The Impact of Mechanical Compression on the Postharvest Quality of ‘Shine Muscat’ Grapes during Short-Term Storage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Grape Samples and Treatments
2.2. Physical Properties
2.2.1. Visual Appearance
2.2.2. Determination of Color
2.3. Cell Structure Changes
2.4. Textural Properties
2.5. Physiological Properties
2.5.1. Total Soluble Solids (TSS), Titratable Acidity (TA) and Ascorbic Acid (AsA)
2.5.2. Weight Loss (WL) and Decay Incidence (DI)
2.5.3. Malondialdehyde (MDA) Content and Relative Conductivity (RC)
2.6. Statistical Analysis
3. Results
3.1. Physical Properties
3.1.1. Visual Appearance
3.1.2. Color
3.2. Cell Structure
3.3. Texture Profile Analysis
3.4. Physiological Properties
3.4.1. Total Soluble Solids (TSS), Titratable Acidity (TA), and Ascorbic Acid (AsA)
3.4.2. Weight Loss (WL) and Decay Incidence (DI)
3.4.3. Malondialdehyde (MDA) and Relative Conductivity (RC)
3.5. Correlation Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- FAO. Grapes Production Quantity and Area Harvested. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 1 February 2023).
- Zhang, L.; Li, X.; Pang, Y.; Cai, X.; Lu, J.; Ren, X.; Kong, Q. Phenolics composition and contents, as the key quality parameters of table grapes, may be influenced obviously and differently in response to short-term high temperature. LWT 2021, 149, 111791. [Google Scholar] [CrossRef]
- Faheem, M.; Liu, J.; Chang, G.; Ahmad, I.; Peng, Y. Hanging force analysis for realizing low vibration of grape clusters during speedy robotic post-harvest handling. Int. J. Agric. Biol. Eng. 2021, 14, 62–71. [Google Scholar] [CrossRef]
- Mohammad Shafie, M.; Rajabipour, A.; Mobli, H. Determination of Bruise Incidence of Pomegranate Fruit under Drop Case. Int. J. Fruit Sci. 2017, 17, 296–309. [Google Scholar] [CrossRef]
- Hussein, Z.; Fawole, O.A.; Opara, U.L. Preharvest factors influencing bruise damage of fresh fruits—A review. Sci.Hortic. 2018, 229, 45–58. [Google Scholar] [CrossRef]
