Structure of Endophytes in the Root, Stem, and Leaf Tissues of Sweetpotato and Their Response to Sweetpotato Scab Disease Caused by Elsinoë batatas
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site and Cultivation Methods
2.2. DNA Extraction
2.3. PCR Amplification
2.4. Illumina Library Construction
2.5. Illumina Sequencing
2.6. High-Throughput Sequencing
2.7. Data Analysis
3. Results
3.1. Sequencing Data and Sequencing Depth Analysis
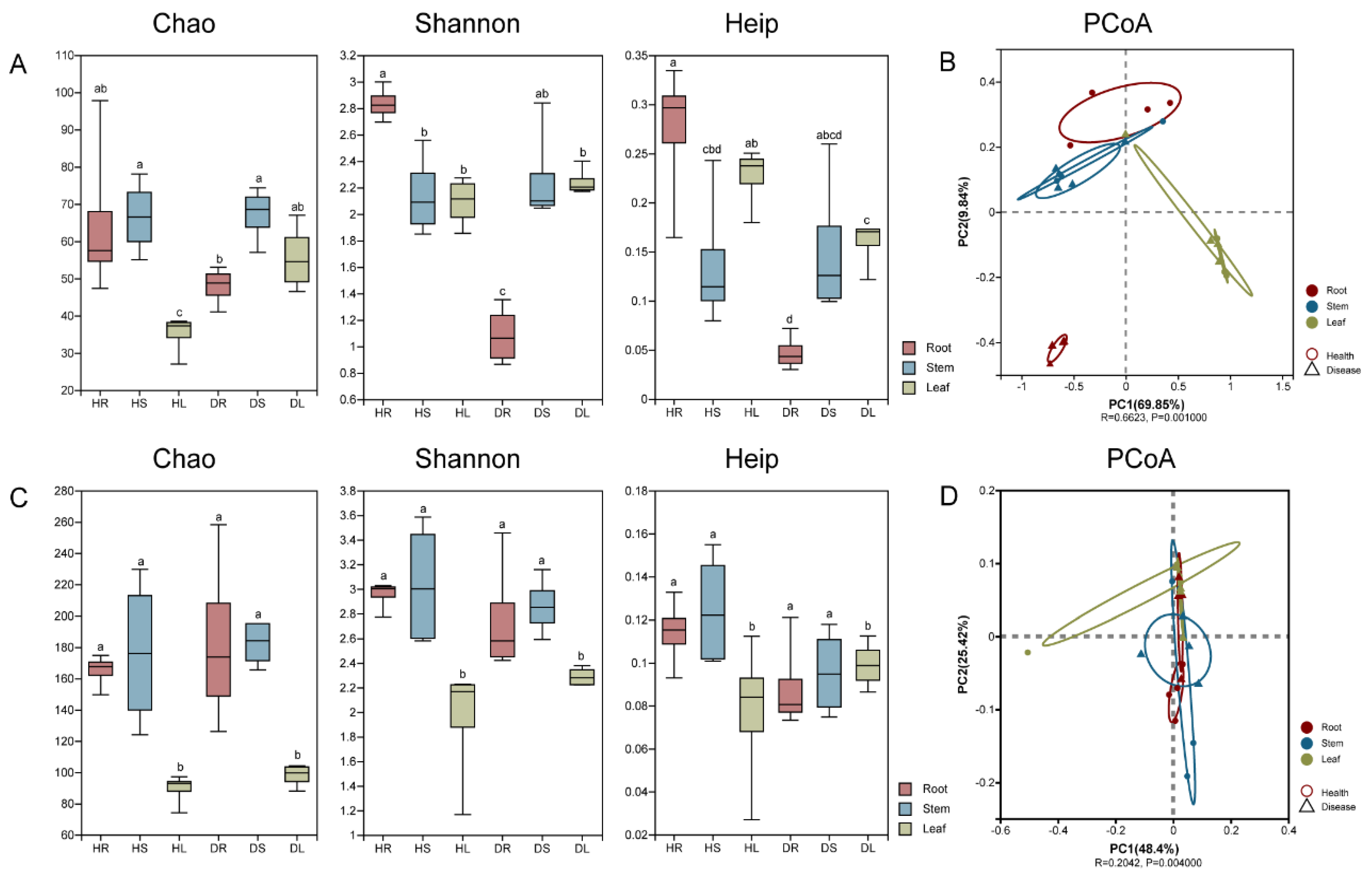
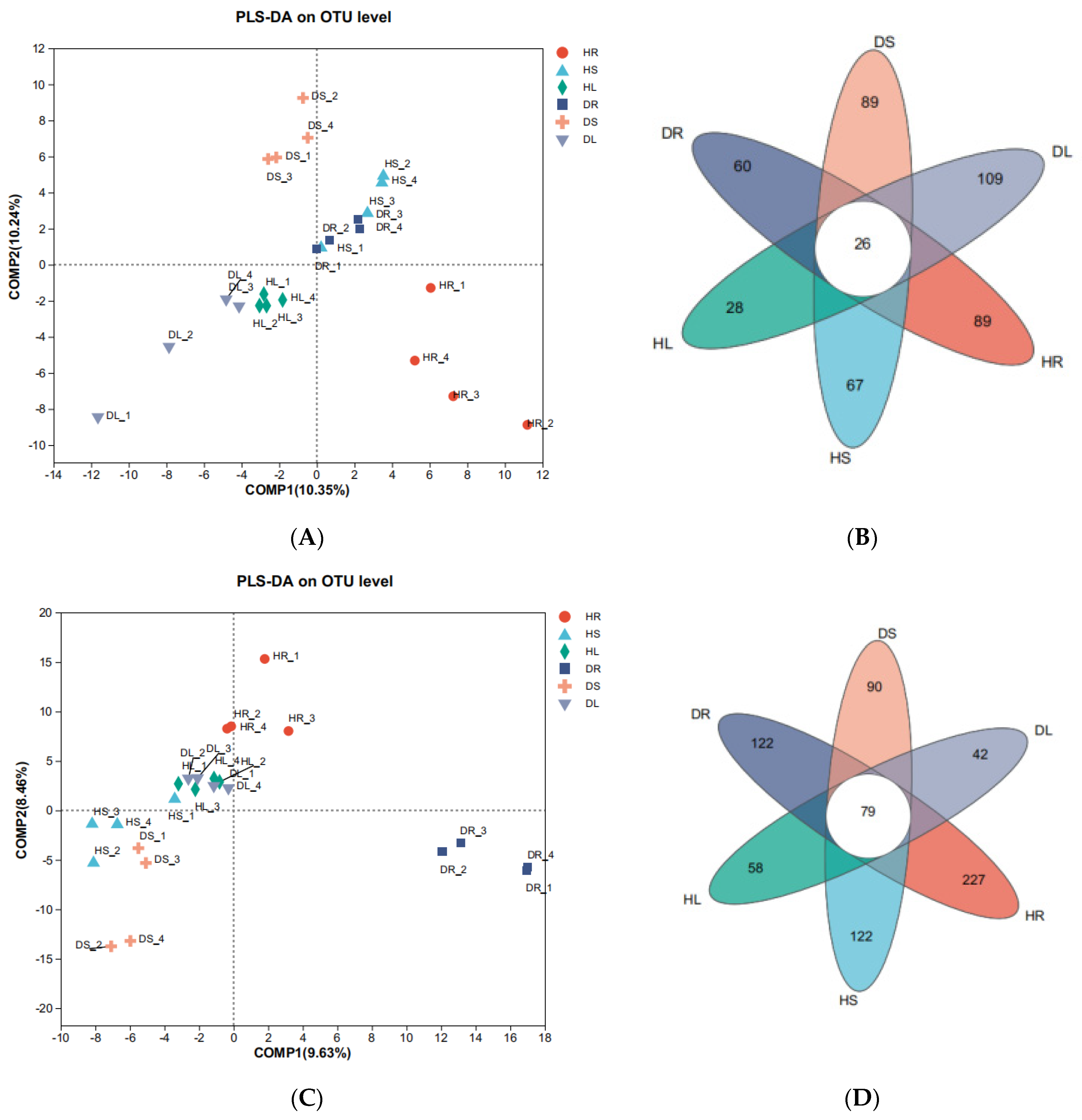
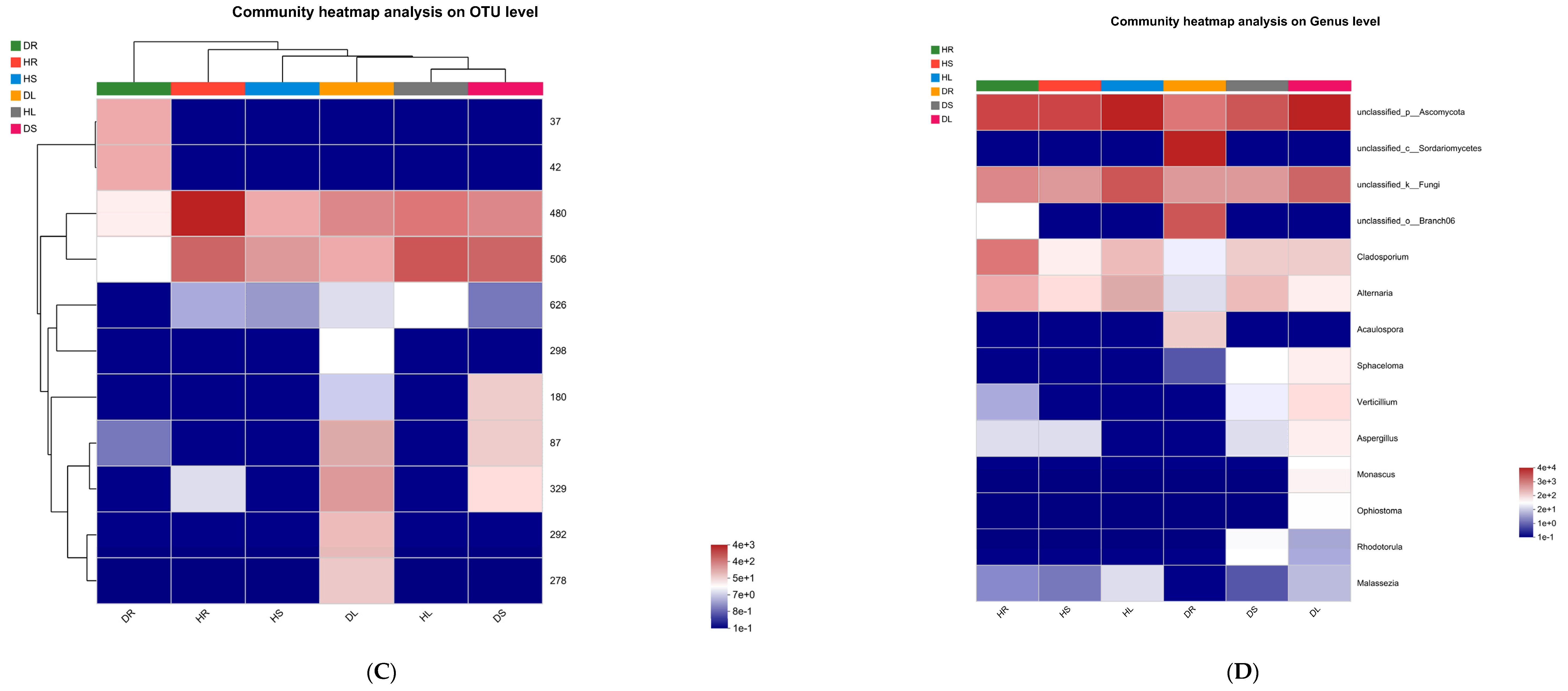
3.2. Distribution and Variation of the Endophytic Species in Different Tissues of Sweetpotato
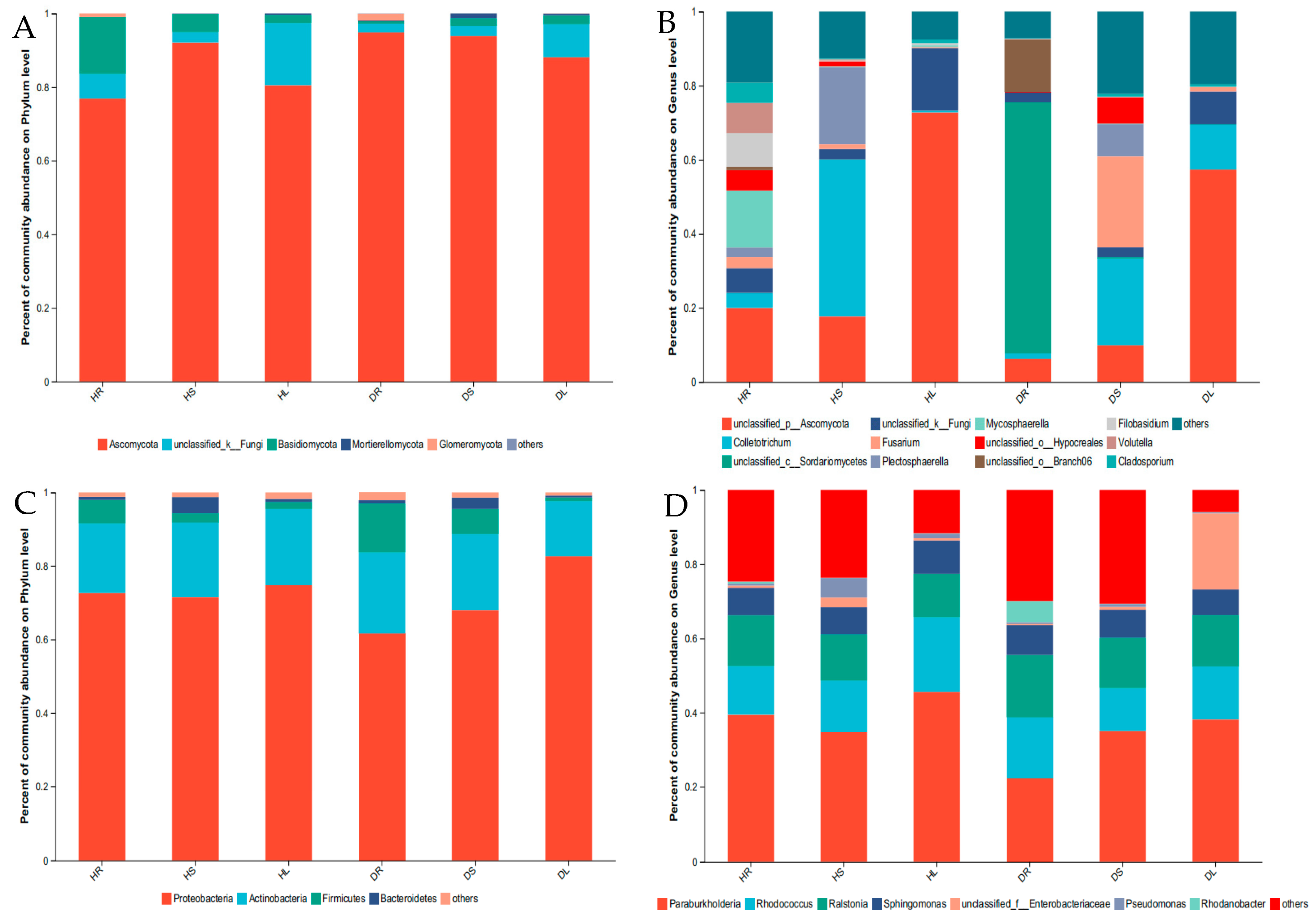
3.3. Composition of the Endophytes in Sweetpotato
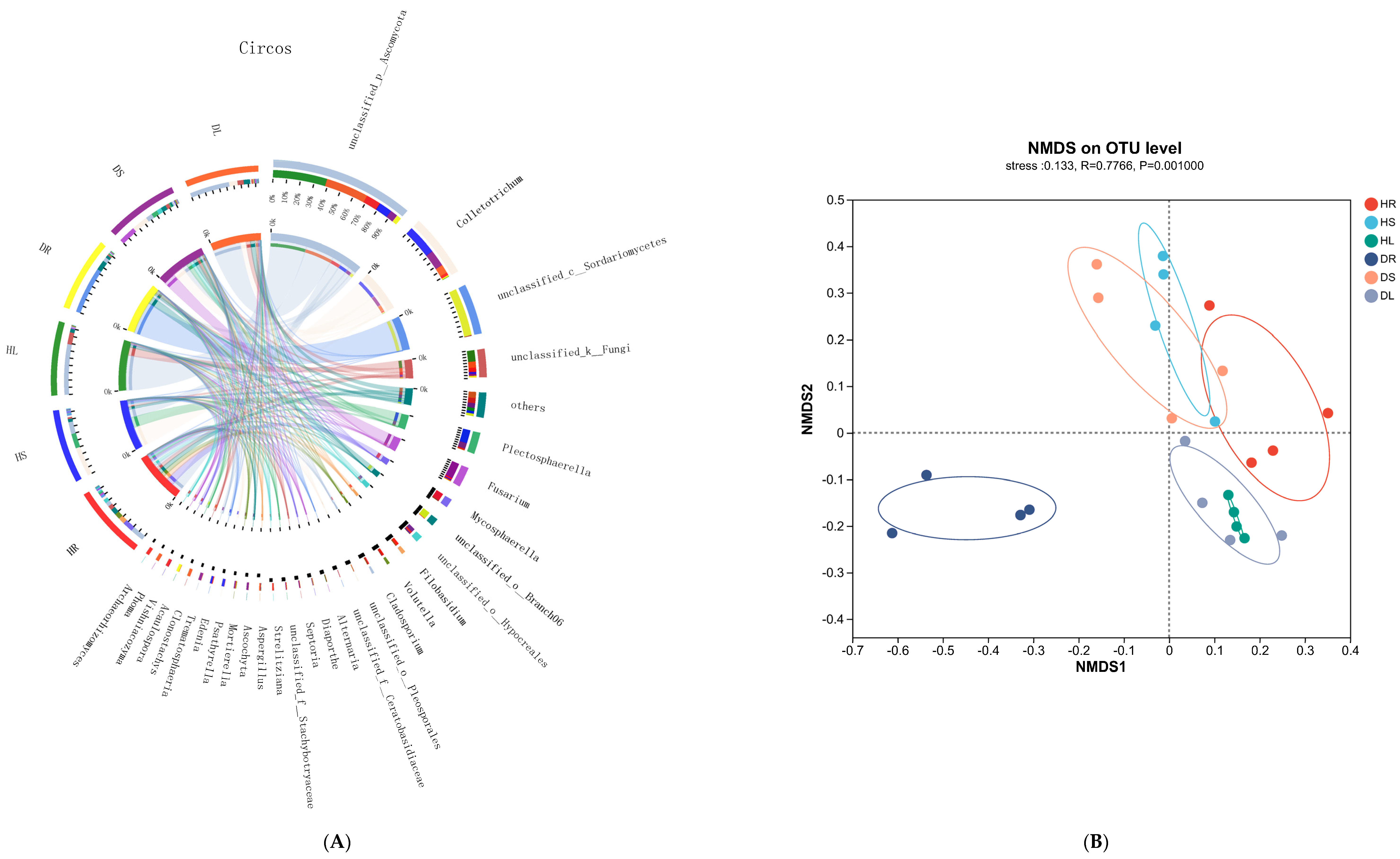
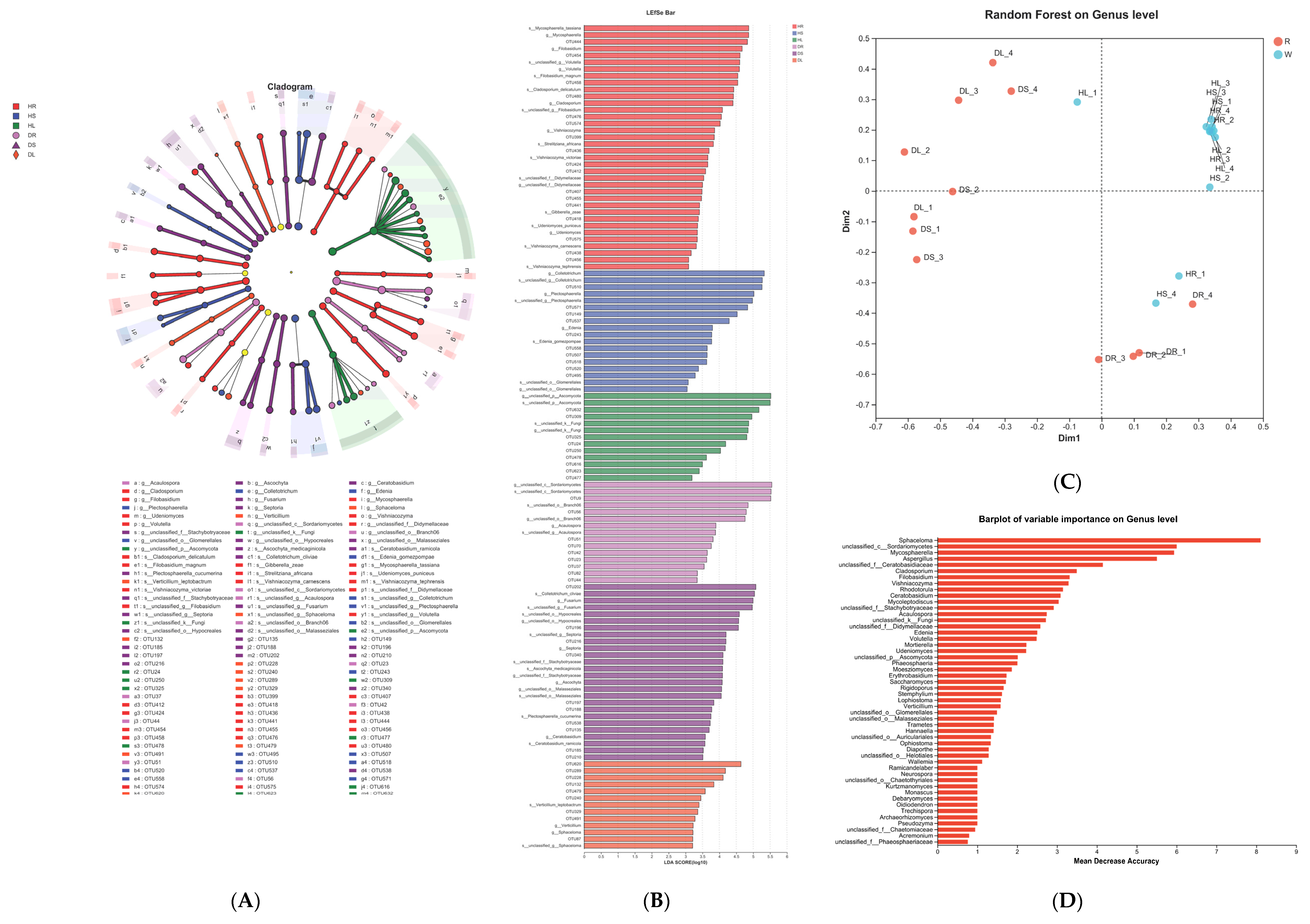
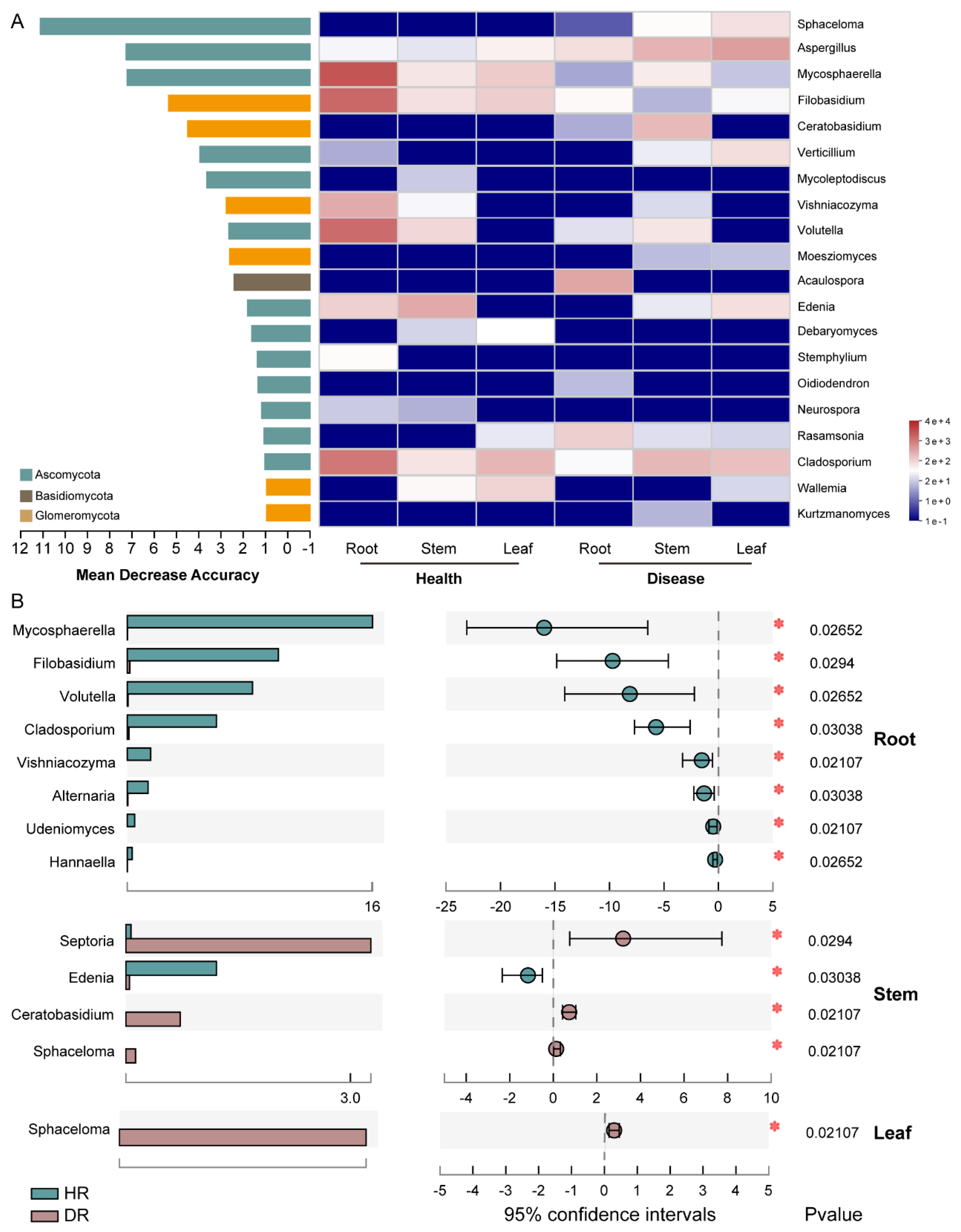
3.4. Analysis of the Endophytic Fungi in Sweetpotato Infected with Scab Disease
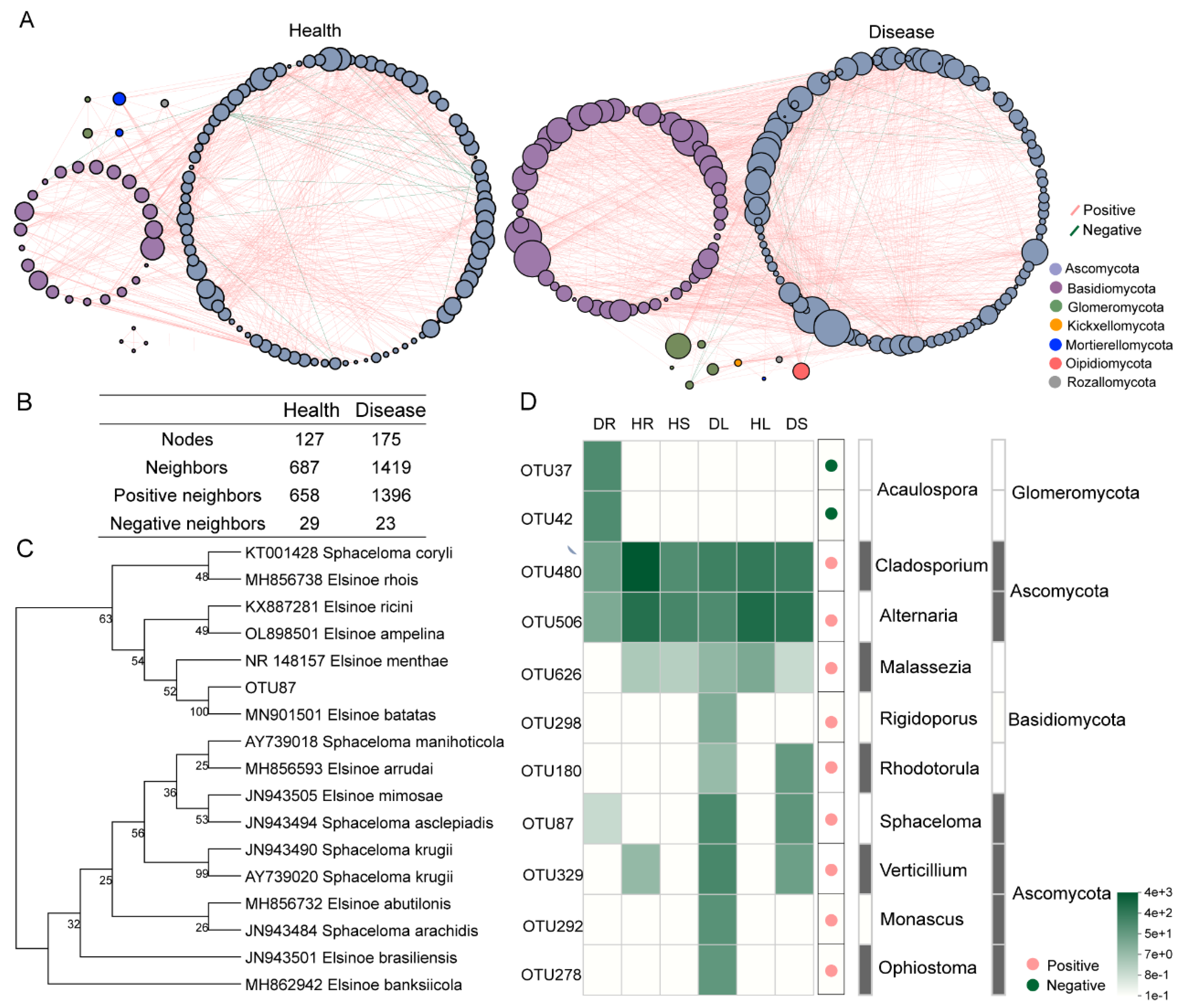
3.5. Relationship Analysis of the Endophytes Pathogenic to Sweetpotato Scab
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nguyen, H.C.; Chen, C.C.; Lin, K.H.; Chao, P.Y.; Lin, H.H.; Huang, M.Y. Bioactive Compounds, Antioxidants, and Health Benefits of Sweet Potato Leaves. Molecules 2021, 26, 1820. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, Q.; Zhou, M.; Yang, X.; Yang, C.; Jiao, C. Sweet potato study in China: Stress response mechanisms, molecular breeding, and productivity. J. Plant Physiol. 2020, 254, 153283. [Google Scholar] [CrossRef] [PubMed]
- Agili, S.; Nyende, B.; Ngamau, K. Selection, yield evaluation, drought tolerance indices of Orange-Flesh Sweet Potato (Ipomoea batatas Lam) Hybrid Clone. J. Nutr. Food Sci. 2012, 3, 138. [Google Scholar] [CrossRef]
