Soybean LEAFY COTYLEDON 1: A Key Target for Genetic Enhancement of Oil Biosynthesis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Gene Identification and Cloning
2.2. Real-Time PCR Analysis
2.3. Production of Transgenic Lines
2.4. Lipid Extraction and Fatty Acid Analysis
2.5. Starch Quantification
3. Results
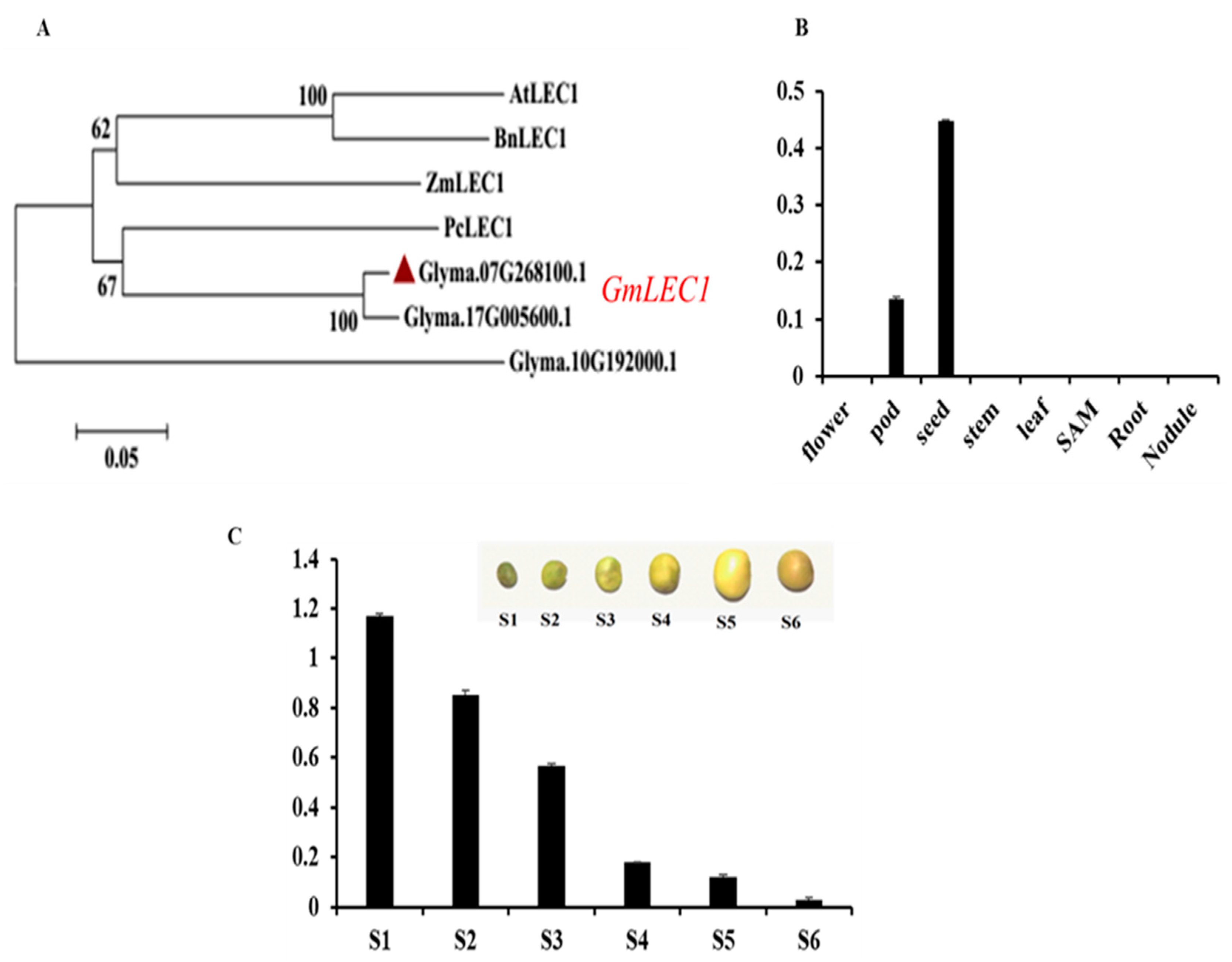
3.1. Gene Identification and Expression in Soybean Plant Tissues
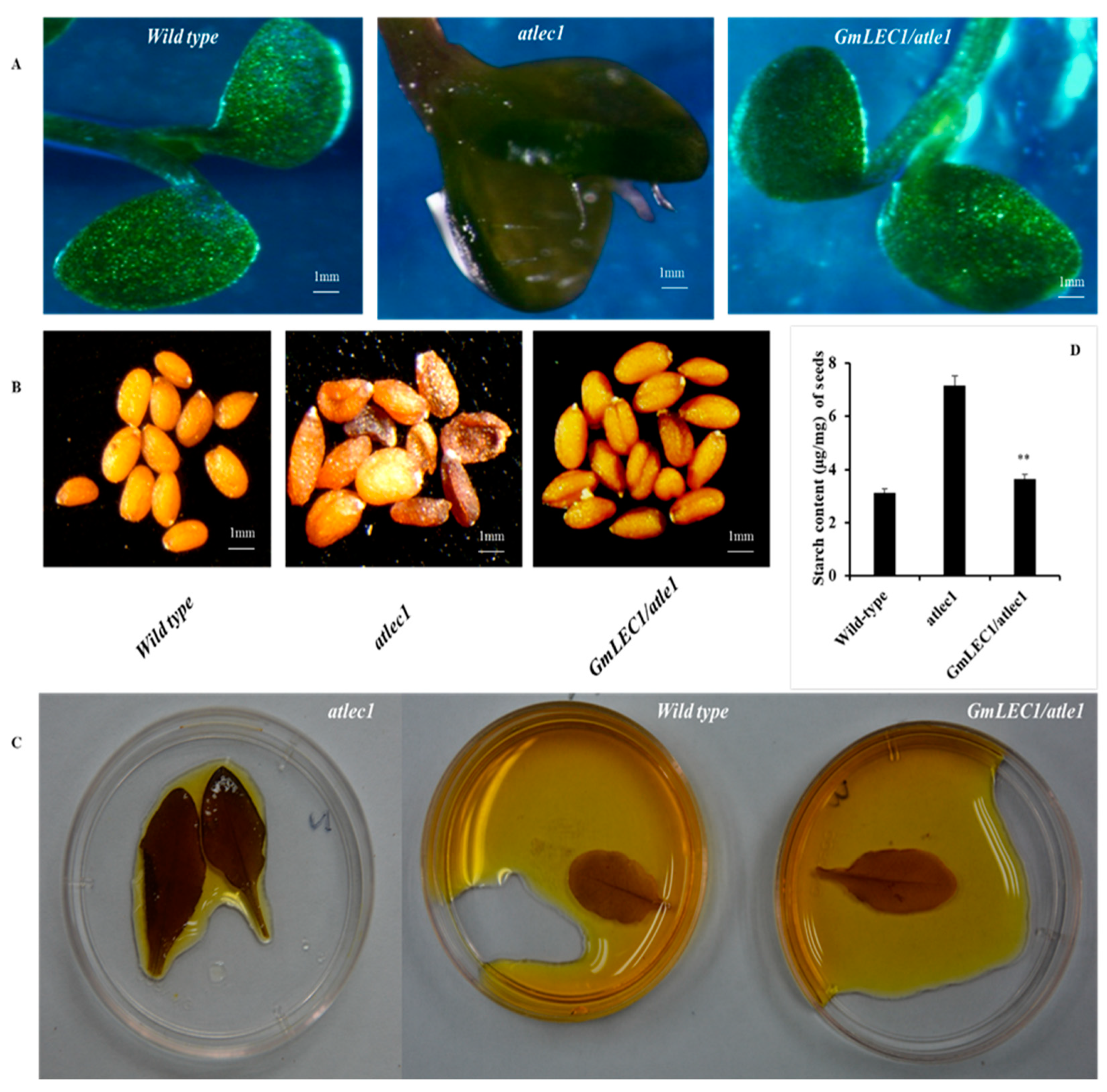
3.2. Plant Development
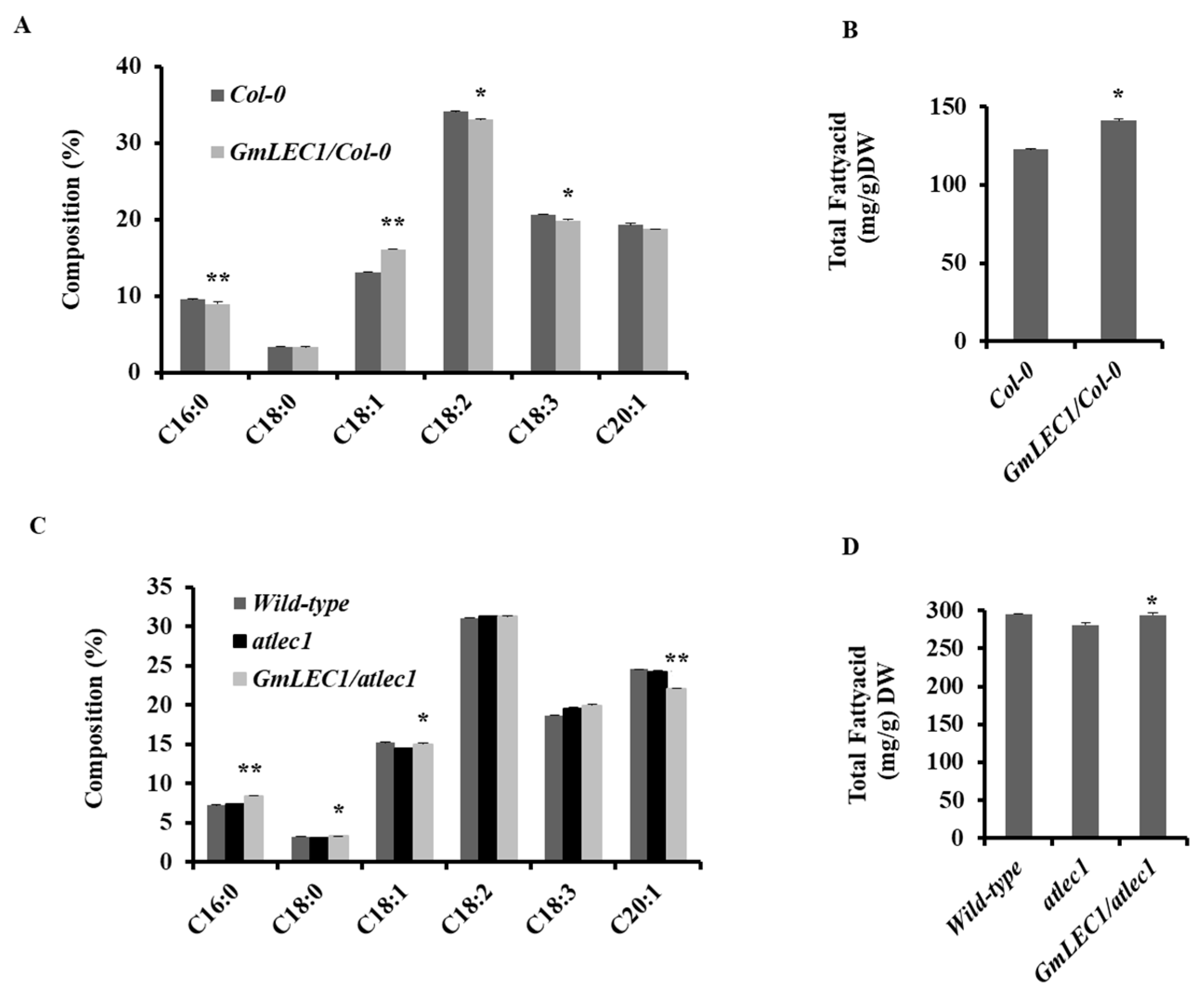
3.3. Impact on Seed TAG Content and Composition
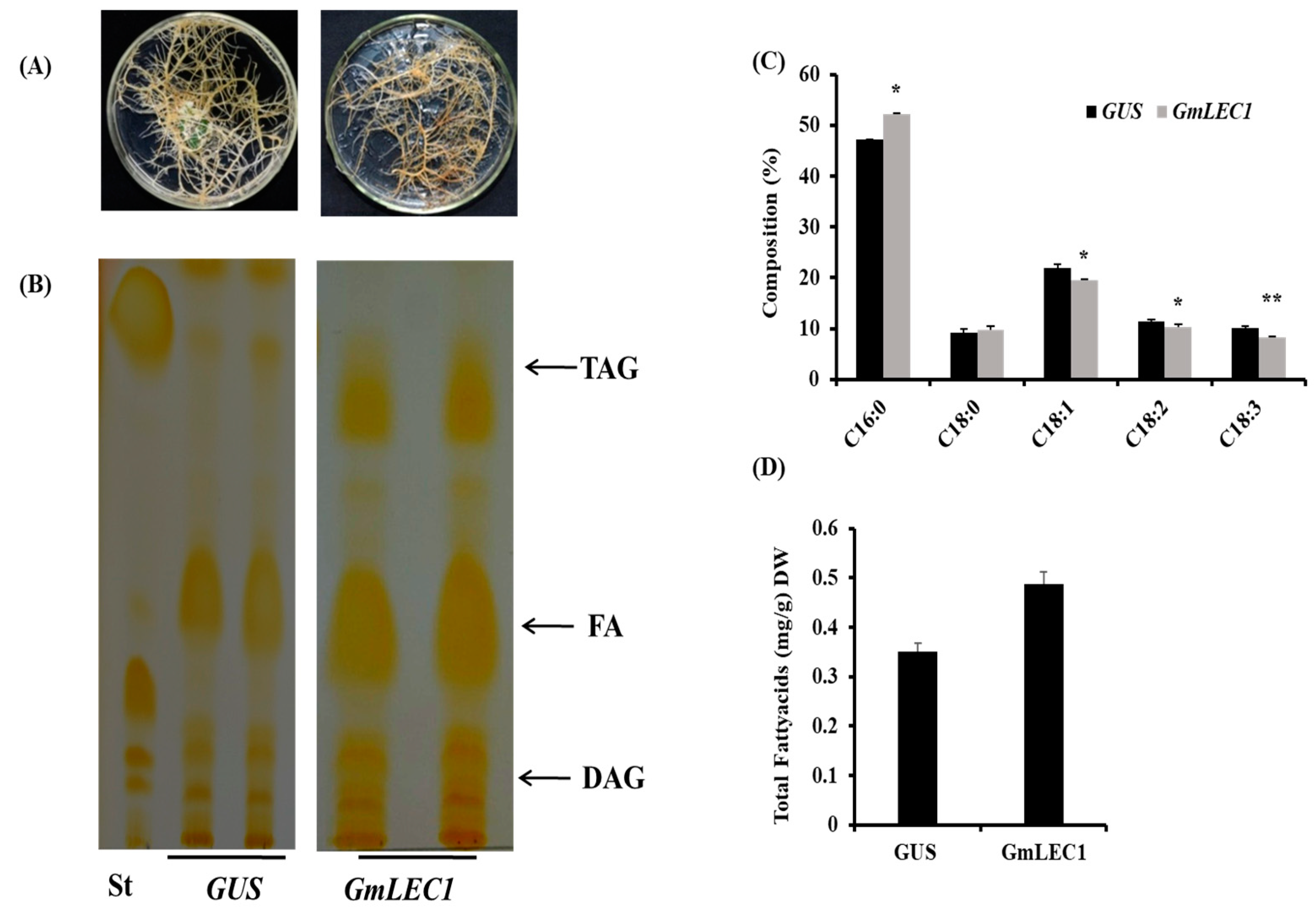
3.4. Ectopic Expression in Soybean Hairy Roots Induces TAG Storage
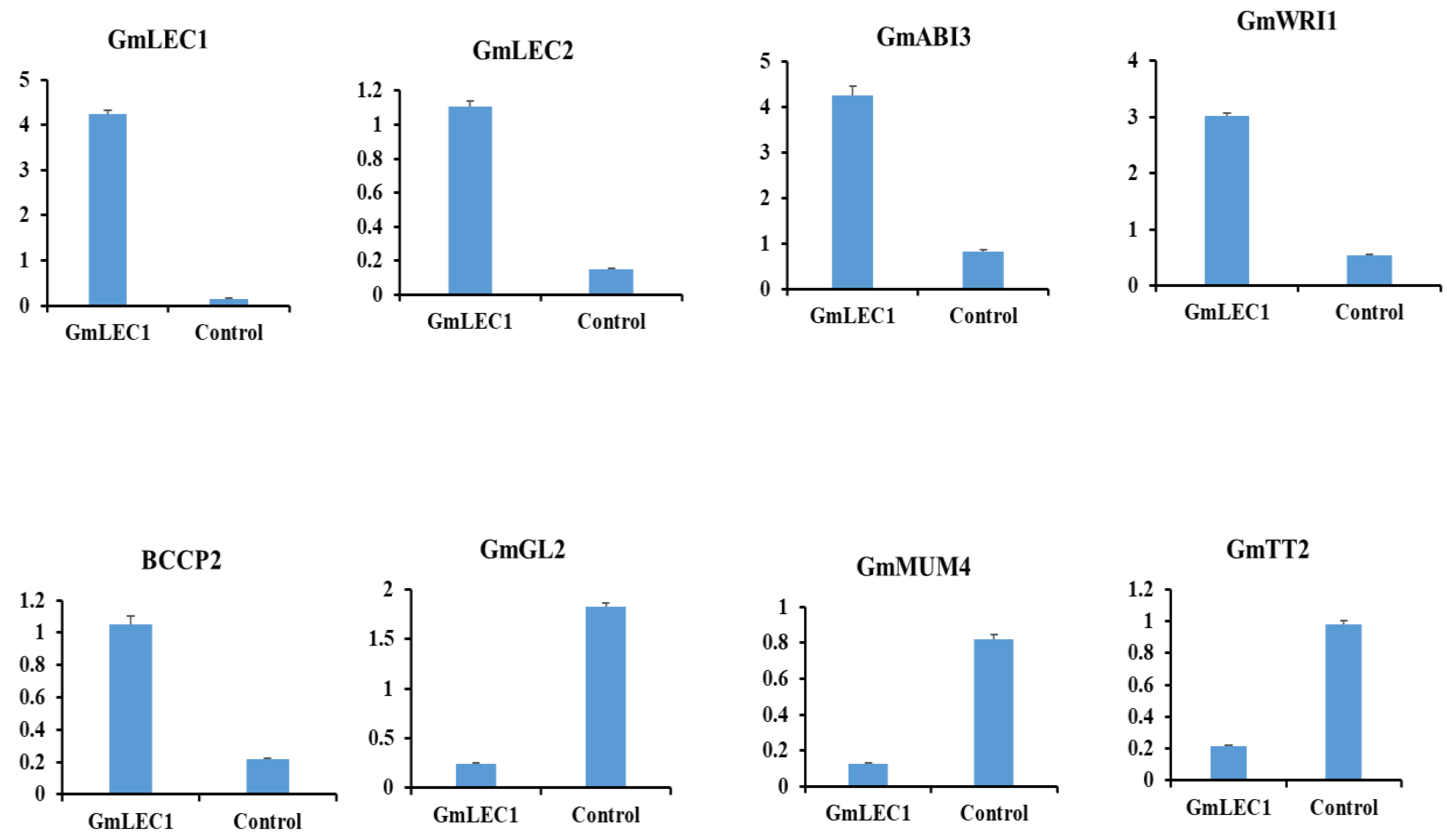
3.5. LEC1 Regulates Key Genes of Lipid Biosynthesis in Soybean
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Junker, A.; Bäumlein, H. Multifunctionality of the LEC1 Transcription Factor during Plant Development. Plant Signal. Behav. 2012, 7, 1718–1720. [Google Scholar] [CrossRef] [PubMed]
- Le, B.H.; Cheng, C.; Bui, A.Q.; Wagmaister, J.A.; Henry, K.F.; Pelletier, J.; Kwong, L.; Belmonte, M.; Kirkbride, R.; Horvath, S.; et al. Global Analysis of Gene Activity during Arabidopsis Seed Development and Identification of Seed-Specific Transcription Factors. Proc. Natl. Acad. Sci. USA 2010, 107, 8063–8070. [Google Scholar] [CrossRef] [PubMed]