- Hussein, Z.; Fawole, O.A.; Opara, U.L. Bruise damage susceptibility of pomegranates (Punica granatum L.) and impact on fruit physiological response during short term storage. Sci. Hortic. 2019, 246, 664–674. [Google Scholar] [CrossRef]
- Opara, U.L.; Pathare, P.B. Bruise damage measurement and analysis of fresh horticultural produce—A review. Postharvest Biol. Technol. 2014, 91, 9–24. [Google Scholar] [CrossRef]
- Fadiji, T.; Coetzee, C.; Chen, L.; Chukwu, O.; Opara, U.L. Susceptibility of apples to bruising inside ventilated corrugated paperboard packages during simulated transport damage. Postharvest Biol. Technol. 2016, 118, 111–119. [Google Scholar] [CrossRef]
- Li, Z.; Thomas, C. Quantitative evaluation of mechanical damage to fresh fruits. Trends Food Sci. Technol. 2014, 35, 138–150. [Google Scholar] [CrossRef]
- Dagdelen, C.; Aday, M.S. The effect of simulated vibration frequency on the physico-mechanical and physicochemical properties of peach during transportation. LWT 2021, 137, 110497. [Google Scholar] [CrossRef]
- Chaiwong, S.; Yoythaisong, P.; Arwatchananukul, S.; Aunsri, N.; Tontiwattanakul, K.; Trongsatitkul, T.; Kitazawa, H.; Saengrayap, R. Vibration damage in guava during simulated transportation assessed by digital image analysis using response surface methodology. Postharvest Biol. Technol. 2021, 181, 111641. [Google Scholar] [CrossRef]
- Sun, Y.; Pessane, I.; Pan, L.; Wang, X. Hyperspectral characteristics of bruised tomatoes as affected by drop height and fruit size. LWT 2021, 141, 110863. [Google Scholar] [CrossRef]
- Lin, M.; Chen, J.; Chen, F.; Zhu, C.; Wu, D.; Wang, J.; Chen, K. Effects of cushioning materials and temperature on quality damage of ripe peaches according to the vibration test. Food Packag. Shelf Life 2020, 25, 100518. [Google Scholar] [CrossRef]
- Xu, F.; Lu, F.; Xiao, Z.; Li, Z. Influence of drop shock on physiological responses and genes expression of apple fruit. Food Chem. 2020, 303, 125424. [Google Scholar] [CrossRef] [PubMed]
- Mir, H.; Shahbazi, F. Simulated transit vibration effects on the postharvest quality of persimmon during storage. Postharvest Biol. Technol. 2022, 189, 111918. [Google Scholar] [CrossRef]
