Valorizing Traditional Greek Wheat Varieties: Phylogenetic Profile and Biochemical Analysis of Their Nutritional Value
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples
2.2. DNA Barcoding
2.3. SSR Analysis
2.4. Extraction of Free and Bound Phenolic Compounds
2.5. Total Phenolic Content (TPC)
2.6. Total Flavonoid Content (TFC)
2.7. Determination of Antioxidant Activity
2.8. Analysis of Fatty-Acid Methyl Esters (FAMEs)
2.9. Statistical Analysis
3. Results
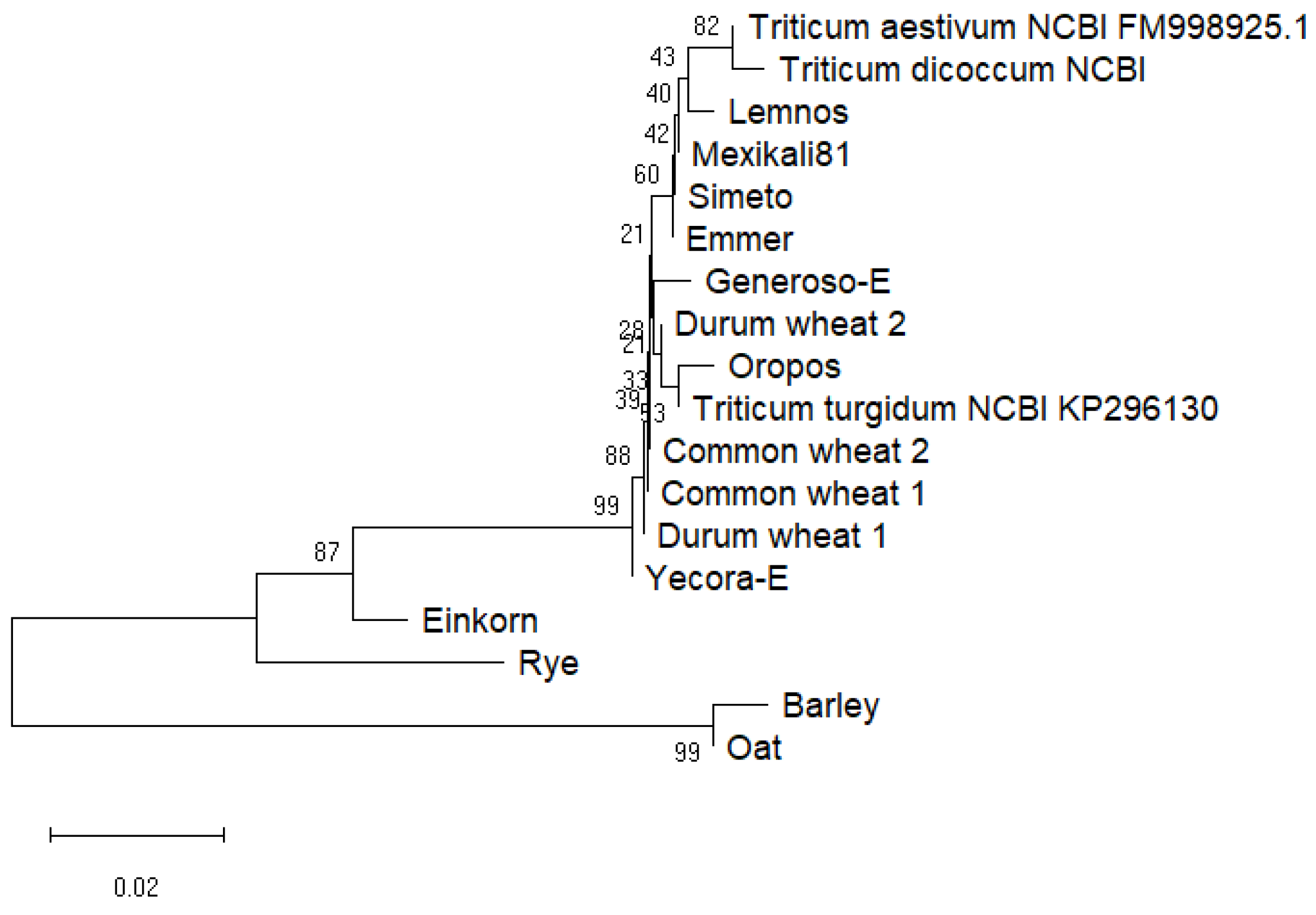
3.1. Phylogenetic Analysis
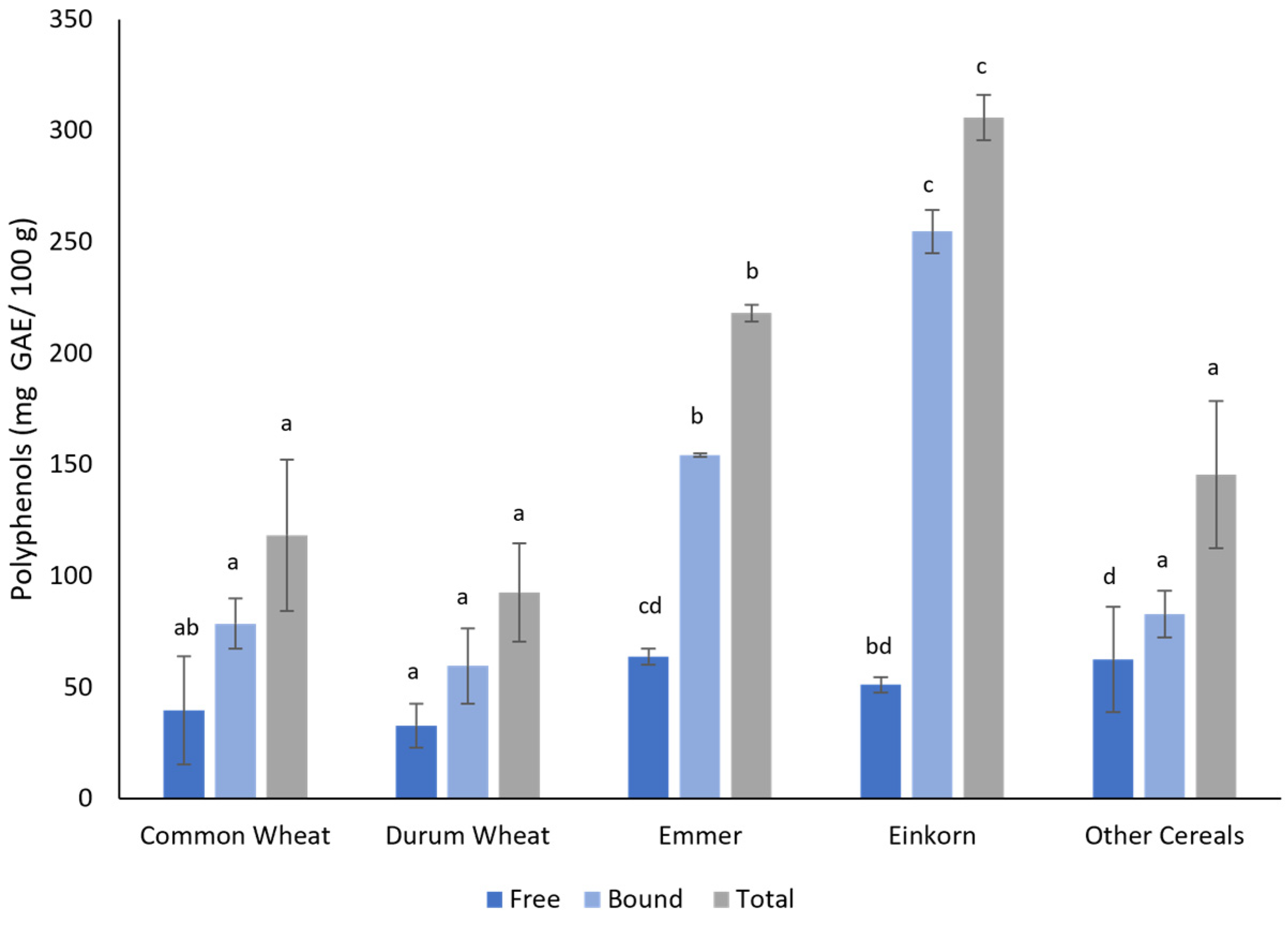
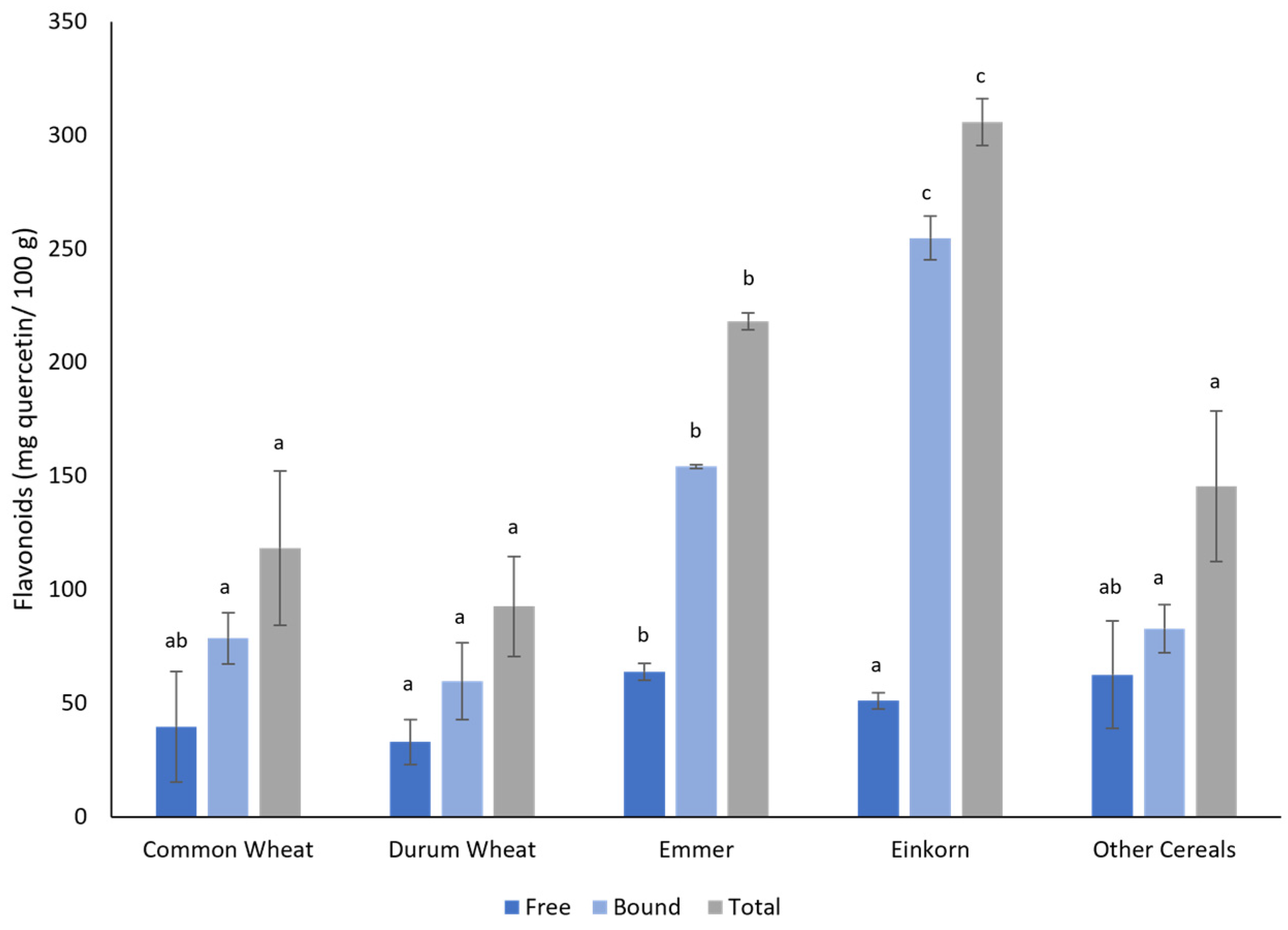
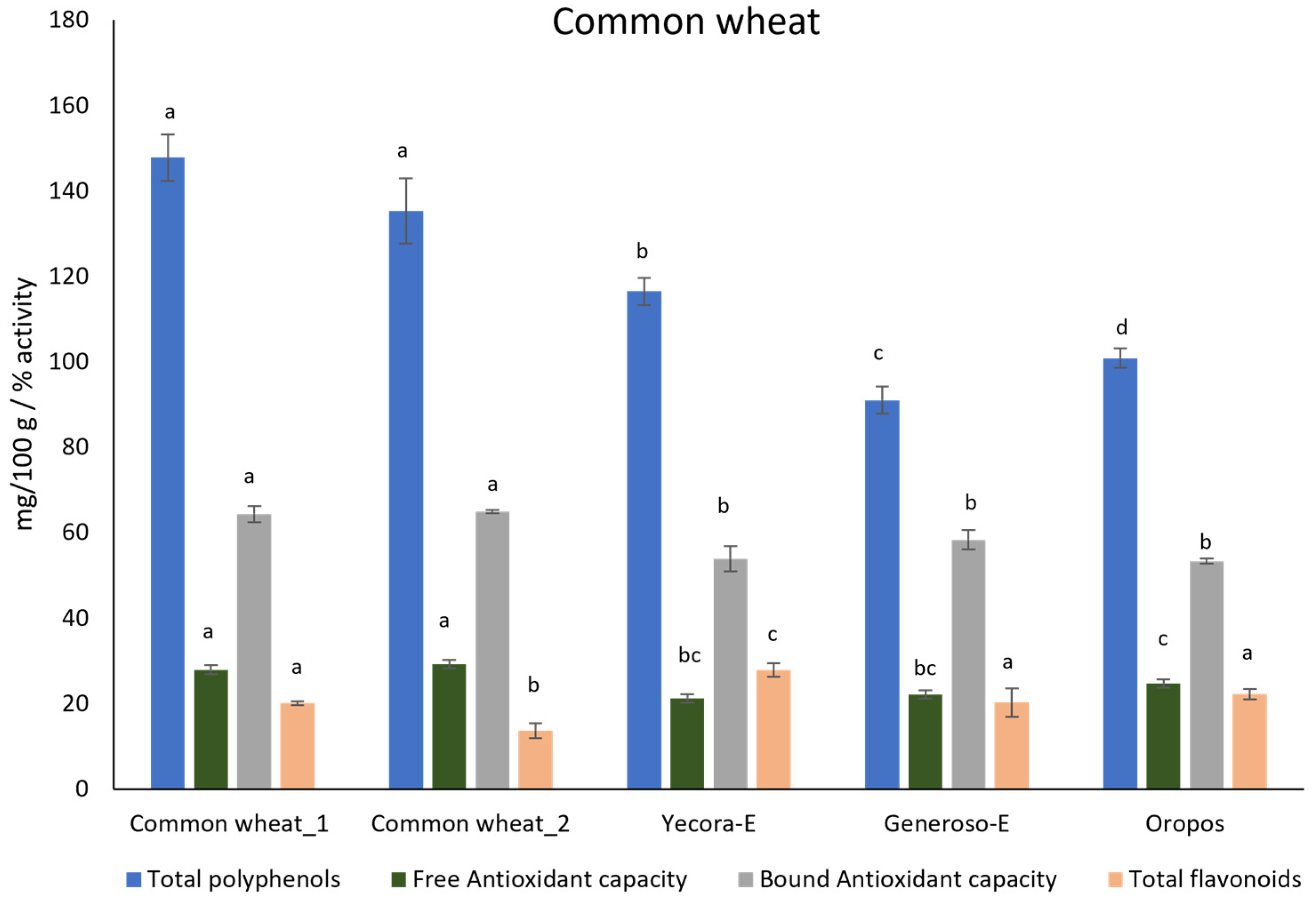
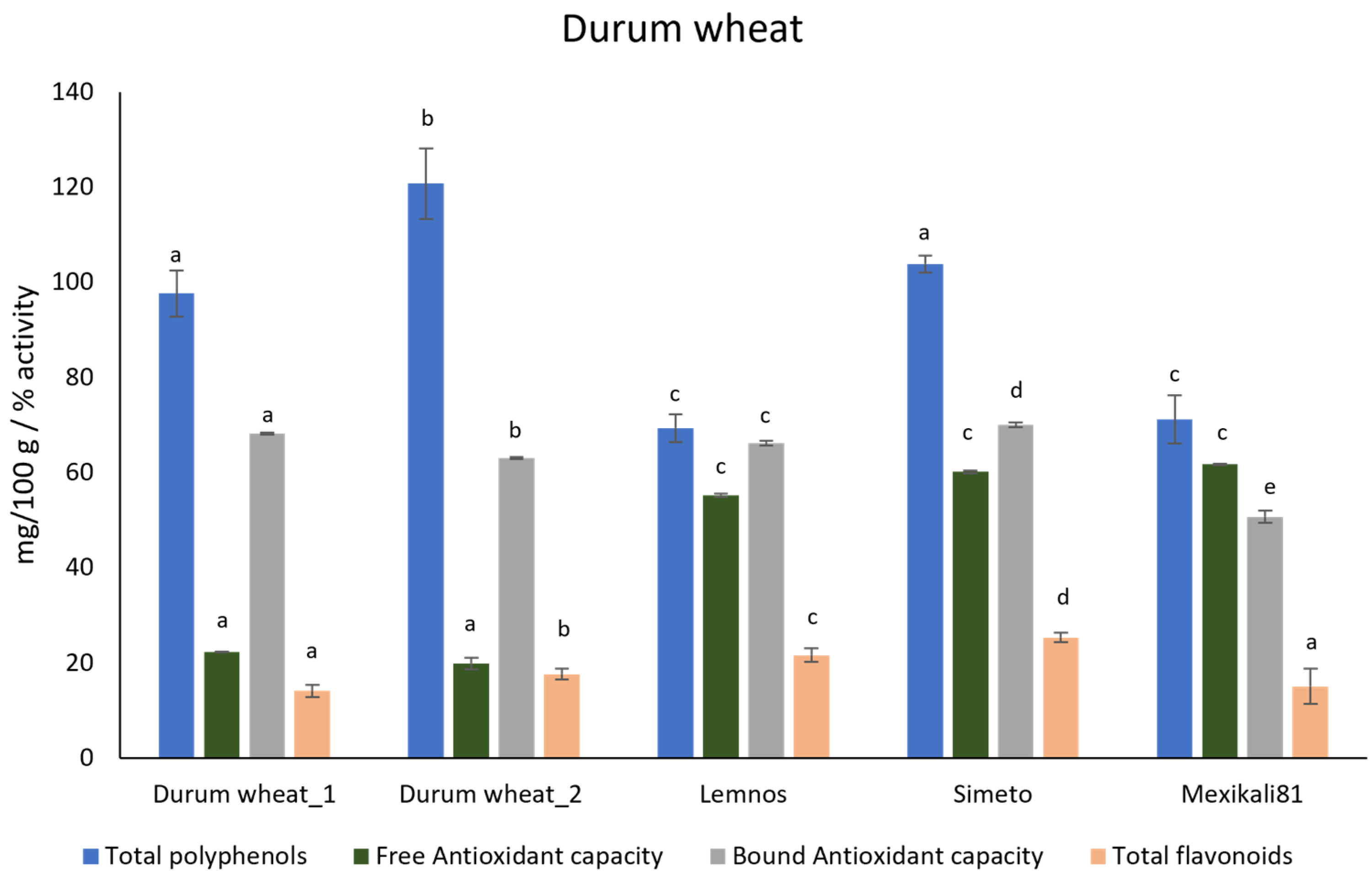
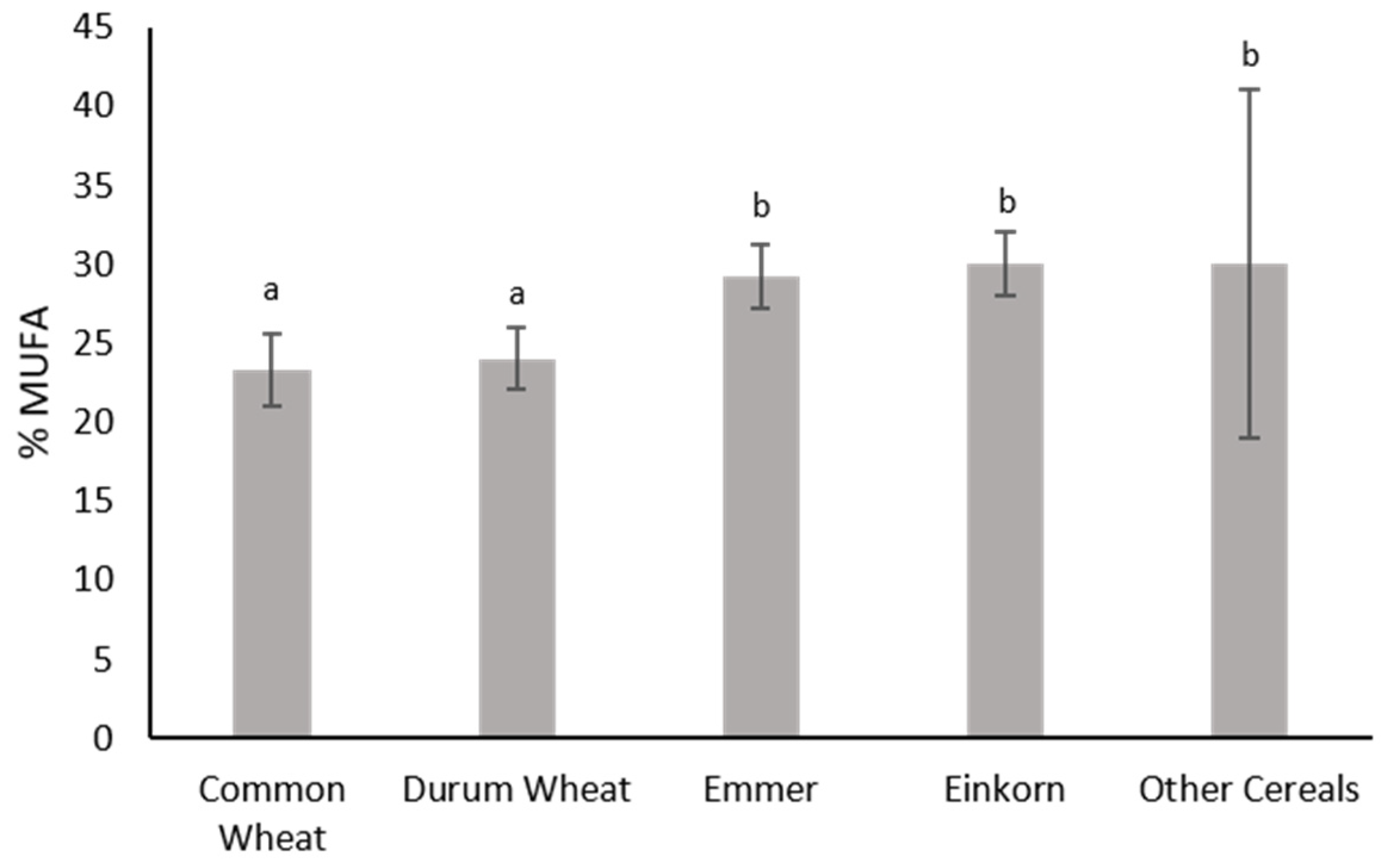
3.2. Biochemical Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abdellatif, K.F.; AbouZeid, H.M. Assessment of Genetic Diversity of Mediterranean Bread Wheat Using Randomly Amplified Polymorphic DNA (RAPD) Markers. J. Genet. Eng. Biotechnol. 2011, 9, 157–163. [Google Scholar] [CrossRef]
- FAOSTAT. Crops and Livestock Products; FAOSTAT: Rome, Italy, 2022. [Google Scholar]
- Lukaszewski, A.J.; Alberti, A.; Sharpe, A.; Kilian, A.; Stanca, A.M.; Keller, B.; Clavijo, B.J.; Friebe, B.; Gill, B.; Wulff, B.; et al. A Chromosome-Based Draft Sequence of the Hexaploid Bread Wheat (Triticum aestivum) Genome. Science 2014, 345, 1251788. [Google Scholar] [CrossRef]
- Kabbaj, H.; Sall, A.T.; Al-Abdallat, A.; Geleta, M.; Amri, A.; Filali-Maltouf, A.; Belkadi, B.; Ortiz, R.; Bassi, F.M. Genetic Diversity within a Global Panel of Durum Wheat (Triticum durum) Landraces and Modern Germplasm Reveals the History of Alleles Exchange. Front. Plant Sci. 2017, 8, 1277. [Google Scholar] [CrossRef]
- Zohary, D.; Hopf, M. Domestication of Plants in the Old World: The Origin and Spread of Cultivated Plants in West Asia, Europe and the Nile Valley, 3rd ed.; Zohary, D., Hopf, M., Eds.; Oxford University Press: Oxford, UK, 2000. [Google Scholar]
- Akhunov, E.D.; Akhunova, A.R.; Dvořák, J. BAC Libraries of Triticum Urartu, Aegilops Speltoides and Ae. Tauschii, the Diploid Ancestors of Polyploid Wheat. Theor. Appl. Genet. 2005, 111, 1617–1622. [Google Scholar] [CrossRef]
- Di Francesco, A.; Cunsolo, V.; Saletti, R.; Svensson, B.; Muccilli, V.; De Vita, P.; Foti, S. Quantitative Label-Free Comparison of the Metabolic Protein Fraction in Old and Modern Italian Wheat Genotypes by a Shotgun Approach. Molecules 2021, 26, 2596. [Google Scholar] [CrossRef]
