Interactions and Regulatory Functions of Phenolics in Soil-Plant-Climate Nexus
Abstract
1. Introduction
2. Bio-Synthesis of Phenolic Compounds in Plant System
3. Antioxidant Properties of Phenols
- I
- 2FlavOH + H2O2 → 2FlavO·+ 2H2O
- II
- 2FlavO + (asc) → 2FlavOH + 2MDA (monodehydroascorbate radical)
- III
- MDA + MDA → (asc) + DHA
- IV
- Summary of the three steps of reaction: H2O2 + (asc) → 2H2O + DHA
4. Role of Phenol and Phenolic Compounds in Plant Systems
5. Regulatory and Inhibitory Interactions of Phenolic Compounds in the Rhizospheric Soil Matrix
6. Phenolic Compounds in Plant-Pathogen Interaction
7. Allelopathic Interactions
8. Phenolic Compounds from Agro-Industrial Byproducts
9. Phenol as Contaminants
10. Response of Phenolics to Climate Change
11. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Choudhary, S.; Singh Tanwer, B.; Vijayvergia, R.; Singh, T. Preliminary phytochemical screening and primary metabolites of Melothria maderaspatana (Linn.) Cong. Int. J. Biol. Pharm. Res. 2013, 4, 168–171. [Google Scholar]
- Wolfe, N.L.; Hoehamer, C.F.; Mccutcheon, S.C.; Schnoor, J.L. Enzymes Used by Plants and Microorganisms to Detoxify Organic Compounds. In A Wiley-Interscience Series of Texts and Monographs; McCutcheon, S.C., Schnoor, J.L., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2004; pp. 159–187. [Google Scholar] [CrossRef]
- Nicholson, R.L.; Hammerschmidt, R. Phenolic Compounds and their Role in Disease Resistance. Annu. Rev. Phytopathol. 2003, 369–389. [Google Scholar] [CrossRef]
- Crozier, A.; Clifford, M.; Ashihara, H. Plant Secondary Metabolites: Occurrence, Structure and Role in The Human Diet. Q. Rev. Biol. 2014, 82, 151. [Google Scholar] [CrossRef]
- Seigler, D.S. Plant Secondary Metabolism; Springer: New York, NY, USA, 1998. [Google Scholar] [CrossRef]
- Hounsome, N.; Hounsome, B.; Tomos, D.; Edwards-Jones, G. Plant Metabolites and Nutritional Quality of Vegetables. J. Food. Sci. 2008, 73, R48–R65. [Google Scholar] [CrossRef]
- Irchhaiya, R.; Kumar, A.; Yadav, A.; Gupta, N.; Kumar, S.; Gupta, N.; Kumar, S.; Yadav, V.; Prakash, A.; Gurjar, H. Metabolites In Plants and Its Classification. World. J. Pharm. Pharm. Sci. 2015, 4, 287–305. [Google Scholar]
- Maffei, M.E.; Mithöfer, A.; Boland, W. Before Gene Expression: Early Events in Plant–Insect Interaction. Trends. Plant. Sci 2007, 12, 310–316. [Google Scholar] [CrossRef]
- Rasmann, S.; Turlings, T.C.J.; Rasmann, S.; Turlings, T.C.J. First Insights into Specificity of Belowground Tritrophic Interactions. Oikos 2008, 117, 362–369. [Google Scholar] [CrossRef]
- Shitan, N. Secondary Metabolites in Plants: Transport and Self-Tolerance Mechanisms. Biosci. Biotechnol. Biochem. 2016, 80, 1283–1293. [Google Scholar] [CrossRef]
- Goldberg, G.; British Nutrition Foundation. Plants: Diet and Health: The Report of a British Nutrition Foundation Task Force; Cambridge University Press: Cambridge, UK, 2003; p. 347. [Google Scholar]
- Maldonado-Bonilla, L.D.; Betancourt-Jiménez, M.; Lozoya-Gloria, E. Local and Systemic Gene Expression of Sesquiterpene Phytoalexin Biosynthetic Enzymes in Plant Leaves. Eur. J. Plant. Pathol. 2008, 121, 439–449. [Google Scholar] [CrossRef]
- Bohlmann, J.; Stauber, E.J.; Krock, B.; Oldham, N.J.; Gershenzon, J.; Baldwin, I.T. Gene Expression of 5-Epi-Aristolochene Synthase and Formation of Capsidiol in Roots of Nicotiana attenuata and N. sylvestris. Phytochemistry 2002, 60, 109–116. [Google Scholar] [CrossRef]
- Marchiosi, R.; dos Santos, W.D.; Constantin, R.P.; de Lima, R.B.; Soares, A.R.; Finger-Teixeira, A.; Mota, T.R.; de Oliveira, D.M.; Foletto-Felipe, M.d.P.; Abrahão, J.; et al. Biosynthesis and Metabolic Actions of Simple Phenolic Acids in Plants. Phytochem. Rev. 2020, 19, 865–906. [Google Scholar] [CrossRef]
- Hättenschwiler, S.; Vitousek, P.M. The Role of Polyphenols in Terrestrial Ecosystem Nutrient Cycling. Trends. Ecol. Evol. 2000, 15, 238–242. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, K.M. The Shikimate Pathway: Early Steps in the Biosynthesis of Aromatic Compounds. Plant. Cell 1995, 7, 907. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Dudareva, N. The Shikimate Pathway and Aromatic Amino Acid Biosynthesis in Plants. Annu. Rev. Plant. Biol. 2012, 63, 73–105. [Google Scholar] [CrossRef]
- Mandal, S.M.; Chakraborty, D.; Dey, S. Phenolic Acids Act as Signaling Molecules in Plant-Microbe Symbioses. Plant. Signal. Behav. 2010, 5, 359–368. [Google Scholar] [CrossRef]
- Koukol, J.; Conn, E.E. The Metabolism of Aromatic Compounds in Higher Plants: IV. Purification and Properties of the Phenylalanine Deaminase of Hordeum vulgare. J. Biol. Chem. 1961, 236, 2692–2698. [Google Scholar] [CrossRef]
- de Oliveira, D.M.; Finger-Teixeira, A.; Rodrigues Mota, T.; Salvador, V.H.; Moreira-Vilar, F.C.; Correa Molinari, H.B.; Craig Mitchell, R.A.; Marchiosi, R.; Ferrarese-Filho, O.; Dantas dos Santos, W. Ferulic Acid: A Key Component in Grass Lignocellulose Recalcitrance to Hydrolysis. Plant. Biotechnol. J. 2015, 13, 1224–1232. [Google Scholar] [CrossRef]