- Wang, F.; Tan, W.F.; Song, W.; Yang, S.T.; Qiao, S. Transcriptome analysis of sweet potato responses to potassium deficiency. BMC Genom. 2022, 15, 655. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Zhu, M.; Gao, S. Genetic and Genomic Research on Sweet Potato for Sustainable Food and Nutritional Security. Genes 2022, 11, 1833. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Ye, S.; Liao, W.; Wu, M.; He, J.; Mateus, N.; Oliveira, H. The botanical profile, phytochemistry, biological activities and protected-delivery systems for purple sweet potato (Ipomoea batatas (L.) Lam.): An up-to-date review. Food Res. Int. 2022, 161, 111811. [Google Scholar] [CrossRef] [PubMed]
- Sheikha, A.F.E.; Ray, R.C. Potential impacts of bioprocessing of sweet potato: Review. Crit. Rev. Food Sci. Nutr. 2017, 57, 455–471. [Google Scholar] [CrossRef]
- Sun, H.; Mu, T.; Xi, L.; Zhang, M.; Chen, J. Sweet potato (Ipomoea batatas L.) leaves as nutritional and functional foods. Food Chem. 2014, 156, 380–389. [Google Scholar] [CrossRef]
- FAO. FAOSTAT. 2022. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 30 October 2023).
- Zhang, X.X.; Chen, J.Y.; Fang, B.P.; Wang, Z.Y.; Yao, Z.F.; Huang, L.F. Identification of the pathogen causing sweet potato scab in Guangdong and resistance evaluation of main domestic leaf-vegetable sweet potato germplasm resources. J. Plant Prot. 2021, 48, 298–304. [Google Scholar] [CrossRef]
- Huang, S.H.; Huang, L.F.; Fang, B.P.; Chen, J.Y.; Zhang, X.J.; Li, Y.J.; Wang, Z.Y.; Luo, Z.X. Identification and control of leaf scab disease of sweetpotato. Guangdong Agric. Sci. 2011, S1, 80–81. [Google Scholar] [CrossRef]
- Clark, C.A.; Ferrin, D.M.; Smith, T.P.; Holmes, G.J. Compendium of Sweetpotato Diseases, Pests, and Disorders, 2nd ed.; American Phytopathological Society: St. Paul, MN, USA, 2013; p. 26. [Google Scholar] [CrossRef]
- Jenkins, A.E.; Viegas, A.P. Stem and foliage scab of sweet potato (Ipomoea batatas). J. Wash. Acad. Sci. 1943, 33, 244–249. Available online: https://biostor.org/reference/134201 (accessed on 25 July 2023).
