The Response of Soil Bacterial Communities to Cropping Systems in Saline–Alkaline Soil in the Songnen Plain
Abstract
:1. Introduction
2. Material and Methods
2.1. Experimental Site
2.2. Experimental Design and Sample Collection
2.3. Determination of Soil Chemical Properties
2.4. Miseq Sequencing
2.5. Bioinformatics Analysis
2.6. Statistical Analysis
3. Results
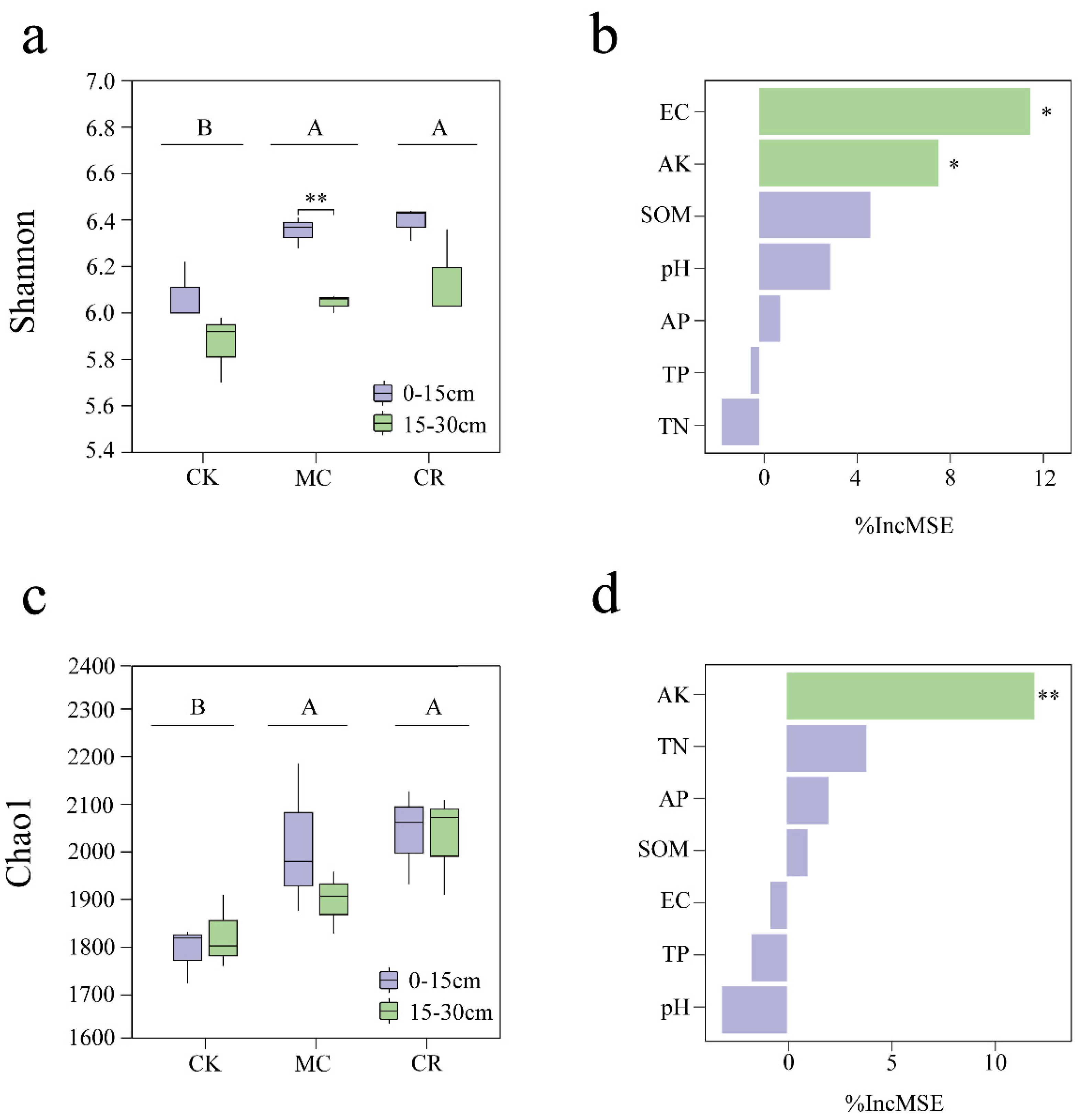
3.1. Bacterial Community Diversity
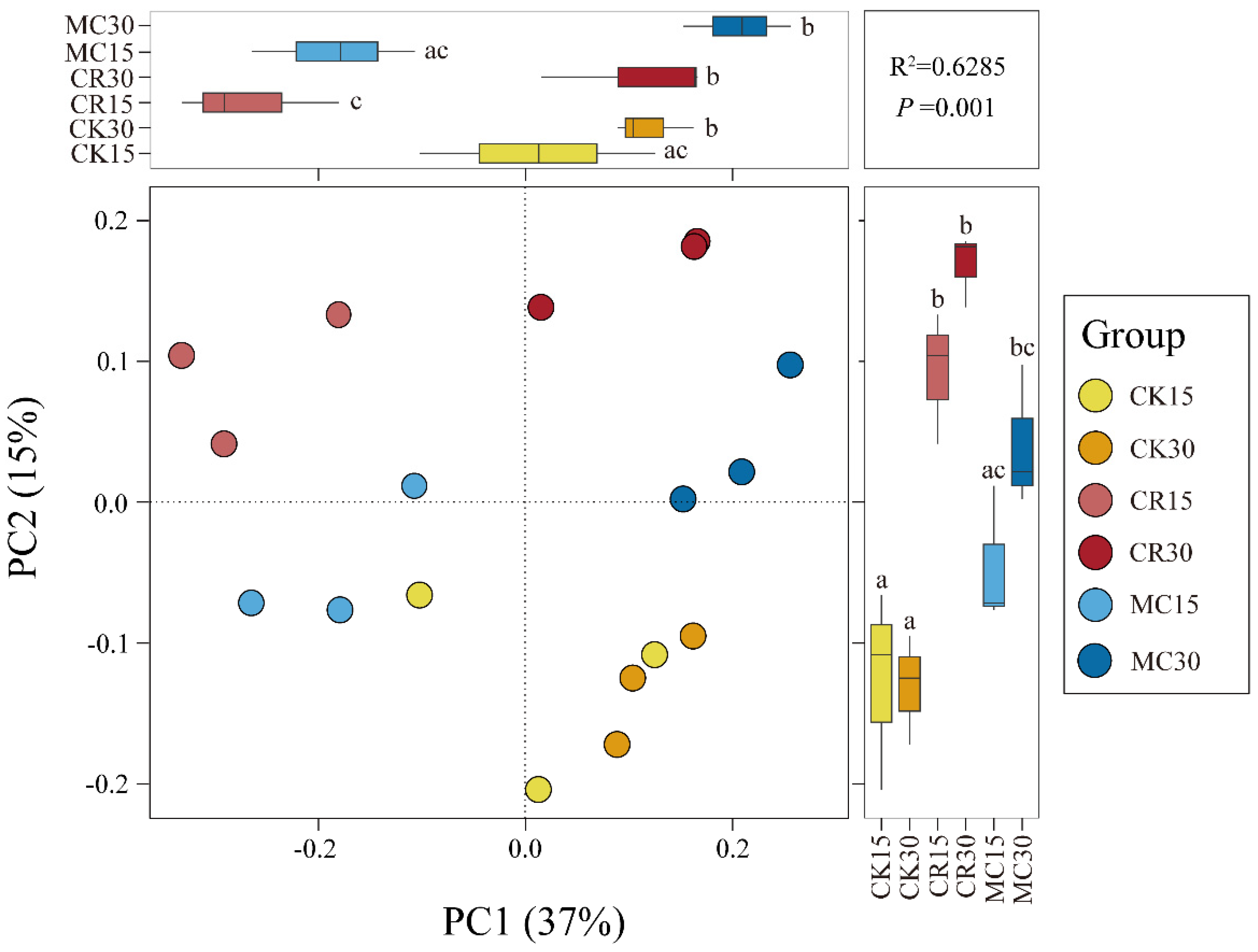
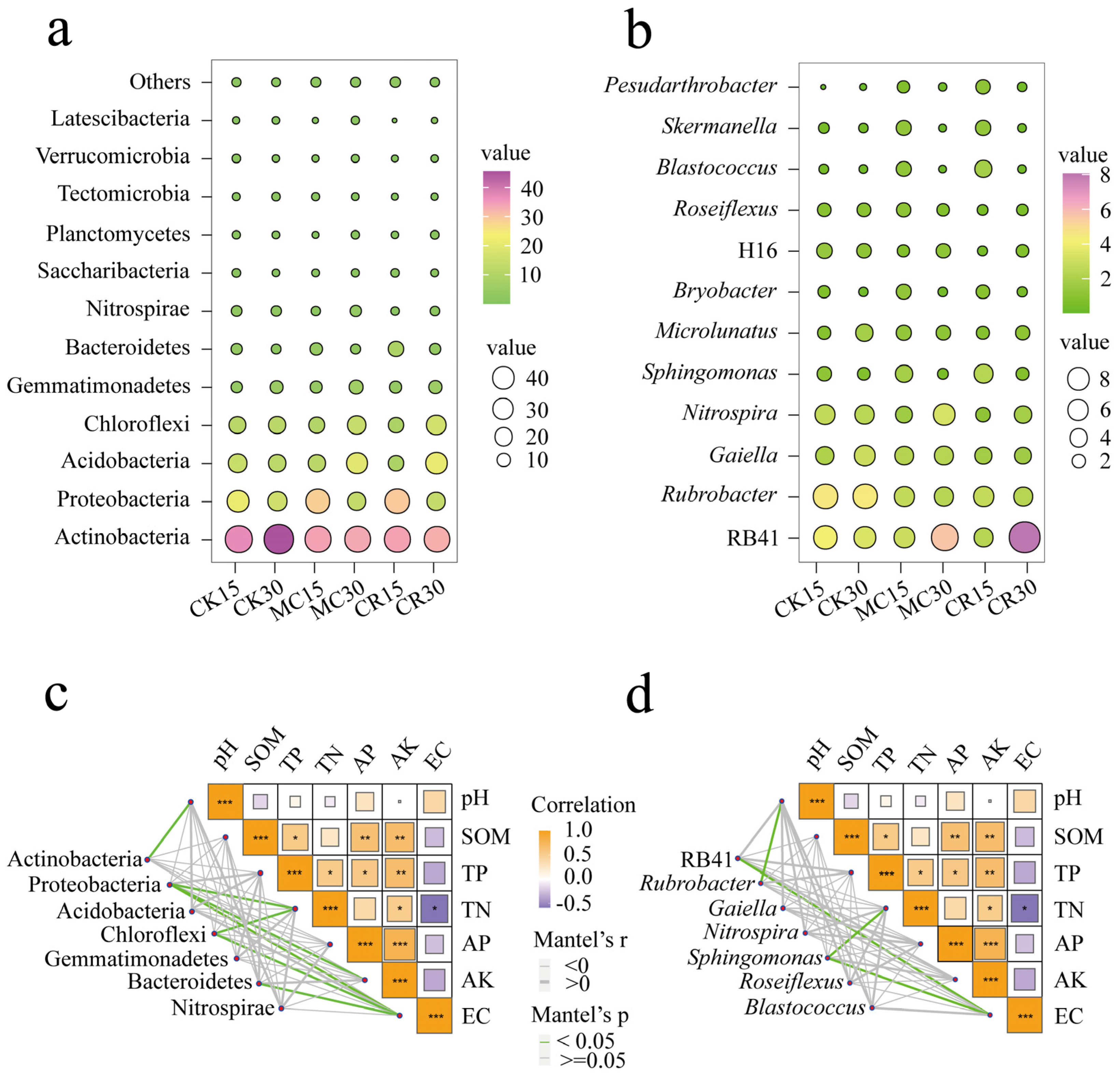
3.2. Bacterial Community Composition
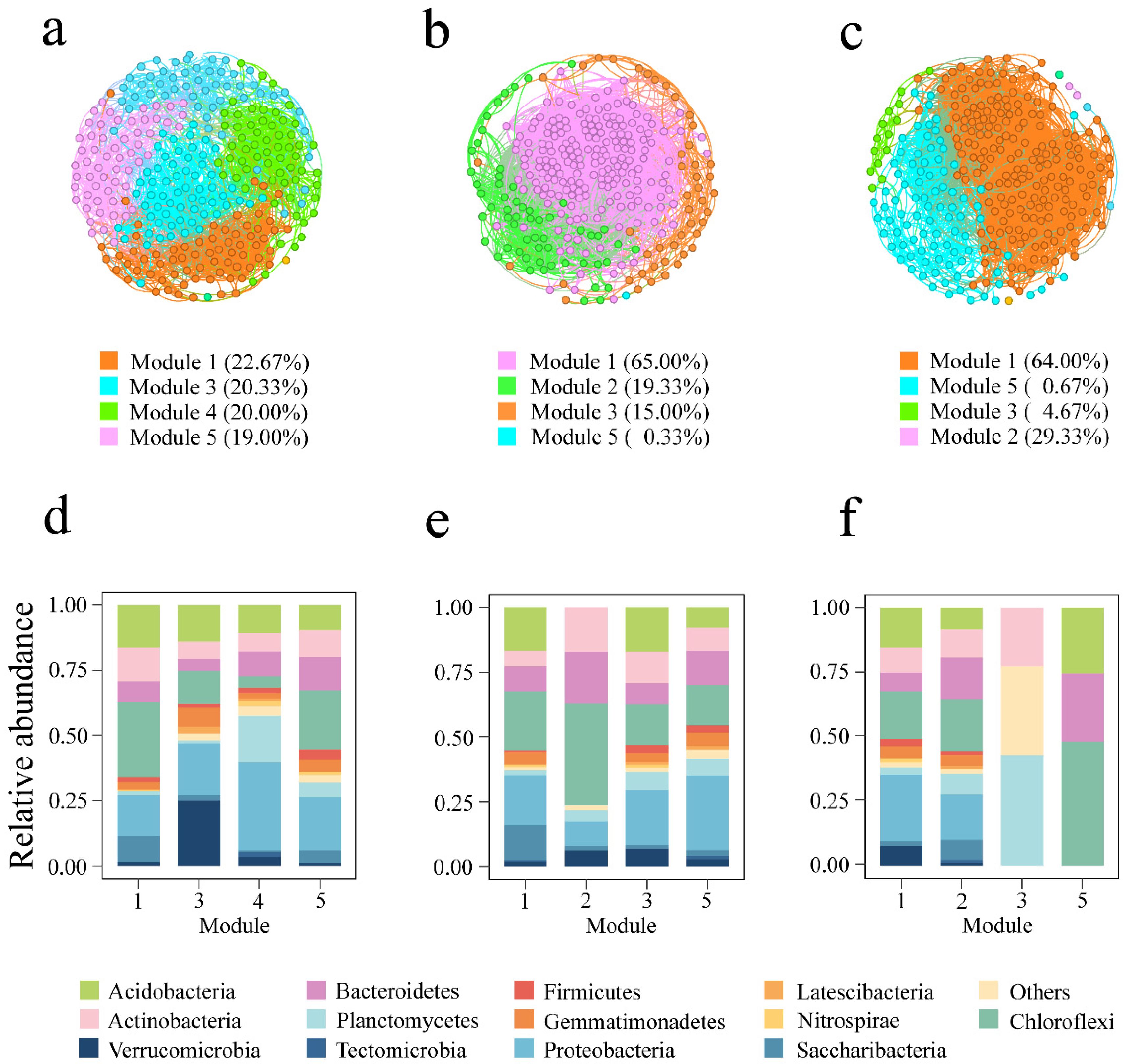
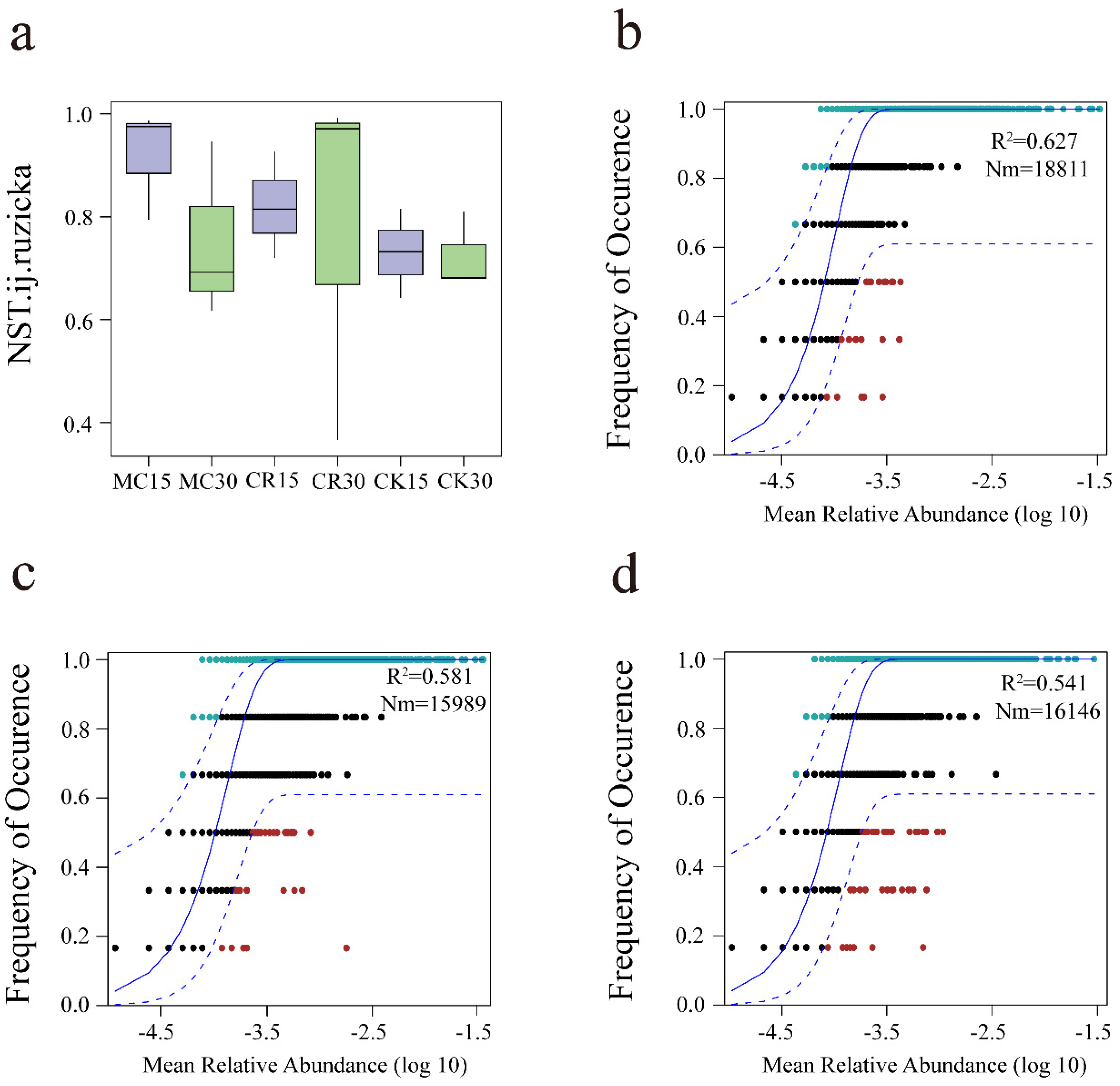
3.3. Co-Occurrence Network Patterns of the Soil Bacterial Community
3.4. The Assembly Process of Soil Bacterial Communities
3.5. Function Prediction of the Soil Bacterial Community
4. Discussion
4.1. Effects of Cropping Systems on Soil Bacteria Diversity
4.2. Effects of Cropping Systems on Soil Bacterial Community Composition
4.3. Effects of Cropping Systems on the Soil Bacterial Community Co-Occurrence Network
4.4. Effects of Cropping Systems on the Soil Bacterial Community Assembly Process
4.5. Effects of Cropping Systems on Soil Bacterial Community Function Prediction
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, J.; Ding, Q.; Wang, Y.; He, M.; Jia, K. Soil quality assessment and constraint diagnosis of salinized farmland in the Yellow River irrigation area in northwestern China. Geoderma Reg. 2023, 34, e00684. [Google Scholar] [CrossRef]
- Mao, W.; Kang, S.; Wan, Y.; Sun, Y.; Li, X.; Wang, Y. Yellow River Sediment as a Soil Amendment for Amelioration of Saline Land in the Yellow River Delta. Land Degrad. Dev. 2014, 27, 1595–1602. [Google Scholar] [CrossRef]
- Wong, V.; Greene, R.; Dalal, R.; Murphy, B.W. Soil carbon dynamics in saline and sodic soils: A review. Soil Use Manag. 2009, 26, 2–11. [Google Scholar] [CrossRef]
