Metagenomics of the Effect of Long-Term Straw Return on the Phosphorus Cycle in Meadow Black Soil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site and Experimental Design
2.2. Analysis of Soil Chemical Properties
2.3. DNA Extraction and Metagenomics Analysis
2.4. Statistical Analyses
3. Results
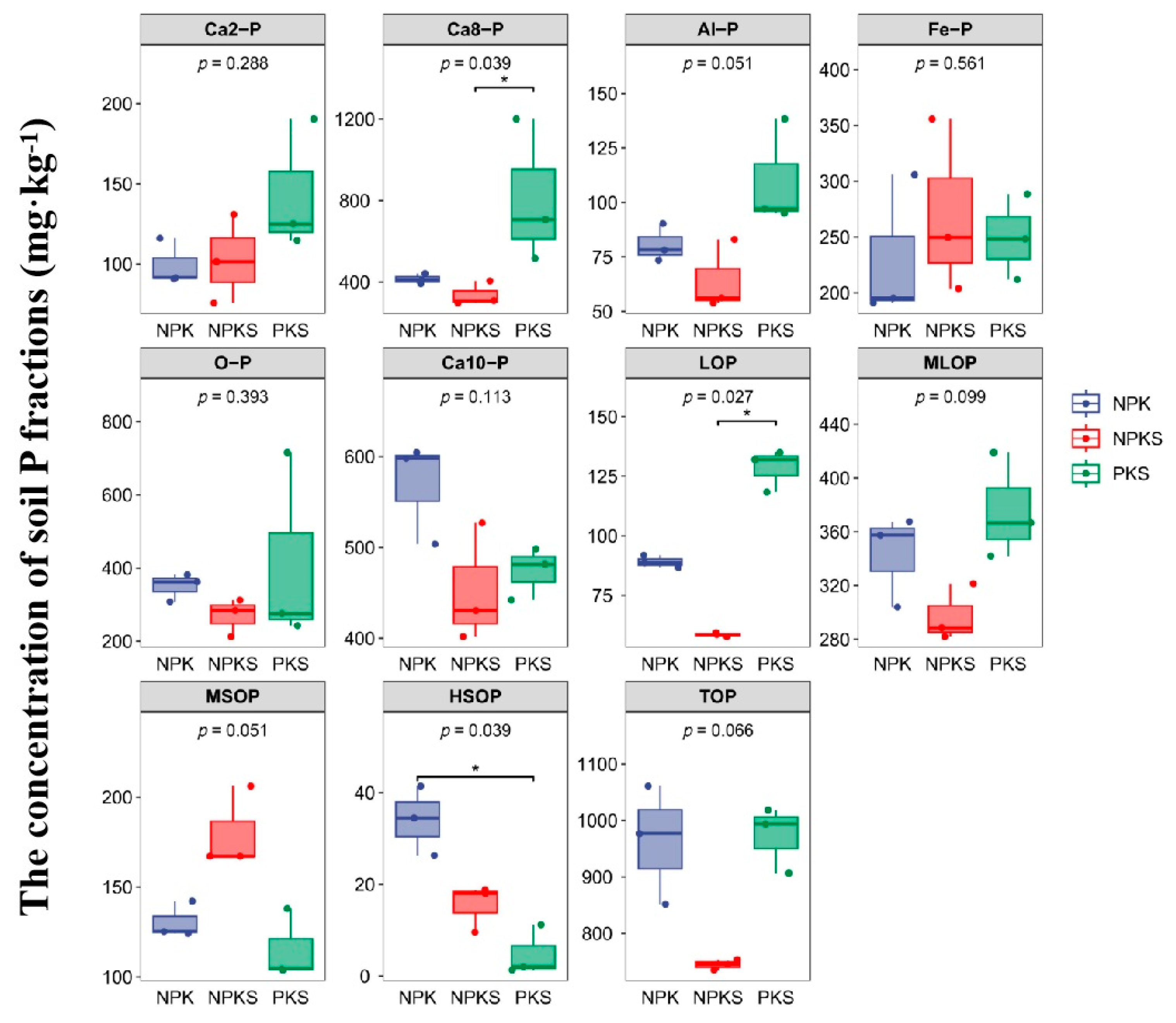
3.1. Changes in Soil Properties and P Fractions
3.2. Soil Microbial Functional Gene Composition for P Cycling
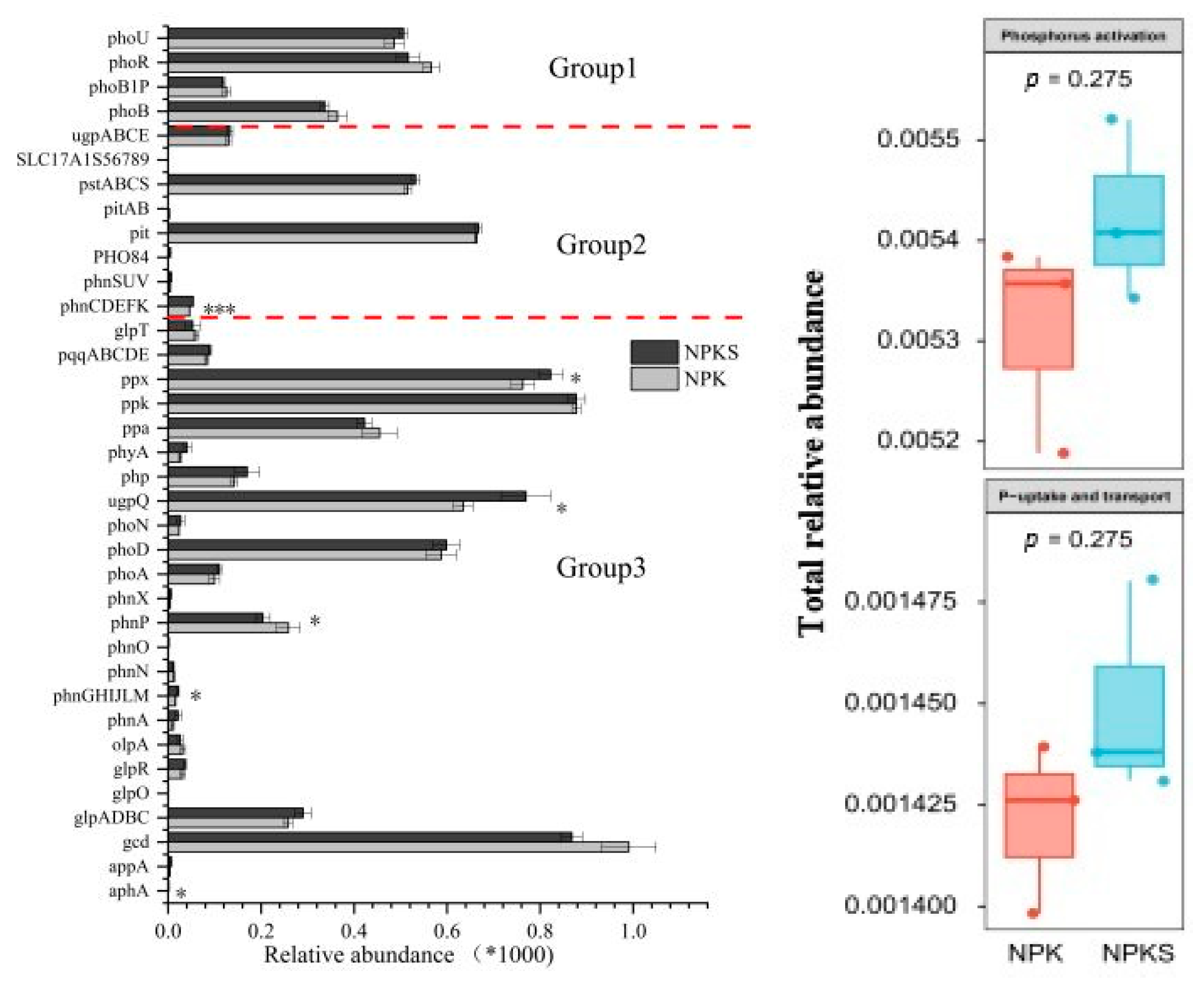
3.3. Relative Abundances of Soil P-Cycling Functional Genes
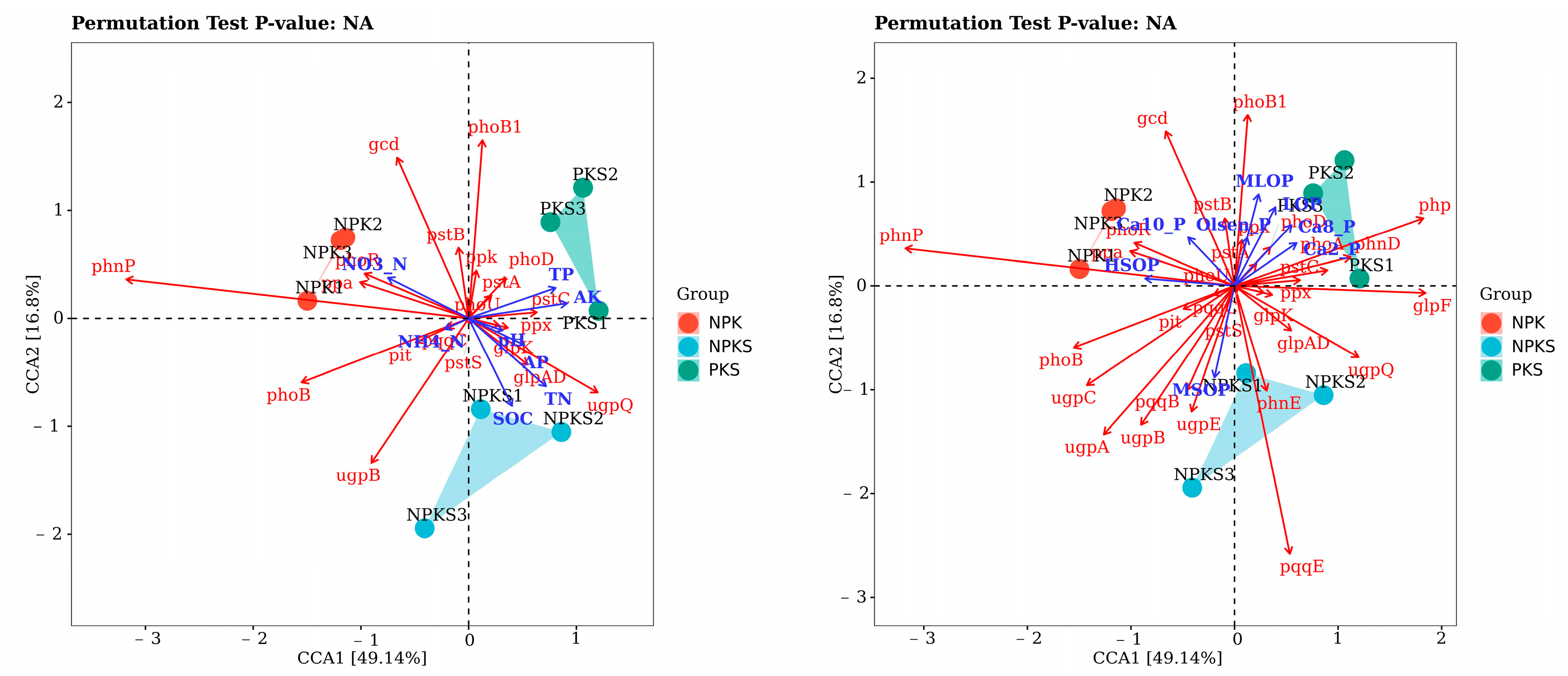
3.4. Linkages between Soil P-Cycling Functional Genes and Soil Properties
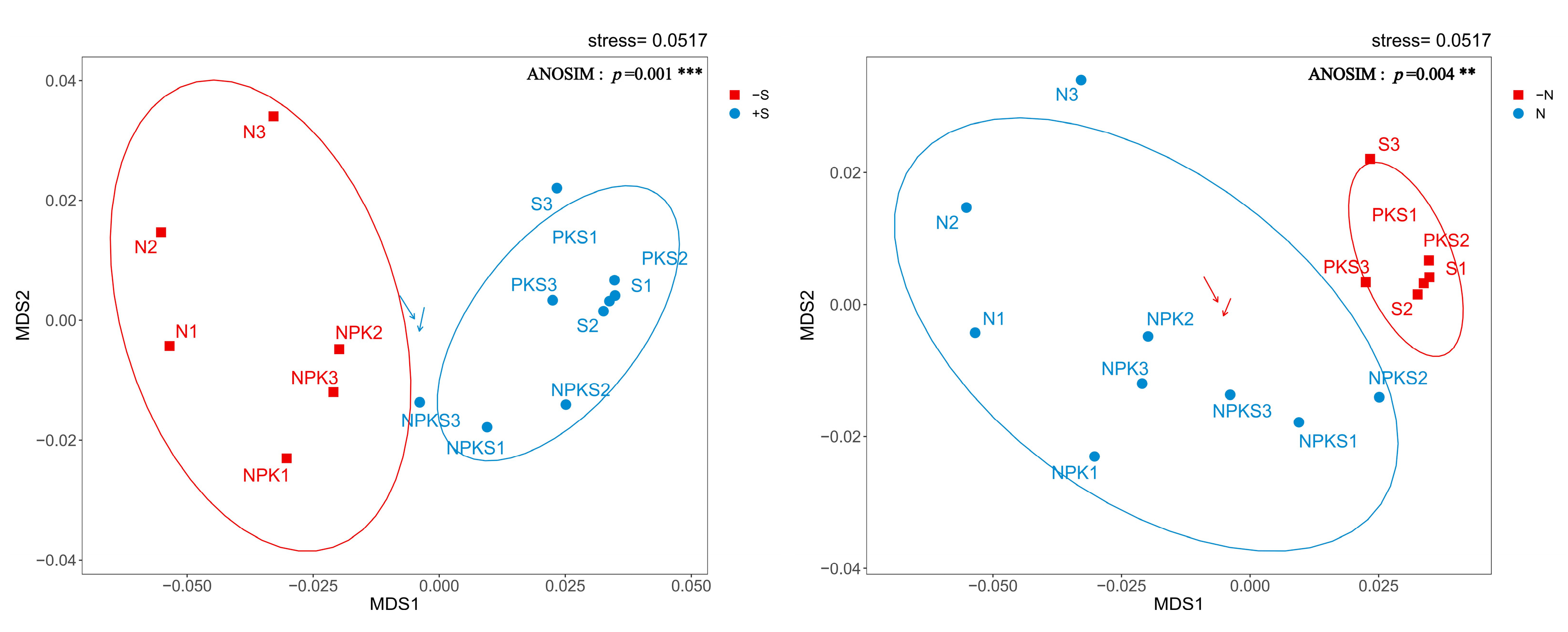
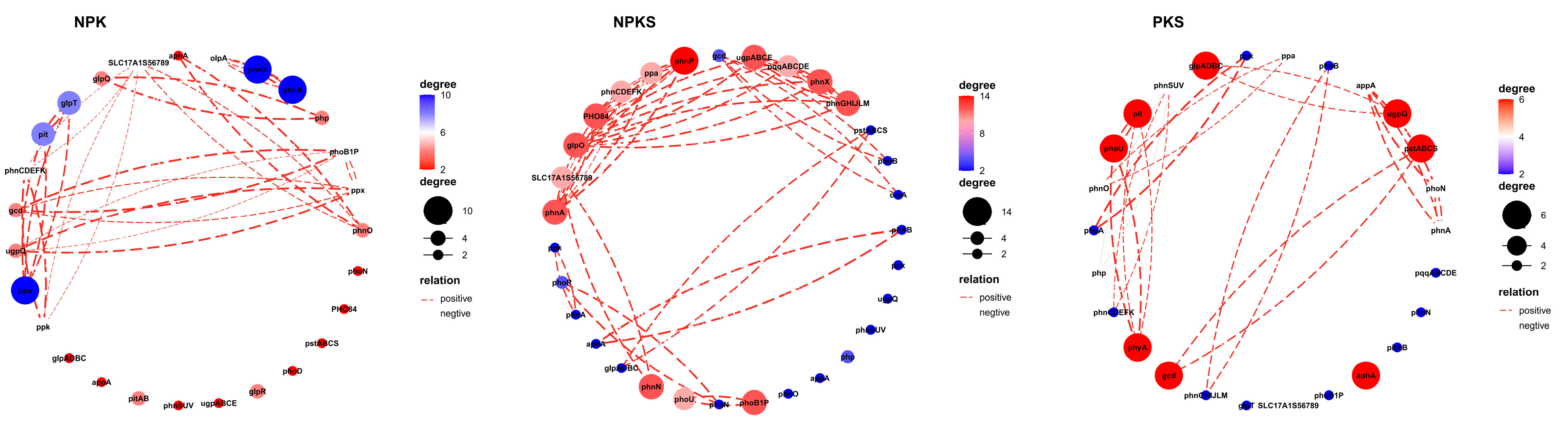
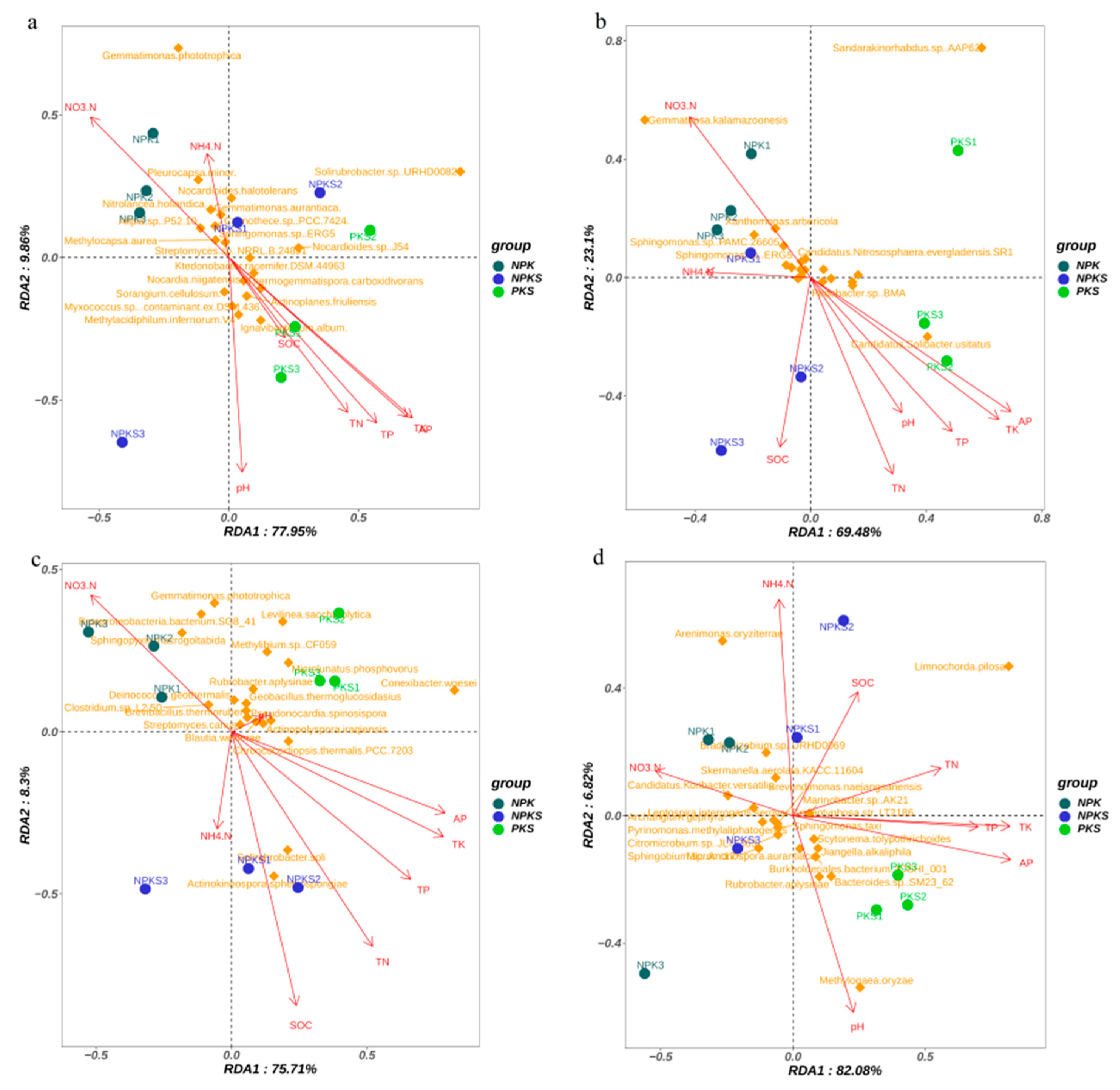
3.5. Straw Input Drives Shifts in the Soil P-Cycling-Related Microbial Community
3.6. Linking Soil Properties, P-Cycling-Related Functional Genes, and Crop Yield
4. Discussion
4.1. Long-Term Straw Returning Enhances Soil P Availability
4.2. Effects of Straw Return on Genes Involved in P Transformation
4.3. Factors Influencing Microbial Communities and Functional Genes Linked to Soil P Cycling under Straw Return
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alori, E.T.; Glick, B.R.; Babalola, O.O. Microbial Phosphorus Solubilization and Its Potential for Use in Sustainable Agriculture. Front. Microbiol. 2017, 8, 971–978. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Ren, T.; Yan, J.; Zhu, D.; Liao, S.; Zhang, Y.; Lu, Z.; Cong, R.; Li, X.; Lu, J. Straw returning mediates soil microbial biomass carbon and phosphorus turnover to enhance soil phosphorus availability in a rice-oilseed rape rotation with different soil phosphorus levels. Agric. Ecosyst. Environ. 2022, 335, 107991–108001. [Google Scholar] [CrossRef]
- Sharma, S.B.; Sayyed, R.Z.; Trivedi, M.H.; Gobi, T.A. Phosphate solubilizing microbes: Sustainable approach for managing phosphorus deficiency in agricultural soils. SpringerPlus 2013, 2, 587–600. [Google Scholar] [CrossRef] [PubMed]
- Krishnaraj, P.U.; Dahale, S. Mineral Phosphate Solubilization: Concepts and Prospects in Sustainable Agriculture. Proc. Indian Natn. Sci. Acad. 2014, 80, 389–405. [Google Scholar] [CrossRef]
- Zhu, J.; Wei, R.; Wang, X.; Zheng, C.; Wang, M.; Yang, Y.; Yang, L. Polyphosphate Accelerates Transformation of Nonstructural Carbohydrates to Improve Growth of ppk-Expressing Transgenic Rice in Phosphorus Deficiency Culture. Rice Sci. 2023, 30, 235–246. [Google Scholar]
