Impact of Fusarium Crown Rot on Root System Area and Links to Genetic Variation within Commercial Wheat Varieties
Abstract
:1. Introduction
2. Materials and Methods
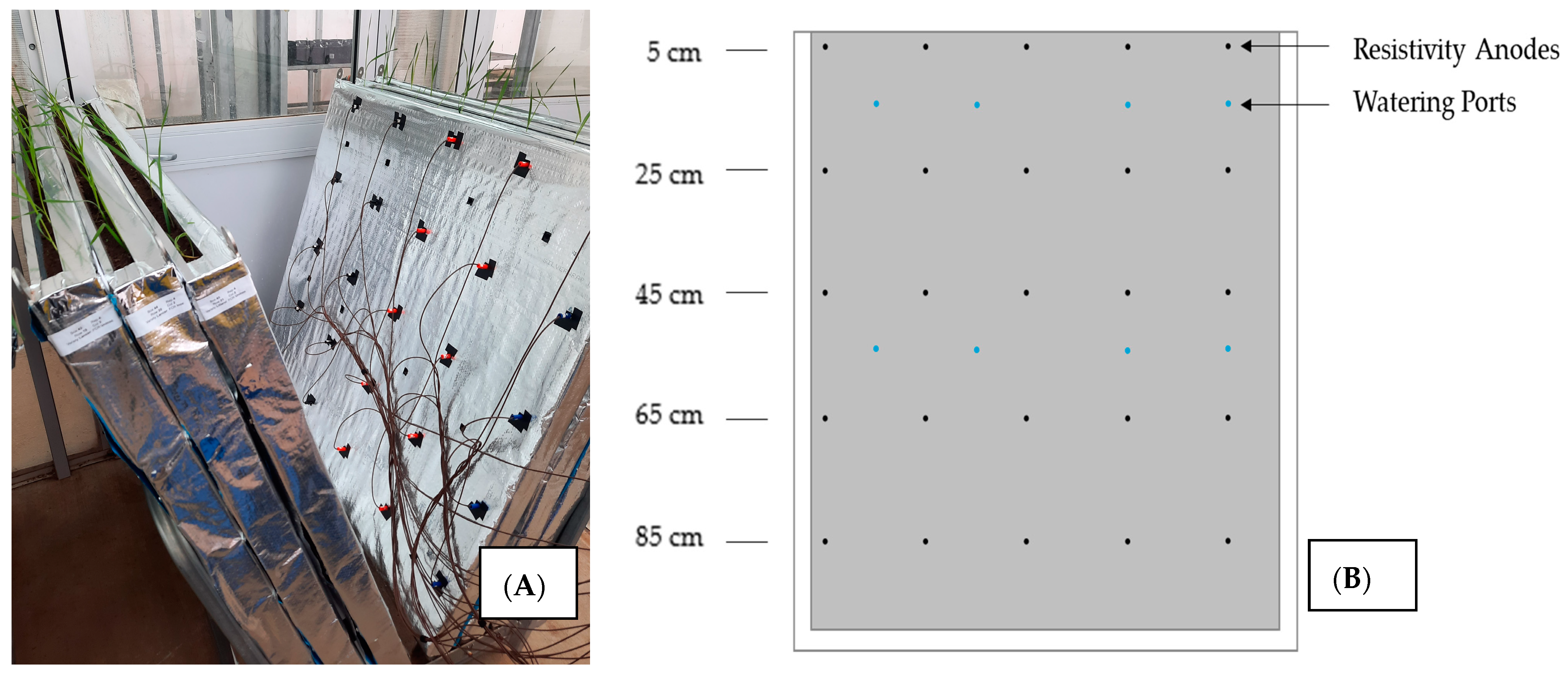
2.1. Soil, Rhizobox Design and FCR Treatments
2.2. Plant Materials and Growing Conditions
2.3. Fertiliser
2.4. In-Crop FCR Measurements
2.5. Root Image Acquisition
2.6. Root Image Processing
2.7. Electrical Resistivity Tomography Measurements and Analysis
2.8. Harvest Assessments
2.9. Statistical Analysis
2.9.1. Harvest Data
2.9.2. Electrical Resistivity Tomography and Root Data
3. Results
3.1. Harvest Measurements
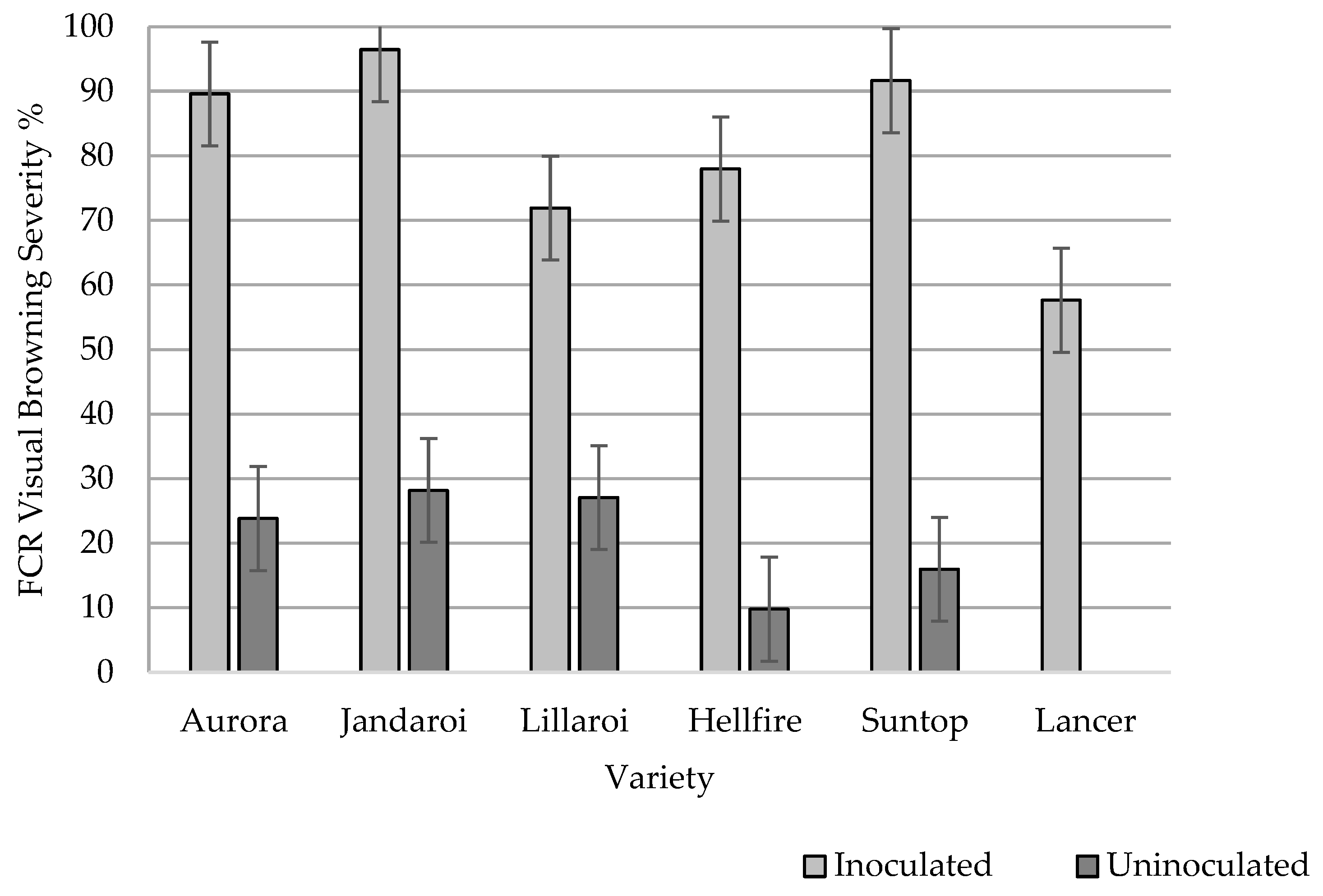
3.1.1. Inoculation Response
3.1.2. Yield and Grain Quality
3.2. Water Measurements
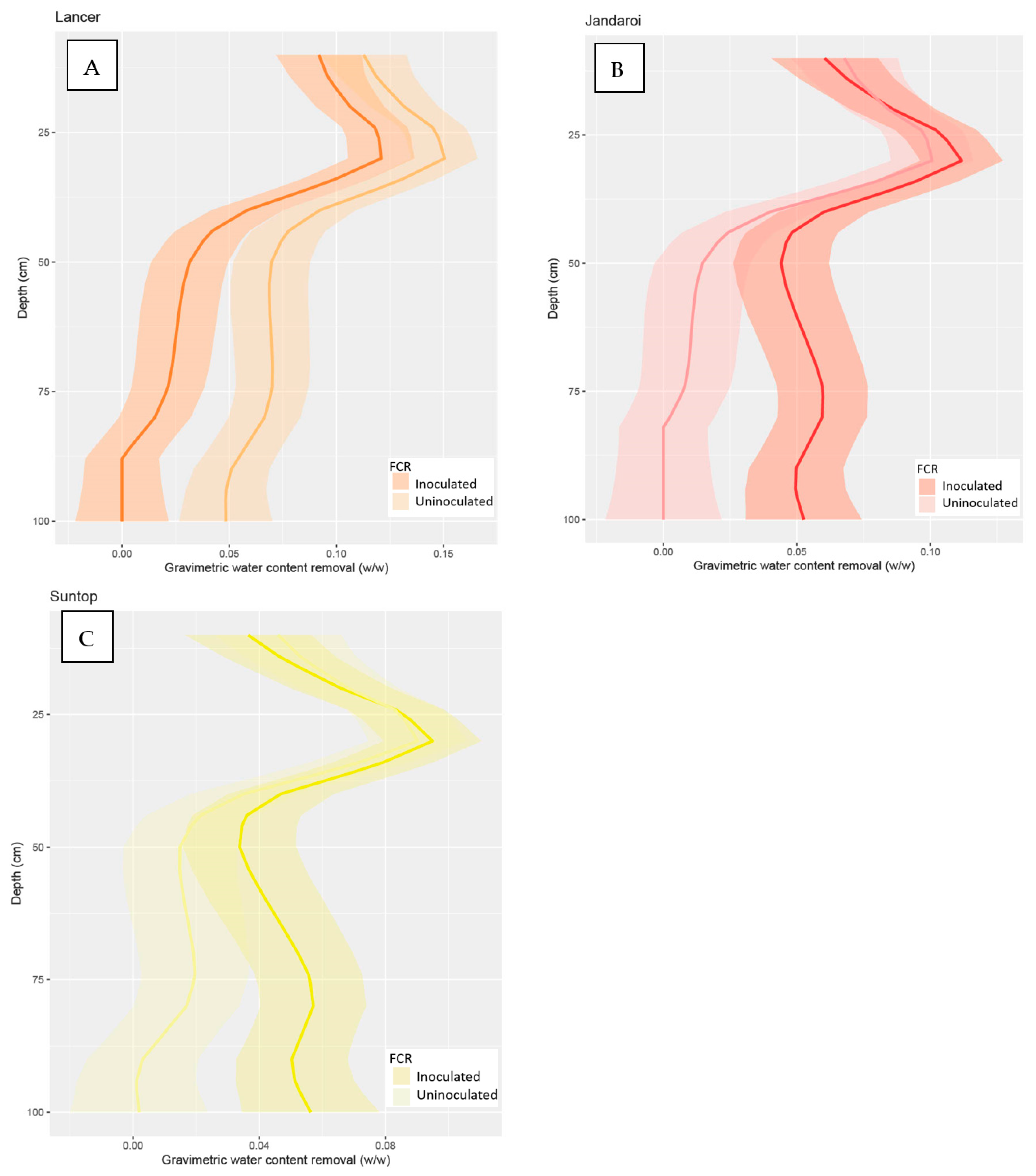
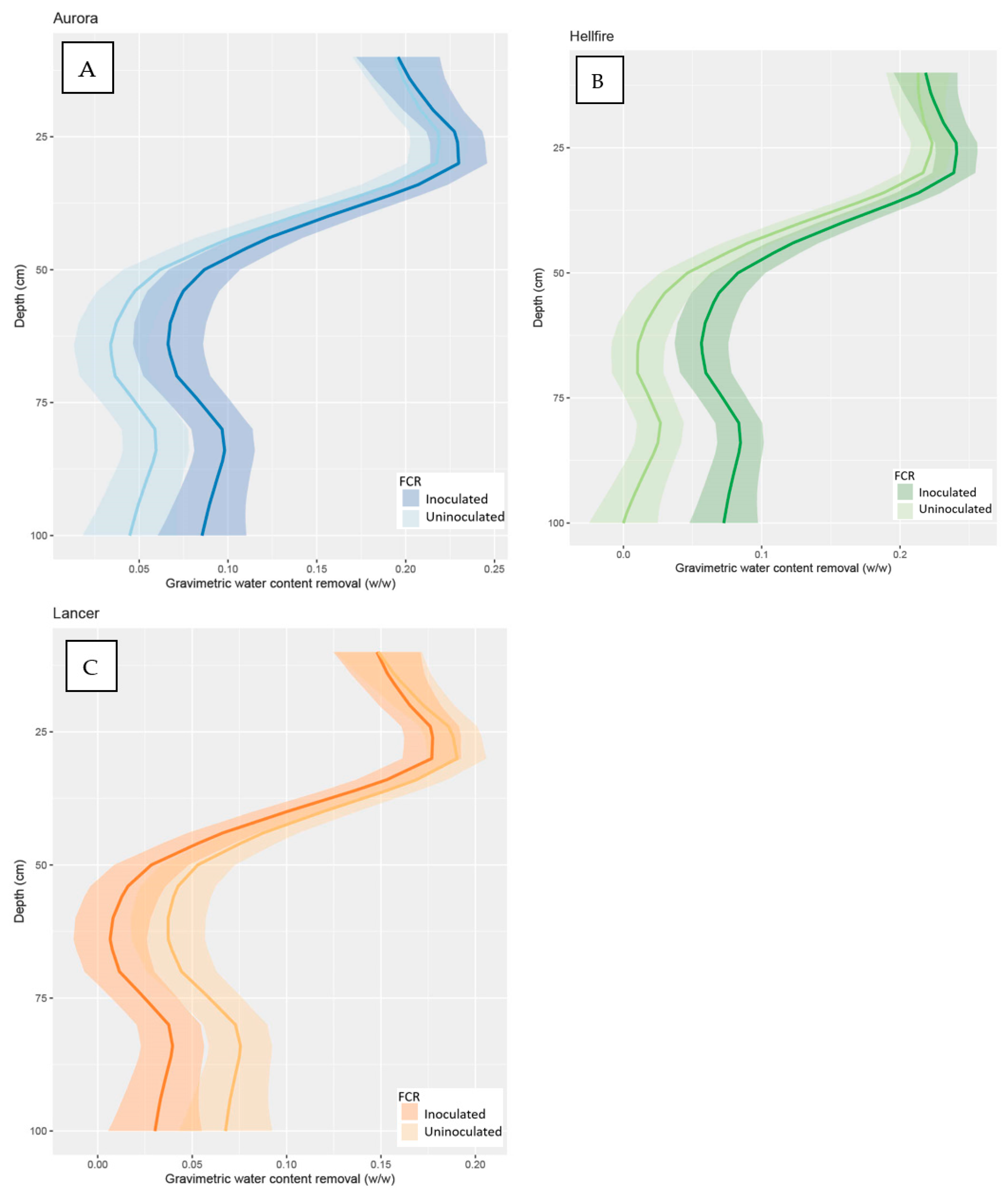
3.2.1. Water Measurements at Stem Elongation (GS 30)
3.2.2. Water Measurements at Flowering (GS 60)
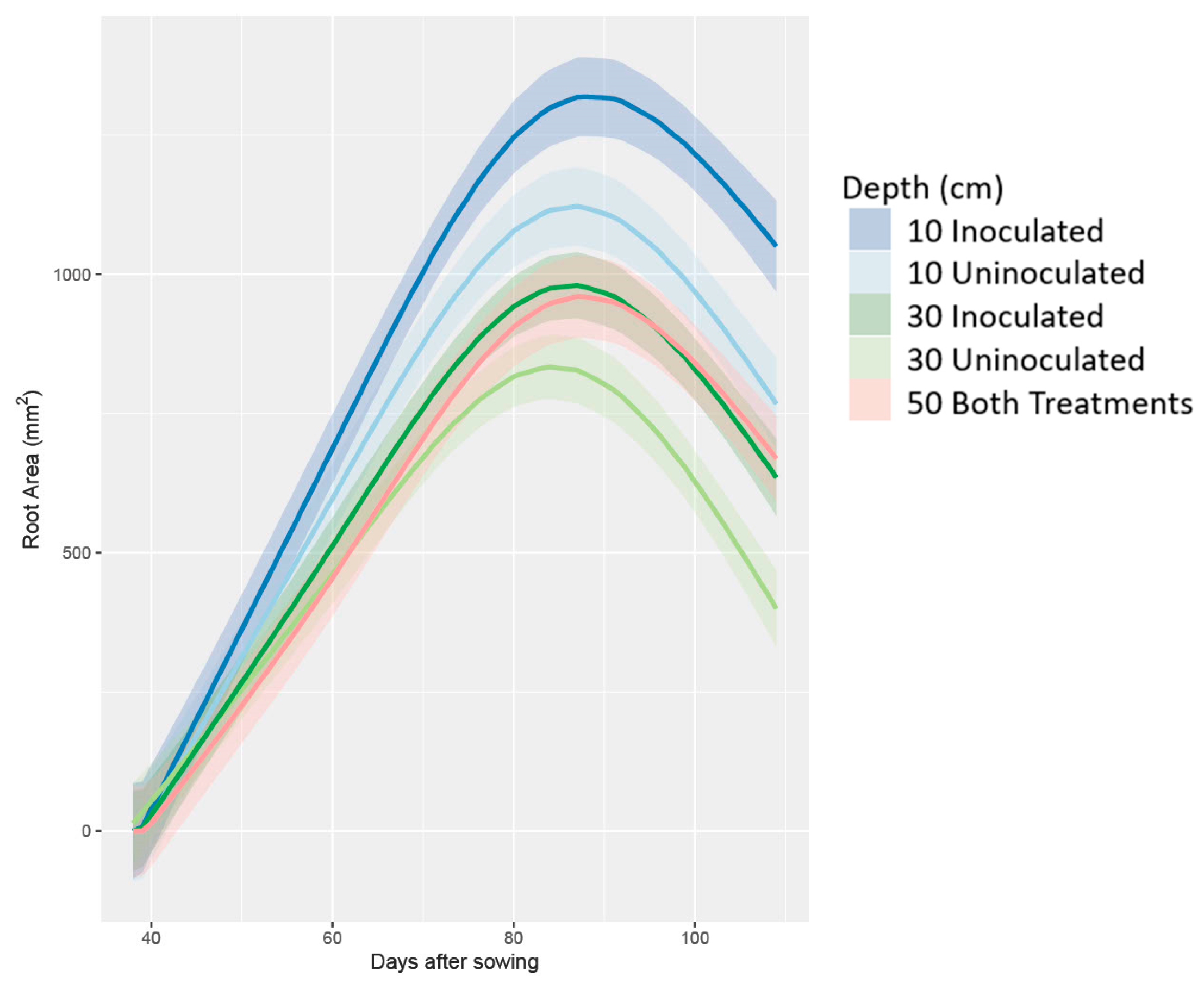
3.3. Root Architecture Measurements