- Yan, W.; Bai, R.; Wang, S.; Tian, X.; Li, Y.; Wang, S.; Yang, F.; Xiao, Y.; Lu, X.; Zhao, F. Antibiotic resistance genes are increased by combined exposure to sulfamethoxazole and naproxen but relieved by low-salinity. Environ. Int. 2020, 139, 105742. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Sun, J. Soil Salinity Drives the Distribution Patterns and Ecological Functions of Fungi in Saline-alkaline Land in the Yellow River Delta, China. Front. Microbiol. 2020, 11, 594284. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Bahadur, A.; Sajjad, W.; Wu, X.; Zhang, G.; Liu, G.; Chen, T. Seasonal Variation in Fungal Community Composition Associated with Tamarix chinensis Roots in the Coastal Saline Soil of Bohai Bay, China. Microb. Ecol. 2021, 82, 652–665. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, P.; Kumar, R. Soil salinity: A serious environmental issue and plant growth promoting bacteria as one of the tools for its alleviation. Saudi J. Biol. Sci. 2015, 22, 123–131. [Google Scholar] [CrossRef]
- Zhao, S.; Zhang, Q.; Liu, M.; Zhou, H.; Ma, C.; Wang, P. Regulation of Plant Responses to Salt Stress. Int. J. Mol. Sci. 2021, 22, 4609. [Google Scholar] [CrossRef]
- Srivastava, P.; Gupta, M.; Shikha; Singh, N.; Tewari, S. Amelioration of Sodic Soil for Wheat Cultivation Using Bioaugmented Organic Soil Amendment. Land Degrad. Dev. 2014, 27, 1245–1254. [Google Scholar] [CrossRef]
- Mbarki, S.; Cerdà, A.; Zivcak, M.; Brestic, M.; Rabhi, M.; Mezni, M.; Jedidi, N.; Abdelly, C.; Pascual, J.A. Alfalfa crops amended with MSW compost can compensate the effect of salty water irrigation depending on the soil texture. Process Saf. Environ. Prot. 2018, 115, 8–16. [Google Scholar] [CrossRef]
- Li, K.-S.; Li, Q.; Geng, Y.; Liu, C. An evaluation of the effects of microstructural characteristics and frost heave on the remediation of saline-alkali soils in the Yellow River Delta, China. Land Degrad. Dev. 2020, 32, 1325–1337. [Google Scholar] [CrossRef]
- Cheng, Z.; Chen, Y.; Zhang, F. Effect of cropping systems after abandoned salinized farmland reclamation on soil bacterial communities in arid northwest China. Soil Tillage Res. 2019, 187, 204–213. [Google Scholar] [CrossRef]
- Williams, A.; Birt, H.W.G.; Raghavendra, A.; Dennis, P.G. Cropping Systems Diversification Influences Soil Microbial Diversity in Subtropical Dryland Farming Systems. Microb. Ecol. 2023, 85, 1473–1484. [Google Scholar] [CrossRef]