- Cordell, D.; Drangert, J.-O.; White, S. The story of phosphorus: Global food security and food for thought. Glob. Environ. Change 2009, 19, 292–305. [Google Scholar] [CrossRef]
- Ahmed, N.; Shahab, S. Phosphate solubilization: Their mechanism genetics and application. Internet J. Microbiol. 2009, 9, 1–19. [Google Scholar]
- Thakur, D.; Kaushal, R.; Shyam, V. Phosphate solubilising microorganisms: Role in phosphorus nutrition of crop plants—A review. Agric. Rev. 2014, 35, 159–173. [Google Scholar] [CrossRef]
- Wu, X.; Rensing, C.; Han, D.; Xiao, K.; Dai, Y.; Tang, Z.; Liesack, W.; Peng, J.; Cui, Z.; Zhang, F. Genome-Resolved Metagenomics Reveals Distinct Phosphorus Acquisition Strategies between Soil Microbiomes. mSystems 2022, 7, e01107-21. [Google Scholar] [CrossRef]
- Meyer, J.; Frapolli, M.; Keel, C.; Maurhofer, M. Pyrroloquinoline quinone biosynthesis gene pqqC, a novel molecular marker for studying the phylogeny and diversity of phosphate-solubilizing pseudomonads. Appl. Environ. Microbiol. 2011, 77, 7345–7354. [Google Scholar] [CrossRef]
- Tan, H.; Barret, M.; Mooij, M.; Rice, J.; Morrissey, O. Long-term phosphorus fertilisation increased the diversity of the total bacterial community and the phoD phosphorus mineraliser group in pasture soils. Biol. Fert. Soils 2013, 49, 661–672. [Google Scholar] [CrossRef]
- Zeng, Q.; Wu, X.; Wen, X. Effects of Soluble Phosphate on Phosphate-Solubilizing Characteristics and Expression of gcd Gene in Pseudomonas frederiksbergensis JW-SD2. Curr. Microbiol. 2016, 72, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Xia, Y.; Sun, Q.; Liu, K.; Chen, X.; Ge, T.; Zhu, B.; Zhu, Z.; Zhang, Z.; Su, Y. Effects of long-term fertilization on phoD–harboring bacterial community in Karst soils. Sci. Total Environ. 2018, 628, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Jiang, N.; Condron, L.; Dunfield, K.; Chen, Z.; Wang, J.; Chen, L. Impact of long-term phosphorus fertilizer inputs on bacterial phoD gene community in a maize field, Northeast China. Sci. Total Environ. 2019, 669, 1011–1018. [Google Scholar] [CrossRef] [PubMed]
- Suleman, R.S.Y. Glucose dehydrogenase gene containing phosphobacteria for biofortification of Phosphorus with growth promotion of rice. Microbiol. Res. 2019, 223–225, 1–12. [Google Scholar]