- Manan, S.; Ahmad, M.Z.; Zhang, G.; Chen, B.; Haq, B.U.; Yang, J.; Zhao, J. Soybean LEC2 Regulates Subsets of Genes Involved in Controlling the Biosynthesis and Catabolism of Seed Storage Substances and Seed Development. Front. Plant Sci. 2017, 8, 1604. [Google Scholar] [CrossRef] [PubMed]
- Brocard-Gifford, I.M.; Lynch, T.J.; Finkelstein, R.R. Regulatory Networks in Seeds Integrating Developmental, Abscisic Acid, Sugar, and Light Signaling. Plant Physiol. 2003, 131, 78–92. [Google Scholar] [CrossRef] [PubMed]
- Mu, J.; Tan, H.; Zheng, Q.; Fu, F.; Liang, Y.; Zhang, J.; Yang, X.; Wang, T.; Chong, K.; Wang, X.-J.; et al. LEAFY COTYLEDON1 Is a Key Regulator of Fatty Acid Biosynthesis in Arabidopsis. Plant Physiol. 2008, 148, 1042–1054. [Google Scholar] [CrossRef]
- Huang, M.; Hu, Y.; Liu, X.; Li, Y.; Hou, X. Arabidopsis LEAFY COTYLEDON1 Controls Cell Fate Determination during Post-Embryonic Development. Front. Plant Sci. 2015, 6, 955. [Google Scholar] [CrossRef] [PubMed]
- Shen, B.; Allen, W.B.; Zheng, P.; Li, C.; Glassman, K.; Ranch, J.; Nubel, D.; Tarczynski, M.C. Expression of ZmLEC1 and ZmWRI1 Increases Seed Oil Production in Maize. Plant Physiol. 2010, 153, 980–987. [Google Scholar] [CrossRef]
- Tan, H.; Yang, X.; Zhang, F.; Zheng, X.; Qu, C.; Mu, J.; Fu, F.; Li, J.; Guan, R.; Zhang, H.; et al. Enhanced Seed Oil Production in Canola by Conditional Expression of Brassica Napus LEAFY COTYLEDON1 and LEC1-LIKE in Developing Seeds. Plant Physiol. 2011, 156, 1577–1588. [Google Scholar] [CrossRef]
