Aboveground Structural Attributes and Morpho-Anatomical Response Strategies of Bromus valdivianus Phil. and Lolium perenne L. to Severe Soil Water Restriction
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation
2.2. Water Restriction Treatments
2.3. Plant Measurement
2.4. Observation of Leaf Surface
2.5. Paraffin Sections
2.6. Statistical Analysis
3. Results
3.1. Herbage and Root Biomass, Water Status, and Photosynthesis
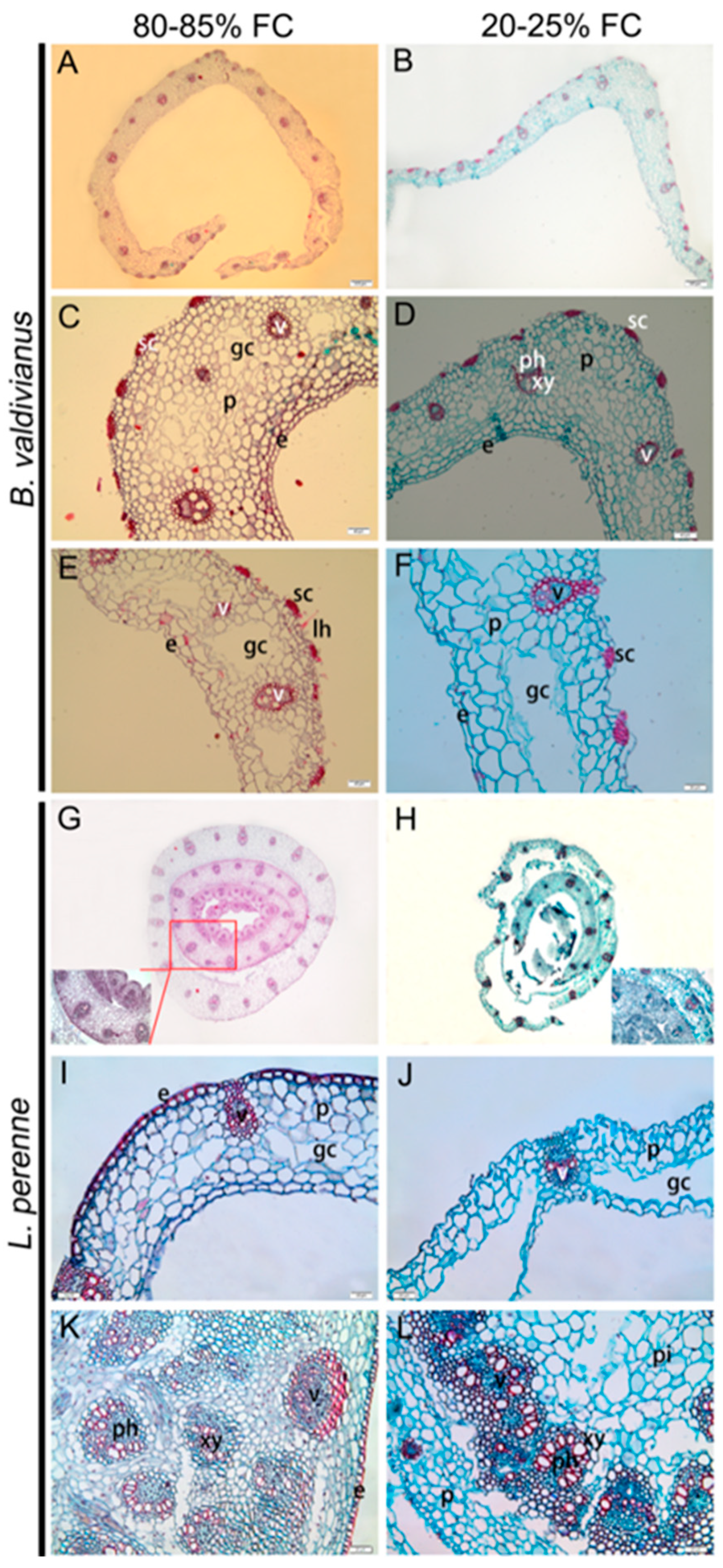
3.2. Traits and Adjustments of Bv and Lp Pseudostems and in Lp Stems
3.3. Leaf Structure Traits and Leaf Adjustments to Water Restriction
3.4. Canonical Variate Analysis of the Pseudostem and Leaf Structure
4. Discussion
4.1. Morpho-Anatomical Traits and Adjustments of Pseudostem (or Stem) Attributes Relevant to Water Movement
4.2. Morpho-Anatomical Traits and Adjustments of Leaf Blade Structures Relevant to Water Evaporation and Transpiration
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Seneviratne, S.I.; Zhang, X.; Adnan, M.; Badi, W.; Dereczynski, C.; Di Luca, A.; Ghosh, S.; Iskandar, I.; Kossin, J.; Lewis, S.; et al. Weather and Climate Extreme Events in a Changing Climate. In Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Masson-Delmotte, V., Zhai, P., Pirani, A., Connors, S.L., Péan, C., Berger, S., Caud, N., Chen, Y., Goldfarb, L., Gomis, M.I., et al., Eds.; Cambridge University Press: Cambridge, UK, 2021; pp. 1513–1766. [Google Scholar] [CrossRef]
- Vicente-Serrano, S.M.; Peña-Angulo, D.; Beguería, S.; Domínguez-Castro, F.; Tomás-Burguera, M.; Noguera, I.; Gimeno-Sotelo, L.; El Kenawy, A. Global drought trends and future projections. Philos. Trans. R. Soc. A 2022, 380, 20210285. [Google Scholar] [CrossRef] [PubMed]
- Malinowski, D.P.; Kigel, J.; Pinchak, W.E. Water deficit, heat tolerance, and persistence of summer-dormant grasses in the U.S. southern plains. Crop Sci. 2009, 49, 2363–2370. [Google Scholar] [CrossRef]
- Lens, F.; Picon-Cochard, C.; Delmas, C.E.L.; Signarbieux, C.; Buttler, A.; Cochard, H.; Jansen, S.; Chauvin, T.; Doria, L.C.; Arco, M.D.; et al. Herbaceous angiosperms are not more vulnerable to drought-induced embolism than angiosperm trees. Plant Physiol. 2016, 172, 661–667. [Google Scholar] [CrossRef] [PubMed]
- Jacob, V.; Choat, B.; Churchill, A.C.; Zhang, H.; Barton, V.M.; Krishnananthaselvan, A.; Post, A.K.; Power, S.A.; Medlyn, B.; Tissue, D.T. High safety margins to drought-induced hydraulic failure found in five pasture grasses. Plant Cell Environ. 2022, 45, 1631–1646. [Google Scholar] [CrossRef] [PubMed]
- Assuero, S.G.; Matthew, C.; Kemp, P.D.; Latch, G.C.M.; Barker, D.J.; Haslett, S.J. Morphological and physiological effects of water deficit and endophyte infection on contrasting tall fescue cultivars. N. Z. J. Agric. Res. 2000, 43, 49–61. [Google Scholar] [CrossRef][Green Version]
- Volaire, F.; Lens, F.; Cochard, H.; Xu, H.; Chacon-Doria, L.; Bristiel, P.; Balachowski, J.; Rowe, N.; Violle, C.; Picon-Cochard, C. Embolism and mechanical resistances play a key role in dehydration tolerance of a perennial grass Dactylis glomerata L. Ann. Bot. 2018, 122, 325–336. [Google Scholar] [CrossRef]
- Ristic, Z.; Jenks, M.A. Leaf cuticle and water loss in maize lines differing in dehydration avoidance. J. Plant Physiol. 2002, 159, 645–651. [Google Scholar] [CrossRef]
- Pitman, W.D.; Holte, C.; Conrad, B.E.; Bashaw, E.C. Histological differences in moisture stressed and non-stressed kleingrass forage. Crop Sci. 1983, 23, 793–795. [Google Scholar] [CrossRef]
