Abstract
The application of nitrogen fertilizer is crucial to the growth and biological nitrogen fixation of peanut, especially in the seedling stage where nodules have not yet formed. However, it is still uncertain how much initial nitrogen fertilizer should be applied to promote peanut root growth, nodule formation, and biological nitrogen fixation (BNF). There, a 2-year pot experiment was conducted using Huayu 22 (HY22, large-grain cultivar) and Huayu 39 (HY39, small-grain cultivar) as experimental materials to research the effects of different initial nitrogen fertilizer application rates on peanut root growth (root weight, root length, root mean diameter, root activity) and biological nitrogen fixation capacity (nodule number, nodule weight, biological nitrogen fixation, and nitrogen fixation potential per plant). N0, as control, four initial nitrogen fertilizer application rates were established: 15 kg·hm−2 (N15), 30 kg·hm−2 (N30), 45 kg·hm−2 (N45), and 60 kg·hm−2 (N60). The present results showed that the nodule number, nodule dry weight, nitrogenase activity, and biological nitrogen fixation of the HY22 cultivar under the N15 treatment were higher compared to those under other treatments over the two growing seasons. In addition, the cultivar of HY39 treated with the N15 treatment also increased the nitrogen fixation potential per plant and BNF relative to other treatments. Although the application of 60 kg·hm−2 nitrogen increased the root surface area and root volume, it decreased the nitrogenase activity, nodule dry weight, and nitrogen fixation potential per plant of HY22 and HY39 varieties in both growing seasons. Above all, an initial nitrogen application of 15 kg·hm−2 may be the optimal treatment for promoting peanut nodule formation and biological nitrogen fixation.
1. Introduction
Nitrogen is a key element in the crop growth process, with sufficient nitrogen to ensure high yields often being provided in the form of industrial nitrogen fertilizer in agricultural settings [1,2]. In contrast to this industrial-grade synthetic nitrogen fertilizer, the symbiotic relationship between leguminous plants and rhizobia offers an alternative mechanism through which these crops can obtain N from atmospheric N2 through biological nitrogen fixation (BNF) [3]. Peanut (Arachis hypogaea L.) is an economically key oil-producing crop in China, and as they are legumes they can also engage in biological nitrogen fixation. However, this BNF activity can only provide an estimated 40–60% of the nitrogen required for optimal plant growth such that exogenous nitrogen is necessary to achieve maximal yields [4,5]. The addition of excessively high nitrogen levels can suppress rhizosphere nitrogen fixation, completely inhibiting both acetylene reduction activity (ARA) and nodulation when these soil nitrogen levels are above a given threshold [6,7]. This inhibition tends to become more severe as soil nitrogen levels rise. Mousumi-Mondal’s study showed that the application of higher nitrogen fertilizers inversely impacted root nodulation and nitrogen fertilizer application efficiency and concluded that 25 kg hm−2 as a base application in combination with Rh inoculant would be very beneficial for irrigated peanut in India [8]. There was also research indicating that 72 kg hm−2 nitrogen fertilizer in combination with Bradyrhizobium sp. increased symbiotic nitrogen fixation in Egypt [9]. In the context of peanut cultivation in China, large amounts of nitrogen are applied as a basal fertilizer that can limit BNF, contributing to lower nitrogen utilization rates that ultimately compromise crop yields and quality while also contributing to the ecological deterioration of the soil. Studies have shown that higher yields and lower environmental pollution can be achieved by optimizing the balance between the application of nitrogen fertilizer and the natural process of biological nitrogen fixation in legumes [10].
Low soil nitrogen concentrations are known to promote soil nodule development, whereas BNF in these nodules is suppressed in response to higher nitrogen concentrations [11,12]. As this symbiotic BNF process requires substantial energy input, leguminous plants will preferentially utilize available soil nitrogen under low-nitrate conditions prior to establishing N2-fixing symbiosis, thereby reducing nitrate source competition between nodules and host plants [13]. These nodules develop high nitrate levels, but excessive nitrate accumulation within the nodules can suppress symbiotic rhizobia-host interactions [14]. Applying appropriate levels of nitrogen in growth can support root nodule formation and development in leguminous crops, with some evidence suggesting that the early stages of growth are the most important period during which to promote increases in root nodule numbers [15]. Given the importance of the early stage of growth as a determinant of root nodule development, appropriate nitrogen supplementation provides an opportunity to improve the uptake of soil and fertilizer-derived nitrogen by host plant root systems while effectively initiating root nodule formation, mainly through an abundant supply of photo-assimilate to the nodules, thus supporting root growth and activity [16].
While the initial nitrogen concentrations applied to peanut seedlings is a key factor in the formation of nodule development and crop yields, studies also proved that the inhibitory effect of one-time application of basal nitrogen on nodulation is greater in the early stage of peanuts. However, split application of controlled release fertilization can slow down the inhibitory effect of nitrogen. It is not clear how much initial nitrogen fertilizer is optimal to apply, making it difficult to accurately match peanut’s nitrogen needs to controlled release fertilization. Some reports have demonstrated that nitrogen can influence the length, surface area, and roots during early phases of growth, in turn influencing BNF activity [17]. Root systems provide a nutrient-rich niche conducive to nodule development, promoting BNF [18]. The close physical, genetic, and biochemical interactions between rhizomes and roots are well documented, particularly in the context of nodule development, symbiotic nitrogen fixation activity, and associated transport activity. However, there have been few efforts to date to simultaneously examine the associations and relationships linking root architecture and BNF. Accordingly, the purpose of the present research was to optimize the initial nitrogen application of two peanut varieties commonly used in production, improve root growth (root weight, root length, root mean diameter, root activity) and biological nitrogen fixation (nodule number, nodule weight, nodule nitrogenase activity, biological nitrogen fixation, and nitrogen fixation potential per plant), and to provide support for realizing the input of nitrogen fertilizer according to the need and breaking through the bottleneck of efficient use of N in peanut.
2. Materials and Methods
2.1. Experimental Design
This research was conducted in 2020 and 2021 under the experimental pot conditions at Shandong Academy of Agricultural Sciences Station (36°43′ N, 117°05′ É) in Jinan, China. Pots (diameter: 30 cm, height: 28 cm, volume: 3.2 dm3) were packed with 10 kg of a quartz and vermiculite mixture (3:1, v/v). The total N content was 2.21 g·kg−1, Experimental crops included the Huayu 22 and Huayu 39 peanut cultivars, which are, respectively, large-grain and small-grain varieties, using seeds that were kindly provided by Shandong Peanut Research Institute, Qingdao, China. Four different nitrogen treatment conditions were established based on the concentration of urea applied, including the N0 (0 kg·hm−2), N15 (15 kg·hm−2), N30 (30 kg·hm−2), N45 (45 kg·hm−2), N60 (60 kg·hm−2) treatments. The arrangements for the 2-year experiment were consistent. Three replicates were established per treatment in a randomized design, with 10 pots per replicate. Three of the pots in each replicate utilized 15N-labeled urea with 10.3% abundance, while the remaining seven pots utilized regular urea. P and K fertilizers were provided in the form of 180 kg·hm−2 potassium phosphate monobasic (KH2PO4), while Ca fertilizer was applied in the form of 150 kg·hm−2 Calcium sulfate (CaSO4). Bradyrhizobium yuanmingense, a rhizobium CCBAU21353 isolated from peanut root nodules.
All fertilizer treatments were applied using the same approach. Briefly, quartz and vermiculite (3:1, v/v) were thoroughly mixed with N, P, and K fertilizers at the defined concentrations on 20 May 2020 and 14 May 2021. Peanut seeds were disinfected for 2 min with 70% ethanol, disinfected for 8 min with 0.1% (w/v) HgCl2, rinsed with sterile water 7–8 times, dried of surface water, inoculated for 2 h with rhizobia, and then sown in 2 holes per pot, with 2 seeds being added per hole. After emergence, 1 plant was cultivated per hole for 2 total peanut plants per pot. The peanuts were watered every 7 days, and irrigation and other management were consistent.
2.2. Morphological and Physiological Characteristic Measurements
Peanut plants grown under various initial nitrogen concentrations were sampled at random 30 days after planting. Measured parameters included stem height, total branch number, number of stem nods, number of stem green leaves, root dry weight (DW), and aboveground DW. A ruler was used when measuring stem height and lateral branch length. Biomass measurements were made by separating roots and aboveground biomass, heating samples for 30 min at 105 °C in an oven, and then drying these samples for 48 h at 75 °C, after which root and aboveground DW was measured and the root/shoot ratio was determined.