- Cooper, R. Re-Discovering Ancient Wheat Varieties as Functional Foods. J. Tradit. Complement Med. 2015, 5, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Shewry, P.R.; Hey, S. Do “Ancient” Wheat Species Differ from Modern Bread Wheat in Their Contents of Bioactive Components? J. Cereal Sci. 2015, 65, 236–243. [Google Scholar] [CrossRef]
- Geisslitz, S.; Longin, C.F.H.; Scherf, K.A.; Koehler, P. Comparative Study on Gluten Protein Composition of Ancient (Einkorn, Emmer and Spelt) and Modern Wheat Species (Durum and Common Wheat). Foods 2019, 8, 409. [Google Scholar] [CrossRef]
- Lachman, J.; Hejtmánková, K.; Kotíková, Z. Tocols and Carotenoids of Einkorn, Emmer and Spring Wheat Varieties: Selection for Breeding and Production. J. Cereal Sci. 2013, 57, 207–214. [Google Scholar] [CrossRef]
- Ruibal-Mendieta, N.L.; Delacroix, D.L.; Mignolet, E.; Pycke, J.M.; Marques, C.; Rozenberg, R.; Petitjean, G.; Habib-Jiwan, J.L.; Meurens, M.; Quetin-Leclercq, J.; et al. Spelt (Triticum aestivum ssp. Spelta) as a Source of Breadmaking Flours and Bran Naturally Enriched in Oleic Acid and Minerals but Not Phytic Acid. J. Agric. Food Chem. 2005, 53, 2751–2759. [Google Scholar] [CrossRef]
- Tsirivakou, A.; Melliou, E.; Magiatis, P. A Method for the Rapid Measurement of Alkylresorcinols in Flour, Bread and Related Products Based on 1H QNMR. Foods 2020, 9, 1025. [Google Scholar] [CrossRef]
- Liu, J.; Hao, Y.; Wang, Z.; Ni, F.; Wang, Y.; Gong, L.; Sun, B.; Wang, J. Identification, Quantification, and Anti-Inflammatory Activity of 5-n-Alkylresorcinols from 21 Different Wheat Varieties. J. Agric. Food Chem. 2018, 66, 9241–9247. [Google Scholar] [CrossRef] [PubMed]
- Agorastos, A.G.; Goulas, C.K. Line Selection for Exploiting Durum Wheat (T. turgidum L. Var. Durum) Local Landraces in Modern Variety Development Program. Euphytica 2005, 146, 117–124. [Google Scholar] [CrossRef]
- Fatkullin, R.; Naumenko, N.; Popova, N.; Ruskina, A.; Kalinina, I.; Potoroko, I. Explaining Consumer Intentions for Foods with Antioxidant Properties: Predictors of Choice and Purchase Barriers. Int. J. Food Sci. 2021, 2021, 9971425. [Google Scholar] [CrossRef] [PubMed]