- Appert, C.; Zoń, J.; Amrhein, N. Kinetic Analysis of the Inhibition of Phenylalanine Ammonia-Lyase by 2-Aminoindan-2-Phosphonic Acid and other Phenylalanine Analogues. Phytochemistry 2003, 62, 415–422. [Google Scholar] [CrossRef]
- Hisaminato, H.; Murata, M.; Homma, S. Relationship between the Enzymatic Browning and Phenylalanine Ammonia-Lyase Activity of Cut Lettuce, and the Prevention of Browning by Inhibitors of Polyphenol Biosynthesis. Biosci. Biotechnol. Biochem. 2001, 65, 1016–1021. [Google Scholar] [CrossRef]
- Keski-Saari, S. Phenolic Compounds in Birch Seedlings during Early Ontogeny: Regulation of Biosynthesis and Accumulation in Response to Nutrient Availability and UV-B Radiation. Ph.D. Thesis, University of Joensuu, Joensuu, Finland, 2005. [Google Scholar]
- Ghasemzadeh, A.; Ghasemzadeh, N. Flavonoids and Phenolic Acids: Role and Biochemical Activity in Plants and Human. J. Med. Plant. Res. 2011, 5, 6697–6703. [Google Scholar] [CrossRef]
- Sies, H.; Jones, D.P. Reactive Oxygen Species (ROS) as Pleiotropic Physiological Signalling Agents. Nat. Rev. Mol. Cell. Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef] [PubMed]
- Michalak, A. Phenolic Compounds and Their Antioxidant Activity in Plants Growing under Heavy Metal Stress. Pol. J. Environ. Stud. 2006, 15, 523–530. [Google Scholar]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive Oxygen Species, Oxidative Damage, and Antioxidative Defense Mechanism in Plants under Stressful Conditions. J. Bot. 2012, 2012, 217037. [Google Scholar] [CrossRef]
- Lea, A.J.; Tung, J.; Zhou, X. A Flexible, Efficient Binomial Mixed Model for Identifying Differential DNA Methylation in Bisulfite Sequencing Data. PLoS Genet 2015, 11, e1005650. [Google Scholar] [CrossRef]
- Urso, M.L.; Clarkson, P.M. Oxidative Stress, Exercise, and Antioxidant Supplementation. Toxicology 2003, 189, 41–54. [Google Scholar] [CrossRef]
- Halliwell, B. Effect of Diet on Cancer Development: Is Oxidative DNA Damage a Biomarker? Free. Radic. Biol. Med. 2002, 32, 968–974. [Google Scholar] [CrossRef]
- Shin, S.Y.; Kim, I.S.; Kim, Y.S.; Lee, H.; Yoon, H.S. Ectopic Expression of Brassica rapa L. MDHAR Increased Tolerance to Freezing Stress by Enhancing Antioxidant Systems of Host Plants. S. Afr. J. Bot. 2013, 88, 388–400. [Google Scholar] [CrossRef]
- Lafka, T.I.; Sinanoglou, V.; Lazos, E.S. On the Extraction and Antioxidant Activity of Phenolic Compounds from Winery Wastes. Food. Chem. 2007, 104, 1206–1214. [Google Scholar] [CrossRef]
- Andarwulan, N.; Fardiaz, D.; Wattimena, G.A.; Shetty, K. Antioxidant Activity Associated with Lipid and Phenolic Mobilization during Seed Germination of Pangium edule Reinw. J. Agric. Food. Chem. 1999, 47, 3158–3163. [Google Scholar] [CrossRef]
- Tsaliki, E.; Lagouri, V.; Doxastakis, G. Evaluation of the Antioxidant Activity of Lupin Seed Flour and Derivatives (Lupinus. albus ssp. graecus). Food. Chem. 1999, 65, 71–75. [Google Scholar] [CrossRef]
- Takahama, U.; Oniki, T. Flavonoids and Some Other Phenolics as Substrates of Peroxidase: Physiological Significance of the Redox Reactions. J. Plant. Res. 2000, 113, 301–309. [Google Scholar] [CrossRef]
- Yamasaki, H.; Sakihama, Y.; Ikehara, N. Flavonoid-Peroxidase Reaction as a Detoxification Mechanism of Plant Cells against H2O2. Plant. Physiol. 1997, 115, 1405–1412. [Google Scholar] [CrossRef] [PubMed]
- Takahama, U.; Oniki, T. A Peroxidase/Phenolics/Ascorbate System can Scavenge Hydrogen Peroxide in Plant Cells. Physiol. Plant 1997, 101, 845–852. [Google Scholar] [CrossRef]
- Sakihama, Y.; Cohen, M.F.; Grace, S.C.; Yamasaki, H. Plant Phenolic Antioxidant and Prooxidant Activities: Phenolics-Induced Oxidative Damage Mediated by Metals in Plants. Toxicology 2002, 177, 67–80. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Mumper, R.J. Plant Phenolics: Extraction, Analysis and their Antioxidant and Anticancer Properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef] [PubMed]
- Inhibition of Lung Carcinogenesis by Black Tea in Fischer Rats Treated with a Tobacco-Specific Carcinogen: Caffeine as an Important Constituent1|Cancer Research|American Association for Cancer Research. Available online: https://aacrjournals.org/cancerres/article/58/18/4096/504549/Inhibition-of-Lung-Carcinogenesis-by-Black-Tea-in (accessed on 31 October 2022).
- Effects of Tea, Decaffeinated Tea, and Caffeine on UVB Light-Induced Complete Carcinogenesis in SKH-1 Mice: Demonstration of Caffeine as a Biologically Important Constitutent of Tea1|Cancer Research|American Association for Cancer Research. Available online: https://aacrjournals.org/cancerres/article/57/13/2623/503367/Effects-of-Tea-Decaffeinated-Tea-and-Caffeine-on (accessed on 31 October 2022).