2.3. Root System Measurement
The peanuts were randomly sampled 30 days after sowing; 6 pots per treatment were selected at random to assess root activity and architecture. the triphenyl tetrazolium chloride (TTC) method was applied to calculate the root activity. In brief, root tip samples of 0.5 g were placed in a beaker, and an equal mixture of 0.4% TTC and phosphate-buffered solution was added in 10 mL; the roots were fully submerged in the solution and kept at 37 °C for 1~3 h without light. Thereafter, 1 mol L−1 sulfuric acid was added in 2 mL in order to stop the reaction. The sample was dried, then extracted via ethyl acetate. The absorbance was detected at 485 nm. Root morphological parameters were evaluated with a scanner (Epson V800 Photo, SEIKO EPSON CORP, Jakarta, Indonesia), and WinRHIZO was applied to determine the root length, and volume. The root activity was calculated with reference to the following formula:
Root activity = amount of TTC reduction (µg)/fresh root weight (g) × time (h)
2.4. Biological Nitrogen Fixation Measurement
The nitrogen fixation number of peanut nodules was calculated by detecting the 15N abundance of plant samples using the 15N labeling tracer method. Peanut plant samples were ground by a plant tissue grinder, then finely ground by a ball mill (MM400, Retsch Crop, Haan, Germany) and passed through a 0.15 mm sieve. Total N levels were measured with an automatic nitrogen analyzer (XY-2 SAMPLER, SEAL, Germany), and 15N abundance was calculated via mass spectrometry (MAT-271, Thermo Finnigan, Germany). The nitrogen derived from nitrogen fertilizer (Ndff) and BNF was calculated with reference to the following formula:
Ndff = (15N atom% excess in plant sample/15N atom% excess in fertilizer) × 100
Total N accumulation (mg/plant) = Plant dry weight × Plant N content
Plant 15N fertilizer absorption(mg/plant) = Plant total N accumulation × Ndff
BNF (mg/plant) = total N accumulation − 15N fertilizer absorption
2.5. Nitrogenase Activity Measurement
Acetylene (C2H2) reduction to ethylene (C2H4) was used to measure nitrogenase activity. Briefly, washed peanut root nodules were added to a 50 mL closed glass bottle. Then, 5 mL of air was removed from each bottle and replaced with 5 mL of C2H2, after which bottles were sealed for 2 h. Ethylene levels were then measured via gas chromatography, and nodule numbers and weight were then assessed after drying, with the nitrogen fixation potential per plant being calculated as follows:
nitrogen fixation potential per plant (mmol·h−1)
= nitrogenase activity (μmol·g−1·h−1) × Root nodule dry weight per plant (g)
= nitrogenase activity (μmol·g−1·h−1) × Root nodule dry weight per plant (g)
2.6. Statistical Analysis
The effects of year, cultivar, N treatment, and their interaction on peanut root development and biological nitrogen fixation characteristic were tested using a two-way ANOVA with SPSS 25.0. The repeated factors “year”, “cultivar” and “N treatment” were combined with the repeated factor “peanut trait.” Using the LSD approach, multiple comparisons of each indicator under various treatments were carried out at a significant probability level of 0.05. We applied DPS (p < 0.05) significance analysis to all the data and processed and plotted results using Origin 2019.
3. Results
3.1. Peanut Root Architecture
Root architecture is a term that is used to refer to root morphology and spatial distributions, directly reflecting the growth of roots in a manner that is responsive to environmental nitrogen levels (Table 1). Analysis of variance (ANOVA) showed that year, cultivar and N treatment had significant effects on peanut root length, volume, and surface area, and the interaction of year and N treatment had a strong influence on root length as well as surface area, whereas the interaction among the three had nonsignificant effects (Table 2). According to the above result, nitrogen application obviously improved root length and surface area, but not root diameter. In 2020 and 2021, the HY22 exhibited a longer root length under N60 conditions relative to the other treatments. We found that the root length and root volume were significantly increased by 12.31% and 7.04% of HY22 in 2020, respectively, compared to N0. In two years, the HY39 in the N45 and N60 group exhibits obviously higher root length, surface area, and volume than those in the other treatments. it can be included that the root length and surface area under N60 were significantly increased by 37.34%, and 25.61% of HY39 in 2021, respectively, compared to N0. These findings indicated that root length and volume for the HY22 and HY39 cultivars can be significantly enhanced under 60 kg·hm−2, thereby improving root absorption area in a manner conducive to peanut root absorption efficiency.

Table 1.
Peanut root architecture in the 2020 and 2021 growing seasons.

Table 2.
Analysis of variance for the effects of year, cultivar and nitrogen on root architecture.
3.2. Peanut Root Activity
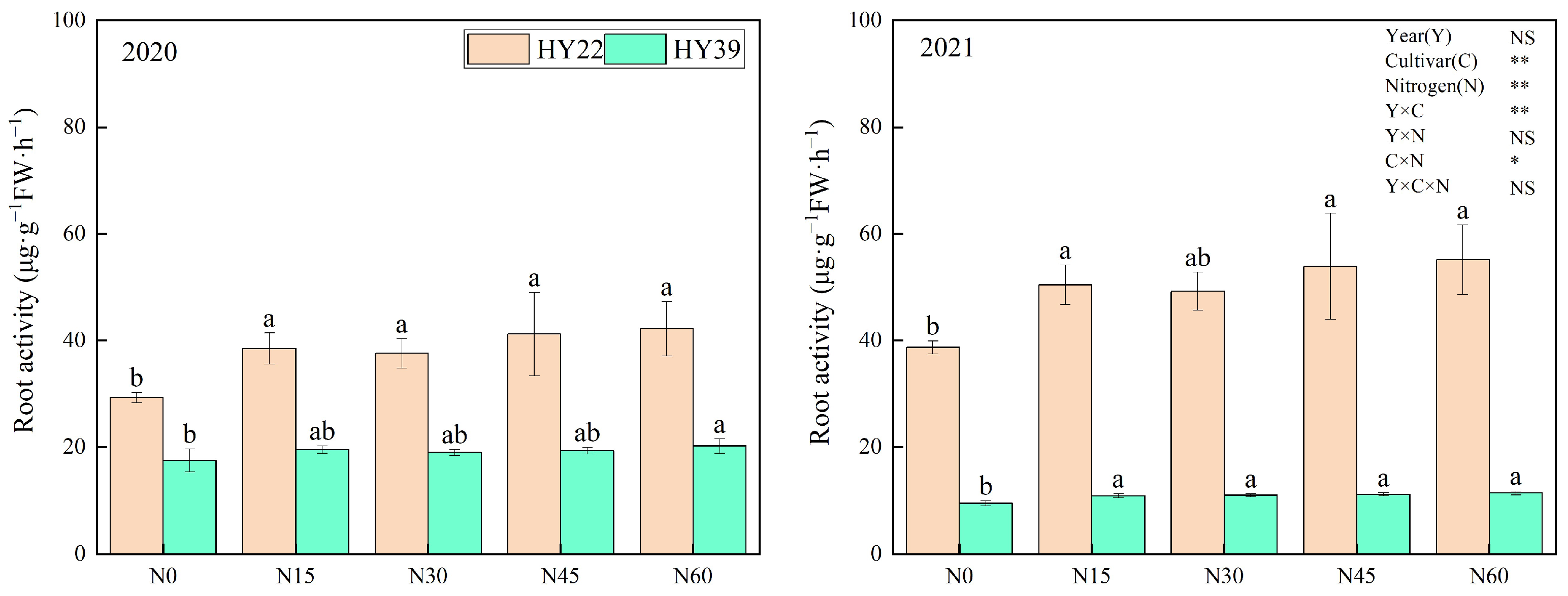
As shown in Figure 1, the cultivar and N treatment had an obvious influence on peanut root activity, and the interaction of year and cultivar and cultivar and nitrogen treatment can significantly impact the peanut root activity. The growth and metabolic level of roots, that is, root activity, is one of the important physiological indicators of plant growth. Plants with strong root activity can better absorb water and nutrients in the soil and provide sufficient nutrient supply. Nitrogen fertilizer application improved root activity over the two years. For the HY22 in 2020 and 2021, higher root activity levels were evident under N15 treatment conditions (31.43% and 30.39% above N0), with no increases as N concentrations were further increased. There were no obvious differences in root activity for the HY39 cultivar among the N15, N30, and N45 treatment conditions over the two years, with increases of 11.72%, 8.69%, and 10.4% in 2020, respectively, and 15.03%, 16.19, and 17.85% in 2021, respectively, compared with the N0. These findings suggested that under N15 treatment conditions, the peanut seedling root system exhibits more vigorous activity and exhibits a higher capacity to engage in nutrient absorption.
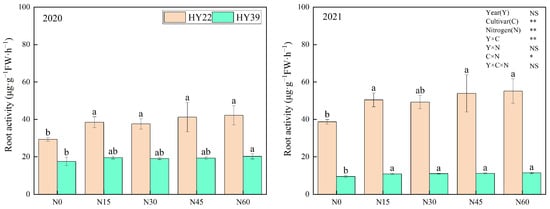
Figure 1.
Peanut root activity. Error bars denote the SD of the mean (SD, n = 6). The letters (a, b) within a column and in the same year indicate significant differences among treatments by Duncan’s range test (p < 0.05). * and ** are significant at the 0.05 and 0.01 levels; NS means not significant.