- Tian, J.; Kuang, X.; Tang, M.; Chen, X.; Cai, K. Biochar application under low phosphorus input promotes soil organic phosphorus mineralization by shifting bacterial phoD gene community composition. Sci. Total Environ. 2021, 779, 146556. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Wu, Y.; Zhou, J.; Yu, D.; Chen, Y. Microorganisms drive stabilization and accumulation of organic phosphorus: An incubation experiment. Soil Biol. Biochem. 2022, 172, 108750. [Google Scholar] [CrossRef]
- Cui, H.; Ou, Y.; Wang, L.; Yan, B.; Guan, F. Phosphorus functional microorganisms and genes: A novel perspective to ascertain phosphorus redistribution and bioavailability during copper and tetracycline-stressed composting. Bioresour. Technol. 2023, 371, 128610. [Google Scholar] [CrossRef]
- Li, H.; Dai, M.; Dai, S.; Dong, X. Current status and environment impact of direct straw return in China’s cropland—A review. Ecotoxicol. Environ. Saf. 2018, 159, 293–300. [Google Scholar] [CrossRef]
- Yin, H.; Zhao, W.; Li, T.; Cheng, X.; Liu, Q. Balancing straw returning and chemical fertilizers in China: Role of straw nutrient resources. Renew. Sustain. Energy Rev. 2018, 81, 2695–2702. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, H.; Zhu, Z.; Xing, S.; Wang, S.; Chen, B. Low-temperature straw biochar: Sustainable approach for sustaining higher survival of B. megaterium and managing phosphorus deficiency in the soil. Sci. Total Environ. 2022, 830, 154790. [Google Scholar] [CrossRef]
- Fei, C.; Zhang, S.; Zhang, L.; Ding, X. Straw is more effective than biochar in mobilizing soil organic phosphorus mineralization in saline-alkali paddy soil. Appl. Soil Ecol. 2023, 186, 104848. [Google Scholar] [CrossRef]
- Miao, S.; Qiao, Y.; Wang, W.; Shi, Y. Priming effect of maize straw addition on soil organic matter in Yellow-brown Soil. Soils 2019, 51, 622–626. [Google Scholar]
- Li, Q.; Wang, Z.; Gu, Z.; Zhang, Y.; Pan, Y. Effect of straw return to soil by effective microorganisms on rice and wheat growth and their straw decay. Soil Environ. Sci. 2001, 10, 124–127. [Google Scholar]
- Cai, L.; Niu, Y.; Luo, Z.; Jun, W.; Yue, D.; Zhou, H.; Dong, B.; Zhang, R. Dynamic characteristics of soil nutrients and soil microbial biomass of field-returned straws at different decay accretion conditions. Chin. J. Eco-Agric. 2014, 22, 1047–1056. [Google Scholar]
- Ma, C.; Wang, X.; Wang, J.; Zhu, X.; Qin, C.; Zeng, Y.; Zhen, W.; Fang, Y.; Shangguan, Z. Interactions of soil nutrients and microbial communities during root decomposition of gramineous and leguminous forages. Land Degrad. Dev. 2023, 34, 3250–3261. [Google Scholar] [CrossRef]