- Eghbaliferiz, S.; Iranshahi, M. Prooxidant Activity of Polyphenols, Flavonoids, Anthocyanins and Carotenoids: Updated Review of Mechanisms and Catalyzing Metals. Phytother. Res. 2016, 30, 1379–1391. [Google Scholar] [CrossRef]
- Curir, P.; VanSumere, C.F.; Termini, A.; Barthe, P.; Marchesini, A.; Dolci, M. Flavonoid Accumulation Is Correlated with Adventitious Roots Formation in Eucalyptus gunnii Hook Micropropagated through Axillary Bud Stimulation. Plant. Physiol. 1990, 92, 1148–1153. [Google Scholar] [CrossRef]
- Kevers, C.; Coumans, M.; Coumans-Gillès, M.-F.; Caspar, T. Physiological and Biochemical Events Leading to Vitrification of Plants Cultured in vitro. Physiol. Plant 1984, 61, 69–74. [Google Scholar] [CrossRef]
- Talaat, I.M.; Khattab, H.I.; Ahmed, A.M. Changes in Growth, Hormones Levels and Essential Oil Content of Ammi. visnaga L. Plants Treated with Some Bioregulators. Saudi. J. Biol. Sci. 2014, 21, 355–365. [Google Scholar] [CrossRef]
- Takahama, U. Oxidation of Vacuolar and Apoplastic Phenolic Substrates by Peroxidase: Physiological Significance of the Oxidation Reactions. Phytochem. Rev. 2004, 3, 207–219. [Google Scholar] [CrossRef]
- Rice, E.L. Allelopathy—An Overview. In Chemically Mediated Interactions between Plants and Other Organismss; Springer: Boston, MA, USA, 1985; pp. 81–105. [Google Scholar] [CrossRef]
- Vidhyasekaran, P. Physiology of Disease Resistance in Plants; CRC Press, Inc.: Boca Raton, FL, USA, 1988; Volume 2. [Google Scholar]
- Waterman, P.G.; Mole, S. Extrinsic Factors Influencing Production of Secondary Metabolites in Plants. In Insect-Plant Interactions; CRC Press: Boca Raton, FL, USA, 2019; pp. 107–134. [Google Scholar] [CrossRef]
- Akhtar, M.; Malik, A. Roles of Organic Soil Amendments and Soil Organisms in the Biological Control of Plant-Parasitic Nematodes: A Review. Bioresour. Technol. 2000, 74, 35–47. [Google Scholar] [CrossRef]
- Lawson, C.G.R.; Rolfe, B.G.; Djordjevic, M.A. Rhizobium Inoculation Induces Condition-Dependent Changes in the Flavonoid Composition of Root Exudates from Trifolium subterraneum. Funct. Plant. Biol. 1996, 23, 93–101. [Google Scholar] [CrossRef]
- Patrick, Z.A.; Patrick, A.Z. Phytotoxic Substances Associated with the Decomposition in Soil of Plant Residues. Soils 1971, 111, 13–18. [Google Scholar] [CrossRef]
- Lodhi, M.A.K. Soil-Plant Phytotoxicity and its Possible Significance in Patterning of Herbaceous Vegetation in a Bottomland Forest. Am. J. Bot. 1975, 62, 618–622. [Google Scholar] [CrossRef]
- Chou, C.H.; Lin, H.J. Autointoxication Mechanism of Oryza sativa I. Phytotoxic Effects of Decomposing Rice Residues in Soil. J. Chem. Ecol. 1976, 2, 353–367. [Google Scholar] [CrossRef]
- Elliott, L.F.; Cheng, H.H. Assessment of Allelopathy among Microbes and Plants; American Chemical Society: Washington, DC, USA, 1987; pp. 504–515. [Google Scholar] [CrossRef]
- Makoi, J.H.J.R.; Ndakidemi, P.A. Biological, Ecological and Agronomic Significance of Plant Phenolic Compounds in Rhizosphere of the Symbiotic Legumes. Afr. J. Biotechnol. 2010, 6, 1358–1368. [Google Scholar] [CrossRef]
- Fog, K. The Effect of Added Nitrogen on the Rate of Decomposition of Organic Matter. Biol. Rev. 1988, 63, 433–462. [Google Scholar] [CrossRef]
- Castells, E. Indirect Effects of Phenolics on Plant Performance by Altering Nitrogen Cycling: Another Mechanism of Plant-Plant Negative Interactions. In Allelopathy in Sustainable Agriculture and Forestry; Springer: New York, NY, USA, 2008; pp. 137–156. [Google Scholar] [CrossRef]
- Phoenix, A Model of The Dynamics of Carbon and Nitrogen in Grassland Soils on JSTOR. Available online: https://www.jstor.org/stable/45128653#metadata_info_tab_contents (accessed on 25 December 2022).
- Kuiters, A.T. Role of Phenolic Substances from Decomposing Forest Litter in Plant-Soil Interactions. Acta. Bot. Neerl. 1990, 39, 329–348. [Google Scholar] [CrossRef]
- Das, N.; Chandran, P. Microbial Degradation of Petroleum Hydrocarbon Contaminants: An Overview. Biotechnol. Res. Int. 2011, 2011, 941810. [Google Scholar] [CrossRef]
- Bennett, R.N.; Wallsgrove, R.M. Secondary Metabolites in Plant Defence Mechanisms. New. Phytol. 1994, 127, 617–633. [Google Scholar] [CrossRef]
- The Chemical Defenses of Plants to Pathogens and Herbivores on JSTOR. Available online: https://www.jstor.org/stable/2096863#metadata_info_tab_contents (accessed on 25 December 2022).
- Yoneyama, K.; Natsume, M. Allelochemicals for Plant–Plant and Plant–Microbe Interactions. Compr. Nat. Prod. II. Chem. Biol. 2010, 4, 539–561. [Google Scholar] [CrossRef]
- Dakora, F.D. Defining New Roles for Plant and Rhizobial Molecules in Sole and Mixed Plant Cultures Involving Symbiotic Legumes. New. Phytol. 2003, 158, 39–49. [Google Scholar] [CrossRef]
- Singh, R.K.; Kumar, D.P.; Singh, P.; Solanki, M.K.; Srivastava, S.; Kashyap, P.L.; Kumar, S.; Srivastava, A.K.; Singhal, P.K.; Arora, D.K. Multifarious Plant Growth Promoting Characteristics of Chickpea Rhizosphere Associated Bacilli Help to Suppress Soil-Borne Pathogens. Plant. Growth. Regul. 2014, 73, 91–101. [Google Scholar] [CrossRef]
- Min, K.; Freeman, C.; Kang, H.; Choi, S.U. The Regulation by Phenolic Compounds of Soil Organic Matter Dynamics under a Changing Environment. Biomed. Res. Int. 2015, 2015. [Google Scholar] [CrossRef]
- Schmidt, M.A.; Halvorson, J.J.; Hagerman, A.E.; Gonzalez, J.M. Macronutrients and Metals Released from Soils by Solutions of Naturally Occurring Phenols. J. Plant. Nutr. Soil. Sci. 2017, 180, 544–553. [Google Scholar] [CrossRef]
- Grilli, E.; di Resta, E.; Scognamiglio, M.; Pacifico, S.; Fiorentino, A.; Nogueira, T.A.R.; Vigliotti, R.C.; Ganga, A. Soil Phenolic Compound Variability in Two Mediterranean Olive Groves. Plant. Soil. Environ. 2020, 66, 207–215. [Google Scholar] [CrossRef]
- Schmidt, M.W.I.; Torn, M.S.; Abiven, S.; Dittmar, T.; Guggenberger, G.; Janssens, I.A.; Kleber, M.; Kögel-Knabner, I.; Lehmann, J.; Manning, D.A.C.; et al. Persistence of Soil Organic Matter as an Ecosystem Property. Nature 2011, 478, 49–56. [Google Scholar] [CrossRef]
- Rani, P.U.; Jyothsna, Y. Biochemical and Enzymatic Changes in Rice Plants as a Mechanism of Defense. Acta. Physiol. Plant 2010, 32, 695–701. [Google Scholar] [CrossRef]
- Suflita, J.M.; Bollag, J.-M. Polymerization of Phenolic Compounds by a Soil-Enzyme Complex. Soil. Sci. Soc. Am. J. 1981, 45, 297–302. [Google Scholar] [CrossRef]
- Bajaj, M.; Gallert, C.; Winter, J. Phenol Degradation Kinetics of an Aerobic Mixed Culture. Biochem. Eng. J. 2009, 46, 205–209. [Google Scholar] [CrossRef]
- Biodegradation Kinetics of Benzene, Toluene, and Phenol as Single and Mixed Substrates for Pseudomonas putida F1.|Semantic Scholar. Available online: https://www.semanticscholar.org/paper/Biodegradation-kinetics-of-benzene%2C-toluene%2C-and-as-Reardon-Mosteller/d1f912457207a500e71c334fd48b07ea2f126a6c (accessed on 24 October 2022).
