1. Introduction
The grafting technique for vegetable crops is a highly effective method for controlling pests and diseases and for promoting plant growth and development. This technique renders the fusion of two distinct plant genotypes at the grafting point possible, with the aim of enhancing the scion attributes of rusticity and vigor [
1] This ancient practice involves the fusion of the tissues of two different plant varieties to create a single, stronger organism that can control pests and diseases better, thereby improving crop yield and produce quality [
1,
2,
3]. Grafting is a widely adopted global practice for the management of soil-borne pests and diseases, particularly those that can substantially impact the cultivation of
Solanaceae and
Cucurbitaceae plant families [
4,
5,
6].
This technique is intricately linked to water use efficiency (WUE), which is a measure that elucidates how effectively a plant utilizes water resources for producing biomass and product yield [
7,
8]. WUE represents the amount of water required to generate the obtained yield. It can also be evidenced by the ratio of biomass obtained under ordinary irrigation regimes, considering the amount of water consumed through plant evapotranspiration during plant growth and development processes, compared to the deficit irrigation ones. Furthermore, WUE quantifies how efficiently plants convert water into useful outputs in terms of growth and crop production [
9,
10]. It is a crucial parameter in agriculture and ecology because it reflects a plant’s ability to thrive in water-limited environments, which have an impact on photosynthetic activity [
11,
12,
13]. The exploitation of the WUE trait is particularly valuable in regions where water resources are limited, or in the face of changing climate conditions where droughts are more frequent [
14,
15,
16]. Drought stress can impact not only the quantity of crop yield but also its quality, potentially altering the production of metabolites [
16,
17,
18]. The improvement of the crop WUE is a key objective in agricultural research as it can lead to more sustainable and resilient agricultural practices. It can reduce water waste and increase crop productivity in water-stressed areas. Consequently, both grafting techniques and strategies aimed at enhancing WUE hold significant promise for adoption in organic farming conditions, contributing to more efficient and environmentally friendly agricultural practices [
19,
20,
21,
22,
23]. Within this context, organic farming needs the development of suitable genetic materials and cultivars capable of thriving under organic farming. This often requires the use of biotechnological tools [
24,
25].
While the primary purpose of grafting is often to combine desirable traits, such as disease resistance or improved fruit amount and quality, its connection to water use efficiency cannot be understood. One of the most critical aspects of grafting in relation to WUE is the choice of rootstock. The rootstock, often chosen for its adaptability to specific environmental conditions, plays a pivotal role in regulating water uptake and distribution within the grafted plant [
26,
27]. Different rootstocks can exhibit varied levels of drought tolerance, water absorption efficiency, and resistance to waterborne diseases. By carefully selecting a rootstock that suits the local climate and soil conditions, growers can optimize water utilization. Moreover, grafting can enhance a plant’s WUE by reducing the overall water demand of the plant [
28,
29,
30]. When grafting is successful, the scion can benefit from the well-established root system of the rootstock. This means that the scion may require less water to sustain itself since it can draw upon the rootstock’s water reserves. As a result, grafted plants can thrive in conditions where non-grafted counterparts might struggle due to limited water availability [
31,
32].
In the present study, the tested eggplant genotypes, encompassing different combinations of rootstocks and scions (including self and non-grafted plants), were evaluated under three levels of irrigation management. The different irrigation regimes consisted of a 50% deficit in water volume (IR50), a doubling of irrigation volume (IR200), and normal watering (IR100). Hence, a primary objective of this study was to assess how plant growth and development are influenced by altering the water volume, specifically by using either half or double the normal irrigation amount. This evaluation took into account various combinations of rootstocks known for their ability to access water from deeper soil layers. Another objective of this study was to identify the most effective combinations under conditions of water deficiency, with the aim of improving water use efficiency (WUE). This study aimed to develop novel agricultural protocols specifically designed for organic farming. Bio-morphometric analysis of the plants was carried out, including the analysis of fruits’ quality traits. Moreover, leaf chromatic parameters and water potential were evaluated to determine how leaf tissue responded in accordance with the plant’s hydration status under the three distinct irrigation regimes studied.
2. Materials and Methods
2.1. Plant Material
The trial was conducted in a cold greenhouse located in Marina di Ragusa (RG, Sicily, Italy). This area was chosen for its representativeness of Sicilian greenhouse cultivation, primarily due to the presence of rot-knot nematodes. The geographical coordinates of the greenhouse were 36°47′15.5″ N, 14°33′18.6″ E.