4. Discussion
4.1. Electrical Resistivity Tomography and Spatial Water Use
4.2. Spatial Root Area and Its Importance in Tolerance
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kazan, K.; Gardiner, D.M. Fusarium crown rot caused by Fusarium pseudograminearum in cereal crops: Recent progress and future prospects. Mol. Plant Pathol. 2018, 19, 1547–1562. [Google Scholar] [CrossRef]
- Petronaitis, T.; Simpfendorfer, S.; Hüberli, D. Importance of Fusarium spp. in Wheat to Food Security: A Global Perspective. In Plant Diseases and Food Security in the 21st Century; Scott, P., Strange, R., Korsten, L., Gullino, M.L., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 127–159. [Google Scholar]
- Murray, G.M.; Brennan, J.P. Estimating disease losses to the Australian wheat industry. Australas. Plant Pathol. 2009, 38, 558–570. [Google Scholar] [CrossRef]
- Buster, M.; Simpfendorfer, S.; Guppy, C.; Sissons, M.; Flavel, R.J. Fusarium Crown Rot Reduces Water Use and Causes Yield Penalties in Wheat under Adequate and above Average Water Availability. Agronomy 2022, 12, 2616. [Google Scholar] [CrossRef]
- Buster, M.; Simpfendorfer, S.; Guppy, C.; Sissons, M.; Flavel, R.J. Interactions of Fusarium Crown Rot of Wheat with Nitrogen. Plants 2023, 12, 533. [Google Scholar] [CrossRef]
- Ehdaie, B.; Layne, A.P.; Waines, J.G. Root system plasticity to drought influences grain yield in bread wheat. Euphytica 2012, 186, 219–232. [Google Scholar] [CrossRef]
- Kadam, N.N.; Yin, X.; Bindraban, P.S.; Struik, P.C.; Jagadish, K.S.V. Does Morphological and Anatomical Plasticity during the Vegetative Stage Make Wheat More Tolerant of Water Deficit Stress Than Rice? Plant Physiol. 2015, 167, 1389–1401. [Google Scholar] [CrossRef]
- Chen, Y.; Palta, J.; Prasad, P.V.V.; Siddique, K. Phenotypic variability in bread wheat root systems at the early vegetative stage. BMC Plant Biol. 2020, 20, 185. [Google Scholar] [CrossRef]