- Yamamoto, A.; Kagaya, Y.; Toyoshima, R.; Kagaya, M.; Takeda, S.; Hattori, T. Arabidopsis NF-YB Subunits LEC1 and LEC1-LIKE Activate Transcription by Interacting with Seed-Specific ABRE-Binding Factors. Plant J. 2009, 58, 843–856. [Google Scholar] [CrossRef]
- Brand, A.; Quimbaya, M.; Tohme, J.; Chavarriaga-Aguirre, P. Arabidopsis LEC1 and LEC2 Orthologous Genes Are Key Regulators of Somatic Embryogenesis in Cassava. Front. Plant Sci. 2019, 10, 673. [Google Scholar] [CrossRef]
- To, A.; Joubès, J.; Barthole, G.; Lécureuil, A.; Scagnelli, A.; Jasinski, S.; Lepiniec, L.; Baud, S. WRINKLED Transcription Factors Orchestrate Tissue-Specific Regulation of Fatty Acid Biosynthesis in Arabidopsis[W]. Plant Cell 2012, 24, 5007–5023. [Google Scholar] [CrossRef] [PubMed]
- Roscoe, T.T.; Guilleminot, J.; Bessoule, J.-J.; Berger, F.; Devic, M. Complementation of Seed Maturation Phenotypes by Ectopic Expression of ABSCISIC ACID INSENSITIVE3, FUSCA3 and LEAFY COTYLEDON2 in Arabidopsis. Plant Cell Physiol. 2015, 56, 1215–1228. [Google Scholar] [CrossRef] [PubMed]
- Qin, P.; Wang, T.; Luo, Y. A Review on Plant-Based Proteins from Soybean: Health Benefits and Soy Product Development. J. Agric. Food Res. 2022, 7, 100265. [Google Scholar] [CrossRef]
- Qu, G.Z.; Zheng, T.; Liu, G.; Wang, W.; Zang, L.; Liu, H.; Yang, C. Overexpression of a MADS-Box Gene from Birch (Betula platyphylla) Promotes Flowering and Enhances Chloroplast Development in Transgenic Tobacco. PLoS ONE 2013, 8, e63398. [Google Scholar] [CrossRef]