- Sultan, S.E. Evolutionary implications of phenotypic plasticity in plants. Evol. Biol. 1987, 21, 127–178. [Google Scholar]
- Begon, M.; Harper, J.L.; Townsend, C.R. Ecology: Individuals, Populations and Communities; Blackwell Science: Oxford, UK, 1996. [Google Scholar]
- López, I.F.; Balocchi, O.A.; Kemp, P.D.; Valdés, C. Phenotypic variability in Holcus lanatus L. in Southern Chile: A strategy that enhances plant survival and pasture stability. Crop Pasture Sci. 2009, 60, 768–777. [Google Scholar] [CrossRef]
- Makbul, S.; Coşkunçelebi, K.; Türkmen, Z.; Beyazoglu, O. Morphology and anatomy of Scrophularia L. (Scrophulariaceae) taxa from NE Anatolia. Acta Biol. Cracoviensia Ser. Bot. 2006, 48, 33–43. [Google Scholar] [CrossRef]
- Makbul, S.; Türkmen, Z.; Coşkunçelebi, K.; Beyazoglu, O. Anatomical and pollen characters in the genus Epilobium L. (Onagraceae) from northeast Anatolia. Acta Biol. Cracoviensia Ser. Bot. 2008, 50, 51–62. [Google Scholar]
- Oliveira, I.; Meyer, A.; Afonso, S.; Gonalves, B. Compared leaf anatomy and water relations of commercial and traditional Prunus dulcis (Mill.) cultivars under rain-fed conditions. Sci. Hortic. 2018, 229, 226–232. [Google Scholar] [CrossRef]
- Brent, D.B.; David, K.; Adam, J.L.; James, B. Evaluation of turf-type interspecific hybrids of meadow fescue with perennial ryegrass for improved stress tolerance. Crop Sci. 2014, 54, 355–365. [Google Scholar] [CrossRef]
- López, I.F.; Kemp, P.D.; Dörner, J.; Descalzi, C.A.; Balocchi, O.A.; García, S. Competitive strategies and growth of neighbouring Bromus valdivianus Phil. And Lolium perenne L. plants under water restriction. J. Agron. Crop Sci. 2013, 199, 449–459. [Google Scholar] [CrossRef]
- Ordóñez, I.; López, I.F.; Kemp, P.D.; Descalzi, C.A.; Horn, R.; Zúñiga, F.; Dorota, D.; Dörner, J. Effect of pasture improvement managements on physical properties and water content dynamics of a volcanic ash soil in southern Chile. Soil Tillage Res. 2018, 178, 55–64. [Google Scholar] [CrossRef]
- Calvache, I.; Balocchi, O.; Alonso, M.; Keim, J.P.; López, I. Water-soluble carbohydrate recovery in pastures of perennial ryegrass (Lolium perenne L.) and pasture brome (Bromus valdivianus Phil.) under two defoliation frequencies determined by thermal time. Agriculture 2020, 10, 563–574. [Google Scholar] [CrossRef]
- Ordóñez, I.P.; López, I.F.; Kemp, P.D.; Donaghy, D.J.; Herrmann, P.; Hernández, F.; Bhatia, S. Pasture brome (Bromus valdivianus) leaf growth physiology: A six leaf grass species. Agron. N. Z. 2017, 47, 13–22. [Google Scholar]
- López, I.; Balocchi, O.; Lailhacar, P.; Oyarzún, C. Characterization of the growing sites of six native and naturalized species in the Humid Dominion of Chile. Agro Sur 1997, 25, 62–80. [Google Scholar] [CrossRef]
- Olmos, E.; Sanchez-Blanco, M.J.; Fernandez, T.; Alarcón, J.J. Subcellular effects of drought stress in Rosmarinus officinalis. Plant Biol. 2007, 9, 77–84. [Google Scholar] [CrossRef]
- Makbul, S.; Güler, N.S.; Durmus, N.; Güven, S. Changes in anatomical and physiological parameters of soybean under drought stress. Turk. J. Bot. 2011, 35, 369–377. [Google Scholar] [CrossRef]
- Saha, P.; Sade, N.; Arzani, A.; Wilhelmi, M.M.R.; Coe, K.M.; Li, B.; Blumwald, E. Effects of abiotic stress on physiological plasticity and water use of Setaria viridis (L.) P. Beauv. Plant Sci. 2016, 251, 128–138. [Google Scholar] [CrossRef] [PubMed]
- Ordóñez, I.P.; López, I.F.; Kemp, P.D.; Donaghy, D.J.; Zhang, Y.; Herrmann, P. Response of Bromus valdivianus (pasture brome) growth and physiology to defoliation frequency based on leaf stage development. Agronomy 2021, 11, 2058–2075. [Google Scholar] [CrossRef]