- Song, K.; Zhou, C.; Li, H.; Zhou, Z.; Ni, G.; Yin, X. Effects of rumen microorganisms on straw returning to soil at different depths. Eur. J. Soil Biol. 2023, 114, 103454. [Google Scholar] [CrossRef]
- Chen, X.; Han, X.; Wang, X.; Guo, Z.; Yan, J.; Lu, X.; Zou, W. Inversion tillage with straw incorporation affects the patterns of soil microbial co-occurrence and multi-nutrient cycling in a Hapli-Udic Cambisol. J. Integr. Agric. 2023, 22, 1546–1559. [Google Scholar] [CrossRef]
- Cao, N.; Zhi, M.; Zhao, W.; Pang, J.; Hu, W.; Zhou, Z.; Meng, Y. Straw retention combined with phosphorus fertilizer promotes soil phosphorus availability by enhancing soil P–related enzymes and the abundance of phoC and phoD genes. Soil Till. Res. 2022, 220, 105390–105398. [Google Scholar] [CrossRef]
- Yang, C.; Lu, S. Straw and straw biochar differently affect phosphorus availability, enzyme activity and microbial functional genes in an Ultisol. Sci. Total Environ. 2022, 805, 150325–150333. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Ling, J.; Zhao, D.; Liu, Z.; Xu, Y.; Kuzyakov, Y.; Marsden, K.; Wen, Y.; Zhou, S. Straw return counteracts the negative effects of warming on microbial community and soil multifunctionality. Agric. Ecosyst. Environ. 2023, 352, 108508. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, W.; Luan, H.; Tang, J.; Li, R.; Li, M.; Zhang, H.; Huang, S. Long-term straw addition promotes moderately labile phosphorus formation, decreasing phosphorus downward migration and loss in greenhouse vegetable soil. J. Integr. Agric. 2022, 21, 2734–2749. [Google Scholar] [CrossRef]
- Maarastawi, S.; Frindte, K.; Bodelier, P.; Knief, C. Rice straw serves as additional carbon source for rhizosphere microorganisms and reduces root exudate consumption. Soil Biol. Biochem. 2019, 135, 235–238. [Google Scholar] [CrossRef]
- Cao, N.; Wang, J.; Pang, J.; Hu, W.; Bai, H.; Zhou, Z.; Meng, Y.; Wang, Y. Straw retention coupled with mineral phosphorus fertilizer for reducing phosphorus fertilizer input and improving cotton yield in coastal saline soils. Field Crops Res. 2021, 274, 108309. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, Y.; Zhao, Z.; Xu, S.; Cai, S.; Zhu, H.; Rengel, Z.; Kuzyakov, Y. Carbon-Phosphorus Coupling Governs Microbial Effects on Nutrient Acquisition Strategies by Four Crops. Front. Plant Sci. 2022, 13, 924154. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; He, P.; Zhang, Z.; You, M.; Wu, X.; Li, L. Straw return, rather than warming, alleviates microbial phosphorus limitation in a cultivated Mollisol. Appl. Soil Ecol. 2023, 186, 104821. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, Q.; Liu, S.; Li, J.; Geng, J.; Wang, L. Key soil properties influencing infiltration capacity after long-term straw incorporation in a wheat (Triticum aestivum L.)–maize (Zea mays L.) rotation system. Agric. Ecosyst. Environ. 2023, 344, 108301. [Google Scholar] [CrossRef]
- Li, S.; Chi, S.; Lin, C.; Cai, C.; Yang, L.; Peng, K.; Huang, X.; Liu, J. Combination of biochar and AMF promotes phosphorus utilization by stimulating rhizosphere microbial co-occurrence networks and lipid metabolites of Phragmites. Sci. Total Environ. 2022, 845, 157339. [Google Scholar] [CrossRef]
- Xie, J.; Evgenia, B.; Zhang, Y.; Wan, Y.; Hu, Q.; Zhang, C.; Wang, J.; Zhang, Y.; Shi, X. Substituting nitrogen and phosphorus fertilizer with optimal amount of crop straw improves rice grain yield, nutrient use efficiency and soil carbon sequestration. J. Integr. Agric. 2022, 21, 3345–3355. [Google Scholar] [CrossRef]
- Li, D.; Wang, Y.; Lu, D.; Chen, X.; Cui, Z.; Chen, X.; Lu, J.; Nie, J.; Wang, H.; Zhou, J. Bio-straw resource recycling systems: Agricultural productivity and green development. Resour. Conserv. Recycl. 2023, 190, 106844. [Google Scholar] [CrossRef]
- Wang, C.; Zhou, X.; Guo, D.; Zhao, J.; Yan, L.; Feng, G.; Gao, Q.; Yu, H.; Zhao, L. Soil pH is the primary factor driving the distribution and function of microorganisms in farmland soils in northeastern China. Ann. Microbiol. 2019, 69, 1461–1473. [Google Scholar] [CrossRef]
- Hedley, M.; Stewart, J.; Chauhan, B. Changes in Inorganic and Organic Soil Phosphorus Fractions Induced by Cultivation Practices and by Laboratory Incubations. Soil Sci. Soc. Am. J. 1982, 46, 970–976. [Google Scholar] [CrossRef]
- Bownan, R.; Cole, C. An exploratory method for fractionation of organic phosphorus from grassland soils. Soil Sci. 1978, 125, 95–101. [Google Scholar] [CrossRef]
- Darilek, J.L.; Huang, B.; Li, D.; Wang, Z.; Zhao, Y.; Sun, W.; Shi, X. Effect of Land Use Conversion from Rice Paddies to Vegetable Fields on Soil Phosphorus Fractions. Pedosphere 2010, 20, 137–145. [Google Scholar] [CrossRef]
- Murphy, J.; Riley, J. A modified single solution method for the determination of phosphate in natural waters. Anal. Chim. Acta 1962, 27, 678–681. [Google Scholar] [CrossRef]