- Xu, F.; Liu, S.; Liu, Y.; Wang, S. Effect of mechanical vibration on postharvest quality and volatile compounds of blueberry fruit. Food Chem. 2021, 349, 129216. [Google Scholar] [CrossRef] [PubMed]
- Solairaj, D.; Yang, Q.; Guillaume Legrand, N.N.; Routledge, M.N.; Zhang, H. Molecular explication of grape berry-fungal infections and their potential application in recent postharvest infection control strategies. Trends Food Sci. Technol. 2021, 116, 903–917. [Google Scholar] [CrossRef]
- Miraei Ashtiani, S.-H.; Sadrnia, H.; Mohammadinezhad, H.; Aghkhani, M.H.; Khojastehpour, M.; Abbaspour-Fard, M.H. FEM-based simulation of the mechanical behavior of grapefruit under compressive loading. Sci. Hortic. 2019, 245, 39–46. [Google Scholar] [CrossRef]
- Al-Dairi, M.; Pathare, P.B.; Al-Yahyai, R.; Opara, U.L. Mechanical damage of fresh produce in postharvest transportation: Current status and future prospects. Trends Food Sci. Technol. 2022, 124, 195–207. [Google Scholar] [CrossRef]
- Hussein, Z.; Fawole, O.A.; Opara, U.L. Harvest and Postharvest Factors Affecting Bruise Damage of Fresh Fruits. Hortic. Plant J. 2020, 6, 1–13. [Google Scholar] [CrossRef]
- Faheem, M.; Liu, J.; Chang, G.; Abbas, I.; Xie, B.; Shan, Z.; Yang, K. Experimental Research on Grape Cluster Vibration Signals during Transportation and Placing for Harvest and Post-Harvest Handling. Agriculture 2021, 11, 902. [Google Scholar] [CrossRef]
- Champa, W.A.H.; Gill, M.I.S.; Mahajan, B.V.C.; Arora, N.K. Postharvest treatment of polyamines maintains quality and extends shelf-life of table grapes (Vitis vinifera L.) cv. Flame Seedless. Postharvest Biol. Technol. 2014, 91, 57–63. [Google Scholar] [CrossRef]
- Najafi Marghmaleki, S.; Mortazavi, S.M.H.; Saei, H.; Mostaan, A. The Effect of Alginate-Based Edible Coating Enriched with Citric Acid and Ascorbic Acid on Texture, Appearance and Eating Quality of Apple Fresh-Cut. Int. J. Fruit Sci. 2020, 21, 40–51. [Google Scholar] [CrossRef]
- Dhital, R.; Joshi, P.; Becerra-Mora, N.; Umagiliyage, A.; Chai, T.; Kohli, P.; Choudhary, R. Integrity of edible nano-coatings and its effects on quality of strawberries subjected to simulated in-transit vibrations. LWT 2017, 80, 257–264. [Google Scholar] [CrossRef]