- López, I.F.; Balocchi, O.A.; Alvarez, X.; Flores, P.; Latrille, L. Selectivity of Bromus valdivianus Phil., Lolium perenne L. and Agrostis capillaris L. by grazing dairy cows. Agro Sur 2016, 44, 53–65. [Google Scholar] [CrossRef]
- Fulkerson, W.J.; Slack, K. Leaf number as a criterion for determining defoliation time for Lolium perenne, 1. Effect of water-soluble carbohydrates and senescence. Grass Forage Sci. 1994, 49, 373–377. [Google Scholar] [CrossRef]
- Fulkerson, W.J.; Donaghy, D.J. Plant-soluble carbohydrate reserves and senescence—Key criteria for developing an effective grazing management system for ryegrass-based pastures: A review. Aust. J. Exp. Agric. 2001, 41, 261–275. [Google Scholar] [CrossRef]
- García-Favre, J.; López, I.F.; Cranston, L.M.; Donaghy, D.J.; Kemp, P.D.; Ordóñez, I.P. Functional contribution of two perennial grasses to enhance pasture production and drought resistance under a leaf regrowth stage defoliation criterion. J. Agron. Crop Sci. 2022, 209, 144–160. [Google Scholar] [CrossRef]
- Steel, R.G.D.; Torrie, J.H.; Dickey, D.A. Principles and Procedures of Statistics: A Biometrical Approach; McGraw-Hill: New York, NY, USA, 1997. [Google Scholar]
- Descalzi, C.; Balocchi, O.; López, I.; Kemp, P.; Dörner, J. Different soil structure and water conditions affect the growing response of Lolium perenne L. and Bromus valdivianus Phil. Growing alone or in mixture. J. Soil Sci. Plant Nutr. 2018, 18, 617–635. [Google Scholar] [CrossRef]
- Zhang, Y.; Ma, H.; Calderón-Urrea, A.; Tian, C.; Bai, X.; Wei, J. Anatomical changes to protect organelle integrity account for tolerance to alkali and salt stress in Melilotus offincinalis. Plant Soil 2016, 406, 327–340. [Google Scholar] [CrossRef]
- Ruzin, S.E. Plant Micro Technique and Microscopy; Oxford University Press: Oxford, UK, 1999. [Google Scholar]
- Elliot, J.; Marsh, C. Exploring Data: An Introduction to Data Analysis for Social Scientists; Polity Press: Cambridge, UK, 2008; ISBN-13: 978-0-7456-2282-8. [Google Scholar]
- Haffani, S.; Mezni, M.; Nasri, M.B.; Chaibi, W. Comparative leaf water relations and anatomical responses of three vetch species (Vicia narbonensis L., V. sativa L. and V. villosa Roth.) to cope with water stress. Crop Pasture Sci. 2017, 68, 691–702. [Google Scholar] [CrossRef]
- Volaire, F. A unified framework of plant adaptive strategies to drought: Crossing scales and disciplines. Glob. Chang. Biol. 2018, 24, 2929–2938. [Google Scholar] [CrossRef] [PubMed]
- Farquhar, G.D.; Sharkey, T.D. Stomatal conductance and photosynthesis. Ann. Rev. Plant Physiol. 1982, 33, 317–345. [Google Scholar] [CrossRef]
- Crang, R.; Lyons-Sobaski, S.; Wise, R. Plant Anatomy: A Concept-Based Approach to the Structure of Seed Plants; Springer Nature: Cham, Switzerland, 2018. [Google Scholar]
- Yang, J.Z.; Matthew, C.; Rowland, R.E. Tiller axis observations for perennial ryegrass (Lolium perenne) and tall fescue (Festuca arundinacea): Number of active phytomers, probability of tiller appearance, and frequency of root appearance per phytomer for three cutting heights. N. Z. J. Agric. Res. 1998, 41, 11–17. [Google Scholar] [CrossRef]
- Barceló, A.R. Xylem parenchyma cells deliver the H2O2 necessary for lignification in differentiating xylem vessels. Planta 2005, 220, 747–756. [Google Scholar] [CrossRef] [PubMed]