- Li, Y.; Wang, C.; Wu, J.; Zhang, Y.; Li, Q.; Liu, S.; Gao, Y. The Effects of Localized Plant–Soil–Microbe Interactions on Soil Nitrogen Cycle in Maize Rhizosphere Soil under Long-Term Fertilizers. Agronomy 2023, 13, 2114. [Google Scholar] [CrossRef]
- Li, Y.; Wang, C.; Chang, H.; Zhang, Y.; Liu, S.; He, W. Metagenomics reveals the effect of long-term fertilization on carbon cycle in the maize rhizosphere. Front. Microbiol. 2023, 14, 1170214. [Google Scholar] [CrossRef]
- Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet. J. 2011, 17, 10–12. [Google Scholar] [CrossRef]
- Lapidus, A.; Korobeynikov, A. Metagenomic data assembly-the way of decoding unknown microorganisms. Front. Microbiol. 2021, 12, 613791. [Google Scholar] [CrossRef]
- Elisa, B.; Marc, L.; Stefano, A.; Alessandra, C.; Elena, M. Non-metric multidimensional scaling. Psychometrika. 2014, 29, 115–129. [Google Scholar]
- Tripathi, L.; Chen, Y.; Mizuguchi, K.; Murakami, Y. Network-based analysis for biological discovery. Encycl. Bioinform. Comput. Biol. 2019, 283–291. [Google Scholar]
- Huang, X.; Liao, W.; Liu, J.; Jia, L.; Liu, M.; Yang, Y.; Yang, L. Response of long–term phosphorus fertilization and straw return to various fractions of phosphorus in typical soil of Hebei province. Soil Fert. Sci. 2017, 49, 30–36. (In Chinese) [Google Scholar]
- Reddy, D.; Rao, S.; Singh, M. Changes in P fractions and sorption in an Alfisol following crop residues application. J. Plant Nutr. Soil Sci. 2005, 168, 241–247. [Google Scholar] [CrossRef]
- Gupta, R.; Yadvinder-Singh; Ladha, J.; Bijay-Singh; Singh, J.; Singh, G.; Pathak, H. Yield and Phosphorus Transformations in a Rice-Wheat System with Crop Residue and Phosphorus Management. Soil Sci. Soc. Am. J. 2007, 71, 1500–1507. [Google Scholar] [CrossRef]
- Gichangi, E.; Mnkeni, P.; Brookes, P. Effects of goat manure and inorganic phosphate addition on soil inorganic and microbial biomass phosphorus fractions under laboratory incubation conditions. Soil Sci. Plant Nutrit. 2009, 55, 764–771. [Google Scholar] [CrossRef]
- Sun, G.; Jin, J.; Shi, Y. Research advance on soil phosphorous forms and their availability to crops in soil. Soil Fert. Sci. 2011, 2, 1–9. (In Chinese) [Google Scholar]
- Zhou, G.; Gao, S.; Lu, Y.; Liao, Y.; Nie, J.; Cao, W. Co–incorporation of green manure and rice straw improves rice production, soil chemical, biochemical and microbiological properties in a typical paddy field in southern China. Soil Till. Res. 2020, 197, 104499. [Google Scholar] [CrossRef]
