Emergence and Early Growth of Four Desmanthus Species in Three Alkaline Clay Soils
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Growth Conditions
2.2. Harvest and Measurements
2.3. Statistical Analyses
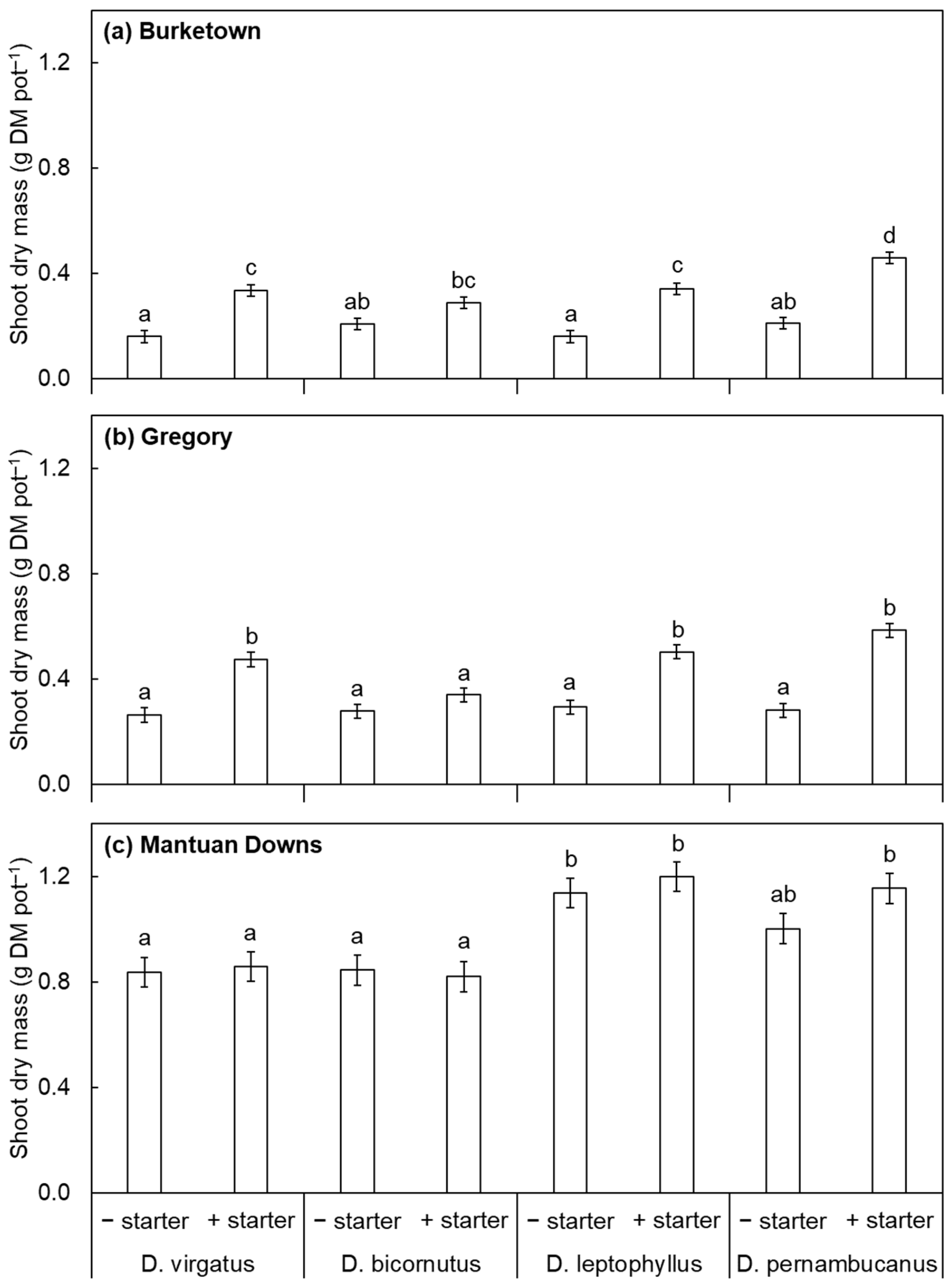
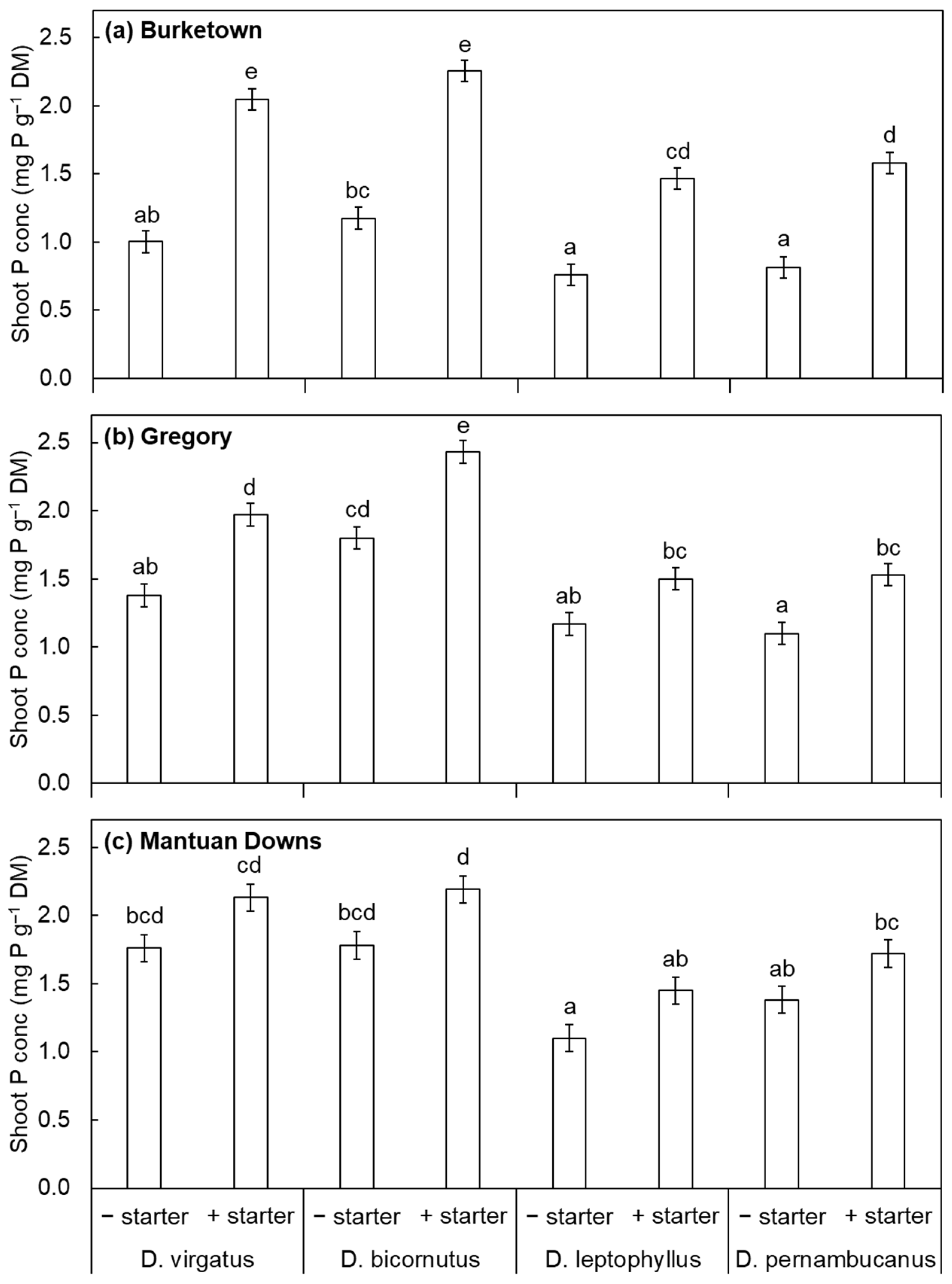
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Robertson, F.A.; Myers, R.J.K.; Saffigna, P.G. Nitrogen cycling in brigalow clay soils under pasture and cropping. Aust. J. Soil Res. 1997, 35, 1323–1340. [Google Scholar] [CrossRef]
- Jones, R.M.; McDonald, C.K.; Silvey, M.W. Permanent pastures on a brigalow soil: The effect of nitrogen fertiliser and stocking rate on pastures and liveweight gain. Trop. Grassl. 1995, 29, 193–209. [Google Scholar]
- Graham, T.W.G.; Webb, A.A.; Waring, S.A. Soil nitrogen status and pasture productivity after clearing of brigalow (Acacia harpophylla). Aust. J. Exp. Agric. Anim. Husb. 1981, 21, 109–118. [Google Scholar] [CrossRef]
- Jones, R.M.; Rees, M.C. Evaluation of tropical legumes on clay soils at four sites in southern inland Queensland. Trop. Grassl. 1997, 31, 95–106. [Google Scholar]
- Clem, R.L.; Hall, T.J. Persistence and productivity of tropical pasture legumes on three cracking clay soils (Vertisols) in north-eastern Queensland. Aust. J. Exp. Agric. 1994, 34, 161–171. [Google Scholar] [CrossRef]