- Séne, M.; Thévenot, C.; Prioul, J.L. Simultaneous Spectrophotometric Determination of Amylose and Amylopectin in Starch from Maize Kernel by Multi-Wavelength Analysis. J. Cereal Sci. 1997, 26, 211–221. [Google Scholar] [CrossRef]
- Tsai, A.Y.L.; Gazzarrini, S. AKIN10 and FUSCA3 Interact to Control Lateral Organ Development and Phase Transitions in Arabidopsis. Plant J. 2012, 69, 809–821. [Google Scholar] [CrossRef] [PubMed]
- Gazzarrini, S.; Tsuchiya, Y.; Lumba, S.; Okamoto, M.; McCourt, P. The Transcription Factor FUSCA3 Controls Developmental Timing in Arabidopsis through the Hormones Gibberellin and Abscisic Acid. Dev. Cell 2004, 7, 373–385. [Google Scholar] [CrossRef] [PubMed]
- Braybrook, S.A.; Harada, J.J. LECs Go Crazy in Embryo Development. Trends Plant Sci. 2008, 13, 624–630. [Google Scholar] [CrossRef]
- Angeles-Núñez, J.G.; Tiessen, A. Mutation of the Transcription Factor LEAFY COTYLEDON 2 Alters the Chemical Composition of Arabidopsis Seeds, Decreasing Oil and Protein Content, While Maintaining High Levels of Starch and Sucrose in Mature Seeds. J. Plant Physiol. 2011, 168, 1891–1900. [Google Scholar] [CrossRef]
- Chen, B.; Wang, J.; Zhang, G.; Liu, J.; Manan, S.; Hu, H.; Zhao, J. Two Types of Soybean Diacylglycerol Acyltransferases Are Differentially Involved in Triacylglycerol Biosynthesis and Response to Environmental Stresses and Hormones. Sci. Rep. 2016, 6, 28541. [Google Scholar] [CrossRef]