- Wu, G.; Wei, K.; Chen, Z.; Jiang, D.; Xie, H.; Jiang, N.; Chen, L. Crop residue application at low rates could improve soil phosphorus cycling under long–term no–tillage management. Biol. Fert. Soils. 2021, 57, 499–511. [Google Scholar] [CrossRef]
- Luo, G.; Li, L.; Friman, V.; Guo, J.; Guo, S.; Shen, Q.; Ling, N. Organic amendments increase crop yields by improving microbe-mediated soil functioning of agroecosystems: A meta-analysis. Soil Biol. Biochem. 2018, 124, 105–115. [Google Scholar] [CrossRef]
- Li, Y.; Yang, R.; Gao, R.; Wei, H.; Chen, A.; Li, Y. Effects of long-term phosphorus fertilization and straw incorporation on phosphorus fractions in subtropical paddy soil. J. Integr. Agric. 2015, 14, 365–373. [Google Scholar] [CrossRef]
- Dai, Z.; Liu, G.; Chen, H.; Chen, C.; Wang, J.; Ai, S.; Wei, D.; Li, D.; Ma, B.; Tang, C.; et al. Long–term nutrient inputs shift soil microbial functional profiles of phosphorus cycling in diverse agroecosystems. ISME J. 2020, 14, 757–770. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liang, B.; Bao, H.; Chen, Q.; Cao, Y.; He, Y.; Li, L. Potential of crop straw incorporation for replacing chemical fertilizer and reducing nutrient loss in Sichuan Province, China. Environ. Pollut. 2023, 230, 121034–121042. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, Y.; Wanner, B. Global regulation by the seven–component Pi signaling system. Curr. Opin. Microbiol. 2010, 13, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Ragot, S.; Kertesz, M.; Bunemann, E. phoD alkaline phosphatase gene diversity in soil. Appl. Environ. Microbiol. 2015, 81, 7281–7289. [Google Scholar] [CrossRef] [PubMed]
- Ragot, S.; Kertesz, M.; Meszaros, E.; Frossard, E.; Bunemann, E. Soil phoD and phoX alkaline phosphatase gene diversity responds to multiple environmental factors. FEMS Microbiol. Ecol. 2017, 97, 118–120. [Google Scholar]
- Sebastian, M.; Ammerman, J. Role of the phosphatase PhoX in the phosphorus metabolism of the marine bacterium Ruegeria pomeroyi DSS–3. Environ. Microbiol. Rep. 2011, 3, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Ma, Q.; Hui, X.; Ran, J.; Ma, Q.; Wang, X.; Wang, Z. Long-term high-P fertilizer input decreased the total bacterial diversity but not phoD-harboring bacteria in wheat rhizosphere soil with available–P deficiency. Soil Biol. Biochem. 2020, 149, 107918. [Google Scholar] [CrossRef]