- Ma, F.; Shi, S.N.; Sun, T.H.; Li, A.; Zhou, J.T.; Qu, Y.Y. Biotransformation of Benzene and Toluene to Catechols by Phenol Hydroxylase from Arthrobacter sp. W1. Appl. Microbiol. Biotechnol. 2013, 97, 5097–5103. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, L.; Jin, Q.; Su, C.; Zhao, L.; Liu, X.; Kou, S.; Wang, Y.; Xiao, M. Bioremediation of Phenol in Soil through Using a Mobile Plant-Endophyte System. Chemosphere 2017, 182, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, Y.; Yan, N.; Chen, J.; Rittmann, B.E. Enhanced Phenol Bioavailability by Means of Photocatalysis. Biodegradation 2013, 24, 597–602. [Google Scholar] [CrossRef] [PubMed]
- Grunditz, C.; Dalhammar, G. Development of Nitrification Inhibition Assays Using Pure Cultures of Nitrosomonas and Nitrobacter. Water. Res. 2001, 35, 433–440. [Google Scholar] [CrossRef]
- McCarty, G.W.; Bremner, J.M.; Schmidt, E.L. Effects of Phenolic Acids on Ammonia Oxidation by Terrestrial Autotrophic Nitrifying Microorganisms. FEMS Microbiol. Lett. 1991, 85, 345–349. [Google Scholar] [CrossRef]
- Chen, L.C.; Guan, X.; Wang, Q.K.; Yang, Q.P.; Zhang, W.D.; Wang, S.L. Effects of Phenolic Acids on Soil Nitrogen Mineralization over Successive Rotations in Chinese Fir Plantations. J. For. Res. (Harbin.) 2020, 31, 303–311. [Google Scholar] [CrossRef]
- Hu, H.; Tang, C.; Rengel, Z. Influence of Phenolic Acids on Phosphorus Mobilisation in Acidic and Calcareous Soils. Plant. Soil. 2005, 268, 173–180. [Google Scholar] [CrossRef]
- Li, J.; Liu, J.; Lu, H.; Jia, H.; Yu, J.; Hong, H.; Yan, C. Influence of the Phenols on the Biogeochemical Behavior of Cadmium in the Mangrove Sediment. Chemosphere 2016, 144, 2206–2213. [Google Scholar] [CrossRef]
- Adeboye, P.T.; Bettiga, M.; Olsson, L. The Chemical Nature of Phenolic Compounds Determines Their Toxicity and Induces Distinct Physiological Responses in Saccharomyces cerevisiae in Lignocellulose Hydrolysates. AMB Express 2014, 4, 46. [Google Scholar] [CrossRef]
- War, A.R.; Paulraj, M.G.; War, M.Y.; Ignacimuthu, S. Jasmonic Acid-Mediated-Induced Resistance in Groundnut (Arachis Hypogaea L.) Against Helicoverpa armigera (Hubner) (Lepidoptera: Noctuidae). J. Plant. Growth. Regul. 2011, 30, 512–523. [Google Scholar] [CrossRef]
- Sharma, H.C.; Sujana, G.; Manohar Rao, D. Morphological and Chemical Components of Resistance to Pod Borer, Helicoverpa. armigera in Wild Relatives of Pigeon pea. Arthropod. Plant. Interact 2009, 3, 151–161. [Google Scholar] [CrossRef]
- Barakat, A.; Bagniewska-Zadworna, A.; Frost, C.J.; Carlson, J.E. Phylogeny and Expression Profiling of CAD and CAD-like Genes in Hybrid Populus (P. deltoides × P. nigra): Evidence from Herbivore Damage for Subfunctionalization and Functional Divergence. BMC Plant. Biol. 2010, 10, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.T.J.; Smith, S.D.; Rausher, M.D. Plant Sex and the Evolution of Plant Defenses against Herbivores. Proc. Natl. Acad. Sci. USA 2009, 106, 18079–18084. [Google Scholar] [CrossRef] [PubMed]
- Bhonwong, A.; Stout, M.J.; Attajarusit, J.; Tantasawat, P. Defensive Role of Tomato Polyphenol Oxidases against Cotton Bollworm (Helicoverpa. armigera) and Beet Armyworm (Spodoptera exigua). J. Chem. Ecol. 2009, 35, 28–38. [Google Scholar] [CrossRef]
- Duffey, S.S.; Stout, M.J. Antinutritive and Toxic Components of Plant Defense Against Insects. Arch. Insect. Biochem. Physiol. 1996, 32, 3. [Google Scholar] [CrossRef]
- Simmonds, M.S.J. Flavonoid-Insect Interactions: Recent Advances in Our Knowledge. Phytochemistry 2003, 64, 21–30. [Google Scholar] [CrossRef]
- Abebe, W. Review on Plant Defense Mechanisms Against Insect Pests. Int. J. Nov. Res. Interdiscip. Stud. 2021, 8, 15–39. [Google Scholar]
- Cheeke, P.R. Toxicants of Plant Origin; CRC Press: Boca Raton, FL, USA, 1989; ISBN 9780849369933. [Google Scholar]