- Jarecki, M.; Grant, B.; Smith, W.; Deen, B.; Drury, C.; VanderZaag, A.; Qian, B.; Yang, J.; Wagner-Riddle, C. Long-term Trends in Corn Yields and Soil Carbon under Diversified Crop Rotations. J. Environ. Qual. 2018, 47, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Agomoh, I.; Drury, C.; Phillips, L.; Reynolds, D.; Yang, X. Increasing crop diversity in wheat rotations increases yields but decreases soil health. Soil Sci. Soc. Am. J. 2020, 84, 170–181. [Google Scholar] [CrossRef]
- Stanger, T.F.; Lauer, J.G. Corn Grain Yield Response to Crop Rotation and Nitrogen over 35 Years. Agron. J. 2008, 100, 643–650. [Google Scholar] [CrossRef]
- Esmaeilzadeh-Salestani, K.; Bahram, M.; Ghanbari Moheb Seraj, R.; Gohar, D.; Tohidfar, M.; Eremeev, V.; Talgre, L.; Khaleghdoust, B.; Mirmajlessi, S.M.; Luik, A.; et al. Cropping systems with higher organic carbon promote soil microbial diversity. Agric. Ecosyst. Environ. 2021, 319, 107521. [Google Scholar] [CrossRef]
- Liu, C.L.C.; Kuchma, O.; Krutovsky, K.V. Mixed-species versus monocultures in plantation forestry: Development, benefits, ecosystems services and perspectives for the future. Glob. Ecol. Conserv. 2018, 15, e00419. [Google Scholar] [CrossRef]
- Ishaq, S.L.; Johnson, S.P.; Miller, Z.J.; Lehnhoff, E.A.; Olivo, S.; Yeoman, C.J.; Menalled, F.D. Impact of Cropping Systems, Soil Inoculum, and Plant Species Identity on Soil Bacterial Community Structure. Microb. Ecol. 2017, 73, 417–434. [Google Scholar] [CrossRef]
- Chen, G.; Hu, Y.-G.; Lin, Y.-C.; Lu, J.-S.; Wang, X.; Yuan, X.-L.; Zeng, Z.-H.; Zhu, B. Effects of oat mixed with common vetch on the microorganism populations in rhizosphere soil. Acta Prataculturae Sin. 2009, 18, 151–157. [Google Scholar]
- Xuan, D.T.; Guong, V.T.; Rosling, A.; Alström, S.; Chai, B.; Högberg, N. Different crop rotation systems as drivers of change in soil bacterial community structure and yield of rice, Oryza sativa. Biol. Fertil. Soils 2012, 48, 217–225. [Google Scholar] [CrossRef]
- Gorbacheva, M.A.; Melnikova, N.V.; Chechetkin, V.R.; Kravatsky, Y.V.; Tchurikov, N.A. DNA sequencing and metagenomics of cultivated and uncultivated chernozems in Russia. Geoderma Reg. 2018, 14, e00180. [Google Scholar] [CrossRef]
- Li, C.; Li, X.; Kong, W.; Wu, Y.; Wang, J. Effect of monoculture soybean on soil microbial community in the Northeast China. Plant Soil 2010, 330, 423–433. [Google Scholar] [CrossRef]