- Abouzied, H.; Eldemery, S.; Abdellatif, K. SSR-Based Genetic Diversity Assessment in Tetraploid and Hexaploid Wheat Populations. Br. Biotechnol. J. 2013, 3, 390–404. [Google Scholar] [CrossRef]
- Xynias, I.N.; Mylonas, I.; Korpetis, E.G.; Ninou, E.; Tsaballa, A.; Avdikos, I.D.; Mavromatis, A.G. Durum Wheat Breeding in the Mediterranean Region: Current Status and Future Prospects. Agronomy 2020, 10, 432. [Google Scholar] [CrossRef]
- Yao, H.; Song, J.; Liu, C.; Luo, K.; Han, J.; Li, Y.; Pang, X.; Xu, H.; Zhu, Y.; Xiao, P.; et al. Use of ITS2 Region as the Universal DNA Barcode for Plants and Animals. PLoS ONE 2010, 5, e13102. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Saitou, N.; Nei, M. The Neighbor-Joining Method: A New Method for Reconstructing Phylogenetic Trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar] [CrossRef]
- Röder, M.S.; Korzun, V.; Wendehake, K.; Plaschke, J.; Tixier, M.H.; Leroy, P.; Ganal, M.W. A Microsatellite Map of Wheat. Genetics 1998, 149, 2007. [Google Scholar] [CrossRef]
- Gupta, P.K.; Balyan, H.S.; Edwards, K.J.; Isaac, P.; Korzun, V.; Röder, M.; Gautier, M.F.; Joudrier, P.; Schlatter, A.R.; Dubcovsky, J.; et al. Genetic Mapping of 66 New Microsatellite (SSR) Loci in Bread Wheat. Theor. Appl. Genet. 2002, 105, 413–422. [Google Scholar] [CrossRef] [PubMed]
- Peakall, R.; Smouse, P.E. GenAlEx 6.5: Genetic Analysis in Excel. Population Genetic Software for Teaching and Research—An Update. Bioinform. Appl. 2012, 28, 2537–2539. [Google Scholar] [CrossRef] [PubMed]
- Roldán-Ruiz, I.; Dendauw, J.; Van Bockstaele, E.; Depicker, A.; De Loose, M. AFLP Markers Reveal High Polymorphic Rates in Ryegrasses (Lolium spp.). Mol. Breed. 2000, 6, 125–134. [Google Scholar] [CrossRef]
- Kaur, N.; Singh, B.; Kaur, A.; Yadav, M.P.; Singh, N.; Ahlawat, A.K.; Singh, A.M. Effect of Growing Conditions on Proximate, Mineral, Amino Acid, Phenolic Composition and Antioxidant Properties of Wheatgrass from Different Wheat (Triticum aestivum L.) Varieties. Food Chem. 2021, 341, 128201. [Google Scholar] [CrossRef]
- Adom, K.K.; Sorrells, M.E.; Liu, R.H. Phytochemical Profiles and Antioxidant Activity of Wheat Varieties. J. Agric. Food Chem. 2003, 51, 7825–7834. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Liu, M.; Li, X.Q.; Weber, C.; Lee, C.Y.; Brown, J.; Liu, R.H. Antioxidant and Antiproliferative Activities of Raspberries. J. Agric. Food Chem. 2002, 50, 2926–2930. [Google Scholar] [CrossRef]