- Epifanio, P.S.; de Pinho Costa, K.A.; da Costa Severiano, E.; de Souza, W.F.; Teixeira, D.A.A.; da Silva, J.T.; de Moura Aquino, M. Productive and nutritional characteristics of Brachiaria brizantha cultivars intercropped with Stylosanthes cv. Campo Grande in different forage systems. Crop Pasture Sci. 2019, 70, 718–729. [Google Scholar] [CrossRef]
- McIvor, J.G.; Guppy, C.; Probert, M.E. Phosphorus requirements of tropical grazing systems: The northern Australian experience. Plant Soil 2011, 349, 55–67. [Google Scholar] [CrossRef]
- Shaw, R.; Brebber, L.; Ahern, C.; Weinand, M. A review of sodicity and sodic soil behaviour in Queensland. Aust. J. Soil Res. 1994, 32, 143–172. [Google Scholar] [CrossRef]
- Gardiner, C.; Kempe, N.; Hannah, I.; McDonald, J. PROGARDES: A legume for tropical/subtropical semi-arid clay soils. Trop. Grassl.-Forrajes Trop. 2013, 1, 78–80. [Google Scholar] [CrossRef]
- Hall, T.J.; Walker, R.W. Pasture legume adaptation to six environments of the seasonally dry tropics of north Queensland. Trop. Grassl. 2005, 39, 182–196. [Google Scholar]
- Spies, P.R.; Brandon, N.J.; Date, R.A.; Bahnisch, L.M.; George, D. Nutrient limitations of clay soils for Desmanthus virgatus. II. A glasshouse study of 7 soils. Trop. Grassl. 1998, 32, 6–12. [Google Scholar]
- Bell, A.; Sangster, N. Research, development and adoption for the north Australian beef cattle breeding industry: An analysis of needs and gaps. Anim. Prod. Sci. 2022, 63, 1–40. [Google Scholar] [CrossRef]