- Awad, M.A.; Al-Qurashi, A.D.; Mohamed, S.A. Postharvest trans -resveratrol and glycine betaine treatments affect quality, antioxidant capacity, antioxidant compounds and enzymes activities of ‘El-Bayadi’ table grapes after storage and shelf life. Sci. Hortic. 2015, 197, 350–356. [Google Scholar] [CrossRef]
- Xiao, J.; Gu, C.; Zhu, D.; Huang, Y.; Luo, Y.; Zhou, Q. Development and characterization of an edible chitosan/zein-cinnamaldehyde nano-cellulose composite film and its effects on mango quality during storage. LWT 2021, 140, 110809. [Google Scholar] [CrossRef]
- Min, D.; Dong, L.; Shu, P.; Cui, X.; Zhang, X.; Li, F. The application of carbon dioxide and 1-methylcyclopropene to maintain fruit quality of ‘Niuxin’ persimmon during storage. Sci. Hortic. 2018, 229, 201–206. [Google Scholar] [CrossRef]
- Cui, K.; Yang, L.; Shu, C.; Liu, J.; Zhu, Z.; Yang, Z.; Zhu, X.; Jiang, W. Near freezing temperature storage alleviates cell wall polysaccharide degradation and softening of apricot (Prunus armeniaca L.) fruit after simulated transport vibration. Sci. Hortic. 2021, 288, 110296. [Google Scholar] [CrossRef]
- Stopa, R.; Szyjewicz, D.; Komarnicki, P.; Kuta, Ł. Limit values of impact energy determined from contours and surface pressure distribution of apples under impact loads. Comput. Electron. Agric. 2018, 154, 1–9. [Google Scholar] [CrossRef]
- Abbasi, N.; Ali, I.; Hafiz, I.; Alenazi, M.; Shafiq, M. Effects of Putrescine Application on Peach Fruit during Storage. Sustainability 2019, 11, 2013. [Google Scholar] [CrossRef]
- Spricigo, P.C.; Freitas, T.P.; Purgatto, E.; Ferreira, M.D.; Correa, D.S.; Bai, J.; Brecht, J.K. Visually imperceptible mechanical damage of harvested tomatoes changes ethylene production, color, enzyme activity, and volatile compounds profile. Postharvest Biol. Technol. 2021, 176, 111503. [Google Scholar] [CrossRef]