- Chen, L.; Zheng, Y.; Dong, Z.; Meng, F.; Sun, X.; Fan, X.; Zhang, Y.; Wang, M.; Wang, S. Soybean (Glycine Max) WRINKLED1 Transcription Factor, GmWRI1a, Positively Regulates Seed Oil Accumulation. Mol. Genet. Genom. 2018, 293, 401–415. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Y.; Shang, P.; Yang, C.; Yang, M.; Huang, J.; Ren, B.; Zuo, Z.; Zhang, Q.; Li, W.; et al. Overexpression of Soybean GmWRI1a Stably Increases the Seed Oil Content in Soybean. Int. J. Mol. Sci. 2022, 23, 5084. [Google Scholar] [CrossRef] [PubMed]
- Manan, S.; Zhao, J. Role of Glycine Max ABSCISIC ACID INSENSITIVE 3 (GmABI3) in Lipid Biosynthesis and Stress Tolerance in Soybean. Funct. Plant Biol. 2021, 48, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Perianez-Rodriguez, J.; Manzano, C.; Moreno-Risueno, M.A. Post-Embryonic Organogenesis and Plant Regeneration from Tissues: Two Sides of the Same Coin? Front. Plant Sci. 2014, 5, 219. [Google Scholar] [CrossRef] [PubMed]
- Manan, S.; Chen, B.; She, G.; Wan, X.; Zhao, J. Transport and Transcriptional Regulation of Oil Production in Plants. Crit. Rev. Biotechnol. 2017, 37, 641–655. [Google Scholar] [CrossRef]
- Yang, Y.; Kong, Q.; Lim, A.R.Q.; Lu, S.; Zhao, H.; Guo, L.; Yuan, L.; Ma, W. Transcriptional Regulation of Oil Biosynthesis in Seed Plants: Current Understanding, Applications, and Perspectives. Plant Commun. 2022, 3, 100328. [Google Scholar] [CrossRef]