3.3. Nodulation and BNF Activity
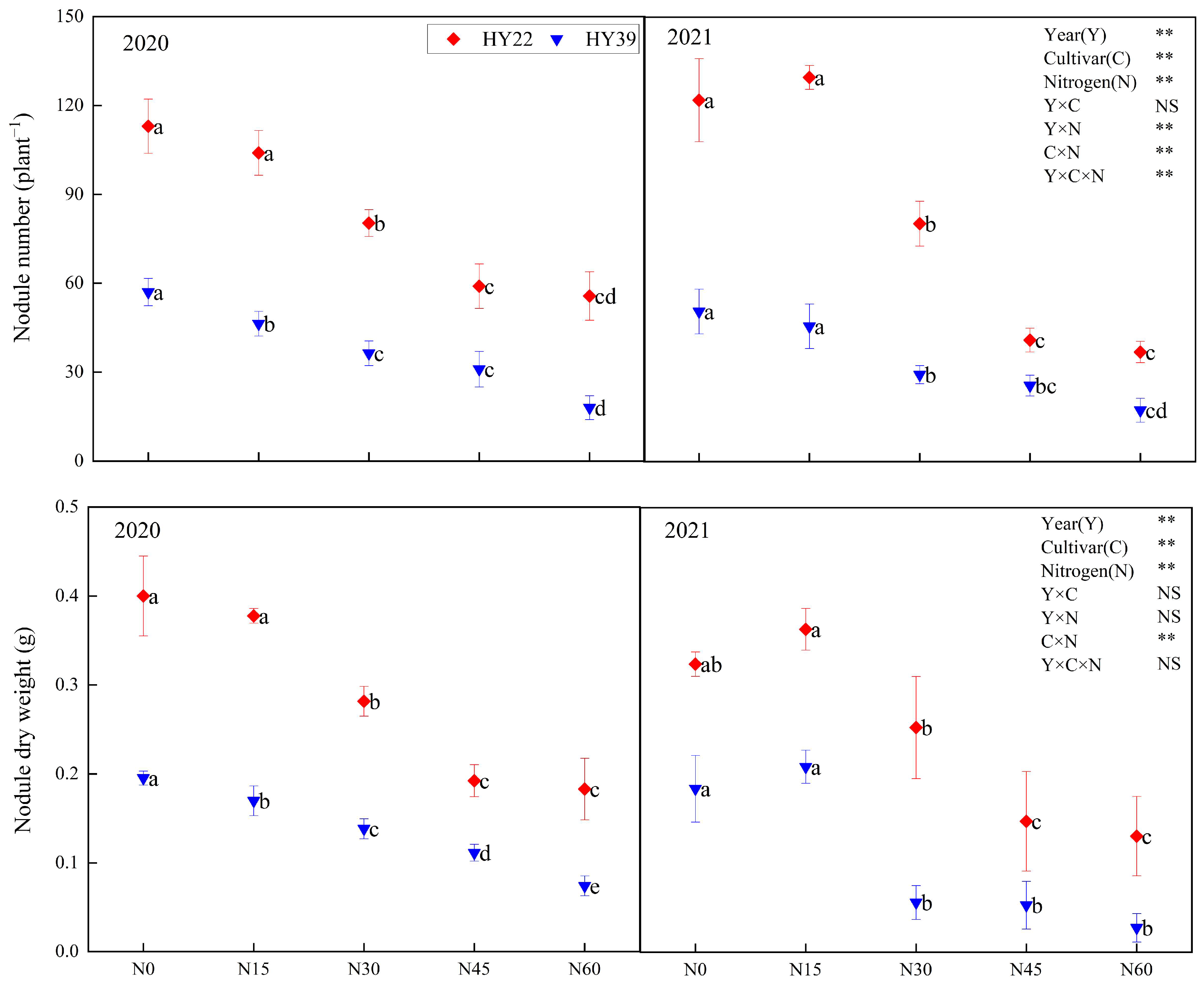
As shown in Figure 2, Analysis of variance (ANOVA) showed that year, cultivar, N treatment, and the interaction of cultivar and N treatment obviously impacted the nodule number and dry weight, whereas the interaction among the year and cultivar had nonsignificant effects. Root nodule numbers and dry weight showed a continuous decreasing trend with increasing N input in both years. From 2020 to 2021, N30, N45, and N60 treatments reduced the nodule number and dry weight by 28.91–68.42% and 29.2–62.07%, respectively, among the 2 cultivars compared with N0. Compared with other nitrogen treatments, N15 treatment significantly increased the nodule number and dry weight, but it was not significantly different from N0. Nitrogen application higher than N15 had a significant inhibitory effect on the peanut nodulation ability, and the higher the nitrogen level, the stronger the inhibitory effect. So N15 treatment had stronger nodulation ability in both varieties.
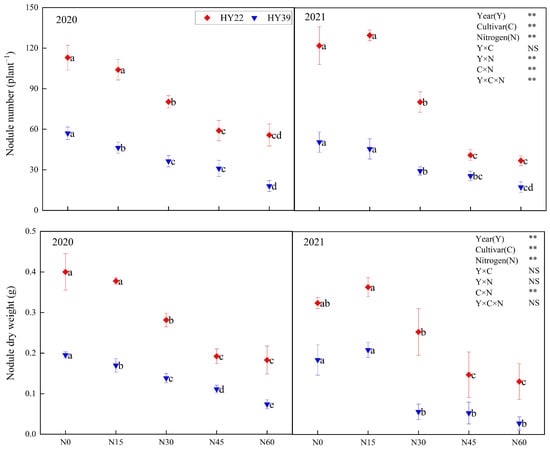
Figure 2.
Peanut nodule number as well as dry weight in the 2020 and 2021 growing seasons. Error bars denote the standard deviation of the mean (SD, n = 6). The letters (a, b, c, d, e) within a column and in the same year indicate significant differences among treatments by Duncan’s range test (p < 0.05). ** are significant at the 0.01 levels; NS means not significant.
The result of nitrogenase activity and BNF potential per plant in various nitrogen levels for the two years can be seen from Table 3. Analysis of variance (ANOVA) showed that year, N treatment and the interaction of year and cultivar had a significant influence on nitrogenase activity, whereas the interaction among the cultivar and N treatment had nonsignificant effects. Analysis of variance (ANOVA) showed that nitrogen fixation potential per plant was affected by the interaction among the three (Table 4). Both the HY22 and HY39 exhibited peak nitrogenase activity under N15 treatment conditions over the two years, with 25.88% and 11.9% above N0 levels, respectively, in 2020. The BNF potential per plant is obviously higher in N15 compared to that in other N treatments. The single-plant nitrogen fixation potential of the HY22 under N15 conditions was increased by 18.85% and 13.86% during 2020 and 2021, respectively, relative to the N0 condition, whereas for the HY39, this potential increased by 28.57% relative to N0 condition in 2021. Accordingly, the initial nitrogen concentrations for the small-grain HY22 and the large-grain HY39 should, respectively, be 15 kg·hm−2, which exhibited a more notably increased single-plant nitrogen fixation potential than the other treatments, thereby enhanced the nodulation capacity of peanut.