- Bhinder, S.; Singh, B.; Kaur, A.; Singh, N.; Kaur, M.; Kumari, S.; Yadav, M.P. Effect of Infrared Roasting on Antioxidant Activity, Phenolic Composition and Maillard Reaction Products of Tartary Buckwheat Varieties. Food Chem. 2019, 285, 240–251. [Google Scholar] [CrossRef]
- O’Fallon, J.V.; Busboom, J.R.; Nelson, M.L.; Gaskins, C.T. A Direct Method for Fatty Acid Methyl Ester Synthesis: Application to Wet Meat Tissues, Oils, and Feedstuffs. J. Anim. Sci. 2007, 85, 1511–1521. [Google Scholar] [CrossRef]
- Avise, J. Molecular Markers, Natural History and Evolution; Springer Science and Business Media, LLC: New York, NY, USA, 1994; ISBN 0-412-03771-8. [Google Scholar]
- Harris, A.M.; DeGiorgio, M. An Unbiased Estimator of Gene Diversity with Improved Variance for Samples Containing Related and Inbred Individuals of Any Ploidy. G3 2017, 7, 671–691. [Google Scholar] [CrossRef]
- Leoncini, E.; Prata, C.; Malaguti, M.; Marotti, I.; Segura-Carretero, A.; Catizone, P.; Dinelli, G.; Hrelia, S. Phytochemical Profile and Nutraceutical Value of Old and Modern Common Wheat Cultivars. PLoS ONE 2012, 7, e45997. [Google Scholar] [CrossRef]
- Ruisi, P.; Frangipane, B.; Amato, G.; Frenda, A.S.; Plaia, A.; Giambalvo, D.; Saia, S. Nitrogen Uptake and Nitrogen Fertilizer Recovery in Old and Modern Wheat Genotypes Grown in the Presence or Absence of Interspecific Competition. Front. Plant Sci. 2015, 6, 185. [Google Scholar] [CrossRef] [PubMed]
- Hollingsworth, P.M.; Graham, S.W.; Little, D.P. Choosing and Using a Plant DNA Barcode. PLoS ONE 2011, 6, e19254. [Google Scholar] [CrossRef]
- Raveendar, S.; Lee, G.A.; Lee, K.J.; Shin, M.J.; Kim, S.H.; Lee, J.R.; Cho, G.T.; Hyun, D.Y. DNA Barcoding for Efficient Identification of Triticum Subspecies: Evaluation of Four Candidate Loci on Phylogenetic Relationships. Plant Breed. Biotechnol. 2019, 7, 220–228. [Google Scholar] [CrossRef]
- Ganopoulos, I.; Kapazoglou, A.; Bosmali, I.; Xanthopoulou, A.; Nianiou-Obeidat, I.; Tsaftaris, A.; Madesis, P. Application of the Its2 Region for Barcoding Plants of the Genus Triticum L. and Aegilops L. Cereal Res. Commun. 2017, 45, 381–389. [Google Scholar] [CrossRef]
- Roussel, V.; Leisova, L.; Exbrayat, F.; Stehno, Z.; Balfourier, F. SSR Allelic Diversity Changes in 480 European Bread Wheat Varieties Released from 1840 to 2000. Theor. Appl. Genet. 2005, 111, 162–170. [Google Scholar] [CrossRef]