- Wójcicka, A. Introduction Cereal Phenolic Compounds as Biopesticides of Cereal Aphids. J. Environ. Stud. 2010, 19, 1337–1343. [Google Scholar]
- Luczynski, A.; Isman, M.B.; Raworth, D.A. Strawberry Foliar Phenolics and Their Relationship to Development of the Twospotted Spider Mite. J. Econ. Entomol. 1990, 83, 557–563. [Google Scholar] [CrossRef]
- Maxwell, F.G.; Lafever, H.N.; Jenkins, J.N. Blister Beetles on Glandless Cotton. J. Econ. Entomol. 1965, 58, 792–793. [Google Scholar] [CrossRef]
- Baldwin, I.T. Mechanism of Damage-Induced Alkaloid Production in Wild Tobacco. J. Chem. Ecol. 1989, 15, 1661–1680. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, A.; Yazaki, K. Root Exudates of Legume Plants and Their Involvement in Interactions with Soil Microbes; Springer: Berlin/Heidelberg, Germany, 2012; pp. 27–48. [Google Scholar] [CrossRef]
- Berestetskiy, A.O. A Review of Fungal Phytotoxins: From Basic Studies to Practical Use. Appl. Biochem. Microbiol. 2008, 44, 453–465. [Google Scholar] [CrossRef]
- Polyak, Y.M.; Sukcharevich, V.I. Allelopathic Interactions between Plants and Microorganisms in Soil Ecosystems. Biol. Bull. Rev. 2020, 9, 562–574. [Google Scholar] [CrossRef]
- Daguerre, Y.; Siegel, K.; Edel-Hermann, V.; Steinberg, C. Fungal Proteins and Genes Associated with Biocontrol Mechanisms of Soil-Borne Pathogens: A Review. Fungal. Biol. Rev. 2014, 28, 97–125. [Google Scholar] [CrossRef]
- Chatterton, S.; Punja, Z.K.; Chatterton, S. Chitinase and Beta-1,3-Glucanase Enzyme Production by the Mycoparasite Clonostachys. rosea f. catenulata against Fungal Plant Pathogens. Can. J. Microbiol. 2009, 55, 356–367. [Google Scholar] [CrossRef]
- Inderjit. Plant Phenolics in Allelopathy. Botanical. Rev. 1996, 62, 186–202. [Google Scholar] [CrossRef]
- Li, H.H.; Inoue, M.; Nishimura, H.; Mizutani, J.; Tsuzuki, E. Interactions of trans-Cinnamic Acid, Its Related Phenolic Allelochemicals, and Abscisic Acid in Seedling Growth and Seed Germination of Lettuce. J. Chem. Ecol. 1993, 19, 1775–1787. [Google Scholar] [CrossRef]
- John, J.; Sarada, S. Role of Phenolics in Allelopathic Interactions “Statistical Models for Profit Maximization of Homesteads in Kerala” View Project Nutrient Budgeting, Bio-Resource Recycling, Microbial Dynamics and Disease Mapping in Integrated Farming Systems of Wayanad District View Project Role of Phenolics in Allelopathic Interactions. Allelopath. J. 2012, 29, 215–230. [Google Scholar]
- Rice, E.L. Some Roles of Allelopathic Compounds in Plant Communities. Biochem. Syst. Ecol 1977, 5, 201–206. [Google Scholar] [CrossRef]
- Bhatla, S.C.; Lal, M.A. Plant Physiology, Development and Metabolism; Springer: Berlin/Heidelberg, Germany, 2018; pp. 1–1237. [Google Scholar] [CrossRef]
- Hoang Anh, L.; van Quan, N.; Tuan Nghia, L.; Dang Xuan, T. Phenolic Allelochemicals: Achievements, Limitations, and Prospective Approaches in Weed Management. Weed. Biol. Manag. 2021, 21, 37–67. [Google Scholar] [CrossRef]
- Chai, T.T.; Ooh, K.F.; Ooi, P.W.; Chue, P.S.; Wong, F.C. Leucaena leucocephala Leachate Compromised Membrane Integrity, Respiration and Antioxidative Defence of Water Hyacinth Leaf Tissues. Bot. Stud. 2013, 54, 8. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, S.; Asami, T.; Kawano, T.; Yoneyama, K.; Crow, W.D.; Paton, D.M.; Takahashi, N. Photosynthetic Inhibitors in Eucalyptus grandis. Phytochemistry 1988, 27, 1943–1946. [Google Scholar] [CrossRef]
- Moure, A.; Cruz, J.M.; Franco, D.; Manuel Domínguez, J.; Sineiro, J.; Domínguez, H.; Núñez, M.J.; Carlos Parajó, J. Natural Antioxidants from Residual Sources. Food. Chem. 2001, 72, 145–171. [Google Scholar] [CrossRef]
- Ramarathnam, N.; Osawa, T.; Namiki, M.; Kawakishi, S. Chemical Studies on Novel Rice Hull Antioxidants. 2. Identification of Isovitexin, A C-Glycosyl Flavonoid. J. Agric. Food. Chem. 1989, 37, 316–319. [Google Scholar] [CrossRef]