- Lienhard, P.; Terrat, S.; Prévost-Bouré, N.C.; Nowak, V.; Régnier, T.; Sayphoummie, S.; Panyasiri, K.; Tivet, F.; Mathieu, O.; Levêque, J.; et al. Pyrosequencing evidences the impact of cropping on soil bacterial and fungal diversity in Laos tropical grassland. Agron. Sustain. Dev. 2014, 34, 525–533. [Google Scholar] [CrossRef]
- Lladó, S.; López-Mondéjar, R.; Baldrian, P. Forest Soil Bacteria: Diversity, Involvement in Ecosystems Processes, and Response to Global Change. Microbiol. Mol. Biol. Rev. 2017, 81, e00063-16. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Liang, K.; Huang, G.; Wang, X.; Lin, M.; Chen, Y.; Zhou, Z. Soil Bacterial Community Shifts Are Driven by Soil Nutrient Availability along a Teak Plantation Chronosequence in Tropical Forests in China. Biology 2021, 10, 1329. [Google Scholar] [CrossRef] [PubMed]
- Lupi, A.; Steinbach, H.S.; Ciarlo, E.; Romaniuk, R.; Cosentino, V.R.N.; Rimski-Korsakov, H.; Alvarez, C.R. Organic carbon stored in soils under different land uses and soil textures in southeast Argentinean Mesopotamia. Geoderma Reg. 2021, 27, e00435. [Google Scholar] [CrossRef]
- Zhang, K.; Shi, Y.; Cui, X.; Yue, P.; Li, K.; Liu, X.; Tripathi, B.M.; Chu, H. Salinity Is a Key Determinant for Soil Microbial Communities in a Desert Ecosystems. mSystems 2019, 4, e00225-18. [Google Scholar] [CrossRef]
- Fang, J.; Deng, Y.; Che, R.; Han, C.; Zhong, W. Bacterial community composition in soils covered by different vegetation types in the Yancheng tidal marsh. Environ. Sci. Pollut. Res. 2020, 27, 21517–21532. [Google Scholar] [CrossRef]
- Alizadeh, H.; Kandula, D.R.W.; Hampton, J.G.; Stewart, A.; Leung, D.W.M.; Edwards, Y.; Smith, C. Urease producing microorganisms under dairy pasture management in soils across New Zealand. Geoderma Reg. 2017, 11, 78–85. [Google Scholar] [CrossRef]
- Bremner, J.M.; Shaw, K. Denitrification in soil. I. Methods of investigation. J. Agric. Sci. 1958, 51, 22–39. [Google Scholar] [CrossRef]
- Olsen, S.R. Estimation of Available Phosphorus in Soils by Extraction with Sodium Bicarbonate; US Department of Agriculture: Washington, DC, USA, 1954.