Table 3.
Peanut nitrogenase activity in the 2020 and 2021 growing seasons.

Table 4.
Analysis of variance for the effects of year, cultivar and nitrogen on nitrogenase activity.
3.4. Peanut Seedling Growth
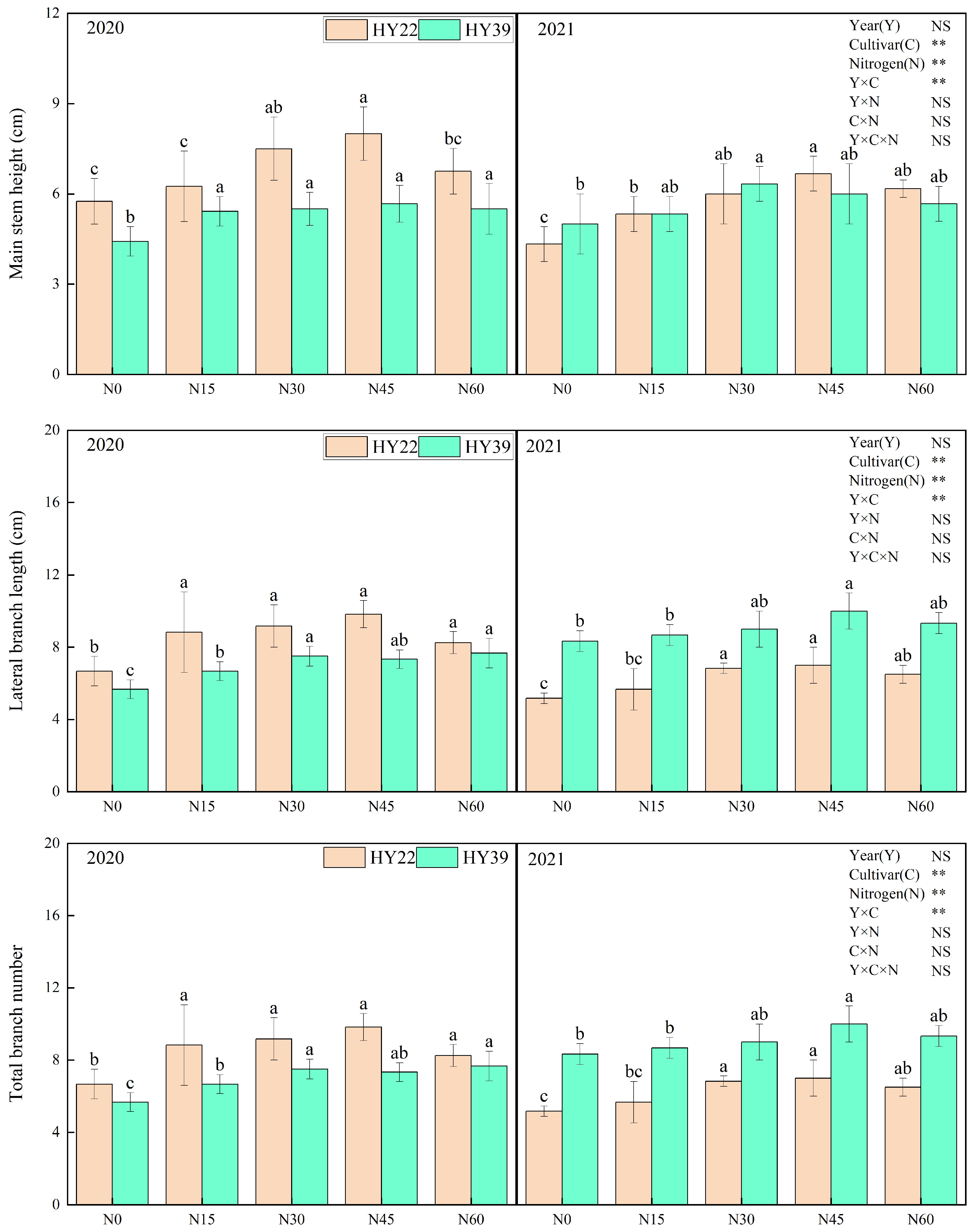
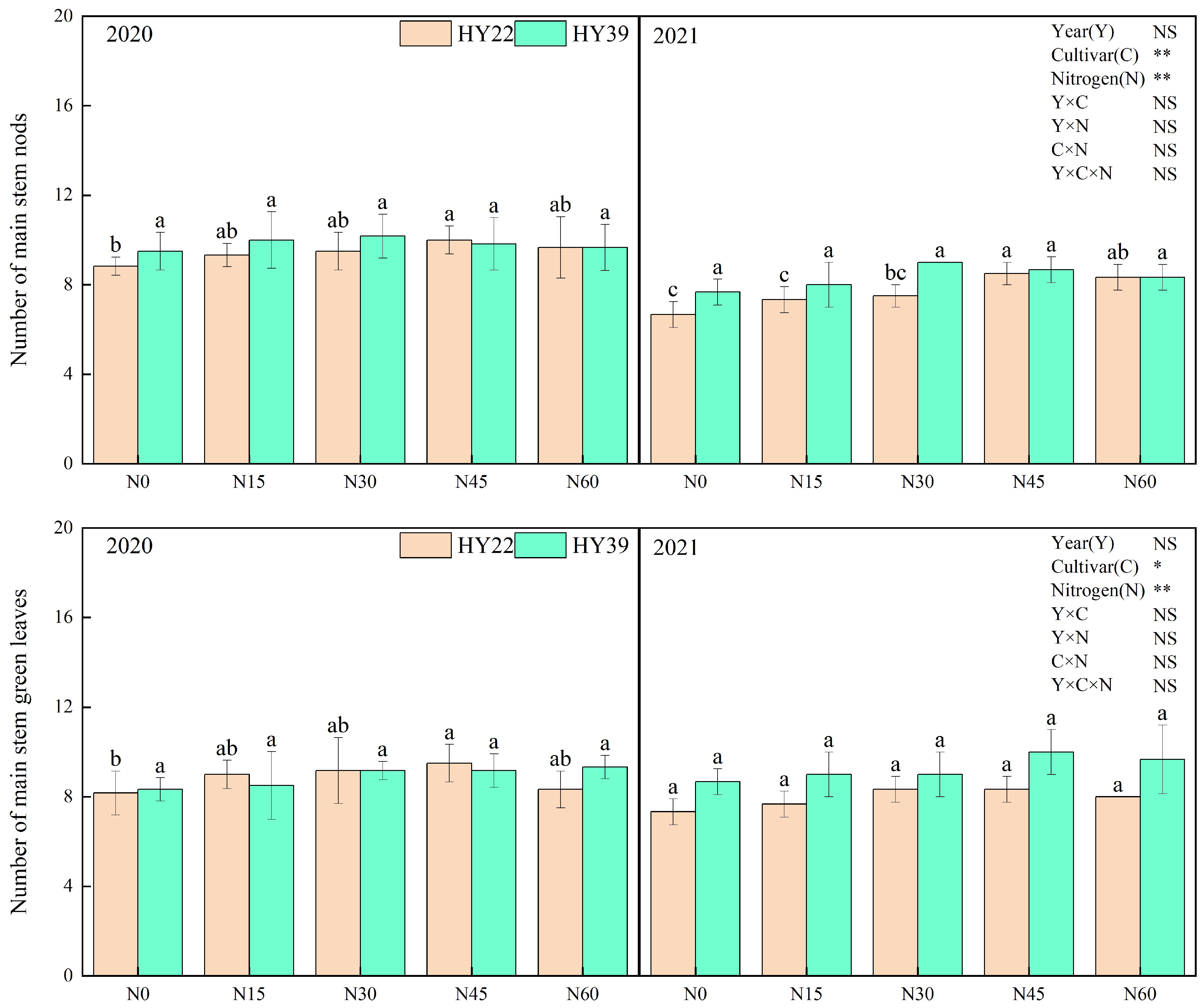
Analysis of variance (ANOVA) showed that cultivars and N treatment had obvious influence on the main stem height, total branch number, main stem nod number, and main stem green leaves, whereas the interaction among the year and N treatment had nonsignificant effects. For both experimented peanut cultivars, initial nitrogen application strongly accelerated the growth of the peanut (Figure 3). Nitrogen inputs to both varieties significantly increased the main stem height, total branch number, main stem nods number, and main stem green leaves. The HY22 cultivar with the N45 treatment increased the main stem height, main stem nod number, and main stem green leaves compared with other treatments during 2020 to 2021. For HY39, nitrogen fertilizer inputs did not significantly increase the main stem nod number and main stem green leaves. However, the lateral branch length under N30, N45, and N60 was significantly higher than in the other treatments during both years.
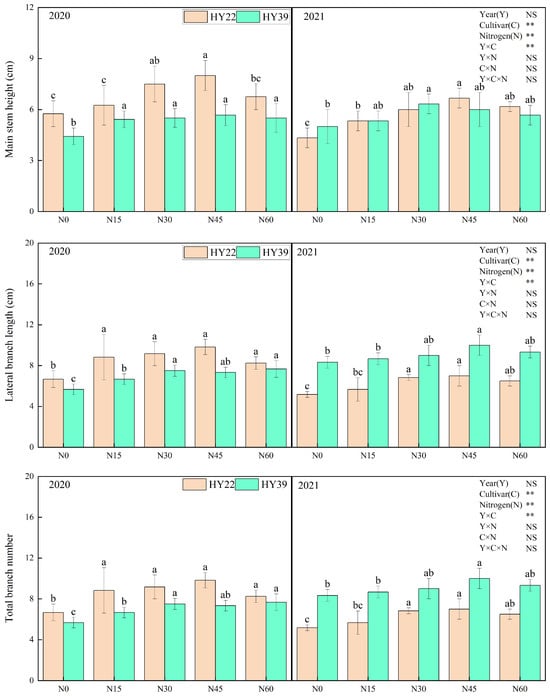
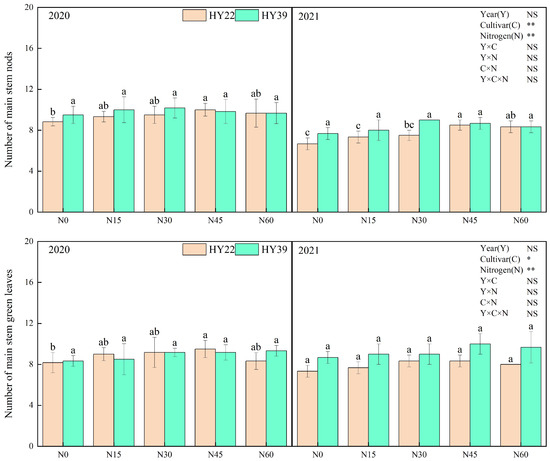
Figure 3.
Peanut plant agronomic characteristics in the 2020 and 2021 growing seasons. Note: values represent the means ± standard deviation (SD, n = 8). The letters (a, b, c) within a column and in the same year indicate significant differences among treatments by Duncan’s range test (p < 0.05). * and ** refer to the same meaning as above; NS means not significant.
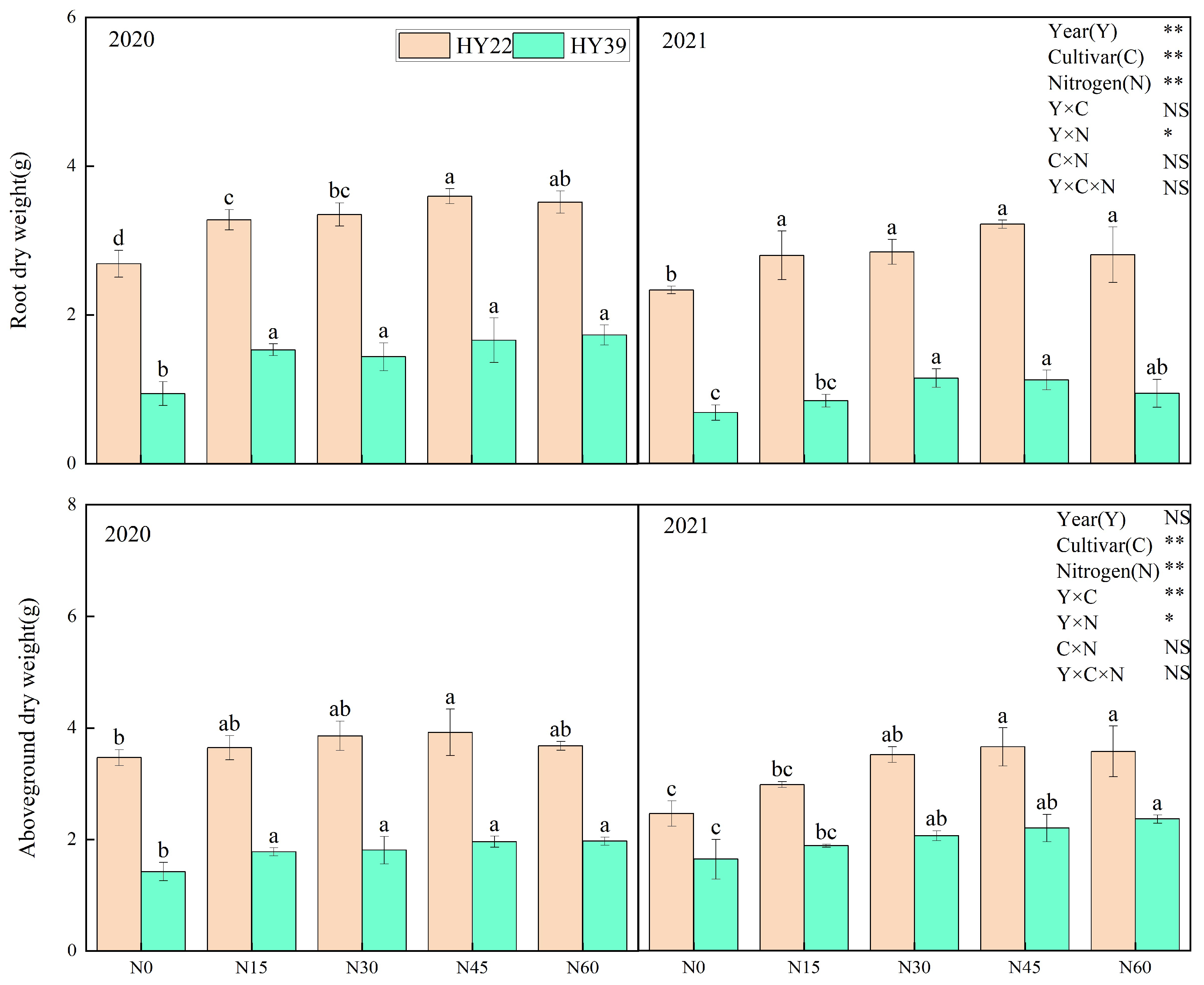
Analysis of variance (ANOVA) showed that year, cultivars, and N treatment had obvious effects on root dry weight, while interaction among the year and N treatment had significant effects. It was clear that the maximum root dry weight in the large-grain HY22 peanut cultivar was N45 conditions over both years and in the order of N45 > N60 > N30 > N15 > N0 in 2020 (Figure 4). The root dry weight of the small-grain HY39 cultivar was significantly decreased under the N0 treatment relative to other treatments in 2020. In the 2021 growing season, the root dry weight under the N30 and N45 treatments was obviously higher compared to the other treatments. Root growth inevitably influences aboveground plant growth. As shown in Figure 4, the aboveground dry weight of these two peanut cultivars rose continuously with increasing nitrogen application to a peak value under the N45 treatment of the HY22 cultivar and under the N60 treatment of the HY39 cultivar.