- Watanabe, M.; Ohshita, Y.; Tsushida, T. Antioxidant Compounds from Buckwheat (Fagopyrum esculentum Möench) Hulls. J. Agric. Food. Chem. 1997, 45, 1039–1044. [Google Scholar] [CrossRef]
- Takeoka, G.R.; Dao, L.T. Antioxidant Constituents of Almond [Prunus dulcis (Mill.) D.A. Webb] Hulls. J. Agric. Food. Chem. 2003, 51, 496–501. [Google Scholar] [CrossRef]
- Bryngelsson, S.; Mannerstedt-Fogelfors, B.; Kamal-Eldin, A.; Andersson, R.; Dimberg, L.H. Lipids and Antioxidants in Groats and Hulls of Swedish Oats (Avena sativa L.). J. Sci. Food. Agric. 2002, 82, 606–614. [Google Scholar] [CrossRef]
- Balasundram, N.; Sundram, K.; Samman, S. Phenolic Compounds in Plants and Agri-Industrial by-Products: Antioxidant Activity, Occurrence, and Potential Uses. Food. Chem. 2006, 99, 191–203. [Google Scholar] [CrossRef]
- Gorinstein, S.; Martín-Belloso, O.; Park, Y.S.; Haruenkit, R.; Lojek, A.; Íž, M.; Caspi, A.; Libman, I.; Trakhtenberg, S. Comparison of Some Biochemical Characteristics of Different Citrus Fruits. Food. Chem. 2001, 74, 309–315. [Google Scholar] [CrossRef]
- Ćetković, G.; Savatović, S.; Čanadanović-Brunet, J.; Djilas, S.; Vulić, J.; Mandić, A.; Četojević-Simin, D. Valorisation of Phenolic Composition, Antioxidant and Cell Growth Activities of Tomato Waste. Food. Chem. 2012, 133, 938–945. [Google Scholar] [CrossRef]
- Volpi, C.; Bartolini, D.; Brighenti, V.; Galli, F.; Tiecco, M.; Pellati, F.; Clementi, C.; Sardella, R. Antioxidant Power on Dermal Cells by Textiles Dyed with an Onion (Allium cepa L.) Skin Extract. Antioxidants 2021, 10, 1655. [Google Scholar] [CrossRef] [PubMed]
- Wijngaard, H.H.; Ballay, M.; Brunton, N. The Optimisation of Extraction of Antioxidants from Potato Peel by Pressurised Liquids. Food. Chem. 2012, 133, 1123–1130. [Google Scholar] [CrossRef]
- Rodis, P.S.; Karathanos, V.T.; Mantzavinou, A. Partitioning of Olive Oil Antioxidants between Oil and Water Phases. J. Agric. Food. Chem. 2002, 50, 596–601. [Google Scholar] [CrossRef]
- Ranalli, A.; Lucera, L.; Contento, S. Antioxidizing Potency of Phenol Compounds in Olive Oil Mill Wastewater. J. Agric. Food. Chem. 2003, 51, 7636–7641. [Google Scholar] [CrossRef] [PubMed]
- Obied, H.K.; Allen, M.S.; Bedgood, D.R.; Prenzler, P.D.; Robards, K.; Stockmann, R. Bioactivity and Analysis of Biophenols Recovered from Olive Mill Waste. J. Agric. Food. Chem. 2005, 53, 823–837. [Google Scholar] [CrossRef] [PubMed]
- Benavente-García, O.; Castillo, J.; Lorente, J.; Ortuño, A.; del Rio, J.A. Antioxidant Activity of Phenolics Extracted from Olea. europaea L. Leaves. Food. Chem. 2000, 68, 457–462. [Google Scholar] [CrossRef]
- Sánchez-Moreno, C.; Larrauri, J.A.; Saura-Calixto, F. Free Radical Scavenging Capacity and Inhibition of Lipid Oxidation of Wines, Grape Juices and Related Polyphenolic Constituents. Food. Res. Int. 1999, 32, 407–412. [Google Scholar] [CrossRef]
- Tepe, O.; Dursun, A.Y. Combined Effects of External Mass Transfer and Biodegradation Rates on Removal of Phenol by Immobilized Ralstonia eutropha in a Packed Bed Reactor. J. Hazard. Mater 2008, 151, 9–16. [Google Scholar] [CrossRef]
- Stefanakis, A.; Akratos, C.S.; Tsihrintzis, V.A. Vertical Flow Constructed Wetlands: Eco-Engineering Systems for Wastewater and Sludge Treatment. In Vertical Flow Constructed Wetlands: Eco-engineering Systems for Wastewater and Sludge Treatment; Elsevier: Amsterdam, The Netherlands, 2014; pp. 1–378. [Google Scholar] [CrossRef]
- Abdelwahab, O.; Amin, N.K.; El-Ashtoukhy, E.S.Z. Electrochemical Removal of Phenol from Oil Refinery Wastewater. J. Hazard. Mater. 2009, 163, 711–716. [Google Scholar] [CrossRef]
- Costa, C.R.; Botta, C.M.R.; Espindola, E.L.G.; Olivi, P. Electrochemical Treatment of Tannery Wastewater Using DSA Electrodes. J. Hazard. Mater. 2008, 153, 616–627. [Google Scholar] [CrossRef]