- Walker, J.M.; Barber, S.A. Absorption of potassium and rubidium from the soil by corn roots. Plant Soil 1962, 17, 243–259. [Google Scholar] [CrossRef]
- Wolf, A.M.A.; Baker, D.E. Comparisons of soil test phosphorus by Olsen, Bray P1, Mehlich I and Mehlich III methods. Commun. Soil Sci. Plant Anal. 1985, 16, 467–484. [Google Scholar] [CrossRef]
- Mori, H.; Maruyama, F.; Kato, H.; Toyoda, A.; Dozono, A.; Ohtsubo, Y.; Nagata, Y.; Fujiyama, A.; Tsuda, M.; Kurokawa, K. Design and Experimental Application of a Novel Non-Degenerate Universal Primer Set that Amplifies Prokaryotic 16S rRNA Genes with a Low Possibility to Amplify Eukaryotic rRNA Genes. DNA Res. 2013, 21, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.-J.; Li, J.-H.; Ross Friedman, C.; Wang, H.-F. Variation of Soil Bacterial Communities in a Chronosequence of Rubber Tree (Hevea brasiliensis) Plantations. Front. Plant Sci. 2017, 8, 849. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Liu, J.; Wu, F. Soil microbial communities in cucumber monoculture and rotation systems and their feedback effects on cucumber seedling growth. Plant Soil 2017, 415, 507–520. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [PubMed]
- Schloss, P.D.; Westcott, S.L.; Ryabin, T.; Hall, J.R.; Hartmann, M.; Hollister, E.B.; Lesniewski, R.A.; Oakley, B.B.; Parks, D.H.; Robinson, C.J.; et al. Introducing mothur: Open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microbiol. 2009, 75, 7537–7541. [Google Scholar] [CrossRef]
- Liu, W.; Qiu, K.; Xie, Y.; Huang, Y.; Wang, R.; Li, H.; Meng, W.; He, Y.; Li, Y.; Li, H.; et al. High-Throughput Absolute Quantification Sequencing Reveals that a Combination of Leguminous Shrubs Is Effective in Driving Soil Bacterial Diversity During the Process of Desertification Reversal. Microb. Ecol. 2023, 86, 1145–1163. [Google Scholar] [CrossRef]
- Mendes, L.W.; Raaijmakers, J.M.; de Hollander, M.; Mendes, R.; Tsai, S.M. Influence of resistance breeding in common bean on rhizosphere microbiome composition and function. ISME J. 2018, 12, 212–224. [Google Scholar] [CrossRef]
- Burns, A.R.; Stephens, W.Z.; Stagaman, K.; Wong, S.; Rawls, J.F.; Guillemin, K.; Bohannan, B.J.M. Contribution of neutral processes to the assembly of gut microbial communities in the zebrafish over host development. ISME J. 2016, 10, 655–664. [Google Scholar] [CrossRef] [PubMed]
- Ning, D.; Deng, Y.; Tiedje, J.M.; Zhou, J. A general framework for quantitatively assessing ecological stochasticity. Proc. Natl. Acad. Sci. USA 2019, 116, 16892–16898. [Google Scholar] [CrossRef] [PubMed]
- Deepak, G.; Yogeshvari, K.; Harsha, N.; Rajababu, V. Microorganisms for Green Revolution Volume 2: Microbes for Sustainable Agro-Ecosystems; Department of Agricultural Microbiology, BA College of Agriculture Anand Agricultural University Anand: Gujarat, India, 2018. [Google Scholar]
- Song, B.; Li, Y.; Yang, L.; Shi, H.; Li, L.; Bai, W.; Zhao, Y. Soil Acidification Under Long-Term N Addition Decreases the Diversity of Soil Bacteria and Fungi and Changes Their Community Composition in a Semiarid Grassland. Microb. Ecol. 2023, 85, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Luo, G.; Jin, T.; Zhang, H.; Peng, J.; Zuo, N.; Huang, Y.; Han, Y.; Tian, C.; Yang, Y.; Peng, K.; et al. Deciphering the diversity and functions of plastisphere bacterial communities in plastic-mulching croplands of subtropical China. J. Hazard. Mater. 2022, 422, 126865. [Google Scholar] [CrossRef] [PubMed]
- Luo, G.; Xue, C.; Jiang, Q.; Xiao, Y.; Zhang, F.; Guo, S.; Shen, Q.; Ling, N. Soil Carbon, Nitrogen, and Phosphorus Cycling Microbial Populations and Their Resistance to Global Change Depend on Soil C:N:P Stoichiometry. mSystems 2020, 5, e00162-20. [Google Scholar] [CrossRef] [PubMed]
- Woo, S.L.; De Filippis, F.; Zotti, M.; Vandenberg, A.; Hucl, P.; Bonanomi, G. Pea-Wheat Rotation Affects Soil Microbiota Diversity, Community Structure, and Soilborne Pathogens. Microorganisms 2022, 10, 370. [Google Scholar] [CrossRef] [PubMed]
- Venter, Z.S.; Jacobs, K.; Hawkins, H.-J. The impact of crop rotation on soil microbial diversity: A meta-analysis. Pedobiologia 2016, 59, 215–223. [Google Scholar] [CrossRef]
- Lavelle, P. Invertebrate biodiversity as bioindicators of sustainable landscapes. Plant Sci. 2001, 160, 1069–1070. [Google Scholar] [CrossRef]
- Benitez, M.-S.; Ewing, P.M.; Osborne, S.L.; Lehman, R.M. Rhizosphere microbial communities explain positive effects of diverse crop rotations on maize and soybean performance. Soil Biol. Biochem. 2021, 159, 108309. [Google Scholar] [CrossRef]
- Liu, B.; Arlotti, D.; Huyghebaert, B.; Tebbe, C.C. Disentangling the impact of contrasting agricultural management practices on soil microbial communities—Importance of rare bacterial community members. Soil Biol. Biochem. 2022, 166, 108573. [Google Scholar] [CrossRef]
- Kuerban, M.; Cong, W.-F.; Jing, J.; Bezemer, T.M. Microbial soil legacies of crops under different water and nitrogen levels determine succeeding crop performance. Plant Soil 2023, 485, 167–180. [Google Scholar] [CrossRef]
- Brockett, B.F.T.; Prescott, C.E.; Grayston, S.J. Soil moisture is the major factor influencing microbial community structure and enzyme activities across seven biogeoclimatic zones in western Canada. Soil. Biol. Biochem. 2012, 44, 9–20. [Google Scholar] [CrossRef]
- Song, X.; Tao, B.; Guo, J.; Li, J.; Chen, G. Changes in the Microbial Community Structure and Soil Chemical Properties of Vertisols under Different Cropping Systems in Northern China. Front. Environ. Sci. 2018, 6, 132. [Google Scholar] [CrossRef]
- Thomas, G.A.; Dalal, R.C.; Standley, J. No-till effects on organic matter, pH, cation exchange capacity and nutrient distribution in a Luvisol in the semi-arid subtropics. Soil Tillage Res. 2007, 94, 295–304. [Google Scholar] [CrossRef]