- McLachlan, J.W.; Guppy, C.N.; Flavel, R.J. Differences in phosphorus acquisition and critical phosphorus requirements among nine Desmanthus spp. genotypes. Crop Pasture Sci. 2021, 72, 742–753. [Google Scholar] [CrossRef]
- Amar, A.L.; Congdon, R.A.; Gardiner, C.P.; Coventry, R.J. Quality of seed produced by tropical forage legumes on low fertility soils. Agroland Agric. Sci. J. 2016, 3, 1–13. [Google Scholar] [CrossRef][Green Version]
- Brandon, N.J.; Jones, R.M. The effect of sowing depth and duration of watering on emergence of tropical legumes in clay soil in growth cabinets. Trop. Grassl. 1998, 32, 81–88. [Google Scholar]
- Hopkinson, J.M.; English, B.H. Germination and hardseededness in desmanthus. Trop. Grassl. 2004, 38, 1–16. [Google Scholar]
- Coombes, N.E. DiGGer. 2019. Available online: https://www.nswdpibiom.org/austatgen/software/ (accessed on 6 November 2023).
- Irving, G.C.; McLaughlin, M.J. A rapid and simple field test for phosphorus in Olsen and Bray No. 1 extracts of soil. Commun. Soil Sci. Plant Anal. 1990, 21, 2245–2255. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023. [Google Scholar]
- Lenth, R. Emmeans: Estimated Marginal Means, Aka Least-Squares Means. R Package Version 1.5.0. 2020. Available online: https://CRAN.R-project.org/package=emmeans (accessed on 6 November 2023).