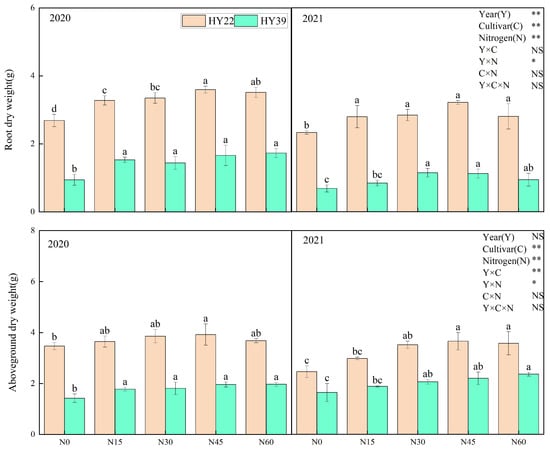
Figure 4.
Peanut root dry weight and aboveground dry weight in the 2020 to 2021 growing seasons. Error bars denote the standard deviation of the mean (SD, n = 6). The letters (a, b, c, d) within a column and in the same year indicate significant differences among treatments by Duncan’s range test (p < 0.05). * and ** are significant at the 0.05 and 0.01 levels; NS means not significant.
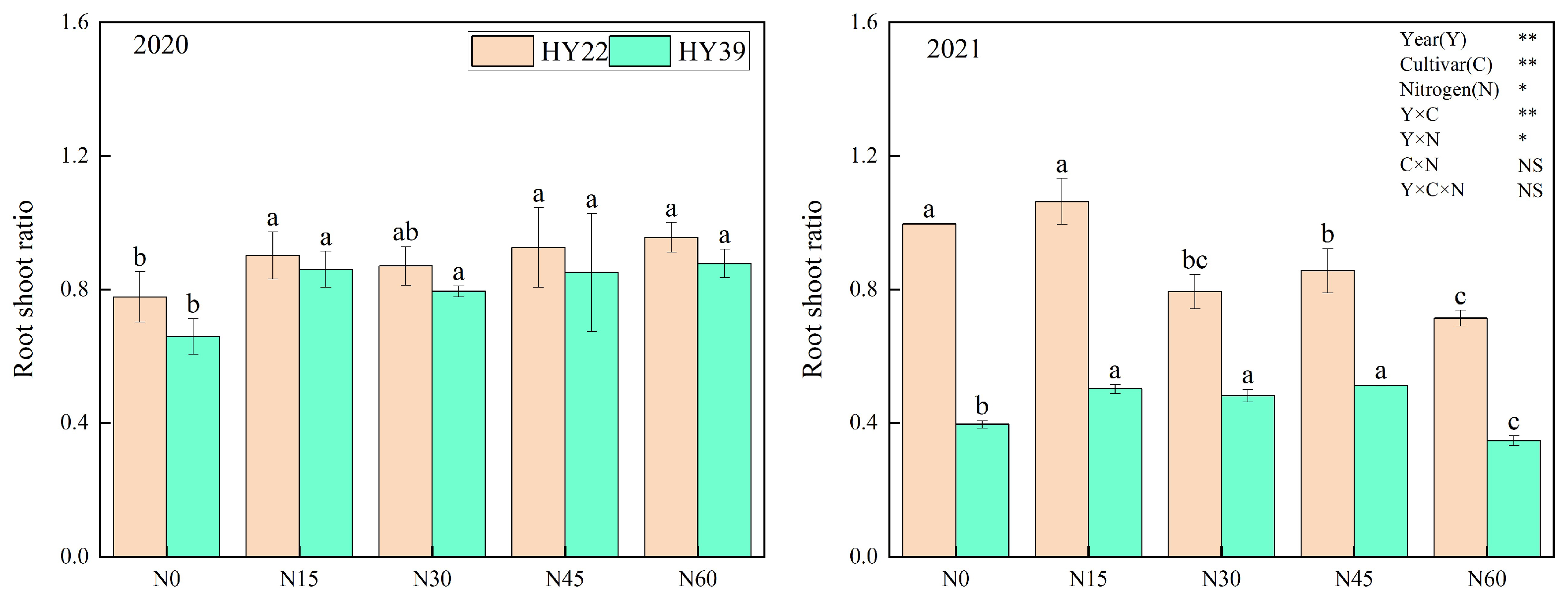
3.5. Peanut Root Shoot Ratio
Analysis of variance (ANOVA) showed that year, cultivar, and N treatment had an obvious influence on root shoot ratio, and the interaction of year and cultivar and N treatment had remarkable effects, whereas the interaction among the cultivar and N treatment had nonsignificant effects. In our study, the root shoot ratio of HY22 and HY39 was higher in N15 than in other treatments (Figure 5). This may be due to the root-foraging response such that it increased dry matter distribution to the belowground root system.
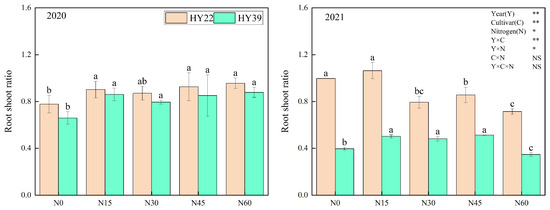
Figure 5.
Peanut root shoot ratio in the 2020 and 2021 growing seasons. Error bars denote the standard deviation of the mean (SD, n = 6). The letters (a, b, c) within a column and in the same year indicate significant differences among treatments by Duncan’s range test (p < 0.05). * and ** are significant at the 0.05 and 0.01 levels; NS means not significant.
3.6. Peanut Nitrogen Content and Ndff
Analysis of variance (ANOVA) showed that the year, cultivar, and N treatment had strong effects on the N content and Ndff of various organs, except for no interaction between year and cultivar; the interaction between the other two significantly affected Ndff. Nitrogen fertilizer inputs significantly increased nitrogen content and Ndff in all organs of peanut compared with the N0 treatment, reaching the maximum at the N60 (Table 5). Compared with N0, the N15, N30, N45, and N60 treatments of HY22 and HY39 in 2020 showed an increase of 6.2%, 8.34%, 15.61%, 20.87% and 2.72%, 7.82%, 27.56%, 32.1% in root nitrogen content, which was consistent with the results of 2021. Ndff represents the percentage of plant nitrogen from 15N-labeled fertilizer and can be used to reflect the competitive ability of plant organs to absorb fertilizer nitrogen. The Ndff of the root, stem, and leaves receiving the different N conditions differed in the following order: N60 > N45 > N30 > N15> N0. The results of 2 years were consistent.

Table 5.
Peanut nitrogen content and Ndff in different organs in the 2020 and 2021 growing seasons.