- Luduena, L.; Anzuay, M.; Magallanes–Noguera, C.; Tonelli, M.; Ibanez, F.; Angelini, J.; Fabra, A.; McIntosh, M.; Taurian, T. Effects of P limitation and molecules from peanut root exudates on pqqE gene expression and pqq promoter activity in the phosphate–solubilizing strain Serratia sp. S119. Res. Microbiol. 2017, 168, 710–721. [Google Scholar] [CrossRef]
- Fraser, T.; Lynch, D.; Bent, E.; Entz, M.; Dunfield, K. Soil bacterial phoD gene abundance and expression in response to applied phosphorus and long-term management. Soil Biol. Biochem. 2015, 88, 137–147. [Google Scholar] [CrossRef]
- Jiang, Y.; Yang, X.; Ni, K.; Ma, L.; Shi, Y.; Wang, Y.; Cai, Y.; Ma, Q.; Ruan, J. Nitrogen addition reduces phosphorus availability and induces a shift in soil phosphorus cycling microbial community in a tea (Camellia sinensis L.) plantation. J. Environ. Manag. 2023, 342, 118207–118215. [Google Scholar] [CrossRef]
- Hossain, M.; Tani, C.; Suzuki, T.; Taguchi, F.; Ezawa, T.; Ichinose, Y. Polyphosphate kinase is essential for swarming motility, tolerance to environmental stresses, and virulence in Pseudomonas syringae pv. tabaci 6605. Physiol. Mol. Plant Pathol. 2008, 72, 122–127. [Google Scholar] [CrossRef]
- Seufferheld, M.; Alvarez, H.M.; Farias, M. Role of Polyphosphates in Microbial Adaptation to Extreme Environments. Appl. Environ. Microbiol. 2008, 74, 5867–5874. [Google Scholar] [CrossRef]
- Brzoska, P.; Boos, W. Characteristics of a ugp-encoded and phoB-dependent glycerophosphoryl diester phosphodiesterase which is physically dependent on the ugp transport system of Escherichia coli. J. Bacteriol. 1988, 170, 4125–4135. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.; Wang, D.; Li, Y.; Liu, S. Metagenomics of the Effect of Long-Term Straw Return on the Phosphorus Cycle in Meadow Black Soil. Agronomy 2023, 13, 3003. https://doi.org/10.3390/agronomy13123003
Wang C, Wang D, Li Y, Liu S. Metagenomics of the Effect of Long-Term Straw Return on the Phosphorus Cycle in Meadow Black Soil. Agronomy. 2023; 13(12):3003. https://doi.org/10.3390/agronomy13123003
Chicago/Turabian StyleWang, Chengyu, Dong Wang, Yanan Li, and Shuxia Liu. 2023. "Metagenomics of the Effect of Long-Term Straw Return on the Phosphorus Cycle in Meadow Black Soil" Agronomy 13, no. 12: 3003. https://doi.org/10.3390/agronomy13123003
APA StyleWang, C., Wang, D., Li, Y., & Liu, S. (2023). Metagenomics of the Effect of Long-Term Straw Return on the Phosphorus Cycle in Meadow Black Soil. Agronomy, 13(12), 3003. https://doi.org/10.3390/agronomy13123003