- Zhang, X.X.; Zou, H.D.; Yang, Y.L.; Fang, B.P.; Huang, L.F. Genome Resource for Elsinoë batatas, the Causal Agent of Stem and Foliage Scab Disease of Sweet Potato. Phytopathology 2022, 112, 973–975. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.L.; Barreto, R.W.; Groenewald, J.Z.; Bezerra, J.D.P.; Pereira, O.L.; Cheewangkoon, R.; Mostert, L.; Tian, C.M.; Crous, P.W. Phylogeny and taxonomy of the scab and spot anthracnose fungus Elsinoë (Myriangiales, Dothideomycetes). Stud. Mycol. 2017, 87, 1–41. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.Y.; Fang, B.P.; Zhang, X.J.; Huang, L.F.; Wang, Z.Y.; Luo, Z.X. A New Leaf-vegetable Sweet Potato Cultivar ‘Guangcaishu 5’. Acta Hortic. Sin. 2021, 48, 2861–2862. [Google Scholar] [CrossRef]
- Mendes, R.; Garbeva, P.; Raaijmakers, J.M. The rhizosphere microbiome: Significance of plant beneficial, plant pathogenic, and human pathogenic microorganisms. FEMS Microbiol. Rev. 2013, 37, 634–663. [Google Scholar] [CrossRef] [PubMed]
- Qu, Q.; Zhang, Z.; Peijnenburg, W.J.G.M.; Liu, W.; Qian, H. Rhizosphere Microbiome Assembly and Its Impact on Plant Growth. J. Agric. Food Chem. 2020, 68, 5024–5038. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, P.; Leach, J.E.; Tringe, S.G.; Sa, T.; Singh, B.K. Plant-microbiome interactions: From community assembly to plant health. Nat. Rev. Microbiol. 2020, 18, 607–621. [Google Scholar] [CrossRef]
- Matsumoto, H.; Fan, X.; Wang, Y.; Kusstatscher, P.; Duan, J.; Wu, S.; Chen, S.; Qiao, K.; Wang, Y.; Ma, B.; et al. Bacterial seed endophyte shapes disease resistance in rice. Nat. Plants 2021, 7, 60–72. [Google Scholar] [CrossRef]
- Yan, L.; Zhu, J.; Zhao, X.; Shi, J.; Jiang, C.; Shao, D. Beneficial effects of endophytic fungi colonization on plants. Appl. Microbiol. Biotechnol. 2019, 103, 3327–3340. [Google Scholar] [CrossRef]
- Padder, S.A.; Mansoor, S.; Bhat, S.A.; Baba, T.R.; Rather, R.A.; Wani, S.M.; Popescu, S.M.; Sofi, S.; Aziz, M.A.; Hefft, D.I.; et al. Bacterial Endophyte Community Dynamics in Apple (Malus domestica Borkh.) Germplasm and Their Evaluation for Scab Management Strategies. J. Fungi 2021, 7, 923. [Google Scholar] [CrossRef]