- Xu, B.; Yang, G.; Lehmann, A.; Riedel, S.; Rillig, M.C. Effects of perfluoroalkyl and polyfluoroalkyl substances (PFAS) on soil structure and function. Soil Ecol. Lett. 2023, 5, 108–117. [Google Scholar] [CrossRef]
- Xu, R.; Tao, W.; Lin, H.; Huang, D.; Su, P.; Gao, P.; Sun, X.; Yang, Z.; Sun, W. Effects of Perfluorooctanoic Acid (PFOA) and Perfluorooctane Sulfonic Acid (PFOS) on Soil Microbial Community. Microb. Ecol. 2022, 83, 929–941. [Google Scholar] [CrossRef]
- Rath, K.M.; Fierer, N.; Murphy, D.V.; Rousk, J. Linking bacterial community composition to soil salinity along environmental gradients. ISME J. 2019, 13, 836–846. [Google Scholar] [CrossRef]
- Wang, S.; Sun, L.; Ling, N.; Zhu, C.; Chi, F.; Li, W.; Hao, X.; Zhang, W.; Bian, J.; Chen, L.; et al. Exploring Soil Factors Determining Composition and Structure of the Bacterial Communities in Saline-alkaline Soils of Songnen Plain. Front. Microbiol. 2020, 10, 2902. [Google Scholar] [CrossRef]
- Yan, N.; Marschner, P.; Cao, W.; Zuo, C.; Qin, W. Influence of salinity and water content on soil microorganisms. Int. Soil Water Conserv. Res. 2015, 3, 316–323. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, Z.; Jia, H.; Li, L.; Wu, F. Continuously Monocropped Jerusalem Artichoke Changed Soil Bacterial Community Composition and Ammonia-Oxidizing and Denitrifying Bacteria Abundances. Front. Microbiol. 2018, 9, 705. [Google Scholar] [CrossRef]
- Liu, J.; Shu, A.; Song, W.; Shi, W.; Li, M.; Zhang, W.; Li, Z.; Liu, G.; Yuan, F.; Zhang, S.; et al. Long-term organic fertilizer substitution increases rice yield by improving soil properties and regulating soil bacteria. Geoderma 2021, 404, 115287. [Google Scholar] [CrossRef]
- Li, Z.; Jiao, Y.; Yin, J.; Li, D.; Wang, B.; Zhang, K.; Zheng, X.; Hong, Y.; Zhang, H.; Xie, C.; et al. Productivity and quality of banana in response to chemical fertilizer reduction with bio-organic fertilizer: Insight into soil properties and microbial ecology. Agric. Ecosyst. Environ. 2021, 322, 107659. [Google Scholar] [CrossRef]
- Fischer, C.; Thürmer, A.; Wollherr, A.; Nacke, H.; Herold, N.; Schrumpf, M.; Gutknecht, J.; Wubet, T.; Buscot, F.; Daniel, R. Horizon-Specific Bacterial Community Composition of German Grassland Soils, as Revealed by Pyrosequencing-Based Analysis of 16S rRNA Genes. Appl. Environ. Microbiol. 2010, 76, 6751–6759. [Google Scholar]
- Zhang, L.; Huang, W.; Xiao, W.; Hu, D.; Shao, J.; Yao, B. Comparison of soil enzyme activity and microbial community structure between rapeseed-rice and rice-rice plantings. Int. J. Agric. Biol. 2018, 20, 1801–1808. [Google Scholar]
- Wagg, C.; Hautier, Y.; Pellkofer, S.; Banerjee, S.; Schmid, B.; van der Heijden, M.G. Diversity and asynchrony in soil microbial communities stabilizes ecosystems functioning. eLife 2021, 10, e62813. [Google Scholar] [CrossRef] [PubMed]
- Goldfarb, K.C.; Karaoz, U.; Hanson, C.A.; Santee, C.A.; Bradford, M.A.; Treseder, K.K.; Wallenstein, M.D.; Brodie, E.L. Differential growth responses of soil bacterial taxa to carbon substrates of varying chemical recalcitrance. Front. Microbiol. 2011, 2, 94. [Google Scholar] [CrossRef]
- Griffith, J.C.; Lee, W.G.; Orlovich, D.A.; Summerfield, T.C. Contrasting bacterial communities in two indigenous Chionochloa (Poaceae) grassland soils in New Zealand. PLoS ONE 2017, 12, e0179652. [Google Scholar] [CrossRef]
- Fierer, N.; Allen, A.; Schimel, J.; Holden, P. Controls on microbial CO2 production: A comparison of surface and subsurface soil horizons. Glob. Chang. Biol. 2003, 9, 1322–1332. [Google Scholar] [CrossRef]
- Fierer, N.; Morse, J.L.; Berthrong, S.T.; Bernhardt, E.S.; Jackson, R.B. Environmental controls on the landscape-scale biogeography of stream bacterial communities. Ecology 2007, 88, 2162–2173. [Google Scholar] [CrossRef]
- Kim, J.M.; Roh, A.-S.; Choi, S.-C.; Kim, E.-J.; Choi, M.-T.; Ahn, B.-K.; Kim, S.-K.; Lee, Y.-H.; Joa, J.-H.; Kang, S.-S.; et al. Soil pH and electrical conductivity are key edaphic factors shaping bacterial communities of greenhouse soils in Korea. J. Microbiol. 2016, 54, 838–845. [Google Scholar] [CrossRef]
- Li, X.; Wang, A.; Wan, W.; Luo, X.; Zheng, L.; He, G.; Huang, D.; Chen, W.; Huang, Q. High Salinity Inhibits Soil Bacterial Community Mediating Nitrogen Cycling. Appl. Environ. Microbiol. 2021, 87, e0136621. [Google Scholar] [CrossRef]
- Li, X.; Meng, D.; Li, J.; Yin, H.; Liu, H.; Liu, X.; Cheng, C.; Xiao, Y.; Liu, Z.; Yan, M. Response of soil microbial communities and microbial interactions to long-term heavy metal contamination. Environ. Pollut. 2017, 231, 908–917. [Google Scholar] [CrossRef]
- Samaei-Nouroozi, A.; Rezaei, S.; Khoshnevis, N.; Doosti, M.; Hajihoseini, R.; Khoshayand, M.R.; Faramarzi, M.A. Medium-based optimization of an organic solvent-tolerant extracellular lipase from the isolated halophilic Alkalibacillus salilacus. Extremophiles 2015, 19, 933–947. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, N.; Marschner, P.; Burns, R.G. Soil microbial activity and community composition: Impact of changes in matric and osmotic potential. Soil Biol. Biochem. 2011, 43, 1229–1236. [Google Scholar] [CrossRef]
- Wichern, J.; Wichern, F.; Joergensen, R.G. Impact of salinity on soil microbial communities and the decomposition of maize in acidic soils. Geoderma 2006, 137, 100–108. [Google Scholar] [CrossRef]
- Hansel, C.M.; Fendorf, S.; Jardine, P.M.; Francis, C.A. Changes in bacterial and archaeal community structure and functional diversity along a geochemically variable soil profile. Appl. Environ. Microbiol. 2008, 74, 1620–1633. [Google Scholar] [CrossRef] [PubMed]
- Schlatter, D.C.; Kahl, K.; Carlson, B.; Huggins, D.R.; Paulitz, T. Soil acidification modifies soil depth-microbiome relationships in a no-till wheat cropping systems. Soil Biol. Biochem. 2020, 149, 107939. [Google Scholar] [CrossRef]
- Battistuzzi, F.U.; Hedges, S.B. A major clade of prokaryotes with ancient adaptations to life on land. Mol. Biol. Evol. 2009, 26, 335–343. [Google Scholar] [CrossRef]