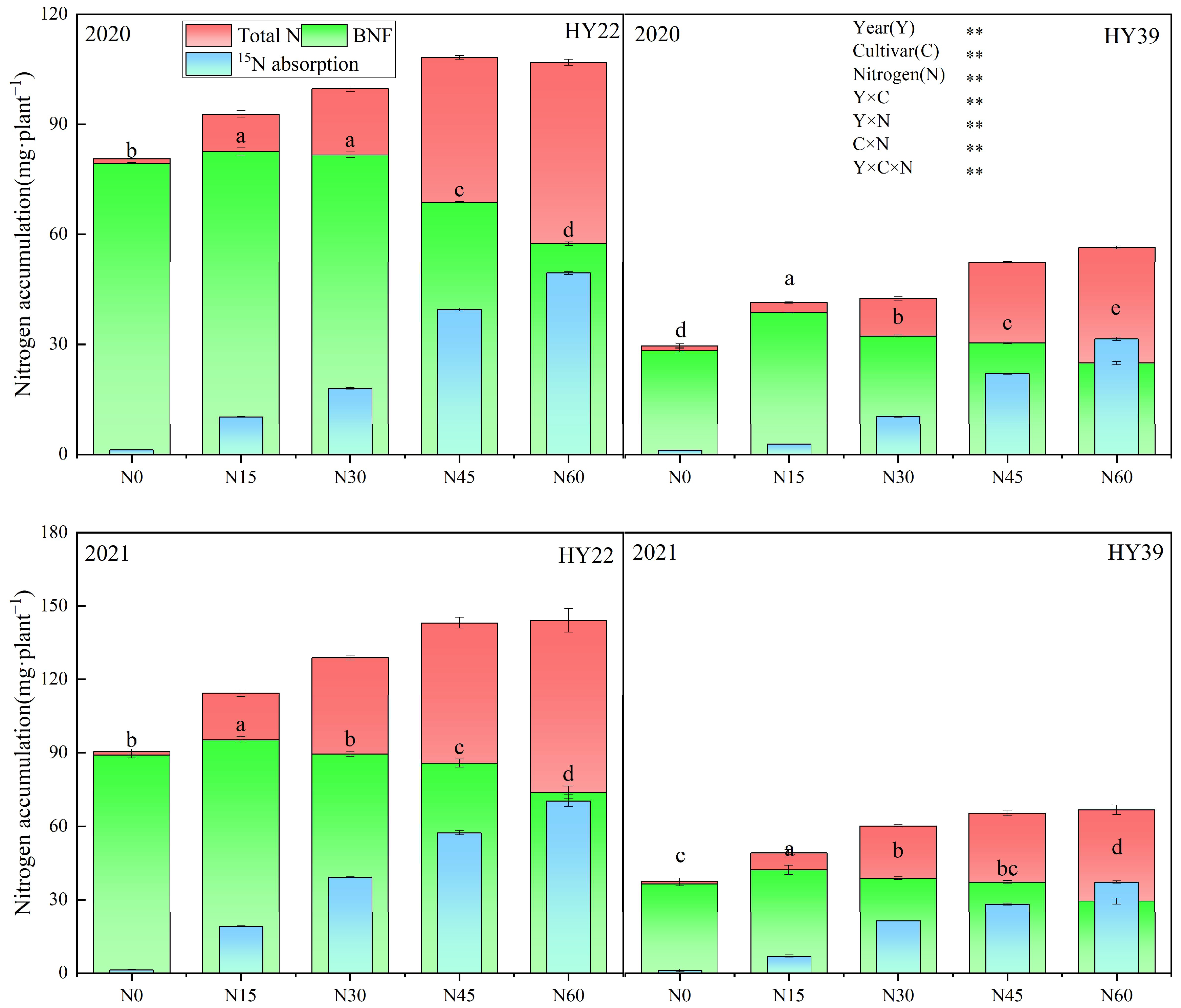
To confirm the above results, the impact of nitrogen levels on nitrogen supply characteristics for the two sources of nitrogen was also assessed in 2020 and 2021 (Figure 6), Analysis of variance (ANOVA) showed that the year, cultivars, and N treatment strongly impacted the BNF, and the interaction between the two and three also had a significant effect. The BNF of HY39 was obviously higher in the N15 treatment, and in the order of N15 > N30 > N45 > N0 > N60. We found that BNF was significantly increased by 10.07%, 16.77%, 24.99% and 42.55% under N15 treatments in 2020, respectively, compared to N30, N45, N0 and N60. And it was increased by 8.86%, 13.6%, 15.69% and 43.36% in 2021, respectively. The HY22 showed a significantly higher BNF under N15 and N30 in 2020, with an observed BNF of 82.58 and 81.66 mg·plant−1, respectively. But the maximum BNF was observed under the N15 treatment in 2021. Accordingly, these results suggest that smaller (N15) initial nitrogen concentrations can help increase the proportion of nitrogen fixed by peanut root nodules.
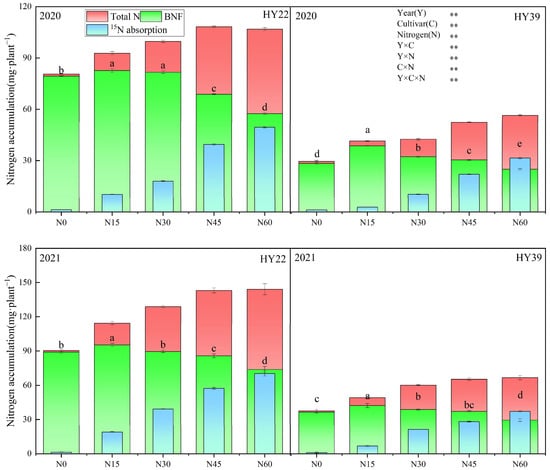
Figure 6.
Peanut nitrogen accumulation from different nitrogen sources in the 2020 and 2021 growing seasons. Error bars denote the SD of the mean (SD, n = 6). The letters (a, b, c, d, e) within a column and in the same year indicate significant differences among treatments by Duncan’s range test (p < 0.05). ** are significant at the 0.01 levels. Total N, Total N accumulation; BNF, biological nitrogen fixation; 15N absorption, Plant 15N fertilizer absorption.
3.7. Pearson Correlations for Key Indices of Root Growth Strategy and BNF
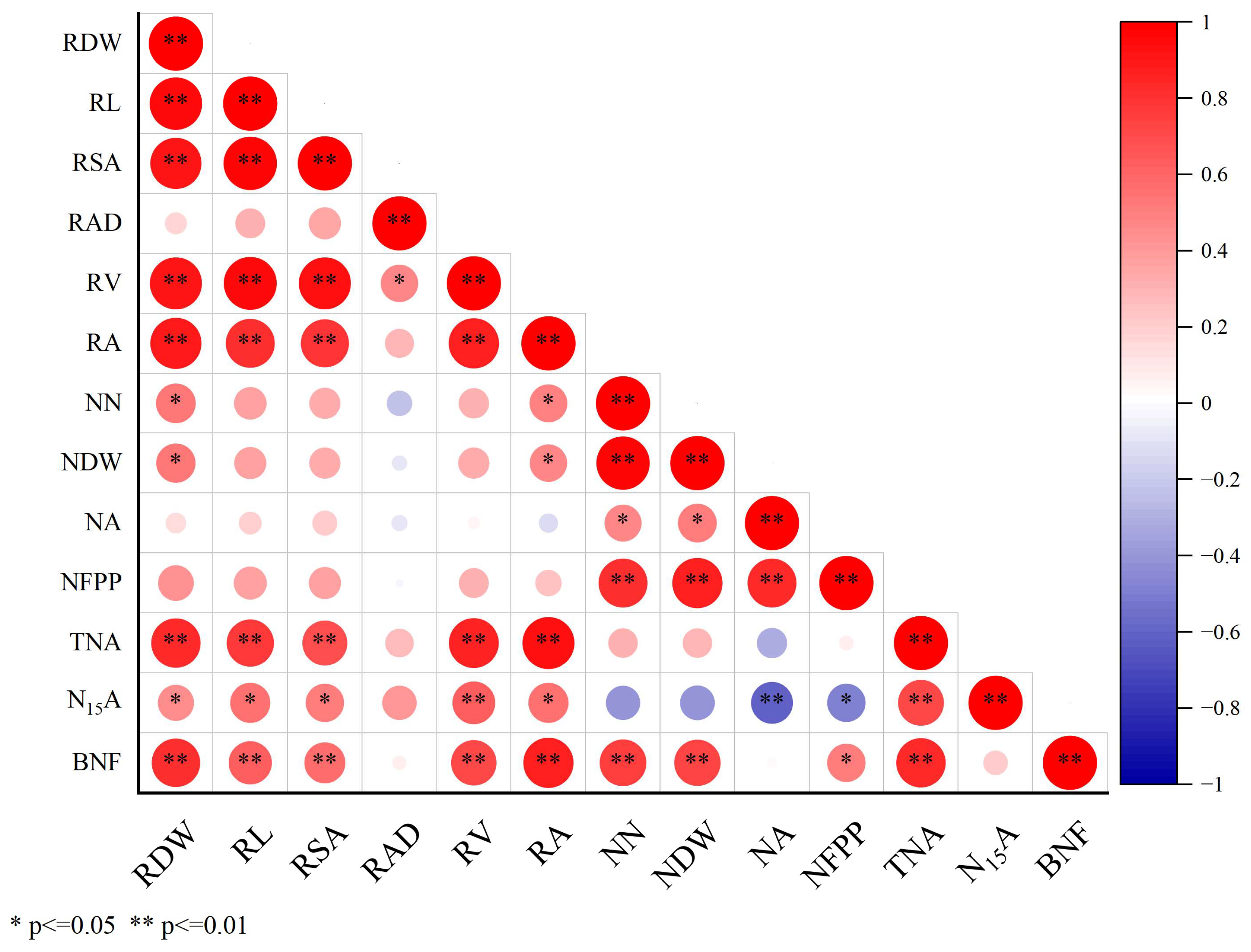
Figure 7 showed that the increase in nodule dry weight was obviously positively associated with both the root dry weight and root activity. The increase in BNF was also significantly positively associated with the root dry weight, root length, root activity, nodule number, nodule dry weight and total nitrogen accumulation. This suggests that variation in the peanut root system may impact nodulation and biological nitrogen fixation. The root system provides both the space and nutrient supply for nodules, thus increasing BNF.
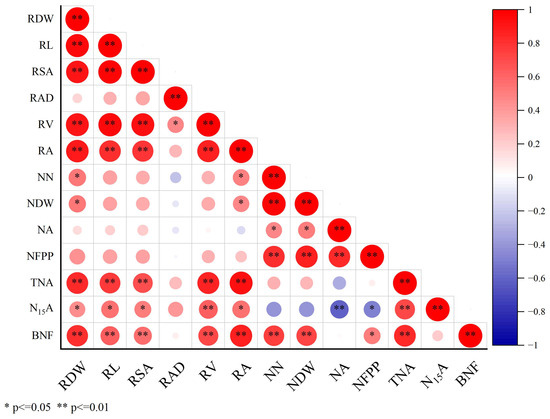
Figure 7.