- Devic, M.; Roscoe, T. Seed Maturation: Simplification of Control Networks in Plants. Plant Sci. 2016, 252, 335–346. [Google Scholar] [CrossRef]
- Santos-Mendoza, M.; Dubreucq, B.; Baud, S.; Parcy, F.; Caboche, M.; Lepiniec, L. Deciphering Gene Regulatory Networks That Control Seed Development and Maturation in Arabidopsis. Plant J. 2008, 54, 608–620. [Google Scholar] [CrossRef]
- Jo, L.; Pelletier, J.M.; Harada, J.J. Central Role of the LEAFY COTYLEDON1 Transcription Factor in Seed Development. J. Integr. Plant Biol. 2019, 61, 564–580. [Google Scholar] [CrossRef]
- Pelletier, J.M.; Kwong, R.W.; Park, S.; Le, B.H.; Baden, R.; Cagliari, A.; Hashimoto, M.; Munoz, M.D.; Fischer, R.L.; Goldberg, R.B.; et al. LEC1 Sequentially Regulates the Transcription of Genes Involved in Diverse Developmental Processes during Seed Development. Proc. Natl. Acad. Sci. USA 2017, 114, E6710–E6719. [Google Scholar] [CrossRef]
- Guo, F.; Zhang, P.; Wu, Y.; Lian, G.; Yang, Z.; Liu, W.; Buerte, B.; Zhou, C.; Zhang, W.; Li, D.; et al. Rice LEAFY COTYLEDON1 Hinders Embryo Greening During the Seed Development. Front. Plant Sci. 2022, 13, 887980. [Google Scholar] [CrossRef] [PubMed]
- Shen, B.; Sinkevicius, K.W.; Selinger, D.A.; Tarczynski, M.C. The Homeobox Gene GLABRA2 Affects Seed Oil Content in Arabidopsis. Plant Mol. Biol. 2006, 60, 377–387. [Google Scholar] [CrossRef] [PubMed]