- Landjeva, S.; Korzun, V.; Ganeva, G. Evaluation of Genetic Diversity among Bulgarian Winter Wheat (Triticum aestivum L.) Varieties during the Period 1925-2003 Using Microsatellites. Genet. Resour. Crop. Evol. 2006, 53, 1605–1614. [Google Scholar] [CrossRef]
- Röder, M.S.; Wendehake, K.; Korzun, V.; Bredemeijer, G.; Laborie, D.; Bertrand, L.; Isaac, P.; Rendell, S.; Jackson, J.; Cooke, R.J.; et al. Construction and Analysis of a Microsatellite-Based Database of European Wheat Varieties. Theor. Appl. Genet. 2002, 106, 67–73. [Google Scholar] [CrossRef]
- Mangini, G.; Taranto, F.; Giove, S.L.; Gadaleta, A.; Blanco, A. Identification of Durum Wheat Cultivars by a Minimum Number of Microsatellite Markers. Cereal Res. Commun. 2010, 38, 155–162. [Google Scholar] [CrossRef]
- Pasqualone, A.; Alba, V.; Mangini, G.; Blanco, A.; Montemurro, C. Durum Wheat Cultivar Traceability in PDO Altamura Bread by Analysis of DNA Microsatellites. Eur. Food Res. Technol. 2010, 230, 723–729. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, C.; Zhang, H.; Yue, Z.; Liu, X.; Ji, W. Genetic Analysis of Wheat (Triticum aestivum L.) and Related Species with SSR Markers. Genet. Resour. Crop. Evol. 2013, 60, 1105–1117. [Google Scholar] [CrossRef]
- Song, Q.J.; Shi, A.J.R.; Singh, A.S.; Fickus, A.E.W.; Costa, J.M.; Lewis, A.J.; Gill, A.B.S.; Ward, A.R.; Cregan, P.B. Development and Mapping of Microsatellite (SSR) Markers in Wheat. Theor. Appl. Genet. 2005, 110, 550–560. [Google Scholar] [CrossRef]
- Torada, A.; Koike, M.; Mochida, K.; Ogihara, Y. SSR-Based Linkage Map with New Markers Using an Intraspecific Population of Common Wheat. Theor. Appl. Genet. 2006, 112, 1042–1051. [Google Scholar] [CrossRef]
- Chao, S.; Zhang, W.; Dubcovsky, J.; Sorrells, M. Evaluation of Genetic Diversity and Genome-Wide Linkage Disequilibrium among U.S. Wheat (Triticum aestivum L.) Germplasm Representing Different Market Classes. Crop. Sci. 2007, 47, 1018–1030. [Google Scholar] [CrossRef]
- FAOSTAT FAO Cereal Supply and Demand Brief | World Food Situation | Food and Agriculture Organization of the United Nations. Available online: https://www.fao.org/worldfoodsituation/csdb/en/ (accessed on 18 July 2022).
- Adom, K.K.; Liu, R.H. Antioxidant Activity of Grains. J. Agric. Food Chem. 2002, 50, 6182–6187. [Google Scholar] [CrossRef]