- Trivedi, P.; Anderson, I.C.; Singh, B.K. Microbial modulators of soil carbon storage: Integrating genomic and metabolic knowledge for global prediction. Trends Microbiol. 2013, 21, 641–651. [Google Scholar] [CrossRef]
- Rime, T.; Hartmann, M.; Brunner, I.; Widmer, F.; Zeyer, J.; Frey, B. Vertical distribution of the soil microbiota along a successional gradient in a glacier forefield. Mol. Ecol. 2015, 24, 1091–1108. [Google Scholar] [CrossRef]
- Mujakić, I.; Piwosz, K.; Koblížek, M. Phylum Gemmatimonadota and Its Role in the Environment. Microorganisms 2022, 10, 151. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chu, L.; Ma, J.; Chi, G.; Lu, C.; Chen, X. Effects of multiple antibiotics residues in broiler manure on composting process. Sci. Total Environ. 2022, 817, 152808. [Google Scholar] [CrossRef] [PubMed]
- Faust, K.; Raes, J. Microbial interactions: From networks to models. Nat. Rev. Microbiol. 2012, 10, 538–550. [Google Scholar] [CrossRef]
- Banerjee, S.; Schlaeppi, K.; van der Heijden, M.G.A. Keystone taxa as drivers of microbiome structure and functioning. Nat. Rev. Microbiol. 2018, 16, 567–576. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Hu, H.-W.; Yang, G.-W.; Cui, Z.-L.; Chen, Y.-L. Crop rotational diversity enhances soil microbiome network complexity and multifunctionality. Geoderma 2023, 436, 116562. [Google Scholar] [CrossRef]
- Steinauer, K.; Chatzinotas, A.; Eisenhauer, N. Root exudate cocktails: The link between plant diversity and soil microorganisms? Ecol. Evol. 2016, 6, 7387–7396. [Google Scholar] [CrossRef]
- D’Acunto, L.; Andrade, J.F.; Poggio, S.L.; Semmartin, M. Diversifying crop rotation increased metabolic soil diversity and activity of the microbial community. Agric. Ecosyst. Environ. 2018, 257, 159–164. [Google Scholar] [CrossRef]
- Wagg, C.; Schlaeppi, K.; Banerjee, S.; Kuramae, E.E.; van der Heijden, M.G.A. Fungal-bacterial diversity and microbiome complexity predict ecosystems functioning. Nat. Commun. 2019, 10, 4841. [Google Scholar] [CrossRef]
- Qiu, L.; Zhang, Q.; Zhu, H.; Reich, P.B.; Banerjee, S.; van der Heijden, M.G.A.; Sadowsky, M.J.; Ishii, S.; Jia, X.; Shao, M.; et al. Erosion reduces soil microbial diversity, network complexity and multifunctionality. ISME J. 2021, 15, 2474–2489. [Google Scholar] [CrossRef]
- Griffiths, B.S.; Philippot, L. Insights into the resistance and resilience of the soil microbial community. FEMS Microbiol. Rev. 2013, 37, 112–129. [Google Scholar] [CrossRef]
- Chase, J.M. Stochastic community assembly causes higher biodiversity in more productive environments. Science 2010, 328, 1388–1391. [Google Scholar] [CrossRef] [PubMed]
- Santolini, M.; Barabási, A.-L. Predicting perturbation patterns from the topology of biological networks. Proc. Natl. Acad. Sci. USA 2018, 115, E6375–E6383. [Google Scholar] [CrossRef]
- Landi, P.; Minoarivelo, H.; Brännström, Å.; Hui, C.; Dieckmann, U. Complexity and Stability of Adaptive Ecological Networks: A Survey of the Theory in Community Ecology. In Systems Analysis Approach for Complex Global Challenges; Springer: Cham, Switzerland, 2018; pp. 209–248. [Google Scholar]
- Allison, S.D.; Martiny, J.B. Colloquium paper: Resistance, resilience, and redundancy in microbial communities. Proc. Natl. Acad. Sci. USA 2008, 105 (Suppl. S1), 11512–11519. [Google Scholar] [CrossRef] [PubMed]
- Fierer, N.; Bradford, M.A.; Jackson, R.B. Toward an ecological classification of soil bacteria. Ecology 2007, 88, 1354–1364. [Google Scholar] [CrossRef] [PubMed]
- Davis, K.E.; Sangwan, P.; Janssen, P.H. Acidobacteria, Rubrobacteridae and Chloroflexi are abundant among very slow-growing and mini-colony-forming soil bacteria. Environ. Microbiol. 2011, 13, 798–805. [Google Scholar] [CrossRef] [PubMed]
- Eo, J.; Park, K.-C. Long-term effects of imbalanced fertilization on the composition and diversity of soil bacterial community. Agric. Ecosyst. Environ. 2016, 231, 176–182. [Google Scholar] [CrossRef]
- Zhang, K.; Maltais-Landry, G.; Liao, H.-L. How soil biota regulate C cycling and soil C pools in diversified crop rotations. Soil Biol. Biochem. 2021, 156, 108219. [Google Scholar] [CrossRef]
- Shi, Y.; Li, Y.; Xiang, X.; Sun, R.; Yang, T.; He, D.; Zhang, K.; Ni, Y.; Zhu, Y.-G.; Adams, J.M.; et al. Spatial scale affects the relative role of stochasticity versus determinism in soil bacterial communities in wheat fields across the North China Plain. Microbiome 2018, 6, 27. [Google Scholar] [CrossRef]
- Zhao, J.; Gao, Q.; Zhou, J.; Wang, M.; Liang, Y.; Sun, B.; Chu, H.; Yang, Y. The scale dependence of fungal community distribution in paddy soil driven by stochastic and deterministic processes. Fungal Ecol. 2019, 42, 100856. [Google Scholar] [CrossRef]
- Jiao, S.; Yang, Y.; Xu, Y.; Zhang, J.; Lu, Y. Balance between community assembly processes mediates species coexistence in agricultural soil microbiomes across eastern China. ISME J. 2020, 14, 202–216. [Google Scholar] [CrossRef]
- Shi, Y.; Dang, K.; Dong, Y.; Feng, M.; Wang, B.; Li, J.; Chu, H. Soil fungal community assembly processes under long-term fertilization. Eur. J. Soil Sci. 2019, 71, 716–726. [Google Scholar] [CrossRef]
- Tripathi, B.M.; Stegen, J.C.; Kim, M.; Dong, K.; Adams, J.M.; Lee, Y.K. Soil pH mediates the balance between stochastic and deterministic assembly of bacteria. ISME J. 2018, 12, 1072–1083. [Google Scholar] [CrossRef] [PubMed]
