibility
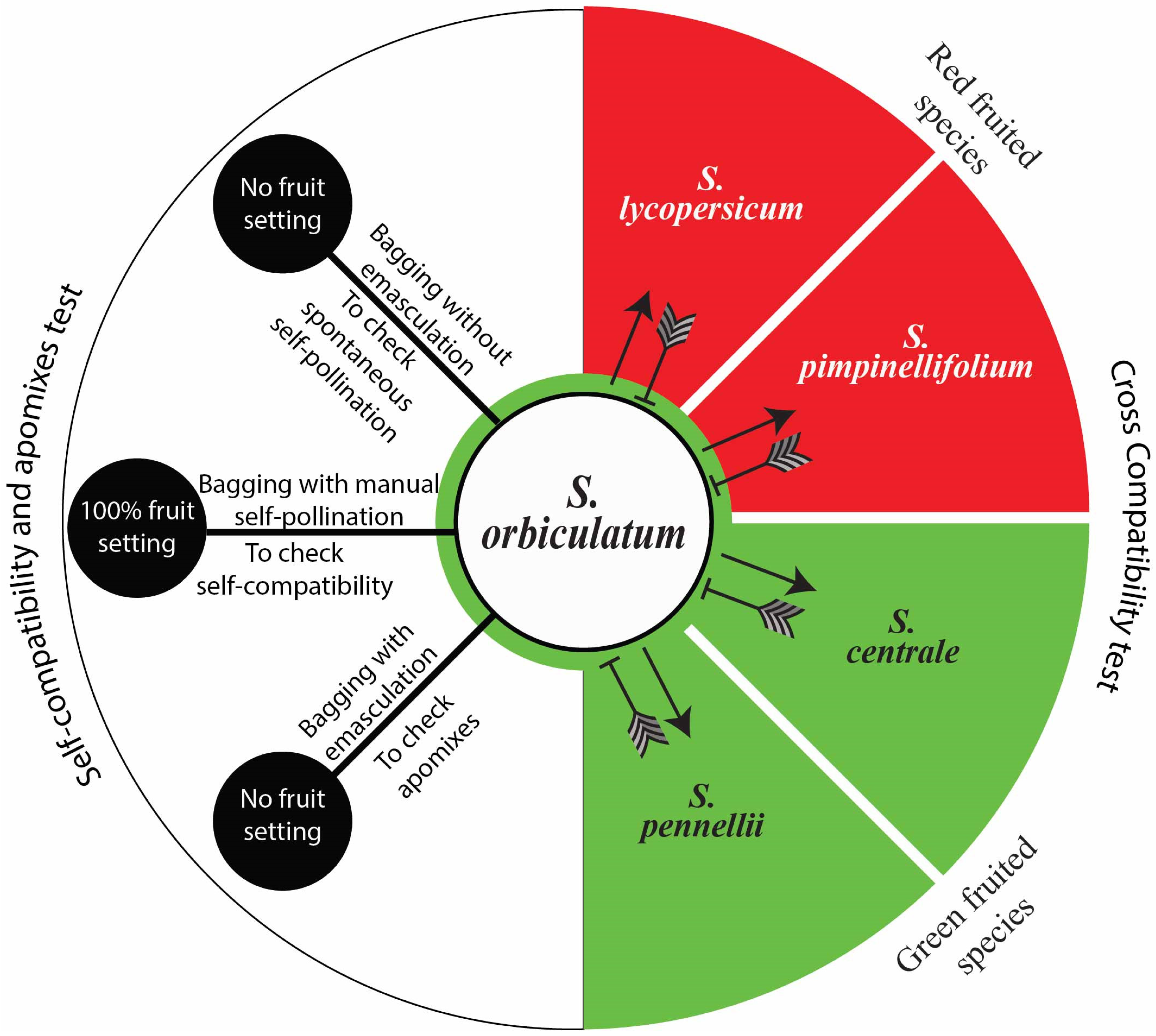
This study found no indication of pre-zygotic reproductive barrier(s) between the studied species when S. orbiculatum was used as a male parent (Figure 8A–D), indicating the potential for the use of interspecific hybridisation in genetic improvement. However, pre-zygotic incompatibility was confirmed when S. orbiculatum was used as a female parent (Figure 8E). This unilateral incompatibility has also been reported for other species of solanum. A simplified illustration of the compatibility tests is presented in Figure 9.