- Laddomada, B.; Caretto, S.; Mita, G. Wheat Bran Phenolic Acids: Bioavailability and Stability in Whole Wheat-Based Foods. Molecules 2015, 20, 15666–15685. [Google Scholar] [CrossRef] [PubMed]
- Anson, N.M.; van den Berg, R.; Havenaar, R.; Bast, A.; Haenen, G.R.M.M. Bioavailability of Ferulic Acid Is Determined by Its Bioaccessibility. J. Cereal Sci. 2009, 49, 296–300. [Google Scholar] [CrossRef]
- Shamloo, M.; Babawale, E.A.; Furtado, A.; Henry, R.J.; Eck, P.K.; Jones, P.J.H. Effects of Genotype and Temperature on Accumulation of Plant Secondary Metabolites in Canadian and Australian Wheat Grown under Controlled Environments. Sci. Rep. 2017, 7, 9133. [Google Scholar] [CrossRef]
- Ayaşan, T.; Kader Esen, V.; Kader Esen, V.; Eseceli, H.; Cabi, E. Arbuscular Mycorrhizae Inoculation of Einkorn Wheat Affects Fatty Acid, Nutrient and Mineral Concentrations. S. Afr. J. Anim. Sci. 2020, 50, 600–606. [Google Scholar] [CrossRef]
- Suchowilska, E.; Wiwart, M.; Borejszo, Z.; Packa, D.; Kandler, W.; Krska, R. Discriminant Analysis of Selected Yield Components and Fatty Acid Composition of Chosen Triticum Monococcum, Triticum Dicoccum and Triticum Spelta Accessions. J. Cereal Sci. 2009, 49, 310–315. [Google Scholar] [CrossRef]
- Gillingham, L.G.; Harris-Janz, S.; Jones, P.J.H. Dietary Monounsaturated Fatty Acids Are Protective against Metabolic Syndrome and Cardiovascular Disease Risk Factors. Lipids 2011, 46, 209–228. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Aal, E.-S.M.; Rabalski, I. Bioactive Compounds and Their Antioxidant Capacity in Selected Primitive and Modern Wheat Species. Open Agric. J. 2008, 2, 7–14. [Google Scholar] [CrossRef]
- Giambanelli, E.; Ferioli, F.; Koçaoglu, B.; Jorjadze, M.; Alexieva, I.; Darbinyan, N.; D’Antuono, L.F. A Comparative Study of Bioactive Compounds in Primitive Wheat Populations from Italy, Turkey, Georgia, Bulgaria and Armenia. J. Sci. Food Agric. 2013, 93, 3490–3501. [Google Scholar] [CrossRef]
- Grausgruber, H.; Scheiblauer, J.; Schonlechner, R.; Ruckenbauer, P.; Berghofer, E. Variability in Chemical Composition and Biologically Active Constituents of Cereals. In Genetic Variation for Plant Breeding; Vollmann, J., Grausgruber, H., Ruckenbauer, P., Eds.; University of Natural Resources and Applied Life Sciences: Vienna, Austria, 2004; pp. 23–26. [Google Scholar]


| Sample | Species | Information | |