- Herouvim, E.; Akratos, C.S.; Tekerlekopoulou, A.; Vayenas, D.v. Treatment of Olive Mill Wastewater in Pilot-Scale Vertical Flow Constructed Wetlands. Ecol. Eng. 2011, 37, 931–939. [Google Scholar] [CrossRef]
- Abira, M.A.; van Bruggen, J.J.A.; Denny, P. Potential of a Tropical Subsurface Constructed Wetland to Remove Phenol from Pre-Treated Pulp and Papermill Wastewater. Water. Sci. Technol. 2005, 51, 173–176. [Google Scholar] [CrossRef]
- Stottmeister, U.; Kuschk, P.; Wiessner, A. Full-Scale Bioremediation and Long-Term Monitoring of a Phenolic Wastewater Disposal Lake. Pure. Appl. Chem. 2010, 82, 161–173. [Google Scholar] [CrossRef]
- Nair, C.I.; Jayachandran, K.; Shashidhar, S. Biodegradation of Phenol. Afr. J. Biotechnol. 2010, 7, 4951–4958. [Google Scholar] [CrossRef]
- Kennedy, L.J.; Vijaya, J.J.; Kayalvizhi, K.; Sekaran, G. Adsorption of Phenol from Aqueous Solutions Using Mesoporous Carbon Prepared by Two-Stage Process. Chem. Eng. J. 2007, 132, 279–287. [Google Scholar] [CrossRef]
- Palma, M.S.A.; Paiva, J.L.; Zilli, M.; Converti, A. Batch Phenol Removal from Methyl Isobutyl Ketone by Liquid–Liquid Extraction with Chemical Reaction. Chem. Eng. Process. Process. Intensif. 2007, 46, 764–768. [Google Scholar] [CrossRef]
- Annadurai, G.; Juang, R.S.; Lee, D.J. Microbiological Degradation of Phenol Using Mixed Liquors of Pseudomonas putida and Activated Sludge. Waste. Manag. 2002, 22, 703–710. [Google Scholar] [CrossRef]
- Mohan, D.; Chander, S. Single Component and Multi-Component Adsorption of Metal Ions by Activated Carbons. Colloids. Surf. A Physicochem. Eng. Asp. 2001, 177, 183–196. [Google Scholar] [CrossRef]
- Dursun, G.; Çiçek, H.; Dursun, A.Y. Adsorption of Phenol from Aqueous Solution by Using Carbonised Beet Pulp. J. Hazard. Mater. 2005, 125, 175–182. [Google Scholar] [CrossRef]
- Sihi, D.; Dari, B.; Kuruvila, A.P.; Jha, G.; Basu, K. Explainable Machine Learning Approach Quantified the Long-Term (1981–2015) Impact of Climate and Soil Properties on Yields of Major Agricultural Crops Across CONUS. Front. Sustain. Food. Syst. 2022, 6, 1–17. [Google Scholar] [CrossRef]
- Hunter, M.D. The Phytochemical Landscape; Princeton University Press: Princeton, NJ, USA, 2016. [Google Scholar]
- Moreira, X.; Roberts, L.A.; Galvez, M.D.H.; González, C.V.; Ramos, I.M.P. Micro-Climatic Effects on Plant Phenolics at the Community Level in a Mediterranean Savanna. Sci. Rep. 2020, 10, 14757. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Li, J.; Jin, P.; Li, X.; Wang, L.; Zheng, Y. The Effect of Temperature on Phenolic Content in Wounded Carrots. Food. Chem. 2017, 215, 116–123. [Google Scholar] [CrossRef]
- Zargoosh, Z.; Ghavam, M.; Bacchetta, G.; Tavili, A. Effects of Ecological Factors on the Antioxidant Potential and Total Phenol Content of Scrophularia striata Boiss. Sci. Rep. 2019, 9, 16021. [Google Scholar] [CrossRef] [PubMed]
- Agati, G.; Tattini, M. Multiple Functional Roles of Flavonoids in Photoprotection. New Phytol. 2010, 186, 786–793. [Google Scholar] [CrossRef] [PubMed]
- Hatier, J.H.B.; Gould, K.S. Foliar Anthocyanins as Modulators of Stress Signals. J. Theor. Biol. 2008, 253, 625–627. [Google Scholar] [CrossRef]
- Varela, M.C.; Arslan, I.; Reginato, M.A.; Cenzano, A.M.; Luna, M.V. Phenolic Compounds as Indicators of Drought Resistance in Shrubs from Patagonian Shrublands (Argentina). Plant. Physiol. Biochem. 2016, 104, 81–91. [Google Scholar] [CrossRef]
- Schmidt, D.D.; Voelckel, C.; Hartl, M.; Schmidt, S.; Baldwin, I.T. Specificity in Ecological Interactions. Attack from the Same Lepidopteran Herbivore Results in Species-Specific Transcriptional Responses in Two Solanaceous Host Plants. Plant. Physiol. 2005, 138, 1763–1773. [Google Scholar] [CrossRef]