- Seeni, S.; Mariappan, T.; Gopalan, G.; Gnanam, A. Mechanical separation of palisade and spongy-parenchyma cells from the leaves of mesomorphic dicotyledons for photosynthetic studies. Planta 1983, 157, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Vasyukova, N.I.; Chalenko, G.I.; Gerasimova, N.G.; Ozeretskovskaya, O.L. Wound repair in plant tissues (Review). Appl. Biochem. Microbiol. 2011, 47, 253–258. [Google Scholar] [CrossRef]
- Soper, K.; Mitchell, K.J. The developmental anatomy of perennial ryegrass (Lolium perenne L.). N. Z. J. Sci. Technol. 1956, 37, 484–504. [Google Scholar]
- Heinen, R.B.; Ye, Q.; Chaumont, F. Role of aquaporins in leaf physiology. J. Exp. Bot. 2009, 60, 2971–2985. [Google Scholar] [CrossRef]
- Shatil-Cohen, A.; Attia, Z.; Moshelion, M. Bundle-sheath cell regulation of xylem-mesophyll water transport via aquaporins under drought stress: A target of xylem-borne ABA? Plant J. 2011, 67, 72–80. [Google Scholar] [CrossRef]
- Vasellati, V.; Oesterheld, M.; Medan, D.; Loret, J. Effects of flooding and drought on the anatomy of Paspalum dilatatum. Ann. Bot. 2001, 88, 355–360. [Google Scholar] [CrossRef]
- Šantruček, J. Transpiration efficiency and apparent cuticular transpiration in some C3 and C4 plants. Biol. Plant. 1991, 33, 192–199. [Google Scholar] [CrossRef]
- Trlica, M.J.; Biondini, M.E. Soil water dynamics, transpiration, and water losses in a crested wheatgrass and native shortgrass ecosystem. Plant Soil 1990, 126, 187–201. [Google Scholar] [CrossRef]
- Charles-Edwards, D.A.; Sant, C.E.I. Models for mesophyll cell arrangement in leaves of ryegrass (Lolium perenne L.). Planta 1972, 104, 297–305. [Google Scholar] [CrossRef]
- Oguchi, R.; Onoda, Y.; Terashima, I.; Tholen, D. Leaf anatomy and function. In The Leaf: A Platform for Performing Photosynthesis; Adams, W.W., III, Terashima, I., Eds.; Springer: Cham, Switzerland, 2018; pp. 97–139. [Google Scholar] [CrossRef]
- Nilsen, E.T.; Forseth, I.N., Jr. The role of leaf movements for optimizing photosynthesis in relation to environmental variation. In The Leaf: A Platform for Performing Photosynthesis; Adams, W.W., III, Terashima, I., Eds.; Springer: Cham, Switzerland, 2018; pp. 401–423. [Google Scholar] [CrossRef]
- Li, K.; Huang, J.; Song, W.; Wang, J.; Wang, X. Automatic segmentation and measurement methods of living stomata of plants based on the CV model. Plant Methods 2019, 15, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Hetherington, A.M.; Woodward, F.I. The role of stomata in sensing and driving environmental change. Nature 2003, 424, 901–908. [Google Scholar] [CrossRef] [PubMed]
- Lyshede, O.B. Xeromorphic features of three stem assimilants in relation to their ecology. Bot. J. Linn. Soc. 1979, 78, 85–98. [Google Scholar] [CrossRef]
- Jäger, K.; Fábián, A.; Eitel, G.; Szabó, L.; Deák, C.; Barnabás, B.; Papp, I. A morpho-physiological approach differentiates bread wheat cultivars of contrasting tolerance under cyclic water stress. J. Plant Physiol. 2014, 171, 1256–1266. [Google Scholar] [CrossRef]
- Hoover, D.; Koriakin, K.; Albrigtsen, J.; Ocheltree, T. Comparing water-related plant functional traits among dominant grasses of the Colorado Plateau: Implications for drought resistance. Plant Soil 2019, 441, 207–218. [Google Scholar] [CrossRef]
- Abernethy, G.A.; Fountain, D.W.; Mcmanus, M.T. Observations on the leaf anatomy of Festuca novae-zelandiae and biochemical responses to a water deficit. N. Z. J. Bot. 1998, 36, 113–123. [Google Scholar] [CrossRef]
- Balsamo, R.A.; Willigen, C.V.; Bauer, A.M.; Farrant, J. Drought tolerance of selected Eragrostis species correlates with leaf tensile properties. Ann. Bot. 2006, 97, 985–991. [Google Scholar] [CrossRef]