A split-plot experimental design was employed with four replicates for each combination of the two experimental factors under consideration. The first experimental factor concerned the various genotypes (GEs), which were represented by the distinct rootstock–scion combinations, and the second experimental factor encompassed the irrigation regime (IR), involving the adoption of three distinct watering levels. As concerns the evaluated GE, the eggplant cultivar Black Bell (Bb) from the seed company PetoSeed was employed as the scion in each combination. The rootstock utilized included the interspecific tomato hybrid F1 Beaufort (Be), the intraspecific tomato hybrid F1 Energy (En), and one accession of the wild species Solanum torvum belonging to the GenBank of vegetables of the Dipartimento di Agricoltura, Alimentazione e Ambiente (Di3A) of the Catania University. In addition to the previously mentioned combinations, the auto-grafted and the non-grafted plants of the cv Black Bell were also evaluated (Bb/Bb and Bb, respectively). Consequently, all the rootstock–scion combinations tested were To/Bb, Be/Bb, En/Bb, the self-grafted Bb/Bb, and the non-grafted Bb.
Sowing was conducted in cellular trays, and the plantlets were transplanted during the second decade of October 2021. All grafting combinations were executed using the oblique cutting method in a specialized nursery. The transplanting occurred within a cold greenhouse that had previously hosted tomato (
Solanum lycopersicum) cultivation in the growing season before the trial. The plants were cultivated at the crop density of 2 plants m
−2 (0.5 m × 1.0 m). Plants were characterized using the morphological traits reported in
Table 1, with their respective codes and units of measure.
During the growing cycle, plants were characterized for their bio morphometric parameters at 21, 42, and 84 days after transplanting (DAT). The traits evaluated during the growing cycle were plant height (PH), the basal diameter of the rootstock and of the scion (PBDR and PBDS, respectively), and the plant diameter at the grafting point (PDGP). These traits were also evaluated at the end of the growing cycle, which was in July 2021. Furthermore, during the growing cycle, the number of days required after transplanting for the first, second, third, and fourth flowers to open was also recorded. During the trial, the leaf chromatic CIEL*a*b* parameters were registered using the colorimeter (Chroma meter CR-200, MINOLTA, Osaka, Japan). In the leaf chromatic analysis performed, L* represented lightness, a* indicated the red/green coordinate, and b* signified the yellow/blue coordinate. The average air temperature throughout the growth cycle ranged from 11.5 °C in the second decade of December 2021 to 30.5 °C at the end of June 2022. The temperatures inside were recorded using the USB data logger (Testo, 174-T, Sparta, NJ, USA).
2.2. Differential Irrigation Set-Up
The second experimental factor was the irrigation regime (IR), which consisted of a 50% deficit in water volume (IR50), a doubling of irrigation volume (IR200), and normal watering (IR100). The total amounts of water per plant provided during the growing period were 46.40 L plant
−1 for IR50, 92.80 L plant
−1 for IR100, and 185.81 L plant
−1 for IR200 (
Table 2). Different irrigation volumes were applied in different phenological stages according to the Biologische Bundesanstalt, Bundessortenamt und Chemische Industrie (BBCH) scale index. The definition of the optimal requirement of water per plant was estimated according to previous works [
33,
34]. Particularly, the irrigation volume employed for the control IR100 was calculated following the model of “FAO irrigation and drainage paper number 56” proposed by Allen et al. [
35]. This model, which is based on the Penman–Monteith formula [
36], considered crop coefficients specific to eggplant cultivation in a cold greenhouse in Sicily, along with global solar radiation. To minimize soil evaporation, plastic mulching was adopted. To ensure the scheduled water supply to the plants, a dripping irrigation system managed by timers was employed. Notably, each irrigation thesis was spatially separated from the others by a border raw.
2.3. Fruit Analysis
Fruit quality traits were assessed, including chromatic parameters (FUL*, FUa*, and FUb*, respectively), longitudinal (FLD) and transversal diameters (FTD), dry matter content (FDM), and soluble solid content (FSSC) (
Table 1). Chromatic CIEL*a*b* parameters of the fruits were measured using a colorimeter (Chroma meter CR-200, MINOLTA, Japan). Among the fruit chromatic parameters analyzed, L* indicated lightness, a* was the red/green coordinate, and b* was the yellow/blue coordinate. The soluble solid content was determined using a digital refractometer (DBX-55A, ATAGO, Italy, Milan). The fruit production components registered were the number of fruits per plant (FP), the weight of individual fruit (FEW), and the total fruit production per plant (FPP) (
Table 1).
2.4. Water Use Efficiency (WUE)
WUE was calculated for the fruit production per plant (FPP) in relation to the volume of water used per plant. The formula used was in accordance with a previous study [
37].
where FPP was the fruit production per plant and IW was the water volume, expressed in m
3, employed for the plant. WUE was expressed in kg m
−3.