- Crawley, M.J. The R Book; John Wiley & Sons Ltd.: Chichester, UK, 2013. [Google Scholar]
- Wallace, A. Use of gypsum on soil where needed can make agriculture more sustainable. Commun. Soil Sci. Plan. 2008, 25, 109–116. [Google Scholar] [CrossRef]
- Farooq, M.; Usman, M.; Nadeem, F.; Rehman, H.; Wahid, A.; Basra, S.M.A.; Siddique, K.H.M. Seed priming in field crops: Potential benefits, adoption and challenges. Crop Pasture Sci. 2019, 70, 731–771. [Google Scholar] [CrossRef]
- Macor, J.P.; Peck, G.; Newman, L.; Taylor, B.; Mclean, A. Impact of phosphorus fertiliser on tropical pasture legume production. In Proceedings of the 20th Australian Society of Agronomy Conference, Toowoomba, Australia, 18–22 September 2022. [Google Scholar]

| Soil | Organic Carbon (%) | pH (CaCl2) | Nitrate N (mg kg−1) | Colwell P (mg kg−1) | PBI | Colwell K (mg kg−1) | KCl40-S (mg kg−1) |
|---|---|---|---|---|---|---|---|
| Burketown | 0.5 | 7.3 | 12 | 3 | 78 | 123 | 4 |
| Gregory | 0.4 | 7.4 | 15 | 3 | 68 | 78 | 3 |
| Mantuan Downs | 1.1 | 7.2 | 4 | 13 | 90 | 274 | 5 |
| Species and Treatment | T90 Emergence (Days) | Final Emergence (%) |
|---|---|---|
| D. virgatus | ||
| unamended | 8.9 (7.5–10.6) | 92 ± 6 bc |
| gypsum | 8.5 (7.5–9.5) | 95 ± 6 bc |
| starter | 9.5 (8.0–10.9) | 76 ± 6 abc |
| gypsum/starter | 8.1 (7.2–9.2) | 84 ± 6 abc |
| Average | 8.7 (8.1–9.5) | 86 ± 3 B |
| D. bicornutus | ||
| unamended | 8.3 (6.7–9.8) | 77 ± 6 abc |
| gypsum | 8.0 (6.7–9.8) | 86 ± 6 abc |
| starter | 8.4 (7.0–9.9) | 77 ± 6 abc |
| gypsum/starter | 7.3 (6.4–8.6) | 99 ± 6 c |
| Average | 7.9 (7.1–8.8) | 85 ± 3 B |
| D. leptophyllus | ||
| unamended | 8.9 (7.5–10.1) | 60 ± 6 a |
| gypsum | 8.0 (6.7–9.6) | 68 ± 6 abc |
| starter | 10.9 (9.1–13.4) | 57 ± 6 a |
| gypsum/starter | 9.2 (7.9–10.4) | 66 ± 6 ab |
| Average | 9.3 (8.4–10.1) | 63 ± 3 A |
| D. pernambucanus | ||
| unamended | 8.6 (6.7–10.4) | 55 ± 6 a |
| gypsum | 8.5 (7.0–9.8) | 70 ± 6 abc |
| starter | 8.9 (7.5–10.2) | 66 ± 6 ab |
| gypsum/starter | 7.3 (6.4–8.2) | 79 ± 6 abc |
| Average | 8.3 (7.5–9.2) | 68 ± 3 A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
McLachlan, J.W.; Gunadasa, S.G.; Guppy, C.N. Emergence and Early Growth of Four Desmanthus Species in Three Alkaline Clay Soils. Agronomy 2023, 13, 2996. https://doi.org/10.3390/agronomy13122996
McLachlan JW, Gunadasa SG, Guppy CN. Emergence and Early Growth of Four Desmanthus Species in Three Alkaline Clay Soils. Agronomy. 2023; 13(12):2996. https://doi.org/10.3390/agronomy13122996
Chicago/Turabian StyleMcLachlan, Jonathan W., Sajanee G. Gunadasa, and Chris N. Guppy. 2023. "Emergence and Early Growth of Four Desmanthus Species in Three Alkaline Clay Soils" Agronomy 13, no. 12: 2996. https://doi.org/10.3390/agronomy13122996
APA StyleMcLachlan, J. W., Gunadasa, S. G., & Guppy, C. N. (2023). Emergence and Early Growth of Four Desmanthus Species in Three Alkaline Clay Soils. Agronomy, 13(12), 2996. https://doi.org/10.3390/agronomy13122996