|---|---|---|---|
| 1 | Common wheat_1 | Triticum aestivum | Local landrace; low yielding |
| 2 | Common wheat_2 | Triticum aestivum | Local landrace; registered in the national list of indigenous landraces |
| 3 | Yecora-E | Triticum aestivum cv. Yecora-E | Individual selection from CIMMYT’s variety Gecora 70 [17] |
| 4 | Generoso-E | Triticum aestivum cv. Generoso-E | Individual selection from the Italian variety Generoso [17] |
| 5 | Oropos | Triticum aestivum cv. Oropos | Greek spring variety (registration no. CV-975, PI639687). Cross of Siete Cerros × YG-3927. |
| 6 | Durum wheat_1 | Triticum turgidum ssp. durum | Local landrace |
| 7 | Durum wheat_2 | Triticum turgidum ssp. durum | Local landrace; registered in the national list of indigenous landraces |
| 8 | Lemnos | Triticum turgidum ssp. durum | Selection from the landrace Akbasak [18] |
| 9 | Simeto | Triticum turgidum ssp. durum cv. Simeto | Italian modern durum wheat, cross of Capeti 8 × Valnova [17] |
| 10 | Mexikali 81 | Triticum turgidum ssp. durum cv. Mexikali 81 | Selection from CIMMYT‘s variety Mexicali 75 [17] |
| 11 | Emmer | Triticum turgidum ssp. dicoccum | Ancient Greek cultivar |
| 12 | Einkorn | Triticum monococcum ssp. Monococcum | Ancient Greek cultivar |
| 13 | Barley | Hordeum vulgare | Local landrace |
| 14 | Oat | Avena sativa | Local landrace |
| 15 | Rye | Secale cereale | Local landrace; registered in the national list of indigenous landraces |
| Precipitation per Month (mm) | Mean Month Temperature (°C) | |
|---|---|---|
| November 2020 | 17.4 | 11.5 |
| December 2020 | 44.8 | 10.0 |
| January 2021 | 85 | 7.5 |
| February 2021 | 32 | 7.9 |
| March 2021 | 89 | 9.3 |
| April 2021 | 25 | 22.8 |
| May 2021 | 34.4 | 21.0 |
| June 2021 | 54.2 | 24.2 |
| Marker | Chromosome | Sequence of f Primer | Sequence of r Primer | Ta |
|---|---|---|---|---|
| Xgwm148 | 2B | GTGAGGCAGCAAGAGAGAAA | CAAAGCTTGACTCAGACCAAA | 55 °C |
| Xgwm153 | 1B | GATCTCGTCACCCGGAATTC | TGGTAGAGAAGGACGGAGAG | 57 °C |
| Xgwm350 | 7B, 7D | ACCTCATCCACATGTTCTACG | GGATGGATAGGACGCCC | 55 °C |
| Xgwm389 | 3B | ATCATGTCGATCTCCTTGACG | TGCCATGCACATTAGCAGAT | 53 °C |
| Xgwm544 | 5B | TAGAATTCTTTATGGGGTCTGC | AGGATTCCAATCCTTCAAAATT | 50 °C |
| Xgwm614 | 2A | GATCACATGCATGCGTCATG | TTTTACCGTTCCGGCCTT | 51 °C |
| Xgwm637 | 4A | AAAGAGGTCTGGCGCTAACA | TATACGGTTTTGTGAGGGGG | 55 °C |