Pearson correlations for key indexes. RDW, root dry weight; RL, root length; RV, root volume; RSA, root surface area; RAD, root average diameter; RA, root activity; NN, nodule number; NA, nitrogenase activity; NDW, nodule dry weight; TNA, total nitrogen accumulation; N15A, 15N absorption; BNF, biological nitrogen fixation; NFPP, nitrogen fixation potential per plant. * and ** are significant at the 0.05 and 0.01 levels.
4. Discussion
4.1. Peanut Root Growth
Nutrient availability is particularly important for root morphological characteristics [19]. Nitrogen is mainly available to plants in the form of NO3--N and NH4+-N, which can influence root surface area, length, and volume in a manner that shapes root morphological development during early growth phases, in turn shaping the efficiency with which roots take up nutrients [20]. In our study, the application of exogenous nitrogen obviously enhanced the root dry weight and volume of the peanut. Studies have shown that the change in root architecture mainly depends on the growth of lateral roots, which can develop in the direction of relative nutrient enrichment in nutrient-poor soils [21]. The peanut root system is not only an important nutrient absorption organ but also a carrier of nodule attachment, which affects peanut nodulation and nitrogen fixation. Meier [22] found that local ammonium supply promotes lateral root emergence. The point to emphasize here is that the key regulatory components of lateral root formation are utilized by legume plants to form nodules [23]. In our study, during the early stage of peanut root growth, 45 kg·hm−2 initial nitrogen application significantly enhanced root dry weight, and volume for the HY22 and HY39. However, 45 kg·hm−2 initial nitrogen application significantly decreased nodule formation. This indicates that when the nitrogen concentration exceeds 15 kg·hm−2, the nitrogen absorbed by peanuts is preferentially allocated to root growth rather than forming nodules. Consequently, 15 kg·hm−2 exogenous nitrogen significantly promoted lateral root elongation, increased root dry matter accumulation, and had a promoting effect on nodule formation.
Our research shows that the root/shoot ratio declined under high levels of nitrogen application, and the root activity, and root/shoot ratios, under the N15 were obviously higher compared to other treatments over the two years. This is generally in line with the findings of others [24]. In general, under nitrogen stress, crops will increase the distribution of assimilates to enhance water and nutrient uptake, thus increasing the relative dry weight of the root system and the root/shoot ratios. On the contrary, nitrogen sufficiency conditions lead to a decrease in the root/shoot ratios [25]. Although the initial nitrogen dose of 45 kg·hm−2 can promote root development, it has already had an inhibitory effect on root nodule formation, but it is obvious that low nitrogen(N15) significantly increases root activity, promotes root nutrient absorption, increases root to shoot ratio, and influences below-ground partitioning to improve root system plasticity. In addition, the nodulation process of legumes is very sensitive to the available nitrogen in the environment. Legumes can adjust their nitrogen fixation efficacy in a timely manner according to the availability of carbon sources in their bodies, thus maintaining the carbon and nitrogen balance in the plant and adapting to changes in the surrounding environment [26]. Root growth inevitably influences aboveground plant growth. Nitrogen application increased the N content and Ndff of stems and leaves, which in turn increased main stem height, total branch number, main stem nods number, and main stem green leaves, and increased the accumulation of aboveground dry matter. This is consistent with previous research [27,28].
4.2. Biological Nitrogen Fixation
Large amounts of nitrogen fertilizer inputs at one time in China can inhibit nitrogen fixation in peanuts, and slow and controlled addition of nitrogen fertilizer can reduce the inhibitory effect of inorganic nitrogen fertilizer on root nodule infection. Analysis of nitrogen supply parameters of different nitrogen sources further showed that BNF was significantly higher in both peanut varieties under N15 treatment. We found that BNF was significantly increased by 10.07–42.55% under N15 treatments in 2020, respectively, compared to other N treatments. Our research shows that the number of root nodules, root dry weight, ARA, and BNF potential per plant under the N15 was obviously higher compared to other N treatments over the two years. The nitrogenase activity under N15 treatment conditions was increased by 11.9–25.88% above N0 levels, and the single-plant nitrogen fixation potential was increased by 13.86–18.85%. These findings suggest that small (N15) doses of nitrogen can improve the proportion of nitrogen fixed by root nodules, thus providing key source of nitrogen for peanut growth. A few research efforts to date have explored the stimulating effects of small nitrogen doses on soybean seedling growth and early nodulation. For example, Gan [29] found that high mineral N concentrations (10 mM) in the form of nitrate, ammonium, or ammonium nitrate resulted in the significant suppression of root nodule numbers and dry weight values, whereas lower concentrations (1 or 3.75 mM) obviously improved nodule numbers, total N2 fixed per plant. Kato [30] additionally determined that lotus root nodule nitrogenase activity were markedly inhibited at 27 h after nitrate addition, with both nitrogenase activity and nodule growth being suppressed by nitrate nitrogen in a manner related to the timing and location of the nitrogen application.
Peanuts grow rapidly during the seedling stage and exhibit some degree of nitrogen requirement during this period despite the fact that associated rhizobia lack the ability to fix nitrogen. Initial nitrogen application above this value of N15 at the seedling stage inhibits nitrogen fixation. In our study, numbers of root nodules and associated dry weight continuously declined as the exogenous initial nitrogen concentration increased, demonstrating that excessive nitrogen can significantly inhibit the BNF capacity of peanut plants in line with prior evidence [31,32]. In conclusion, the present results suggest that the initial nitrogen dose applied for the large-grain HY22 cultivar and small-grain HY39 cultivar should be approximately 15 kg·hm−2. Above these levels, increased nitrogen fertilizer application will adversely impact peanut plant BNF activity.
4.3. The Association between Biological Nitrogen Fixation and Root Growth Strategy
Roots and rhizomes are closely linked physically, the root systems of legumes form symbiotic nodules with rhizobacteria, and the root system provides a nutrient-rich ecological niche for nodules development that promotes biological nitrogen fixation. With both environmental nitrogen and microbial signals regulating the optimal generation of lateral roots and nodules in leguminous crops [33]. Our studies showed that the increase in BNF was obviously positively associated with the root dry weight, root surface area, nodule number, and total nitrogen accumulation. This suggests that changes in the peanut root system may impact nodulation and biological nitrogen fixation. Recent work has shown that nodules also influence changes in root architecture. Root nodules alter root system responses to changes in external nitrate levels [34]. These nodules can both directly influence plant phenotypes through the regulation of plant hormones and other signals that shape changes in plant root systems, while also indirectly influencing them through changes in stress responses as in the case of the shortening of root length due to nitrogen fixation in low nitrogen environments [35].
5. Conclusions
A two-year pot optimization experiment showed that initial nitrogen application of 15 kg·hm−2 could improve the nitrogenase activity, nitrogen fixation potential per plant, and BNF. Therefore, based on the results of this experiment, the N15 treatment may be the most appropriate N concentration to promote the establishment of a symbiotic nitrogen fixation system, especially at the seedling stage of the plant.
Author Contributions
Y.L. (Ying Liu): Conceptualization, methodology, software, formal analysis, investigation, data curation, writing the original draft. Z.Y., J.Z. (Jihao Zhao)., J.W., Y.L. (Yiyang Liu) and J.Z. (Jie Zou): data curation, supervision, visualization. L.L., J.Z. (Jialei Zhang). and S.W.: conceptualization, resources, writing review and editing, supervision, funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
Shandong Province Key Research and Development Program (2022CXPT031, ZFJH202310), Taishan Scholars Program, National Modern Agricultural Industry Technology System Funding (CARS-13), Shandong Province Agricultural Science Major Scientific and Technological Achievements Cultivation Project (CXGC2023E01).