- Shuang, M.; Wang, Y.; Teng, W.; Jin, G. Isolation and identification of an endophytic bacteria Bacillus sp. K-9 exhibiting biocontrol activity against potato common scab. Arch. Microbiol. 2022, 14, 483. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Yang, C.; Wang, Y.; Ma, T.; Cai, F.; Wei, L.; Jin, M.; Osei, R.; Zhang, J.; Tang, M. Potential of an endophytic bacteria Bacillus amyloliquefaciens 3–5 as biocontrol agent against potato scab. Microb. Pathog. 2022, 163, 105382. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, L.; Hatami, R.S.; Etebarian, H.R. Apple Endophytic fungi and their antagonism against apple scab disease. Front. Microbiol. 2022, 13, 1024001. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.J.; Wang, Y.Z.; Chu, Z.H.; Wang, P.S.; Liu, B.Y.; Li, B.Y.; Yu, X.L.; Luan, B.H. Endophytic Bacillus amyloliquefaciens YTB1407 elicits resistance against two fungal pathogens in sweet potato (Ipomoea batatas (L.) Lam.). J. Plant Physiol. 2020, 253, 153260. [Google Scholar] [CrossRef] [PubMed]
- Yuan, T.; Qazi, I.H.; Yang, P.; Zhang, X.; Li, J.; Liu, J. Analysis of endophytic bacterial flora of mulberry cultivars susceptible and resistant to bacterial wilt using metagenomic sequencing and culture-dependent approach. World J. Microbiol. Biotechnol. 2023, 39, 163. [Google Scholar] [CrossRef] [PubMed]
- Yuan, T.; Qazi, I.H.; Li, J.; Yang, P.; Yang, H.; Zhang, X.; Liu, W.; Liu, J. Analysis of changes in bacterial diversity in healthy and bacterial wilt mulberry samples using metagenomic sequencing and culture-dependent approaches. Front. Plant Sci. 2023, 23, 1206691. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.Y.; Fang, B.P.; Li, Y.J.; Zhang, X.J.; Wang, Z.Y.; Huang, L.F.; Luo, Z.X.; Chen, X.L. Breeding of a new variety Guang Cai Shu No.3 for the tips of sweet potato vines as vegetable. Guangdong Agric. Sci. 2013, 40, 2. [Google Scholar] [CrossRef]