- Alvarez, J.M.; Rocha, J.F.; Machado, S.R. Bulliform cells in Loudetiopsis chrysothrix (Nees) Conert and Tristachya leiostachya Nees (Poaceae): Structure in relation to function. Braz. Arch. Biol. Technol. 2008, 51, 113–119. [Google Scholar] [CrossRef]
- Dickison, W.C. Integrative Plant Anatomy; Harcourt Academic Press: New York, NY, USA, 2000. [Google Scholar]
- Grigore, M.N.; Toma, C. Bulliform Cells. In Anatomical Adaptations of Halophytes; Springer: Cham, Switzerland, 2017; pp. 325–338. [Google Scholar] [CrossRef]
- Kim, S.H.; Park, S.H.; Woo, J.H.; Choi, S.Y. Hairs as physical barrier against adhesion of Xanthomonas axonopodis pv. Glycines on soybean leaf. Res. Plant Dis. 2015, 21, 40–43. [Google Scholar] [CrossRef]
- Roy, B.A.; Stanton, M.L.; Eppley, S.M. Effects of environmental stress on leaf hair density and consequences for selection. J. Evol. Biol. 1999, 12, 1089–1103. [Google Scholar] [CrossRef]
- Zhang, W.; Mirlohi, S.S.; Li, X.; He, Y. Identification of functional single-nucleotide polymorphisms affecting leaf hair number in Brassica rapa. Plant Physiol. 2018, 177, 490–503. [Google Scholar] [CrossRef] [PubMed]
- Ehleringer, J.R.; Mooney, H.A. Leaf hairs: Effects on physiological activity and adaptive value to a desert shrub. Oecologia 1978, 37, 183–200. [Google Scholar] [CrossRef] [PubMed]
- Ripley, B.S.; Pammenter, N.W.; Smith, V.R. Function of leaf hairs revisited: The hair layer on leaves Arctotheca populifolia reduces photoinhibition, but leads to higher leaf temperatures caused by lower transpiration rates. J. Plant Physiol. 1999, 155, 78–85. [Google Scholar] [CrossRef]
- Kenzo, T.; Yoneda, R.; Azani, M.A.; Majid, N.M. Changes in leaf water use after removal of leaf lower surface hairs on Mallotus macrostachyus (Euphorbiaceae) in a tropical secondary forest in Malaysia. J. For. Res. 2008, 13, 137–142. [Google Scholar] [CrossRef]
- Shields, L.M. Leaf xeromorphy as related to physiological and structural influences. Bot. Rev. 1950, 16, 399–447. [Google Scholar] [CrossRef]
- Spence, R.D.; Wu, H.; Sharpe, P.J.H.; Clark, K.G. Water stress effects on guard cell anatomy and the mechanical advantage of the epidermal cells. Plant Cell Environ. 1986, 9, 197–202. [Google Scholar] [CrossRef]
- Galmés, J.; Flexas, J.; Savé, R.; Medrano, H. Water relations and stomatal characteristics of Mediterranean plants with different growth forms and leaf habits: Responses to water stress and recovery. Plant Soil 2007, 290, 139–155. [Google Scholar] [CrossRef]
- Franks, P.J.; Beerling, D.J. Maximum leaf conductance driven by CO2 effects on stomatal size and density over geologic time. Proc. Natl. Acad. Sci. USA 2009, 106, 10343–10347. [Google Scholar] [CrossRef] [PubMed]
- Ganeva, T.; Uzunova, K.; Koleva, D. Comparative leaf epidermis investigation in species of genus Crataegus L. (Rosaceae) from Bulgaria. Feddes Repert. 2009, 120, 169–184. [Google Scholar] [CrossRef]
- Bertolino, L.T.; Caine, R.S.; Gray, J.E. Impact of stomatal density and morphology on water-use efficiency in a changing world. Front. Plant Sci. 2019, 10, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Zhou, G. Responses of leaf stomatal density to water status and its relationship with photosynthesis in a grass. J. Exp. Bot. 2008, 59, 3317–3325. [Google Scholar] [CrossRef] [PubMed]
- Heredia, A.; Dominguez, E. The plant cuticle: A complex lipid barrier between the plant and the environment. In Counteraction to Chemical and Biological Terrorism in East European Countries; Dishovsky, C., Pivovarov, A., Eds.; Springer Nature: Cham, Switzerland, 2009; pp. 109–116. [Google Scholar]