2.5. Leaf Water Potential
Leaf water potential (WP) values, measured in -MPa, were recorded on three specific days corresponding to distinct phenological phases, according to the BBCH index. The first assessment took place on January 26 during the phenological phase of the third visible flower bud, with temperatures ranging from 9 to 10 °C and relative humidity levels between 80% and 90%. The second assessment occurred during the fruit development phase, specifically at the first fruit cluster stage, with temperatures ranging from 27 to 32 °C and humidity levels of 40%. Finally, the third assessment was conducted during the phenological phase of fruit ripening, with temperatures ranging from 24.5 to 26 °C. The three assessments were chronologically labeled in the manuscript as A1c, A2h, and A3i, representing the assessments conducted in cold, hot, and intermediate temperature conditions, respectively. WP measurements were performed using the Scholander pressure chamber (PMS Instrument Company, PMS-600, Albany, OR, USA).
2.6. Data Analysis
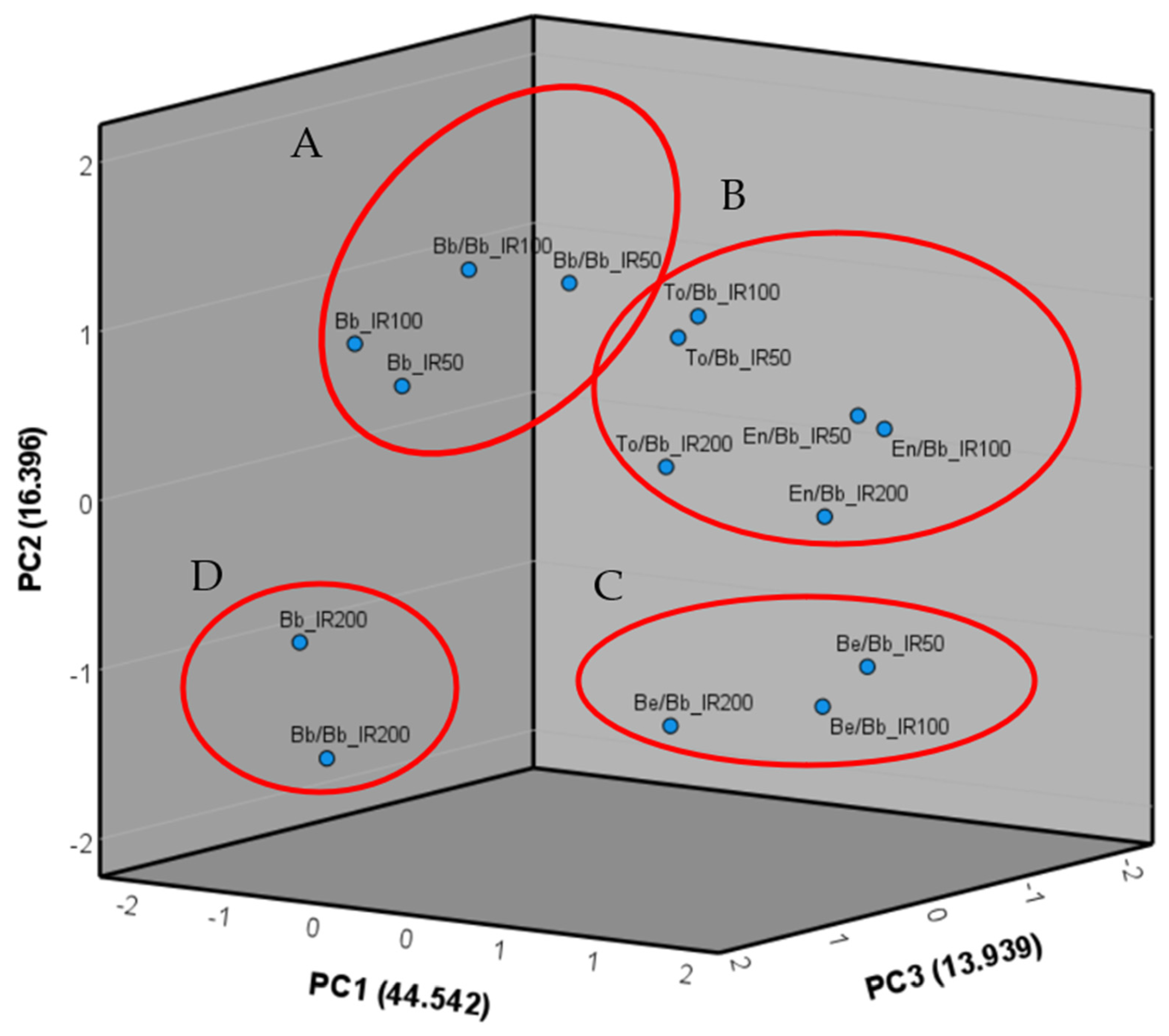
The data were subjected to analysis of variance (ANOVA) using the Newman–Keuls method, and this analysis was conducted using CoStat software version 6.4 (CoHort software, Birmingham, UK). The experimental design included the first factor consisting of four repetitions for each rootstock–scion combination (GE) and the second factor representing the three irrigation levels (IR). Tukey’s multiple comparisons test was also performed by CoStat software to assess significant differences in relation to the experimental factor studied. Subsequently, the means for each repetition were utilized to calculate Pearson’s correlations among all the examined traits, as well as to perform the principal components analysis (PCA) with the extraction of the three main components. Pearson’s correlation and the PCA were carried out using IBM SPSS version 27 software (IBM, Armonk, NY, USA).