- Smith, S.; De Smet, I. Root system architecture: Insights from Arabidopsis and cereal crops. Philos. Trans. R. Soc. B Biol. Sci. 2012, 367, 1441–1452. [Google Scholar] [CrossRef]
- Nagel, K.A.; Kastenholz, B.; Jahnke, S.; van Dusschoten, D.; Aach, T.; Mühlich, M.; Truhn, D.; Scharr, H.; Terjung, S.; Walter, A.; et al. Temperature responses of roots: Impact on growth, root system architecture and implications for phenotyping. Funct. Plant Biol. 2009, 36, 947–959. [Google Scholar] [CrossRef]
- Pandey, R.; Jain, V.; Singh, K. Hydroponics Agriculture: Its Status, Scope and Limitations; Division of Plant Physiology, Indian Agricultural Research Institute: New Delhi, India, 2009; Volume 20. [Google Scholar]
- Chen, Y.L.; Dunbabin, V.M.; Diggle, A.J.; Siddique, K.H.; Rengel, Z. Development of a novel semi-hydroponic phenotyping system for studying root architecture. Funct. Plant Biol. 2011, 38, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Freschet, G.T.; Pagès, L.; Iversen, C.M.; Comas, L.H.; Rewald, B.; Roumet, C.; Klimešová, J.; Zadworny, M.; Poorter, H.; Postma, J.A.; et