- Cheng, C.; Liu, J.; Wang, X.; Wang, Y.; Yuan, Y.; Yang, S. PpERF/ABR1 functions as an activator to regulate PpPG expression resulting in fruit softening during storage in peach (Prunus persica). Postharvest Biol. Technol. 2022, 189, 111919. [Google Scholar] [CrossRef]
- Chea, S.; Yu, D.J.; Park, J.; Oh, H.D.; Chung, S.W.; Lee, H.J. Fruit softening correlates with enzymatic and compositional changes in fruit cell wall during ripening in ‘Bluecrop’ highbush blueberries. Sci. Hortic. 2019, 245, 163–170. [Google Scholar] [CrossRef]
- Chen, Y.; Hung, Y.-C.; Chen, M.; Lin, H. Effects of acidic electrolyzed oxidizing water on retarding cell wall degradation and delaying softening of blueberries during postharvest storage. LWT 2017, 84, 650–657. [Google Scholar] [CrossRef]
- Fan, X.; Jiang, W.; Gong, H.; Yang, Y.; Zhang, A.; Liu, H.; Cao, J.; Guo, F.; Cui, K. Cell wall polysaccharides degradation and ultrastructure modification of apricot during storage at a near freezing temperature. Food Chem. 2019, 300, 125194. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhu, X.; Hou, Y.; Wang, X.; Li, X. Effects of nitric oxide fumigation treatment on retarding cell wall degradation and delaying softening of winter jujube (Ziziphus jujuba Mill. cv. Dongzao) fruit during storage. Postharvest Biol. Technol. 2019, 156, 110954. [Google Scholar] [CrossRef]
- Wang, L.-J.; Zhang, Q.; Song, H.; Wang, Z.-W. Mechanical damage of ‘Huangguan’ pear using different packaging under random vibration. Postharvest Biol. Technol. 2022, 187, 111847. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, W.; Li, X.; Liu, H.; Li, F.; Zhang, X. Combined effects of 1-methylcyclopropene and tea polyphenols coating treatment on the postharvest senescence and reactive oxygen species metabolism of bracken (Pteridium aquilinum var. latiusculum). Postharvest Biol. Technol. 2022, 185, 111813. [Google Scholar] [CrossRef]
- Pintos, F.M.; Lemoine, M.L.; Gergoff Grozeff, G.E.; Hasperué, H.J.; Vicente, A.R.; Rodoni, L.M. Use of riboflavin to reduce decay and extend the shelf-life of fresh-cut sweet pepper. Postharvest Biol. Technol. 2022, 188, 111882. [Google Scholar] [CrossRef]