- Ames, T.; Smit, N.E.J.M.; Braun, A.R.; O’Sullivan, J.N.; Skoglund, L.G. Sweetpotato: Major Pests, Diseases, and Nutritional Disorders; Foliar and stem diseases caused by fungi; International Potato Center (CIP): Lima, Peru, 1997; p. 78. [Google Scholar]
- Ramsey, M.D.; Vawdrey, L.L.; Hardy, J. Scab (Sphaceloma batatas) a new disease of sweet potatoes in Australia: Fungicide and cultivar evaluation. Aust. J. Exp. Agric. 1988, 28, 137–141. [Google Scholar] [CrossRef]
- Fang, S.M.; Ke, Y.Q.; Huang, C.M.; Zhang, Y.J. The evaluation of resistance and analysis of resistant mechanism of sweet potato varieties to leaf scab disease. Acta Phytopathol. 2004, 31, 38–44. [Google Scholar]
- Dubey, A.; Malla, M.A.; Kumar, A.; Dayanandan, S.; Khan, M.L. Plants endophytes: Unveiling hidden agenda for bioprospecting toward sustainable agriculture. Crit. Rev. Biotechnol. 2020, 40, 1210–1231. [Google Scholar] [CrossRef]
- Pandey, S.S.; Jain, R.; Bhardwaj, P.; Thakur, A.; Kumari, M.; Bhushan, S.; Kumar, S. Plant probiotics—Endophytes pivotal to plant health. Microbiol. Res. 2022, 263, 127148. [Google Scholar] [CrossRef] [PubMed]
- Cappelli, S.L.; Domeignoz-Horta, L.A.; Loaiza, V.; Laine, A.L. Laine Plant biodiversity promotes sustainable agriculture directly and via belowground effects. Trends Plant Sci. 2022, 27, 674–687. [Google Scholar] [CrossRef] [PubMed]
- Cai, T. The occurrence pattern and control technology of sweet potato scab disease. Fujian Agric. 2006, 9, 23. [Google Scholar]
- Davide, B.; Klaus, S.; Stijn, S. Structure and Functions of the Bacterial Microbiota of Plants. Annu. Rev. Plant Biol. 2013, 64, 807–838. [Google Scholar] [CrossRef]
- Rosenblueth, M.; Martínez-Romero, E. Rhizobium etli maize populations and their competitiveness for root colonization. Arch. Microbiol. 2004, 181, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Chi, F.; Shen, S.H.; Cheng, H.P.; Jing, Y.X.; Yanni, Y.G.; Dazzo, F.B. Ascending migration of endophytic rhizobia, from roots to leaves, inside rice plants and assessment of benefits to rice growth physiology. Appl. Environ. Microbiol. 2005, 71, 7271–7278. [Google Scholar] [CrossRef] [PubMed]