- Western, T.L.; Young, D.S.; Dean, G.H.; Tan, W.L.; Samuels, A.L.; Haughn, G.W. MUCILAGE-MODIFIED4 Encodes a Putative Pectin Biosynthetic Enzyme Developmentally Regulated by APETALA2, TRANSPARENT TESTA GLABRA1, and GLABRA2 in the Arabidopsis Seed Coat. Plant Physiol. 2004, 134, 296–306. [Google Scholar] [CrossRef]
- Shi, L.; Katavic, V.; Yu, Y.; Kunst, L.; Haughn, G. Arabidopsis Glabra2 Mutant Seeds Deficient in Mucilage Biosynthesis Produce More Oil. Plant J. 2012, 69, 37–46. [Google Scholar] [CrossRef]
- Chen, M.; Wang, Z.; Zhu, Y.; Li, Z.; Hussain, N.; Xuan, L.; Guo, W.; Zhang, G.; Jiang, L. The Effect of TRANSPARENT TESTA2 on Seed Fatty Acid Biosynthesis and Tolerance to Environmental Stresses during Young Seedling Establishment in Arabidopsis. Plant Physiol. 2012, 160, 1023–1036. [Google Scholar] [CrossRef] [PubMed]
- Cheng, T.; Zhao, P.; Ren, Y.; Zou, J.; Sun, M.-X. AtMIF1 Increases Seed Oil Content by Attenuating GL2 Inhibition. New Phytol. 2021, 229, 2152–2162. [Google Scholar] [CrossRef] [PubMed]
- Kanai, M.; Mano, S.; Kondo, M.; Hayashi, M.; Nishimura, M. Extension of Oil Biosynthesis during the Mid-Phase of Seed Development Enhances Oil Content in Arabidopsis Seeds. Plant Biotechnol. J. 2016, 14, 1241–1250. [Google Scholar] [CrossRef]
- Maeo, K.; Tokuda, T.; Ayame, A.; Mitsui, N.; Kawai, T.; Tsukagoshi, H.; Ishiguro, S.; Nakamura, K. An AP2-Type Transcription Factor, WRINKLED1, of Arabidopsis thaliana Binds to the AW-Box Sequence Conserved among Proximal Upstream Regions of Genes Involved in Fatty Acid Synthesis. Plant J. 2009, 60, 476–487. [Google Scholar] [CrossRef]