- Miranda, M.; Spricigo, P.C.; Ferreira, M.D. Mechanical damage during harvest and loading affect orange postharvest quality. Eng. Agríc. 2015, 35, 154–162. [Google Scholar] [CrossRef]
- Montero, C.R.S.; Schwarz, L.L.; Santos, L.C.d.; Andreazza, C.S.; Kechinski, C.P.; Bender, R.J. Postharvest mechanical damage affects fruit quality of ‘Montenegrina’ and ‘Rainha’ tangerines. Pesqui. Agropecu. Bras. 2009, 44, 1636–1640. [Google Scholar] [CrossRef]
- Zheng, Z.; An, Z.; Yang, Y.; Chen, J.; Liu, X.; Lu, L. Metabolic profiling of blueberries (Vaccinium Spp.) to quantitatively and qualitatively assess bruise damage and fruit deterioration. Postharvest Biol. Technol. 2023, 195, 112135. [Google Scholar] [CrossRef]
- Jung, H.M.; Lee, S.; Lee, W.-H.; Cho, B.-K.; Lee, S.H. Effect of vibration stress on quality of packaged grapes during transportation. Eng. Agric. Environ. Food 2018, 11, 79–83. [Google Scholar] [CrossRef]
- Celik, H.K.; Ustun, H.; Erkan, M.; Rennie, A.E.W.; Akinci, I. Effects of bruising of ‘Pink Lady’ apple under impact loading in drop test on firmness, colour and gas exchange of fruit during long term storage. Postharvest Biol. Technol. 2021, 179, 111561. [Google Scholar] [CrossRef]
- Tao, F.; Chen, W.; Jia, Z. Effect of simulated transport vibration on the quality of shiitake mushroom (Lentinus edodes) during storage. Food Sci. Nutr. 2021, 9, 1152–1159. [Google Scholar] [CrossRef]
- Zhao, H.; Liu, B.; Zhang, W.; Cao, J.; Jiang, W. Enhancement of quality and antioxidant metabolism of sweet cherry fruit by near-freezing temperature storage. Postharvest Biol. Technol. 2019, 147, 113–122. [Google Scholar] [CrossRef]
- Wang, H.; Wu, Y.; Yu, R.; Wu, C.; Fan, G.; Li, T. Effects of postharvest application of methyl jasmonate on physicochemical characteristics and antioxidant system of the blueberry fruit. Sci. Hortic. 2019, 258, 108785. [Google Scholar] [CrossRef]
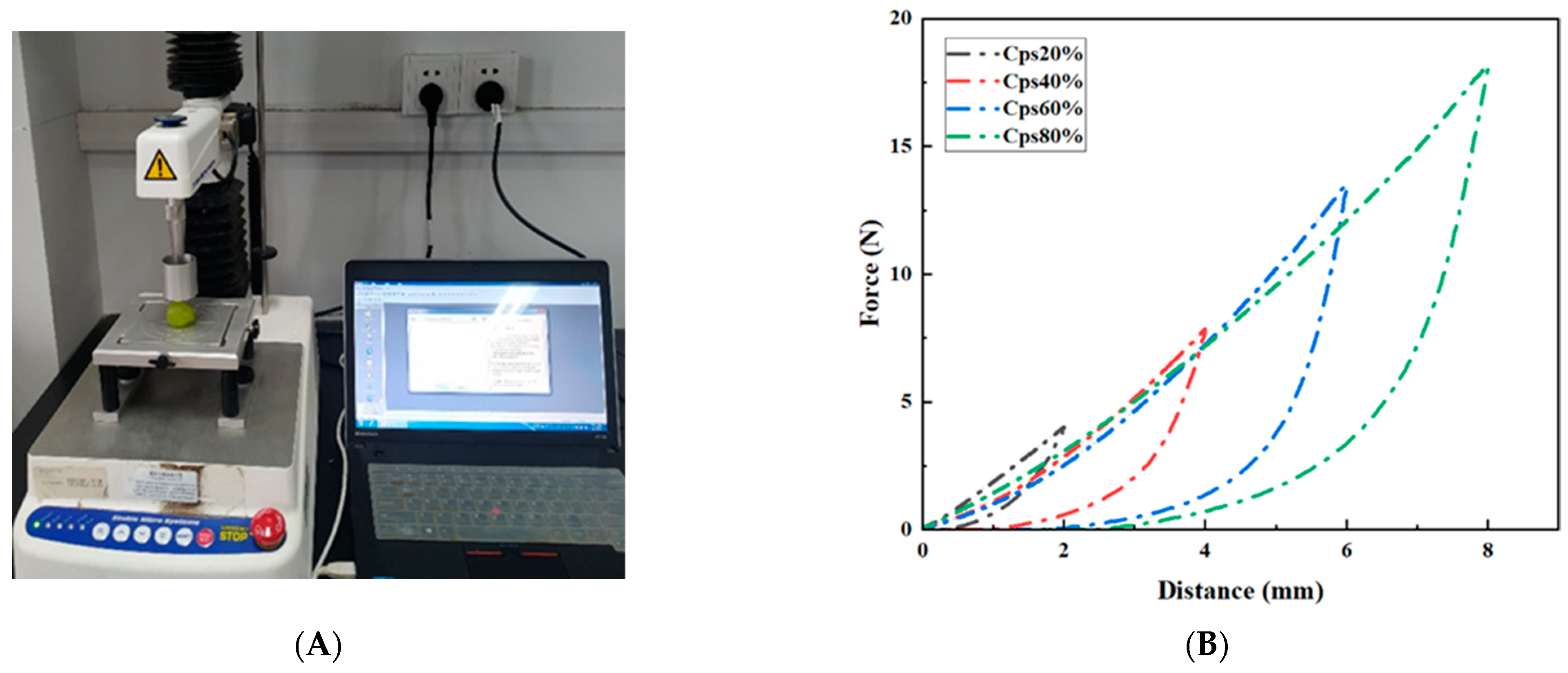


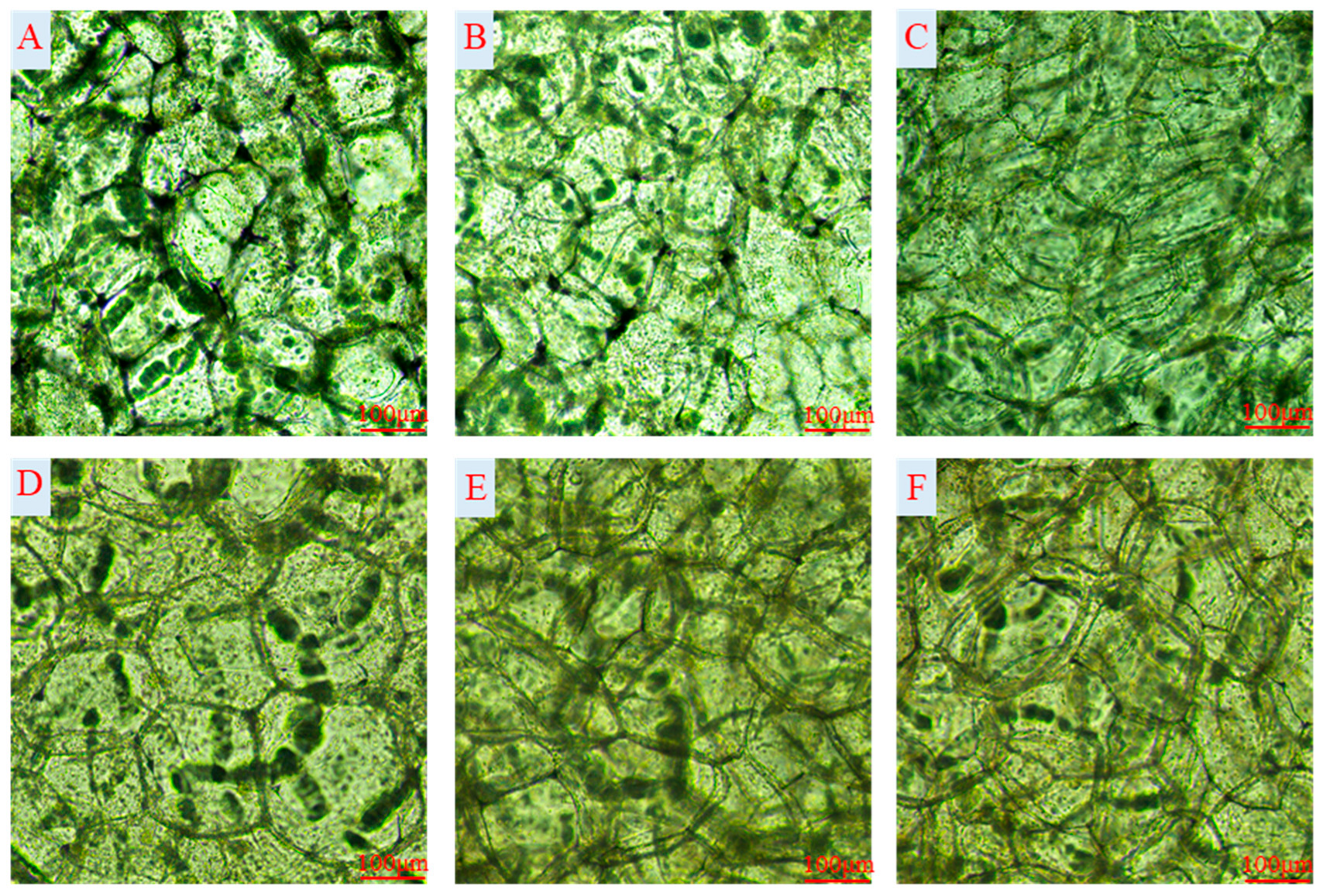

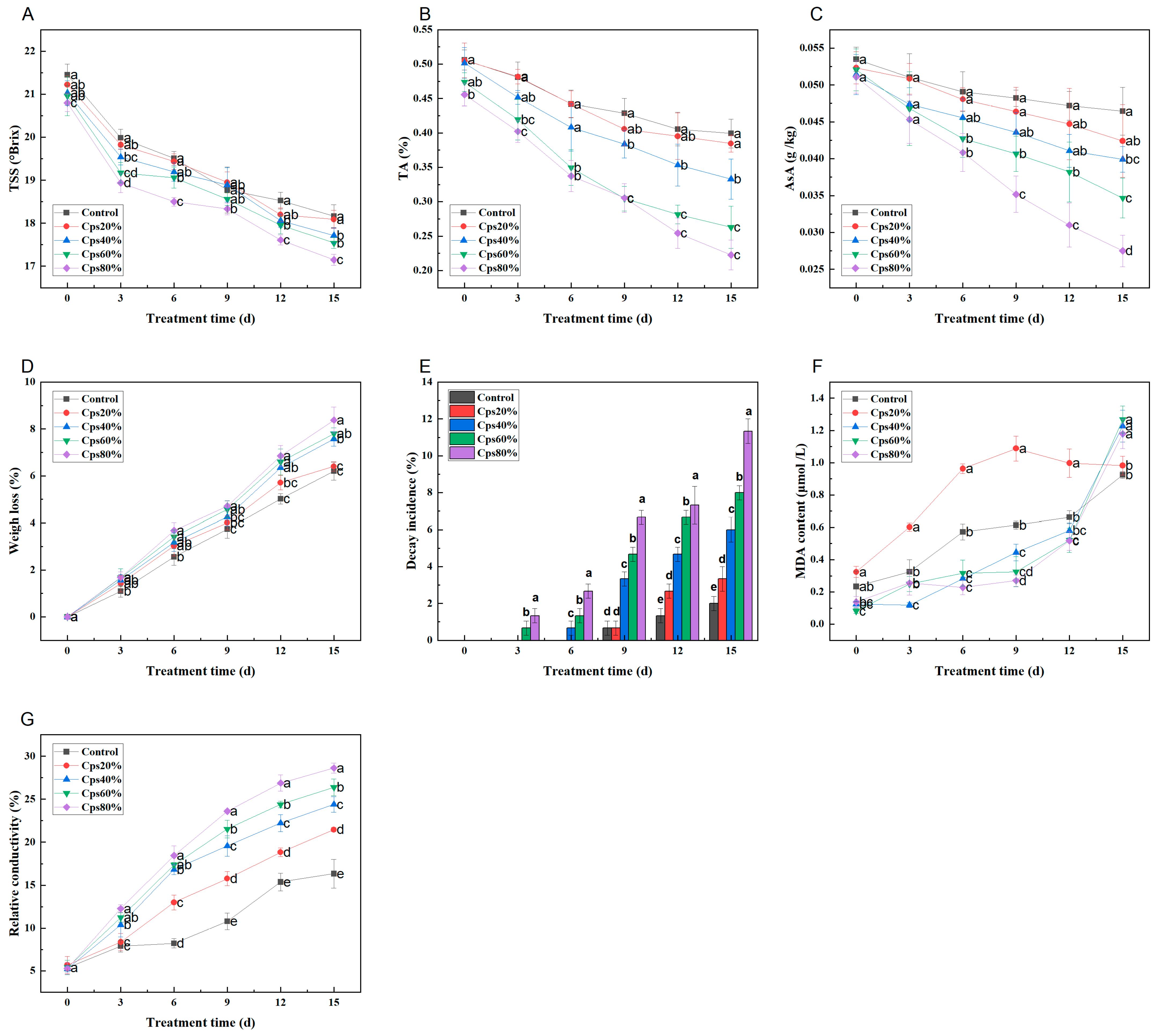

| Index | Treatment | Storage Time (Day) | |||||
|---|---|---|---|---|---|---|---|
| 0 | 3 | 6 | 9 | 12 | 15 | ||