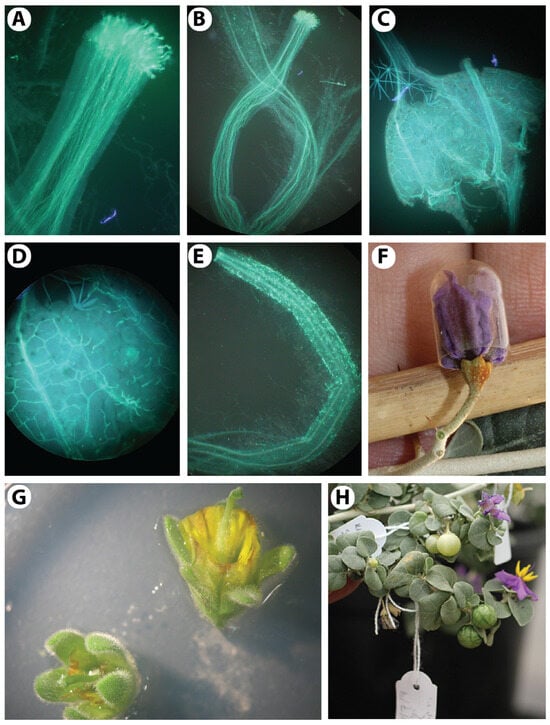
Figure 8.
In vivo and semi-in-vivo self- and cross-compatibility tests. (A–D) High compatibility between the male and female parents, showing pollen tubes growing within styles in (A,B) and entering micropyles of the ovules in (C,D). (E) Incompatibility, as the pollen tubes have stopped growing before reaching the ovary. (F) Covering flowers to test passive autogamy. (G) Semi-in-vivo technique to study compatibility. (H) Fruits resulting from controlled hand pollinations.
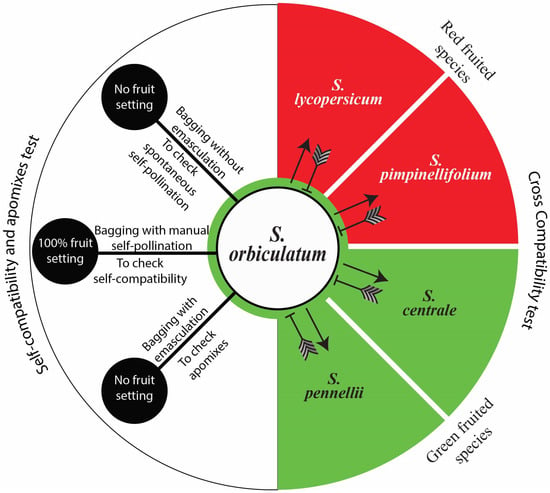
Figure 9.
Solanum orbiculatum self and interspecific compatibilities based on controlled pollination and pollen tube growth behaviour. Arrows, compatible cross pollinations; barred lines, incompatible cross pollinations. No indication of pre-zygotic reproductive barrier(s) when S. orbiculatum was used as the male parent. However, unilateral incompatibility was observed when S. orbiculatum was used as the female parent. No actual fruits or seeds have been produced from compatible crosses, due to post-zygotic barriers. Solanum orbiculatum is self-compatible, with 100% fruit setting obtained from controlled self-pollination. No spontaneous self-pollination or apomixes were observed. Abbreviations: SC, self-compatible.
All of the fruits resulting from the hand cross-pollination of flowers of other solanum species using S. orbiculatum pollen grains have aborted seeds, indicating post-zygotic barriers as genetic isolating mechanisms resulting in the failure of fruit or viable seed production. However, embryo rescue biotechnology may enable us to produce hybrids of S. orbiculatum crossed (as male parent) with other species, or we could possibly use it in bridge crossing.
The fruit setting in all of the hand self-pollinated flowers showed 100% fruit setting. However, no fruiting was observed in the open pollination treatments, in the spontaneous self-pollination, or in the apomixes test.
4. Conclusions
The results have confirmed that S. orbiculatum ssp. orbiculatum is characterised by a broad variation in its floral morphological traits, including the size, number, and colour of the floral organs. On the other hand, studying the floral biology in this species has indicated that the perfect flowers in S. orbiculatum are protogynous. Therefore, based on the floral characteristics and floral biology, S. orbiculatum has a preference for cross pollination. This arrangement is a simple and common way to avoid excessive self-pollination [16,17].