4. Discussion
The primary focus of this research was to determine the optimal rootstock–scion combination in response to varying irrigation levels. In line with this objective, this study aimed to identify the most effective combination capable of thriving with half the usual amount of water, enhancing water use efficiency (WUE) in agriculture, and ensuring optimal agronomic performance. Within this context, the combinations evaluated included the rootstock hybrids F1 Beaufort and Energy, which were already evaluated for their agronomic performance [
38,
39]. Beaufort F1 showed a robust agronomic performance but a low affinity when grafted with eggplant. On the other hand, Energy F1 showed less vigor in comparison to the interspecific rootstock but high mineral uptake.
In our study, the combination of the Black Bell cultivar grafted onto the Beaufort F1 rootstock (Be/Bb) exhibited the highest vegetative growth, as evidenced by parameters like plant height (PH), rootstock basal diameter (PBDR and PBDS), and grafting point diameter. This outcome aligns with previous studies where the Beaufort F1 rootstock has typically shown incompatibility with various eggplant cultivars, resulting in excessive vegetative development [
40,
41,
42]. Conversely, the combination of the
S.
torvum rootstock with the Black Bell scion (To/Bb) displayed substantial vegetative traits, particularly a notable increase in scion basal diameter (PBDS). This can be attributed to the strong compatibility between
S.
torvum tissues, ensuring an efficient vascular connection capable of transferring nutrients and water without causing grafting imbalances [
43,
44,
45].
The combination involving the self-grafted combinations consistently exhibited low values of plant height. This can be attributed to the longer tissue regeneration time of self-grafted plants compared to non-grafted ones. Specifically, self-grafted plants use a rootstock typically chosen for its production-related characteristics. Consequently, after the grafting stress, they may have greater difficulty in regenerating tissues and especially in forming a robust root system. In contrast, F1 hybrid rootstocks like Beaufort, Energy, and
S.
torvum have been selected precisely for their strong adaptability, facilitated by their powerful root systems. Within this context, in line with previous studies [
46,
47], it can be postulated that self-grafted plants require a prolonged adaptation period to achieve the same level of growth performance as their non-grafted counterparts. As a result of these hypotheses, self-grafted plants probably require more time to mitigate the issue of the grafting activity and to regenerate tissues at the grafting point. Building upon our thesis, in a previous work [
48], the self-grafted combinations showed the lowest fruit yield in comparison to the self-rooted and the grafted plants.
It is noteworthy that the non-grafted Black Bell (Bb) exhibited the highest values of plant basal diameter of the scion (PBDS) at 21 and 42 days after transplanting (DAT). The larger stem diameter in non-grafted Bb can be attributed to the absence of stress caused by grafting. Conversely, Bb/Bb exhibited lower PBDS values due to self-grafting. However, after this initial period, PBDS in Bb was surpassed by other rootstock–scion combinations, as they overcame the grafting stress and exhibited greater growth. Furthermore, PBDS significantly increased in the To/Bb combination from 21 to 84 DAT, possibly due to successful grafting compatibility, promoting optimal vegetative growth. In contrast, plants grafted onto Energy and Beaufort F1 rootstocks displayed limited scion growth despite a large diameter at the grafting point (
Figure 1). This could be attributed to the vigorous water uptake by the Beaufort rootstock, resulting in phenomena such as guttation and vitrescence, which we observed in Be/Bb.
Regarding the impact of different irrigation protocols on plant height, our study revealed minimal differences between plant heights under IR50 and IR100, which aligns with the findings of a previous study [
49]. This minimal height difference can be attributed to the development of a robust root system capable of reaching deeper soil layers, ensuring sufficient water and nutrient absorption. In contrast, the excessive water content in the rhizosphere under IR200 may hinder root development, resulting in shorter plants. Similar trends were observed for basal diameters (PBDR and PBDS), which showed no significant variations between deficitary (IR50) and normal (IR100) irrigation, as also noted by previous research [
50].