| L* | Control | 47.67 ± 0.54 a | 46.32 ± 0.88 a | 45.87 ± 0.61 a | 44.83 ± 0.89 a | 44.51 ± 0.89 a | 43.72 ± 0.61 a |
| Cps20% | 46.08 ± 0.35 c | 44.65 ± 1.00 b | 43.83 ± 0.86 bc | 42.91 ± 0.94 b | 41.88 ± 0.99 b | 40.43 ± 0.40 b | |
| Cps40% | 47.27 ± 0.56 ab | 46.07 ± 0.61 ab | 44.48 ± 0.56 b | 42.41 ± 0.46 bc | 40.95 ± 0.90 bc | 39.33 ± 0.21 bc | |
| Cps60% | 46.52 ± 0.51 bc | 46.33 ± 0.65 a | 44.31 ± 0.44 b | 42.87 ± 0.56 cd | 40.03 ± 0.91 cd | 38.82 ± 0.96 c | |
| Cps80% | 46.11 ± 0.46 c | 45.13 ± 0.69 ab | 42.88 ± 0.77 c | 41.44 ± 0.38 d | 38.84 ± 0.91 d | 38.11 ± 0.82 c | |
| a* | Control | −8.04 ± 0.20 a | −8.13 ± 0.41 a | −7.79 ± 0.749 b | −6.51 ± 0.478 b | −5.08 ± 0.32 b | −4.39 ± 0.26 a |
| Cps20% | −8.12 ± 0.119 a | −8.02 ± 0.20 a | −7.22 ± 0.658 b | −6.14 ± 0.5 ab | −5.03 ± 0.39 b | −4.02 ± 0.82 a | |
| Cps40% | −8.12 ± 0.230 a | −7.80 ± 0.56 a | −6.82 ± 0.74 ab | −5.75 ± 0.72 ab | −4.32 ± 0.24 ab | −3.52 ± 0.55 a | |
| Cps60% | −8.03 ± 0.40 a | −7.68 ± 0.64 a | −6.09 ± 0.33 a | −5.54 ± 0.8 ab | −4.27 ± 0.62 ab | −3.33 ± 0.88 a | |
| Cps80% | −8.013 ± 0.37 a | −7.54 ± 0.549 a | −6.05 ± 0.27 a | −5.00 ± 0.44 a | −4.08 ± 0.45 a | −3.28 ± 0.48 a | |
| b* | Control | 10.27 ± 0.21 a | 11.88 ± 0.36 bc | 11.05 ± 0.59 c | 12.72 ± 0.26 b | 13.22 ± 0.66 c | 14.43 ± 0.51 c |
| Cps20% | 10.78 ± 0.73 a | 11.21 ± 0.19 c | 12.34 ± 0.55 b | 13.45 ± 0.54 b | 14.88 ± 0.63 b | 15.91 ± 0.41 b | |
| Cps40% | 10.88 ± 0.65 a | 12.34 ± 0.46 ab | 12.72 ± 0.67 b | 13.41 ± 0.77 b | 14.68 ± 0.55 b | 15.9 ± 0.35 b | |
| Cps60% | 10.91 ± 0.59 a | 12.45 ± 0.44 ab | 13.99 ± 0.66 a | 14.764 ± 0.58 a | 15.465 ± 0.36 b | 16.63 ± 0.39 b | |
| Cps80% | 10.64 ± 0.43 a | 12.98 ± 0.55 a | 14.02 ± 0.37 a | 15.23 ± 0.22 a | 17.35 ± 0.32 a | 17.449 ± 0.33 a | |
| ΔE | Control | 0.61 ± 0.28 a | 2.50 ± 0.86 a | 2.53 ± 0.21 c | 4.51 ± 0.59 c | 5.72 ± 0.07 c | 7.24 ± 0.19 d |
| Cps20% | 1.33 ± 0.19 a | 2.67 ± 0.76 a | 4.04 ± 1.15 b | 5.72 ± 1.18 bc | 7.74 ± 1.21 b | 9.79 ± 0.39 c | |
| Cps40% | 1.10 ± 0.89 a | 3.11 ± 0.11 a | 4.73 ± 0.54 ab | 7.02 ± 1.00 ab | 9.37 ± 0.51 b | 11.52 ± 0.22 ab | |
| Cps60% | 0.89 ± 0.29 a | 2.07 ± 0.16 a | 4.77 ± 0.36 ab | 6.36 ± 1.08 ab | 9.29 ± 1.13 b | 11.23 ± 0.89 b | |