- Holloway, P.J. Structure and histochemistry of plant cuticular membranes: An overview. In The Plant Cuticle; Price, C.E., Ed.; Academic Press: London, UK, 1982; pp. 1–32. [Google Scholar]
- Schweizer, P.; Felix, G.; Buchala, A.; Müller, C.; Métraux, J.P. Perception of free cutin monomers by plant cells. Plant J. 1996, 10, 331–341. [Google Scholar] [CrossRef]
- Suh, M.C.; Samuels, A.L.; Jetter, R.; Kunst, L.; Pollard, M.K.; Pollard, M.; Ohlrogge, J.; Beisson, F. Cuticular lipid composition, surface structure, and gene expression in arabidopsis stem epidermis. Plant Physiol. 2005, 139, 1649–1665. [Google Scholar] [CrossRef] [PubMed]
- Jenks, M.A.; Joly, R.J.; Peters, P.J.; Rich, P.J.; Axtell, J.D.; Ashworth, E.N. Chemically induced cuticle mutation affecting epidermal conductance to water vapor and disease susceptibility in Sorghum bicolor (L.) Moench. Plant Physiol. 1994, 105, 1239–1245. [Google Scholar] [CrossRef]
- Hajibagheri, M.A.; Hall, J.L.; Flowers, T.J. The structure of the cuticle in relation to cuticular transpiration in leaves of the halophyte Suaeda maritima (L.) Dum. New Phytol. 1983, 94, 125–131. [Google Scholar] [CrossRef]
- Descalzi, C.A.; López, I.F.; Kemp, P.D.; Dörner, J.; Ordóñez, I. Pasture restoration improvement methods for temperate degraded pastures and consequences of the climatic seasonality on soil–pasture complex. J. Agron. Crop Sci. 2020, 206, 130–147. [Google Scholar] [CrossRef]




| Tiller Number per Plant | Aerial DM (g/Plant) | Root DM (g/Plant) | Root–Shoot Ratio | |
|---|---|---|---|---|
| Species | ||||
| Bv | 35.90 ± 4.12 | 8.51 ± 1.58 | 2.56 ± 0.42 | 0.38 ± 0.03 |
| Lp | 91.60 ± 10.66 | 7.90 ± 1.65 | 2.40 ± 0.55 | 0.31 ± 0.02 |
| Significance | *** | ns | ns | ns |
| Soil water content | ||||
| 80–85% FC | 85.40 ± 12.67 | 12.97 ± 0.92 | 3.58 ± 0.45 | 0.28 ± 0.02 |
| 20–25% FC | 42.10 ± 6.20 | 3.44 ± 0.18 | 1.37 ± 0.10 | 0.41 ± 0.03 |
| Significance | *** | *** | *** | *** |
| Species × soil water content | ||||
| Bv × 80–85% FC | 48.00 ± 1.05 c | 13.87 ± 0.50 | 3.65 ± 0.42 | 0.29 ± 0.03 b |
| Bv × 20–25% FC | 23.80 ± 1.50 d | 3.16 ± 0.26 | 1.47 ± 0.10 | 0.47 ± 0.02 a |
| Lp × 80–85% FC | 122.80 ± 4.66 a | 12.08 ± 1.87 | 3.51 ± 0.85 | 0.28 ± 0.03 b |
| Lp × 20–25% FC | 60.40 ± 1.83 b | 3.72 ± 0.19 | 1.28 ± 0.16 | 0.34 ± 0.03 b |
| Significance | *** | ns | ns | * |
| Water Potential (Mpa) | Osmotic Potential (Mpa) | Photo (μmCO2/m2s) | Cond (molH2O/m2s) | Ci (μmolCO2/m2s) | Trmmol (mmolH2O/m2s) | Tl-Ta (°C) | H2Odiff (mmolH2O/mol) | RHsfc (%) | |
|---|---|---|---|---|---|---|---|---|---|
| Species | |||||||||
| Bv | −1.86 ± 0.12 | −2.05 ± 0.13 | 12.36 ± 1.22 | 0.18 ± 0.03 | 239.3 ± 10.50 | 3.72 ± 0.46 | −0.05 ± 0.24 | 22.87 ± 0.99 | 44.61 ± 2.19 |
| Lp | −1.76 ± 0.11 | −1.88 ± 0.11 | 9.94 ± 0.39 | 0.15 ± 0.02 | 247.2 ± 13.35 | 3.21 ± 0.26 | 0.21 ± 0.14 | 23.22 ± 0.85 | 42.84 ± 1.45 |
| Significance | ns | ns | ns | ns | ns | ns | ns | ns | ns |
| Soil water content | |||||||||
| 80–85% FC | −1.33 ± 0.12 | −1.67 ± 0.07 | 11.81 ± 1.13 | 0.21 ± 0.03 | 271.6 ± 6.78 | 4.02 ± 0.38 | −0.21 ± 0.20 | 20.68 ± 0.52 | 47.55 ± 1.62 |
| 20–25% FC | −2.29 ± 0.11 | −2.26 ± 0.06 | 10.49 ± 0.83 | 0.12 ± 0.01 | 214.9 ± 8.07 | 2.91 ± 0.28 | 0.37 ± 0.15 | 25.41 ± 0.51 | 39.91 ± 1.12 |
| Significance | *** | *** | ns | * | *** | ns | ns | *** | ** |
| Species × soil water content | |||||||||
| Bv × 80–85% FC | −1.37 ± 0.11 | −1.75 ± 0.13 | 13.55 ± 1.78 | 0.24 ± 0.04 | 262.7 ± 11.30 | 4.35 ± 0.64 | −0.38 ± 0.33 | 20.30 ± 0.93 | 48.92 ± 2.82 |