Data Availability Statement
The collected data are mainly contained in the tables and figures in the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Liu, K.; Meng, M.; Zhang, T.; Chen, Y.; Yuan, H.; Su, T. Quantitative Analysis of Source-Sink Relationships in Two Potato Varieties under Different Nitrogen Application Rates. Agronomy 2023, 13, 1083. [Google Scholar] [CrossRef]
- Tatli, S.; Mirzaee-Ghaleh, E.; Rabbani, H.; Karami, H.; Wilson, A.D. Rapid Detection of Urea Fertilizer Effects on VOC Emissions from Cucumber Fruits Using a MOS E-Nose Sensor Array. Agronomy 2022, 12, 35. [Google Scholar] [CrossRef]
- Roy, S.; Liu, W.; Nandety, R.S.; Crook, A.; Mysore, K.S.; Pislariu, C.I.; Frugoli, J.; Dickstein, R.; Udvardi, M.K. Celebrating 20 Years of Genetic Discoveries in Legume Nodulation and Symbiotic Nitrogen Fixation. Plant Cell 2020, 32, 15–41. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.B.; Zheng, Y.M.; Shen, P.; Zheng, Y.P.; Wu, Z.F.; Sun, X.W.; Yu, T.Y.; Feng, H. Determining N supplied sources and N use efficiency for peanut under applications of four forms of N fertilizers labeled by isotope 15N. J. Integr. Agric. 2016, 15, 432–439. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, L.; Wan, Y.; Liu, F.; Zhang, K. Rational Nitrogen Strategies Can Improve Peanut Source Supply Capacity and Pod Yield. Agron. J. 2017, 109, 2927–2935. [Google Scholar] [CrossRef]
- Lin, J.S.; Li, X.; Luo, Z.; Mysore, K.S.; Wen, J.; Xie, F. NIN interacts with NLPs to mediate nitrate inhibition of nodulation in Medicago truncatula. Nat. Plants 2018, 4, 942–952. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.P.; Miwa, H.; Obirih-Opareh, J.; Suzaki, T.; Yasuda, M.; Okazaki, S. Novel rhizobia exhibit superior nodulation and biological nitrogen fixation even under high nitrate concentrations. FEMS Microbiol. Ecol. 2020, 96, fiz184. [Google Scholar] [CrossRef]
- Mondal, M.; Skalicky, M.; Garai, S.; Hossain, A.; Sarkar, S.; Banerjee, H.; Kundu, R.; Brestic, M.; Barutcular, C.; Erman, M.; et al. Supplementing Nitrogen in Combination with Rhizobium Inoculation and Soil Mulch in Peanut (Arachis hypogaea L.) Production System: Part II. Effect on Phenology, Growth, Yield Attributes, Pod Quality, Profitability and Nitrogen Use Efficiency. Agronomy 2020, 10, 1513. [Google Scholar] [CrossRef]
- Lai, H.; Gao, F.; Su, H.; Zheng, P.; Li, Y.; Yao, H. Nitrogen Distribution and Soil Microbial Community Characteristics in a Legume–Cereal Intercropping System: A Review. Agronomy 2022, 12, 1900. [Google Scholar] [CrossRef]
- Yong, T.W.; Chen, P.; Dong, Q.; Du, Q.; Yang, F.; Wang, X.C. Optimized nitrogen application methods to improve nitrogen use efficiency and nodule nitrogen fixation in a maize-soybean relay intercropping system. J. Integr. Agric. 2018, 17, 664–676. [Google Scholar] [CrossRef]
- Saito, A.; Tanabata, S.; Tanabata, T.; Tajima, S.; Ueno, M.; Ishikawa, S.; Ohtake, N.; Sueyoshi, K.; Ohyama, T. Effect of nitrate on nodule and root growth of soybean (Glycine max (L.) Merr.). Int. J. Mol. Sci. 2014, 15, 4464–4480. [Google Scholar] [CrossRef]
- Wang, Q.; Huang, Y.; Ren, Z.; Zhang, X.; Ren, J.; Su, J.; Zhang, C.; Tian, J.; Yu, Y.; Gao, G.F.; et al. Transfer cells mediate nitrate uptake to control root nodule symbiosis. Nat. Plants 2020, 6, 800–808. [Google Scholar] [CrossRef] [PubMed]
- Bais, J.; Kandel, H.; DeSutter, T.; Deckard, E.; Keene, C. Soybean Response to N Fertilization Compared with Co-Inoculation of Bradyrhizobium japonicum and Azospirillum brasilense. Agronomy 2023, 13, 2022. [Google Scholar] [CrossRef]
- Dumroese, R.K.; Jacobs, D.F.; Davis, A.S. Inoculating Acacia koa with Bradyrhizobium and Applying Fertilizer in the Nursery: Effects on Nodule Formation and Seedling Growth. HortScience 2009, 44, 443–446. [Google Scholar] [CrossRef]
- Shao, Z.Q.; Zheng, C.C.; Johannes, A.P.; Lu, W.L.; Gao, Q.; Gao, Y.Z.; Zhang, J.J. Nitrogen acquisition, fixation and transfer in maize/alfalfa intercrops are increased through root contact and morphological responses to interspecies competition. J. Integr. Agric. 2021, 20, 2240–2254. [Google Scholar] [CrossRef]
- Yang, Y.; Zhao, Q.; Li, X.; Ai, W.; Liu, D.; Qi, W.; Zhang, M.; Yang, C.; Liao, H. Characterization of Genetic Basis on Synergistic Interactions between Root Architecture and Biological Nitrogen Fixation in Soybean. Front. Plant Sci. 2017, 8, 1466. [Google Scholar] [CrossRef]
- Xiu, L.; Zhang, W.; Wu, D.; Sun, Y.; Zhang, H.; Gu, W.; Wang, Y.; Meng, J.; Chen, W. Biochar can improve biological nitrogen fixation by altering the root growth strategy of soybean in Albic soil. Sci. Total Environ. 2021, 773, 144564. [Google Scholar] [CrossRef]
- López-Bucio, J.; Cruz-Ramírez, A.; Herrera-Estrella, L. The role of nutrient availability in regulating root architecture. Curr. Opin. Plant Biol. 2003, 6, 280–287. [Google Scholar] [CrossRef]
- Sinha, S.K.; Kumar, A.; Tyagi, A.; Venkatesh, K.; Paul, D.; Singh, N.K.; Mandal, P.K. Root architecture traits variation and nitrate-influx responses in diverse wheat genotypes under different external nitrogen concentrations. Plant Physiol. Biochem. 2020, 148, 246–259. [Google Scholar] [CrossRef]
- Pereira, E.G.; de Lima, B.R.; Medeiros, L.R.A.; Ribeiro, S.A.; Bucher, C.A.; Santos, L.A.; Fernandes, M.S.; Rossetto, C.A.V. Nutripriming with ammonium nitrate improves emergence and root architecture and promotes an increase in nitrogen content in upland rice seedlings. Biocatal. Agric. Biotechnol. 2022, 42, 102331. [Google Scholar] [CrossRef]
- Giehl, R.F.; Gruber, B.D.; von Wirén, N. It’s time to make changes: Modulation of root system architecture by nutrient signals. J. Exp. Bot. 2014, 65, 769–778. [Google Scholar] [CrossRef] [PubMed]
- Meier, M.; Liu, Y.; Lay-Pruitt, K.S.; Takahashi, H. Auxin-mediated root branching is determined by the form of available nitrogen. Nat. Plants 2020, 6, 1136–1145. [Google Scholar] [CrossRef]
- Soyano, T.; Shimoda, Y.; Kawaguchi, M.; Hayashi, M. A shared gene drives lateral root development and root nodule symbiosis pathways in Lotus. Science 2019, 366, 1021–1023. [Google Scholar] [CrossRef]
- Chen, J.; Liu, L.; Wang, Z.; Zhang, Y.; Sun, H.; Song, S.; Bai, Z.; Lu, Z.; Li, C. Nitrogen Fertilization Increases Root Growth and Coordinates the Root-Shoot Relationship in Cotton. Front. Plant Sci. 2020, 11, 880. [Google Scholar] [CrossRef] [PubMed]
- Gheysari, M.; Mirlatifi, S.M.; Bannayan, M. Interaction of water and nitrogen on maize grown for silage. Agric. Water Manag. 2009, 96, 809–821. [Google Scholar] [CrossRef]
- Ke, X.; Xiao, H.; Peng, Y.; Wang, J.; Lv, Q.; Wang, X. Phosphoenolpyruvate reallocation links nitrogen fixation rates to root nodule energy state. Science 2022, 378, 971–977. [Google Scholar] [CrossRef]
- Yang, Y.; Qin, Q.; Li, Q.; Nangia, V.; Lan, B.; Mo, F.; Liao, Y.; Liu, Y. Effect of Nitrogen Management on Wheat Yield, Water and Nitrogen Utilization, and Economic Benefits under Ridge-Furrow Cropping System with Supplementary Irrigation. Agronomy 2023, 13, 1708. [Google Scholar] [CrossRef]
- Zhou, S.; Liu, K.; Zhuo, X.; Wang, W.; Zhang, W.; Zhang, H.; Gu, J.; Yang, J.; Liu, L. Optimizing Nitrogen Regime Improves Dry Matter and Nitrogen Accumulation during Grain Filling to Increase Rice Yield. Agronomy 2023, 13, 1983. [Google Scholar] [CrossRef]
- Gan, Y.; Stulen, I.; van Keulen, H. Low concentrations of nitrate and ammonium stimulate nodulation and N2 fixation while inhibiting specific nodulation (nodule DW g−1 root dry weight) and specific N2 fixation (N2 fixed g−1 root dry weight) in soybean. Plant Soil. 2004, 258, 281–292. [Google Scholar] [CrossRef]
- Kato, K.; Kanahama, K.; Kanayama, Y. Involvement of nitric oxide in the inhibition of nitrogenase activity by nitrate in Lotus root nodules. J. Plant Physiol. 2010, 167, 238–241. [Google Scholar] [CrossRef]
- Lin, J.; Roswanjaya, Y.P.; Kohlen, W.; Stougaard, J.; Reid, D. Nitrate restricts nodule organogenesis through inhibition of cytokinin biosynthesis in Lotus japonicus. Nat. Commun. 2021, 12, 6544. [Google Scholar] [CrossRef]
- Nishida, H.; Nosaki, S.; Suzuki, T.; Ito, M.; Miyakawa, T.; Nomoto, M.; Tada, Y.; Miura, K.; Tanokura, M.; Kawaguchi, M.; et al. Different DNA-binding specificities of NLP and NIN transcription factors underlie nitrate-induced control of root nodulation. Plant Cell. 2021, 33, 2340–2359. [Google Scholar] [CrossRef] [PubMed]
- Schwember, A.R.; Schulz, J.; Del Pozo, A.; Cabeza, R.A. Regulation of Symbiotic Nitrogen Fixation in Legume Root Nodules. Plants 2019, 8, 333. [Google Scholar] [CrossRef] [PubMed]
- Goh, C.H.; Veliz, V.D. The impact of beneficial plant-associated microbes on plant phenotypic plasticity. J. Chem. Ecol. 2013, 39, 826–839. [Google Scholar] [CrossRef] [PubMed]
- Band, L.R.; Ubeda-Tomas, S.; Dyson, R.J.; Middleton, A.M.; Hodgman, T.C.; Owen, M.R.; Jensen, O.E.; Bennett, M.J.; King, J.R. Growth-induced hormone dilution can explain the dynamics of plant root cell elongation. Proc. Natl. Acad. Sci. USA 2012, 109, 7577–7582. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).