- Compant, S.; Kaplan, H.; Sessitsch, A.; Nowak, J.; Barka, E.A.; Clement, C. Endophytic colonization of Vitis vinifera L. by Burkholderia phytofirmans strain PsJN: From the rhizosphere to inflorescence tissues. FEMS Microbiol. Ecol. 2008, 63, 84–93. [Google Scholar] [CrossRef]
- Hironobu, M.; Hisao, M. Endophytic Bacteria in the Rice Plant. Microbes Environ. 2009, 23, 109–117. [Google Scholar] [CrossRef]
- Beckers, B.; Beeck, M.O.D.; Weyens, N.; Boerjan, W.; Vangronsveld, J. Structural variability and niche differentiation in the rhizosphere and endosphere bacterial microbiome of field-grown poplar trees. Microbiome 2017, 5, 25. [Google Scholar] [CrossRef]
- Tardif, S.; Yergeau, É.; Tremblay, J.; Legendre, P.; Whyte, L.G.; Greer, C.W. The willow microbiome is influenced by soil petroleum-hydrocarbon concentration with plant compartment-specific effects. Front. Microbiol. 2016, 7, 1363. [Google Scholar] [CrossRef]
- Ampt, E.A.; Ruijven, J.V.; Zwart, M.P.; Raaijmakers, J.M.; Termorshuizen, A.J.; Mommer, L. Plant neighbours can make or break the disease transmission chain of a fungal root pathogen. New Phytol. 2022, 233, 1303–1316. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Wang, D.; Mao, Z.; Pan, L.; Liao, J.; Cai, Z. Infection of Plasmodiophora brassicae changes the fungal endophyte community of tumourous stem mustard roots as revealed by high-throughput sequencing and culture-dependent methods. PLoS ONE 2019, 12, e0214975. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Gao, Z.X.; Tian, B.N.; Bi, K.; Chen, T.; Liu, H.Q.; Xie, J.T.; Cheng, J.S.; Fu, Y.P.; Jiang, D.H.; et al. Endosphere microbiome comparison between symptomatic and asymptomatic roots of Brassica napus infected with Plasmodiophora brassicae. PLoS ONE 2017, 12, e0185907. [Google Scholar] [CrossRef] [PubMed]