al. A starting guide to root ecology: Strengthening ecological concepts and standardising root classification, sampling, processing and trait measurements. New Phytol. 2021, 232, 973–1122. [Google Scholar] [CrossRef] [PubMed]
- Saad, A.; Christopher, J.; Martin, A.; McDonald, S.; Percy, C. Fusarium pseudograminearum and F. culmorum affect the root system architecture of bread wheat. Crop J. 2022, 11, 316–321. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, J.; Yan, W.; Yan, G.; Zhou, M.; Wei, Y.; Zheng, Y.; Liu, C. Different Tolerance in Bread Wheat, Durum Wheat and Barley to Fusarium Crown Rot Disease Caused by Fusarium pseudograminearum. J. Phytopathol. 2012, 160, 412–417. [Google Scholar] [CrossRef]
- Liu, C.; Ogbonnaya, F. Resistance to Fusarium crown rot in wheat and barley: A review. Plant Breed. 2015, 134, 365–372. [Google Scholar] [CrossRef]
- Alahmad, S.; El Hassouni, K.; Bassi, F.M.; Dinglasan, E.; Youssef, C.; Quarry, G.; Aksoy, A.; Mazzucotelli, E.; Juhász, A.; Able, J.A. A major root architecture QTL responding to water limitation in durum wheat. Front. Plant Sci. 2019, 10, 436. [Google Scholar] [CrossRef]
- Isbell, R. The Australian Soil Classification; CSIRO publishing: Clayton, Australia, 2016. [Google Scholar]
- Forknall, C.R.; Simpfendorfer, S.; Kelly, A.M. Using Yield Response Curves to Measure Variation in the Tolerance and Resistance of Wheat Cultivars to Fusarium Crown Rot. Phytopathology 2019, 109, 932–941. [Google Scholar] [CrossRef]
- Kadkol, G.P.; Meza, J.; Simpfendorfer, S.; Harden, S.; Cullis, B. Genetic variation for fusarium crown rot tolerance in durum wheat. PLoS ONE 2021, 16, e0240766. [Google Scholar] [CrossRef]
- Simpfendorfer, S. Fusarium Crown Rot Seed Fungicides: Independent Field Evaluation 2018–2020. Available online: https://grdc.com.au/__data/assets/pdf_file/0020/447320/Paper-Simpfendorfer-Fusarium-seed-treatments-June-2021.pdf (accessed on 2 November 2023).