The controlled manual pollination experiments conducted on this species have shown that S. orbiculatum ssp. orbiculatum has very high self-compatibility, with 100% fruit setting when self-pollination is enforced. Moreover, cross-compatibility with other species (both domesticated and wild) was found to be possible when S. orbiculatum was used as the male parent, due to an absence of pre-zygotic barriers. However, no fruits or seeds were produced from these crosses, due to fruit abortion caused by post-zygotic barrier(s).
This study has optimised an in vitro pollen germination technique and a quick viability test, as well as developing an efficient pollination technique.
Author Contributions
Conceptualisation, N.A. and R.T.; methodology, N.A.; formal analysis, N.A. and A.C.; data analysis, N.A. and A.C.; investigation, N.A. and A.C.; writing—original draft preparation, N.A.; writing—review and editing, R.T. and N.A.; supervision, N.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the University of Sydney.
Data Availability Statement
Data are present in the article.
Acknowledgments
The authors acknowledge the contributions made by the staff and students at the Plant Breeding Institute, The University of Sydney, for their help during this study. The authors also thankfully acknowledge the contributions made by TGRC at UC Davis and AVRDC by providing the tomato germplasm.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Moore, P.A. Guide to Plants of Inland Australia; Reed New Holland: Wahroonga, Australia, 2005. [Google Scholar]
- Commander, L.E.; Merritt, D.J.; Rokich, D.P.; Dixon, K.W. Seed biology of Australian arid zone species: Germination of 18 species used for rehabilitation. J. Arid Environ. 2009, 73, 617–625. [Google Scholar] [CrossRef]
- Commander, L.E.; Merritt, D.J.; Rokich, D.P.; Flematti, G.R.; Dixon, K.W. Seed germination of Solanum spp. (Solanaceae) for use in rehabilitation and commercial industries. Aust. J. Bot. 2008, 56, 333–341. [Google Scholar] [CrossRef]
- Symon, D.E. A revision of the genus Solanum in Australia. J. Adel. Bot. Gard. 1981, 4, 1–367. [Google Scholar]
- Milner, K.V.; French, K.; Krix, D.W.; Valenzuela, S.M.; Leigh, A. The effects of spring versus summer heat events on two arid zone plant species under field conditions. Funct. Plant Biol. 2023, 50, 455–469. [Google Scholar] [CrossRef] [PubMed]
- Anderson, G.J.; Symon, D.E. Functional dioecy and andromonoecy in Solanum. Evolution 1989, 43, 204–219. [Google Scholar] [CrossRef] [PubMed]
- Martine, C.T.; Symon, D.E.; Evans, E.C. A new cryptically dioecious species of bush tomato (Solanum) from the Northern Territory, Australia. PhytoKeys 2013, 30, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Stanley, R.G.; Linskens, H.F. Pollen: Biology, Biochemistry, Management; Springer: Berlin/Heidelberg, Germany, 1974; p. ix + 307. [Google Scholar]
- Brewbake, J.L.; Kwack, B.H. Essential role of calcium ion in pollen germination and pollen tube growth. Am. J. Bot. 1963, 50, 859. [Google Scholar] [CrossRef]
- Heslop-Harrison, J.; Heslop-Harrison, Y.; Shivanna, K.R. The evaluation of pollen quality, and a further appraisal of the fluorochromatic (FCR) test procedure. Theor. Appl. Genet. 1984, 67, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Alexander, M.P. Differential staining of aborted and nonaborted pollen. Stain Technol. 1969, 44, 117. [Google Scholar] [CrossRef] [PubMed]
- Lundqvist, A.A. rapid method for the analysis of incompatibilities in grasses. Hereditas 1961, 47, 705–707. [Google Scholar] [CrossRef]
- Martin, F.W. Staining and observing pollen tubes in the style by means of fluorescence. Stain Technol. 1959, 34, 125–128. [Google Scholar] [CrossRef] [PubMed]
- Torres, C. Pollen size evolution: Correlation between pollen volume and pistil length in Asteraceae. Sex. Plant Reprod. 2000, 12, 365–370. [Google Scholar] [CrossRef]
- Aguilar, R.; Bernardello, G.; Galetto, L. Pollen-pistil relationships and pollen size-number trade-off in species of the tribe Lycieae (Solanaceae). J. Plant Res. 2002, 115, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Proctor, M.; Yeo, P.; Lack, A. The Natural History of Pollination; Timber Press: Portland, OR, USA, 1996; p. 479. [Google Scholar]
- Routley, M.B.; Bertin, R.I.; Husband, B.C. Correlated evolution of dichogamy and self incompatibility: A phylogenetic perspective. Int. J. Plant Sci. 2004, 165, 983–993. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).