- Hill, R.; Levicky, Q.; Pitsillides, F.; Junnonen, A.; Arrigoni, E.; Bonnin, J.M.; Kermode, A.; Mian, S.; Leitch, I.J.; Buddie, A.G.; et al. Tapping Culture Collections for Fungal Endophytes: First Genome Assemblies for Three Genera and Five Species in the Ascomycota. Genome Biol. Evol. 2023, 3, evad038. [Google Scholar] [CrossRef] [PubMed]
- Angelini, P.; Rubini, A.; Gigante, D.; Reale, L.; Pagiotti, R.; Venanzoni, R. The endophytic fungal communities associated with the leaves and roots of the common reed (Phragmites australis) in Lake Trasimeno (Perugia, Italy) in declining and healthy stands. Fungal Ecol. 2012, 5, 683–693. [Google Scholar] [CrossRef]
- Challacombe, J.F.; Hesse, C.N.; Bramer, L.M.; McCue, L.A.; Lipton, M.; Purvine, S.; Nicora, C.; Gallegos-Graves, V.; Porras-Alfaro, A.; Kuske, C.R. Genomes and secretomes of Ascomycota fungi reveal diverse functions in plant biomass decomposition and pathogenesis. BMC Genom. 2019, 12, 976. [Google Scholar] [CrossRef]
- Rana, K.L.; Kour, D.; Kaur, T.; Devi, R.; Yadav, A.N.; Yadav, N.; Dhaliwal, H.S.; Saxena, A.K. Endophytic microbes: Biodiversity, plant growth-promoting mechanisms and potential applications for agricultural sustainability. Antonie Van Leeuwenhoek 2020, 113, 1075–1107. [Google Scholar] [CrossRef] [PubMed]
- Crous, P.W.; Wingfield, M.J.; Cheewangkoon, R.; Carnegie, A.J.; Burgess, T.I.; Summerell, B.A.; Edwards, J.; Taylor, P.W.J.; Groenewald, J.Z. Foliar pathogens of eucalypts. Stud. Mycol. 2019, 94, 125–298. [Google Scholar] [CrossRef]
- Li, Z.; Santos, R.F.D.; Gao, L.; Chang, P.; Wang, X. Current status and future prospects of grapevine anthracnose caused by Elsinoë ampelina: An important disease in humid grape-growing regions. Mol. Plant Pathol. 2021, 22, 899–910. [Google Scholar] [CrossRef]
- Lederson, G.B.; Romina, G. Genome Sequence Resource of the Avocado Scab Pathogen Elsinoë perseae. Microbiol. Resour. Announc. 2023, 12, e00190-23. [Google Scholar] [CrossRef]