- Leibold, M.A.; Chase, J.M.; Ernest, S.K. Community assembly and the functioning of ecosystems: How metacommunity processes alter ecosystems attributes. Ecology 2017, 98, 909–919. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Ning, D. Stochastic Community Assembly: Does It Matter in Microbial Ecology? Microbiol. Mol. Biol. Rev. 2017, 81, e00002-17. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Deng, Y.; Zhang, P.; Xue, K.; Liang, Y.; Van Nostrand, J.; Yang, Y.; He, Z.; Wu, L.; Stahl, D.; et al. Stochasticity, succession, and environmental perturbations in a fluidic ecosystems. Proc. Natl. Acad. Sci. USA 2014, 111, E836–E845. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Johnson, N.C.; Mao, L.; Shi, G.; Jiang, S.; Ma, X.; Du, G.; An, L.; Feng, H. Phylogenetic structure of arbuscular mycorrhizal community shifts in response to increasing soil fertility. Soil Biol. Biochem. 2015, 89, 196–205. [Google Scholar] [CrossRef]
- Feng, Y.; Chen, R.; Stegen, J.; Guo, Z.; Zhang, J.; Li, Z.; Lin, X. Two key features influencing community assembly processes at regional scale: Initial state and degree of change in environmental conditions. Mol. Ecol. 2018, 27, 5238–5251. [Google Scholar] [CrossRef]
- Doornbos, R.F.; van Loon, L.C.; Bakker, P.A.H.M. Impact of root exudates and plant defense signaling on bacterial communities in the rhizosphere. A review. Agron. Sustain. Dev. 2012, 32, 227–243. [Google Scholar] [CrossRef]
- Jiang, Y.; Song, H.; Lei, Y.; Korpelainen, H.; Li, C. Distinct co-occurrence patterns and driving forces of rare and abundant bacterial subcommunities following a glacial retreat in the eastern Tibetan Plateau. Biol. Fertil. Soils 2019, 55, 351–364. [Google Scholar] [CrossRef]
- Li, X.; Wan, W.; Zheng, L.; Wang, A.; Luo, X.; Huang, Q.; Chen, W. Community assembly mechanisms and co-occurrence patterns of nitrite-oxidizing bacteria communities in saline soils. Sci. Total Environ. 2021, 772, 145472. [Google Scholar] [CrossRef]
- Liang, S.; Deng, J.; Jiang, Y.; Wu, S.; Zhou, Y.; Zhu, W.-X. Functional Distribution of Bacterial Community under Different Land Use Patterns Based on FaProTax Function Prediction. Pol. J. Environ. Stud. 2019, 29, 1245–1261. [Google Scholar] [CrossRef] [PubMed]
- van der Heijden, M.G.; Bardgett, R.D.; van Straalen, N.M. The unseen majority: Soil microbes as drivers of plant diversity and productivity in terrestrial ecosystems. Ecol. Lett. 2008, 11, 296–310. [Google Scholar] [CrossRef] [PubMed]
- McKinley, K.R.; Wetzel, R.G. Photolithotrophy, photoheterotrophy, and chemoheterotrophy: Patterns of resource utilization on an annual and a diurnal basis within a pelagic microbial community. Microb. Ecol. 1979, 5, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Liu, X.; Liu, J.; Zhang, Z.; Zhang, W.; Qi, Y.; Li, W.; Chen, Y. Changes of soil bacterial community, network structure, and carbon, nitrogen and sulfur functional genes under different land use types. CATENA 2023, 231, 107385. [Google Scholar] [CrossRef]
- Phukongchai, W.; Kaewpradit, W.; Rasche, F. Inoculation of cellulolytic and ligninolytic microorganisms accelerates decomposition of high C/N and cellulose rich sugarcane straw in tropical sandy soils. Appl. Soil Ecol. 2022, 172, 104355. [Google Scholar] [CrossRef]
- Wang, C.; Yao, X.; Li, X.; Wang, Q.; Wang, J.; Zhu, L.; Wang, J. Effects of dibutyl phthalate on microbial community and the carbon cycle in salinized soil. J. Clean. Prod. 2023, 404, 136928. [Google Scholar] [CrossRef]
- Lee, D.Y.; Owens, M.S.; Crump, B.C.; Cornwell, J.C. Elevated microbial CO2 production and fixation in the oxic/anoxic interface of estuarine water columns during seasonal anoxia. Estuar. Coast. Shelf Sci. 2015, 164, 65–76. [Google Scholar] [CrossRef]
- Baggs, E.M.; Smales, C.L.; Bateman, E.J. Changing pH shifts the microbial sourceas well as the magnitude of N2O emission from soil. Biol. Fertil. Soils 2010, 46, 793–805. [Google Scholar] [CrossRef]
- Shu, X.; Daniell, T.J.; Hallett, P.D.; Baggs, E.M.; Griffiths, B.S. Soil pH moderates the resistance and resilience of C and N cycling to transient and persistent stress. Appl. Soil Ecol. 2023, 182, 104690. [Google Scholar] [CrossRef]
- Sun, H.; Peng, Q.; Guo, J.; Zhang, H.; Bai, J.; Mao, H. Effects of short-term soil exposure of different doses of ZnO nanoparticles on the soil environment and the growth and nitrogen fixation of alfalfa. Environ. Pollut. 2022, 309, 119817. [Google Scholar] [CrossRef]
- Sun, Y.; Yan, F.; Liu, F. Drying/rewetting cycles of the soil under alternate partial root-zone drying irrigation reduce carbon and nitrogen retention in the soil–plant systems of potato. Agric. Water Manag. 2013, 128, 85–91. [Google Scholar] [CrossRef]

| Variables | SL | CS | SL*CS | |||
|---|---|---|---|---|---|---|
| F | p | F | p | F | p | |
| Ace | 0.555 | 0.47 | 7.228 | <0.01 ** | 0.804 | 0.470 |
| Chao1 | 0.443 | 0.51 | 8.014 | <0.01 ** | 0.881 | 0.439 |
| Shannon | 20.860 | <0.01 ** | 10.190 | <0.01 ** | 0.283 | 0.758 |
| Simpson | 12.344 | <0.01 ** | 6.182 | <0.05 * | 0.101 | 0.905 |
| Topological Properties | CK | CR | MC |
|---|---|---|---|
| Density | 0.057 | 0.221 | 0.132 |
| Average degree | 17.08 | 66.12 | 39.613 |
| Network diameter | 8.0 | 7.0 | 9.0 |
| Average weighting | 15.217 | 60.606 | 35.744 |
| Average clustering coefficients | 0.532 | 0.608 | 0.584 |
| Average path length | 3.337 | 2.399 | 2.795 |
| Edges | 2562 | 9918 | 5942 |
| Modularization | 0.621 | 0.12 | 0.16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Ding, J.; Li, J.; Zhu, D.; Li, B.; Yan, B.; Mao, L.; Sun, G.; Sun, L.; Li, X. The Response of Soil Bacterial Communities to Cropping Systems in Saline–Alkaline Soil in the Songnen Plain. Agronomy 2023, 13, 2984. https://doi.org/10.3390/agronomy13122984
Liu X, Ding J, Li J, Zhu D, Li B, Yan B, Mao L, Sun G, Sun L, Li X. The Response of Soil Bacterial Communities to Cropping Systems in Saline–Alkaline Soil in the Songnen Plain. Agronomy. 2023; 13(12):2984. https://doi.org/10.3390/agronomy13122984
Chicago/Turabian StyleLiu, Xiaoqian, Junnan Ding, Jingyang Li, Dan Zhu, Bin Li, Bohan Yan, Lina Mao, Guangyu Sun, Lei Sun, and Xin Li. 2023. "The Response of Soil Bacterial Communities to Cropping Systems in Saline–Alkaline Soil in the Songnen Plain" Agronomy 13, no. 12: 2984. https://doi.org/10.3390/agronomy13122984