| Bv × 20–25% FC | −2.35 ± 0.15 | −2.35 ± 0.07 | 11.17 ± 1.51 | 0.13 ± 0.02 | 215.8 ± 8.77 | 3.08 ± 0.45 | 0.28 ± 0.23 | 25.45 ± 0.60 | 40.31 ± 1.60 |
| Lp × 80–85% FC | −1.29 ± 0.03 | −1.58 ± 0.07 | 10.07 ± 0.34 | 0.18 ± 0.01 | 280.6 ± 5.56 | 3.69 ± 0.24 | −0.04 ± 0.13 | 21.06 ± 0.28 | 46.17 ± 0.95 |
| Lp × 20–25% FC | −2.23 ± 0.19 | −2.18 ± 0.06 | 9.81 ± 0.75 | 0.11 ± 0.02 | 213.9 ± 14.7 | 2.73 ± 0.37 | 0.47 ± 0.20 | 25.38 ± 0.91 | 39.51 ± 1.74 |
| Significance | ns | ns | ns | ns | ns | ns | ns | ns | ns |
| Pseudostem T | Epidermis T | Cuticle T | Vascular Bundle | |||||
|---|---|---|---|---|---|---|---|---|
| Xylem D | Vascu D | Vascu Area | Bun sh T | Bun sh Wall | ||||
| μm | μm2 | μm | ||||||
| Species | ||||||||
| Bv | 170.07 ± 14.29 | 9.08 ± 0.62 | 1.22 ± 0.12 | 11.40 ± 0.83 | 52.17 ± 2.31 | 3136 ± 290 | 5.07 ± 0.32 | 1.93 ± 0.10 |
| Lp | 79.42 ± 6.08 | 9.47 ± 0.46 | 1.72 ± 0.19 | 6.05 ± 0.29 | 35.97 ± 1.30 | 1355 ± 102 | 4.87 ± 0.20 | 1.07 ± 0.04 |
| Significance | *** | ns | * | *** | *** | *** | ns | *** |
| Soil water content | ||||||||
| 80–85% FC | 155.63 ± 16.67 | 10.62 ± 0.43 | 1.78 ± 0.20 | 10.06 ± 0.91 | 48.19 ± 2.82 | 2723 ± 341 | 5.17 ± 0.25 | 1.36 ± 0.09 |
| 20–25% FC | 93.87 ± 8.65 | 7.92 ± 0.38 | 1.15 ± 0.10 | 7.39 ± 0.70 | 39.95 ± 2.14 | 1767 ± 190 | 4.76 ± 0.25 | 1.64 ± 0.16 |
| Significance | *** | *** | ** | *** | *** | *** | ns | ** |
| Species × soil water content | ||||||||
| Bv × 80–85% FC | 218.53 ± 9.20 a | 10.50 ± 0.78 | 1.61 ± 0.12 | 13.16 ± 1.21 | 57.71 ± 2.88 | 3918 ± 372 a | 5.61 ± 0.39 | 1.68 ± 0.06 b |
| Bv × 20–25% FC | 121.61 ± 8.73 b | 7.66 ± 0.65 | 0.83 ± 0.08 | 9.63 ± 0.79 | 46.63 ± 2.38 | 2354 ± 223 b | 4.52 ± 0.43 | 2.18 ± 0.14 a |
| Lp × 80–85% FC | 92.73 ± 8.69 c | 10.75 ± 0.53 | 1.96 ± 0.33 | 6.95 ± 0.28 | 38.67 ± 1.89 | 1529 ± 157 c | 4.74 ± 0.30 | 1.04 ± 0.06 c |
| Lp × 20–25% FC | 66.11 ± 4.96 c | 8.19 ± 0.43 | 1.48 ± 0.08 | 5.15 ± 0.26 | 33.28 ± 1.11 | 1181 ± 87 c | 5.01 ± 0.26 | 1.10 ± 0.06 c |
| Significance | *** | ns | ns | ns | ns | ** | ns | * |
| Epidermis T | Stem D | Cortex T | Vascular Bundle | |||
|---|---|---|---|---|---|---|
| Xylem D | Vascu D | Vascu Area | ||||
| μm | μm2 | |||||
| 80–85% FC | 7.44 ± 0.30 | 423.5 ± 49.5 | 107.22 ± 17.83 | 60.94 ± 3.21 | 6.07 ± 0.28 | 3777 ± 396 |
| 20–25% FC | 7.25 ± 0.70 | 432.9 ± 24.2 | 63.09 ± 11.65 | 46.02 ± 1.75 | 6.80 ± 0.35 | 2239 ± 438 |
| Significance | ns | ns | ns | ns | ns | ns |
| Leaf T | Mesophyll T | Epidermis T | Cuticle T | Mid-Vein | Bulliform | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Peak | Valley | Peak | Valley | Upper | Lower | Upper | Lower | |||
| Species | ||||||||||
| Bv | 105.28 ± 3.40 | 105.28 ± 3.40 | 85.37 ± 2.57 | 85.44 ± 2.50 | 10.75 ± 0.33 | 11.83 ± 0.48 | 0.88 ± 0.06 | 1.25 ± 0.06 | 215.91 ± 13.06 | 18.69 ± 0.66 |
| Lp | 126.72 ± 4.69 | 61.13 ± 2.35 | 100.50 ± 4.19 | 35.90 ± 1.37 | 9.90 ± 0.30 | 12.12 ± 0.53 | 0.81 ± 0.04 | 1.41 ± 0.05 | 180.19 ± 7.86 | 15.91 ± 0.98 |
| Significance | *** | *** | ** | *** | ns | ns | ns | * | *** | ** |
| Soil water content | ||||||||||
| 80–85% FC | 124.38 ± 4.72 | 87.68 ± 7.31 | 98.11 ± 3.85 | 64.02 ± 6.83 | 10.86 ± 0.38 | 12.38 ± 0.68 | 0.73 ± 0.04 | 1.25 ± 0.08 | 235.72 ± 12.78 | 17.24 ± 1.18 |
| 20–25% FC | 107.62 ± 3.53 | 78.73 ± 3.83 | 87.76 ± 2.43 | 57.31 ± 4.53 | 9.79 ± 0.25 | 11.57 ± 0.28 | 0.96 ± 0.06 | 1.42 ± 0.04 | 160.38 ± 5.96 | 17.35 ± 0.61 |
| Significance | ** | * | * | * | * | ns | *** | * | *** | ns |
| Species × soil water content | ||||||||||