- Wasson, A.; Bischof, L.; Zwart, A.; Watt, M. A portable fluorescence spectroscopy imaging system for automated root phenotyping in soil cores in the field. J. Exp. Bot. 2016, 67, 1033–1043. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Seethepalli, A.; Dhakal, K.; Griffiths, M.; Guo, H.; Freschet, G.T.; York, L.M. RhizoVision Explorer: Open-source software for root image analysis and measurement standardization. AoB PLANTS 2021, 13, plab056. [Google Scholar] [CrossRef]
- Telford, W.M.; Telford, W.; Geldart, L.; Sheriff, R.E. Applied Geophysics; Cambridge University Press: Cambridge, UK, 1990. [Google Scholar]
- R Development Core Team. R: A Language and Enviroment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- Butler, D. asreml: Fits the Linear Mixed Model, R Package Version 4.1. 0.143. 2020. Available online: https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&cad=rja&uact=8&ved=2ahUKEwiNj4ibz-qCAxU0xzgGHYagDEwQFnoECBUQAQ&url=https%3A%2F%2Fcran.r-project.org%2Fweb%2Fpackages%2FasremlPlus%2FasremlPlus.pdf&usg=AOvVaw0OE71mGPy92nyWKOHu_lb3&opi=89978449(accessed on 2 November 2023).
- Knight, N.L.; Sutherland, M.W. Histopathological Assessment of Fusarium pseudograminearum Colonization of Cereal Culms During Crown Rot Infections. Plant Dis. 2015, 100, 252–259. [Google Scholar] [CrossRef]
- van der Bom, F.J.T.; Williams, A.; Bell, M.J. Root architecture for improved resource capture: Trade-offs in complex environments. J. Exp. Bot. 2020, 71, 5752–5763. [Google Scholar] [CrossRef]
- Graham, R. Fusarium crown rot of wheat-impact on plant available soil water usage. In Proceedings of the 17th Australian Agronomy Conference, Hobart, Australia, 20–24 September 2015; pp. 20–24. [Google Scholar]
- Zhu, Y.; Saltzgiver, M. A systematic analysis of apple root resistance traits to Pythium ultimum infection and the underpinned molecular regulations of defense activation. Hortic. Res. 2020, 7, 62. [Google Scholar] [CrossRef]
- Abd Alhakim, A.; Hashem, A.; Abdelaziz, A.M.; Attia, M.S. Impact of plant growth promoting fungi on biochemical defense performance of tomato under fusarial infection. Egypt. J. Chem. 2022, 65, 291–301. [Google Scholar] [CrossRef]
- Simpfendorfer, S.; McKay, A.; Ophel-Keller, K. New approaches to crop disease management in conservation agriculture. In Australian Agriculture in 2020: From Conservation to Automation; Australia, A., Ed.; Graham Centre for Agricultural Innovation, Charles Sturt University: Wagga Wagga, Australia, 2020; Volume 1, pp. 173–188. [Google Scholar]
- Schmidt, J.E.; Lowry, C.; Gaudin, A.C.M. An Optimized Rhizobox Protocol to Visualize Root Growth and Responsiveness to Localized Nutrients. J. Vis. Exp. 2018, 58674. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buster, M.; Simpfendorfer, S.; Guppy, C.; Sissons, M.; Harden, S.; Flavel, R.J. Impact of Fusarium Crown Rot on Root System Area and Links to Genetic Variation within Commercial Wheat Varieties. Agronomy 2023, 13, 2955. https://doi.org/10.3390/agronomy13122955
Buster M, Simpfendorfer S, Guppy C, Sissons M, Harden S, Flavel RJ. Impact of Fusarium Crown Rot on Root System Area and Links to Genetic Variation within Commercial Wheat Varieties. Agronomy. 2023; 13(12):2955. https://doi.org/10.3390/agronomy13122955
Chicago/Turabian StyleBuster, Mitchell, Steven Simpfendorfer, Christopher Guppy, Mike Sissons, Steven Harden, and Richard J. Flavel. 2023. "Impact of Fusarium Crown Rot on Root System Area and Links to Genetic Variation within Commercial Wheat Varieties" Agronomy 13, no. 12: 2955. https://doi.org/10.3390/agronomy13122955
APA StyleBuster, M., Simpfendorfer, S., Guppy, C., Sissons, M., Harden, S., & Flavel, R. J. (2023). Impact of Fusarium Crown Rot on Root System Area and Links to Genetic Variation within Commercial Wheat Varieties. Agronomy, 13(12), 2955. https://doi.org/10.3390/agronomy13122955