- Chung, K.R. Elsinoë fawcettii and Elsinoë australis: The fungal pathogens causing citrus scab. Mol. Plant Pathol. 2011, 12, 123–135. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Xiao, H.; Ma, X.; Cheng, Q. Elsinoë australis Causing Spot Anthracnose on Poplar in China. Plant Dis. 2020, 104, 2202–2209. [Google Scholar] [CrossRef]
- Termsung, N.; Cheewangkoon, R.; Kunasakdakul, K. First report of Septoria steviae causing stevia leaf spot in Thailand. J. Phytopathol. 2021, 12, 260–268. [Google Scholar] [CrossRef]
- Na, Y.E.; Choi, I.Y.; Abasova, L.; Shin, H.D. First report of leaf spot caused by Septoria erigerontis on Conyza sumatrensis in Korea. Plant Dis. 2023, 107, 561. [Google Scholar] [CrossRef]
- Aktan, Z.C.; Soylu, S.; Çiftçi, O.; Türkölmez, Ş. First report of root rot caused by Ceratobasidium sp. on sweet almond in Turkey. J. Plant Pathol. 2019, 102, 241–242. [Google Scholar] [CrossRef]
- Baiswar, P.; Ngachan, S.V. First Report of Root and Collar Rot of Strawberry (Fragaria × ananassa) Caused by Ceratobasidium sp. AG-B(o) (Binucleate Rhizoctonia) in India. Plant Dis. 2017, 102, 1035. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Ma, T.; Yao, X.; Yao, Z.; Wang, Z.; Dong, Z.; Luo, M.; Huang, L. Structure of Endophytes in the Root, Stem, and Leaf Tissues of Sweetpotato and Their Response to Sweetpotato Scab Disease Caused by Elsinoë batatas. Agronomy 2023, 13, 2965. https://doi.org/10.3390/agronomy13122965
Wang S, Ma T, Yao X, Yao Z, Wang Z, Dong Z, Luo M, Huang L. Structure of Endophytes in the Root, Stem, and Leaf Tissues of Sweetpotato and Their Response to Sweetpotato Scab Disease Caused by Elsinoë batatas. Agronomy. 2023; 13(12):2965. https://doi.org/10.3390/agronomy13122965
Chicago/Turabian StyleWang, Shixin, Tingting Ma, Xiaojian Yao, Zhufang Yao, Zhangying Wang, Zhangyong Dong, Mei Luo, and Lifei Huang. 2023. "Structure of Endophytes in the Root, Stem, and Leaf Tissues of Sweetpotato and Their Response to Sweetpotato Scab Disease Caused by Elsinoë batatas" Agronomy 13, no. 12: 2965. https://doi.org/10.3390/agronomy13122965