Concerning flowering time, we observed a significant reduction in non-grafted plants compared to the self-grafted ones, which exhibited a considerable delay in flowering. Additionally, we noticed a significant reduction in flowering time for the deficitary irrigation regime IR50, as compared to IR100 and IR200. This can be attributed to the reduced water in the flower tissue that led the flower to a faster fruit-setting process. The significant reduction in flowering time for IR50 could have substantial advantages in terms of early fruit production.
Regarding fruit quality traits, grafted plants produced longer fruits compared to the non-grafted ones. This is likely due to grafting enabling a more efficient transport of water and nutrients to the reproductive organs. Specifically, the combinations with the
S.
torvum and Energy F1 rootstocks (To/Bb and En/Bb) showed the highest values for both fruit longitudinal and transversal diameters (FLD and FTD, respectively). However, there was no significant variation in FLD and FTD between the IR50 and IR100 conditions. Reduced values were observed only for IR200, which could be attributed to oxygen deprivation in plants grown under IR200, where the water regime was doubled compared to normal conditions. Oxygen deprivation is typically induced by waterlogging stress and significantly affects physiological and developmental processes, ultimately impacting biomass production [
51,
52].
Regarding the soluble solid content (FSSC), non-grafted plants exhibited significantly higher values. This could be attributed to the lower water uptake capacity of the non-grafted root system compared to the rootstock’s, which might result in a dilution effect on the sugar content in fruits. Surprisingly, we did not observe an increase in FSSC under IR50, as the deficitary water regime we applied did not induce stress during the fruit-setting process.
Furthermore, the number of fruits per plant (FP) significantly varied among the different grafting combinations. Notably, the combination with
S.
torvum rootstock displayed the highest FP value. This higher FP value can be attributed to the strong compatibility between S. melongena grafted onto
S.
torvum rootstock, as supported by various studies [
42,
44,
53]. Additionally, under IR50 conditions, we observed the highest FP value, which is likely due to the reduced vegetative growth of IR50 plants, which addressed a faster transition to the reproductive stage.
The trend in fruit weight (FWE) mirrored that of the number of fruits per plant (FP), with the combination involving S. torvum rootstock registering the highest value. Notably, the highest FWE value was observed under normal irrigation conditions (IR100), exhibiting a significant difference from both IR50 and IR200 conditions. This difference can be attributed to the enhanced water and nutrient uptake facilitated by grafting compatibility.
Regarding water use efficiency (WUE), we noticed a significant variation due to the different irrigation regimes applied. Interestingly, all combinations subjected to reduced irrigation (IR50) displayed the highest WUE values. This characteristic led to a significant reduction in water uptake from the soil, but there was minimal impact on fruit production per plant compared to the control (IR100). Among these combinations, To/Bb exhibited the highest WUE value, which is possibly attributed to the strong compatibility between the rootstock and scion, optimizing nutrient and water conversion into fresh produce. Additionally, the robust root system of the rootstock allowed for more effective exploration of soil layers, enhancing water absorption.
Conversely, our research revealed a notable decrease in WUE values for plants cultivated with double the amount of water (IR200). This can be attributed to the challenges plants face in water absorption due to reduced oxygen availability in waterlogged conditions.
Regarding the physiological analysis of water potential (WP), the highest values observed at A2h suggest that plants were transitioning to the reproductive phase, with resources being allocated to reproductive organs. Furthermore, this value was recorded when plants were under stress, as this assessment was conducted within a temperature range of 27 °C to 32 °C.
Notably, Bb/Bb exhibited the highest water potential values at A2h DAT, while the grafting combinations with the rootstocks Energy F1 and S. torvum showed lower values due to their better grafting compatibility. Conversely, lower water potential values were recorded at A3i DAT, likely reflecting the differentiation of floral buds, as confirmed by flower observations. On the other hand, the higher water potential in IR50 may be explained by the increased pressure required for water to exit the leaves. This higher pressure requirement can be attributed to the reduced water content inside the leaf tissue, which is possibly related to the plants’ efforts to support reproductive growth.
In light of these findings, it becomes imperative to conduct additional assessments that include the modulation of substrate temperature. This is necessary to overcome the limitation posed by the grafting incompatibility observed with the rootstocks F1 Energy and Beaufort. These particular rootstocks resulted in an excessive growth of the Black Bell cultivar used as the scion in the experiment.