- Sun, R.; Ye, R.; Gao, L.; Zhang, L.; Wang, R.; Mao, T.; Zheng, Y.; Li, D.; Lin, Y. Characterization and Ectopic Expression of CoWRI1, an AP2/EREBP Domain-Containing Transcription Factor from Coconut (Cocos nucifera L.) Endosperm, Changes the Seeds Oil Content in Transgenic Arabidopsis thaliana and Rice (Oryza sativa L.). Front. Plant Sci. 2017, 8, 63. [Google Scholar] [CrossRef]
- Boulard, C.; Thévenin, J.; Tranquet, O.; Laporte, V.; Lepiniec, L.; Dubreucq, B. LEC1 (NF-YB9) Directly Interacts with LEC2 to Control Gene Expression in Seed. Biochim. Biophys. Acta Gene Regul. Mech. 2018, 1861, 443–450. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manan, S.; Alabbosh, K.F.; Al-Andal, A.; Ahmad, W.; Khan, K.A.; Zhao, J. Soybean LEAFY COTYLEDON 1: A Key Target for Genetic Enhancement of Oil Biosynthesis. Agronomy 2023, 13, 2810. https://doi.org/10.3390/agronomy13112810
Manan S, Alabbosh KF, Al-Andal A, Ahmad W, Khan KA, Zhao J. Soybean LEAFY COTYLEDON 1: A Key Target for Genetic Enhancement of Oil Biosynthesis. Agronomy. 2023; 13(11):2810. https://doi.org/10.3390/agronomy13112810
Chicago/Turabian StyleManan, Sehrish, Khulood Fahad Alabbosh, Abeer Al-Andal, Waqas Ahmad, Khalid Ali Khan, and Jian Zhao. 2023. "Soybean LEAFY COTYLEDON 1: A Key Target for Genetic Enhancement of Oil Biosynthesis" Agronomy 13, no. 11: 2810. https://doi.org/10.3390/agronomy13112810