| Xwmc254 | 1A | AGTAATCTGGTCCTCTCTTCTTCT | AGGTAATCTCCGAGTGCACTTCAT | 57 °C |
| Xwmc415 | 5B | GATCTCCCATGTCCGCC | CGACAGTCGTCACTTGCCTA | 55 °C |
| Xwmc597 | 2B | AACACACCTTGCTTCTCTGGGA | GACTAGGGTTTCGGTTGTTGGC | 58 °C |
| SSR Marker | Allele Size Range | No. of Bands | Ne | I | Ho | He | PIC | |
|---|---|---|---|---|---|---|---|---|
| Total | Polymorphic | |||||||
| Xgwm148 | 138–167 | 8 | 8 | 5.14 | 1.84 | 0.58 | 0.81 | 0.24 |
| Xgwm153 | 162–210 | 11 | 11 | 7.02 | 2.16 | 0.92 | 0.86 | 0.31 |
| Xgwm350 | 134–148 | 5 | 5 | 3.65 | 1.42 | 0.58 | 0.73 | 0.16 |
| Xgwm389 | 112–141 | 11 | 11 | 8.73 | 2.28 | 0.33 | 0.89 | 0.31 |
| Xgwm544 | 168–198 | 7 | 7 | 5.56 | 1.82 | 0.50 | 0.82 | 0.21 |
| Xgwm614 | 123–148 | 6 | 6 | 1.92 | 1.06 | 0.33 | 0.48 | 0.19 |
| Xgwm637 | 140–176 | 10 | 10 | 8.07 | 2.19 | 0.73 | 0.88 | 0.29 |
| Xwmc254 | 158–188 | 3 | 3 | 2.33 | 0.95 | 0.63 | 0.57 | 0.10 |
| Xwmc415 | 126–134 | 3 | 3 | 2.69 | 1.04 | 0.00 | 0.63 | 0.10 |
| Xwmc597 | 221–243 | 7 | 7 | 2.94 | 1.44 | 0.75 | 0.66 | 0.21 |
| Mean | 7.1 | 7.1 | 4.80 | 1.62 | 0.54 | 0.73 | 0.21 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mougiou, N.; Didos, S.; Bouzouka, I.; Theodorakopoulou, A.; Kornaros, M.; Mylonas, I.; Argiriou, A. Valorizing Traditional Greek Wheat Varieties: Phylogenetic Profile and Biochemical Analysis of Their Nutritional Value. Agronomy 2023, 13, 2703. https://doi.org/10.3390/agronomy13112703
Mougiou N, Didos S, Bouzouka I, Theodorakopoulou A, Kornaros M, Mylonas I, Argiriou A. Valorizing Traditional Greek Wheat Varieties: Phylogenetic Profile and Biochemical Analysis of Their Nutritional Value. Agronomy. 2023; 13(11):2703. https://doi.org/10.3390/agronomy13112703
Chicago/Turabian StyleMougiou, Niki, Spyros Didos, Ioanna Bouzouka, Athina Theodorakopoulou, Michael Kornaros, Ioannis Mylonas, and Anagnostis Argiriou. 2023. "Valorizing Traditional Greek Wheat Varieties: Phylogenetic Profile and Biochemical Analysis of Their Nutritional Value" Agronomy 13, no. 11: 2703. https://doi.org/10.3390/agronomy13112703
APA StyleMougiou, N., Didos, S., Bouzouka, I., Theodorakopoulou, A., Kornaros, M., Mylonas, I., & Argiriou, A. (2023). Valorizing Traditional Greek Wheat Varieties: Phylogenetic Profile and Biochemical Analysis of Their Nutritional Value. Agronomy, 13(11), 2703. https://doi.org/10.3390/agronomy13112703