| Bv × 80–85% FC | 115.64 ± 5.16 | 115.6 ± 5.16 a | 92.55 ± 4.40 | 92.69 ± 4.04 a | 11.51 ± 0.48 | 12.81 ± 0.90 | 0.68 ± 0.06 b | 1.05 ± 0.06 b | 269.1 ± 12.46 a | 20.85 ± 0.50 a |
| Bv × 20–25% FC | 94.93 ± 2.58 | 94.93 ± 2.58 b | 78.19 ± 1.51 | 78.19 ± 1.51 b | 9.99 ± 0.33 | 10.85 ± 0.18 | 1.07 ± 0.07 a | 1.45 ± 0.06 a | 162.7 ± 7.98 c | 16.53 ± 0.58 c |
| Lp × 80–85% FC | 133.13 ± 8.09 | 59.73 ± 4.70 c | 103.68 ± 6.82 | 35.35 ± 2.40 c | 10.21 ± 0.49 | 11.94 ± 1.03 | 0.78 ± 0.04 b | 1.44 ± 0.10 a | 202.3 ± 4.57 b | 13.64 ± 1.44 c |
| Lp × 20–25% FC | 120.31 ± 4.73 | 62.54 ± 1.91 c | 97.33 ± 3.54 | 36.44 ± 1.26 c | 9.59 ± 0.38 | 12.30 ± 0.56 | 0.84 ± 0.07 b | 1.38 ± 0.06 a | 158.1 ± 3.58 c | 18.18 ± 0.99 b |
| Significance | ns | ** | ns | * | ns | ns | ** | ** | *** | *** |
| Vein D | Vein Area | Xylem D | Phloem T | |||||
|---|---|---|---|---|---|---|---|---|
| Big | Small | Big | Small | Big | Small | Big | Small | |
| μm | μm2 | μm | ||||||
| Species | ||||||||
| Bv | 45.14 ± 1.59 | 18.89 ± 0.21 | 1975 ± 113 | 435 ± 18 | 11.18 ± 0.59 | 2.80 ± 0.15 | 16.95 ± 0.45 | 10.54 ± 0.42 |
| Lp | 39.36 ± 2.12 | 17.88 ± 0.69 | 1307 ± 83 | 389 ± 26 | 9.67 ± 0.61 | 2.71 ± 0.16 | 12.77 ± 0.60 | 9.75 ± 0.42 |
| Significance | * | * | *** | ** | * | ns | *** | ns |
| Soil water content | ||||||||
| 80–85% FC | 45.55 ± 1.96 | 19.52 ± 0.30 | 1822 ± 134 | 395 ± 26 | 12.32 ± 0.58 | 3.14 ± 0.12 | 15.18 ± 0.89 | 10.33 ± 0.40 |
| 20–25% FC | 38.95 ± 1.75 | 17.25 ± 0.49 | 1460 ± 118 | 427 ± 19 | 8.53 ± 0.38 | 2.37 ± 0.09 | 14.54 ± 0.72 | 9.97 ± 0.47 |
| Significance | * | *** | * | * | *** | *** | ns | ns |
| Species × soil water content | ||||||||
| Bv × 80–85% FC | 46.70 ± 2.75 | 19.16 ± 0.30 a | 2197 ± 145 | 401 ± 66 a | 13.17 ± 0.76 | 3.21 ± 0.10 | 17.41 ± 0.85 | 10.17 ± 0.66 |
| Bv × 20–25% FC | 43.59 ± 1.90 | 18.63 ± 0.28 a | 1753 ± 145 | 409 ± 93 a | 9.18 ± 0.48 | 2.38 ± 0.16 | 17.69 ± 0.29 | 10.92 ± 0.52 |
| Lp × 80–85% FC | 44.40 ± 2.42 | 19.88 ± 0.47 a | 1447 ± 108 | 276 ± 55 b | 11.47 ± 0.63 | 3.06 ± 0.21 | 12.95 ± 0.68 | 10.48 ± 0.51 |
| Lp × 20–25% FC | 34.32 ± 2.11 | 15.87 ± 0.23 b | 1167 ± 107 | 387 ± 83 a | 7.78 ± 0.54 | 2.37 ± 0.07 | 12.60 ± 1.01 | 9.02 ± 0.53 |
| Significance | ns | *** | ns | ** | ns | ns | ns | ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; García-Favre, J.; Hu, H.; López, I.F.; Ordóñez, I.P.; Cartmill, A.D.; Kemp, P.D. Aboveground Structural Attributes and Morpho-Anatomical Response Strategies of Bromus valdivianus Phil. and Lolium perenne L. to Severe Soil Water Restriction. Agronomy 2023, 13, 2964. https://doi.org/10.3390/agronomy13122964
Zhang Y, García-Favre J, Hu H, López IF, Ordóñez IP, Cartmill AD, Kemp PD. Aboveground Structural Attributes and Morpho-Anatomical Response Strategies of Bromus valdivianus Phil. and Lolium perenne L. to Severe Soil Water Restriction. Agronomy. 2023; 13(12):2964. https://doi.org/10.3390/agronomy13122964
Chicago/Turabian StyleZhang, Yongmei, Javier García-Favre, Haiying Hu, Ignacio F. López, Iván P. Ordóñez, Andrew D. Cartmill, and Peter D. Kemp. 2023. "Aboveground Structural Attributes and Morpho-Anatomical Response Strategies of Bromus valdivianus Phil. and Lolium perenne L. to Severe Soil Water Restriction" Agronomy 13, no. 12: 2964. https://doi.org/10.3390/agronomy13122964
APA StyleZhang, Y., García-Favre, J., Hu, H., López, I. F., Ordóñez, I. P., Cartmill, A. D., & Kemp, P. D. (2023). Aboveground Structural Attributes and Morpho-Anatomical Response Strategies of Bromus valdivianus Phil. and Lolium perenne L. to Severe Soil Water Restriction. Agronomy, 13(12), 2964. https://doi.org/10.3390/agronomy13122964