| Cps80% | 1.04 ± 0.49 a | 3.18 ± 0.65 a | 5.84 ± 0.86 a | 7.99 ± 0.33 a | 11.43 ± 1.01 a | 12.33 ± 0.57 a | |
| Treatment | Index | ||||
|---|---|---|---|---|---|
| Area/μm2 | Perimeter/μm | Major/μm | Minor/μm | Circularity | |
| 0 day | 7805.27 ± 1746.28 c | 335.02 ± 36.30 c | 110.22 ± 11.91 c | 89.57 ± 12.32 c | 0.86 ± 0.02 a |
| 15 day—Control | 11,773.78 ± 862.91 bc | 422.46 ± 31.35 bc | 136.94 ± 11.51 bc | 109.54 ± 1.97 c | 0.83 ± 0.07 ab |
| 15 day—Cps20% | 16,500.66 ± 6654.17 b | 480.31 ± 99.39 b | 163.94 ± 32.30 b | 124.58 ± 30.91 bc | 0.86 ± 0.01 a |
| 15 day—Cps40% | 30,748.30 ± 3259.18 a | 667.03 ± 31.46 a | 221.97 ± 13.01 a | 177.46 ± 28.14 a | 0.86 ± 0.03 a |
| 15 day—Cps60% | 30,997.73 ± 5644.37 a | 690.42 ± 47.69 a | 248.15 ± 7.96 a | 158.55 ± 24.40 ab | 0.81 ± 0.04 ab |
| 15 day—Cps80% | 33,255.06 ± 3023.24 a | 738.23 ± 29.52 a | 253.60 ± 24.42 a | 167.44 ± 13.89 a | 0.76 ± 0.05 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, S.; Liu, J.; Yang, Q.; Jin, Y.; Zhao, S.; Tan, Z.; Qiu, J.; Zhang, H. The Impact of Mechanical Compression on the Postharvest Quality of ‘Shine Muscat’ Grapes during Short-Term Storage. Agronomy 2023, 13, 2836. https://doi.org/10.3390/agronomy13112836
Zhu S, Liu J, Yang Q, Jin Y, Zhao S, Tan Z, Qiu J, Zhang H. The Impact of Mechanical Compression on the Postharvest Quality of ‘Shine Muscat’ Grapes during Short-Term Storage. Agronomy. 2023; 13(11):2836. https://doi.org/10.3390/agronomy13112836
Chicago/Turabian StyleZhu, Shan, Jizhan Liu, Qiya Yang, Yucheng Jin, Shengyi Zhao, Zhuqing Tan, Jieer Qiu, and Hongyin Zhang. 2023. "The Impact of Mechanical Compression on the Postharvest Quality of ‘Shine Muscat’ Grapes during Short-Term Storage" Agronomy 13, no. 11: 2836. https://doi.org/10.3390/agronomy13112836
APA StyleZhu, S., Liu, J., Yang, Q., Jin, Y., Zhao, S., Tan, Z., Qiu, J., & Zhang, H. (2023). The Impact of Mechanical Compression on the Postharvest Quality of ‘Shine Muscat’ Grapes during Short-Term Storage. Agronomy, 13(11), 2836. https://doi.org/10.3390/agronomy13112836