- de Sousa Araújo, T.A.; de Almeida e Castro, V.T.N.; da Silva Solon, L.G.; da Silva, G.A.; das Graças Almeida, M.; da Costa, J.G.M.; de Amorim, E.L.C.; Albuquerque, U.P. Does Rainfall Affect the Antioxidant Capacity and Production of Phenolic Compounds of an Important Medicinal Species? Ind. Crops. Prod. 2015, 76, 550–556. [Google Scholar] [CrossRef]
- Aninbon, C.; Jogloy, S.; Vorasoot, N.; Patanothai, A.; Nuchadomrong, S.; Senawong, T. Effect of End of Season Water Deficit on Phenolic Compounds in Peanut Genotypes with Different Levels of Resistance to Drought. Food. Chem. 2016, 196, 123–129. [Google Scholar] [CrossRef]
- Goudriaan, J.; Unsworth, M.H. Implications of Increasing Carbon Dioxide and Climate Change for Agricultural Productivity and Water Resources. Impact. Carbon. Dioxide. Trace. Gases. Clim. Chang. Glob. Agric. 2016, 53, 111–130. [Google Scholar] [CrossRef]
- Klunklin, W.; Savage, G. Effect on Quality Characteristics of Tomatoes Grown under Well-Watered and Drought Stress Conditions. Foods 2017, 6, 56. [Google Scholar] [CrossRef] [PubMed]
- Rajabbeigi, E.; Eichholz, I.; Beesk, N.; Ulrichs, C.; Kroh, L.W.; Rohn, S.; Huyskens-Keil, S. Interaction of Drought Stress and UV-B Radiation-Impact on Biomass Production and Flavonoid Metabolism in Lettuce (Lactuca sativa L.). J. Appl. Bot. Food. Qual. 2013, 86, 190–197. [Google Scholar] [CrossRef]
- Živanović, B.; Vidović, M.; Milić Komić, S.; Jovanović, L.; Kolarž, P.; Morina, F.; Veljović Jovanović, S. Contents of Phenolics and Carotenoids in Tomato Grown under Polytunnels with Different UV-Transmission Rates. Turk. J. Agric. For. 2017, 41, 113–120. [Google Scholar] [CrossRef]
- Waśkiewicz, A.; Muzolf-Panek, M.; Goliński, P. Phenolic Content Changes in Plants under Salt Stress; Springer: New York, NY, USA, 2013. [Google Scholar]
- Sztatelman, O.; Grzyb, J.; Gabryś, H.; Banaś, A.K. The Effect of UV-B on Arabidopsis Leaves Depends on Light Conditions after Treatment. BMC Plant. Biol. 2015, 15, 281. [Google Scholar] [CrossRef]
- Kusano, M.; Tohge, T.; Fukushima, A.; Kobayashi, M.; Hayashi, N.; Otsuki, H.; Kondou, Y.; Goto, H.; Kawashima, M.; Matsuda, F.; et al. Metabolomics Reveals Comprehensive Reprogramming Involving Two Independent Metabolic Responses of Arabidopsis to UV-B Light. Plant. J. 2011, 67, 354–369. [Google Scholar] [CrossRef]
- Eseberri, I.; Trepiana, J.; Léniz, A.; Gómez-García, I.; Carr-Ugarte, H.; González, M.; Portillo, M.P. Variability in the Beneficial Effects of Phenolic Compounds: A Review. Nutrients 2022, 14, 1925. [Google Scholar] [CrossRef]
- Classen, A.E.T.; Sundqvist, M.K.; Henning, J.A.; Newman, G.S.; Moore, J.A.M.; Cregger, M.A.; Moorhead, L.C.; Patterson, C.M.; Sundqvist, M.K.; Henning, J.A.; et al. Direct and Indirect Effects of Climate Change on Soil Microbial and Soil Microbial-Plant Interactions: What Lies Ahead? Ecosphere 2015, 6, 1–21. [Google Scholar] [CrossRef]
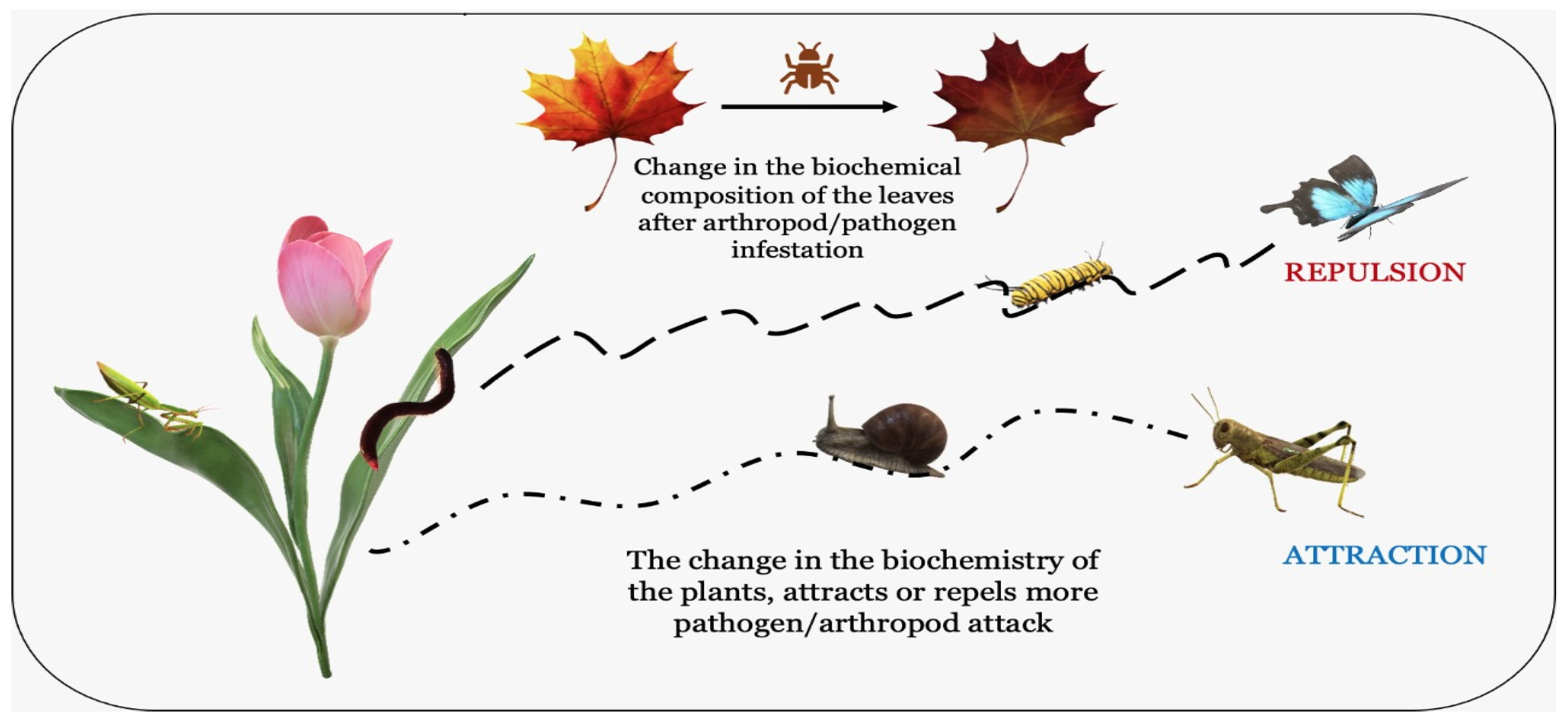

| Phenolic Compounds | Some Examples with Their Structure | Function or Mechanism of Action | Reference |
|---|---|---|---|
| C6 compounds Simple Phenols
| 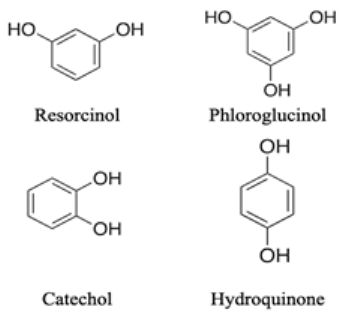 | Have various inhibitory effects against enzyme activities. Catechol acts as inhibitors of phosphorylase. The acylphloroglucinol structure might play a significant role in inhibiting plant transpiration. | [107,108] |
| C6-Cn compounds n = 1
| 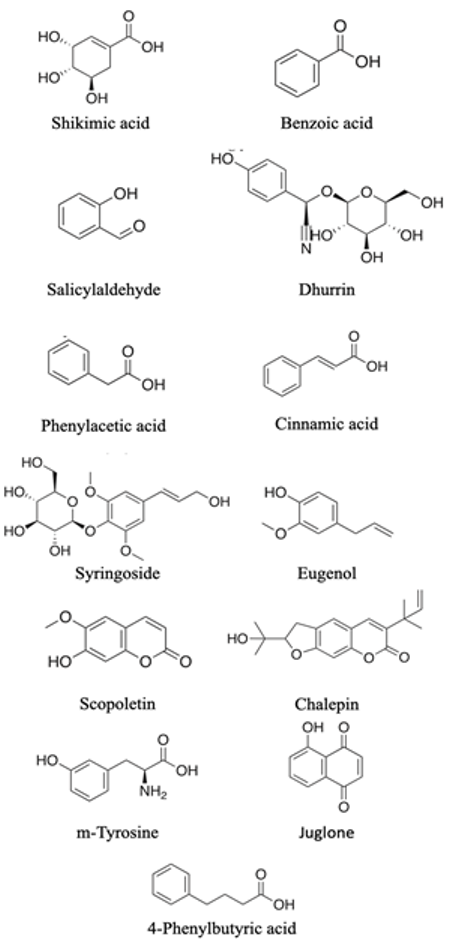 | Effect on membrane permeability hormonal activity, respiration, photosynthetic activity, synthesis of organic compounds and plant growth as a whole | [108] |
C6-Cn-C6 compounds
n = 2
| 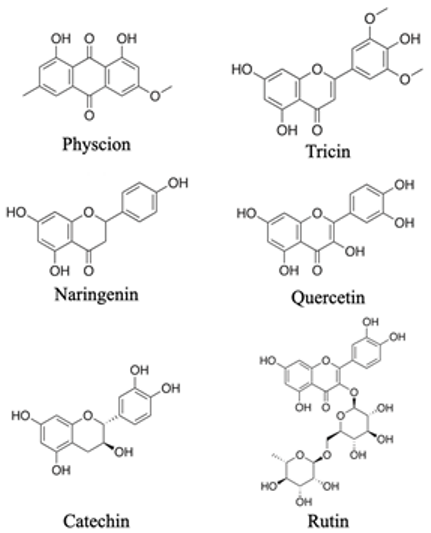 | Inhibit the germination of seeds and growth of plants. Release of flavonoid compounds into the rhizosphere plays an important role in the supply of phosphorus to plants. | [97] |
| Phenolic dimers (C6-C3)2 Compounds
| 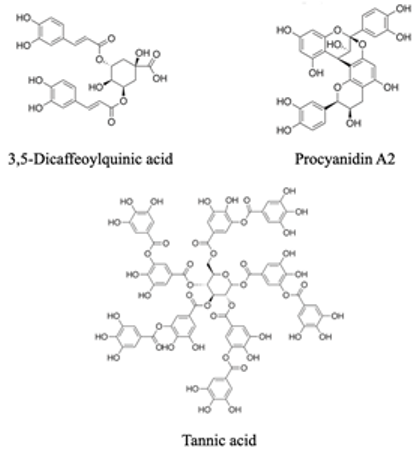 | Tannic acid is an inhibitor of peroxidase, catalase, and cellulase | [97] |
Others
| 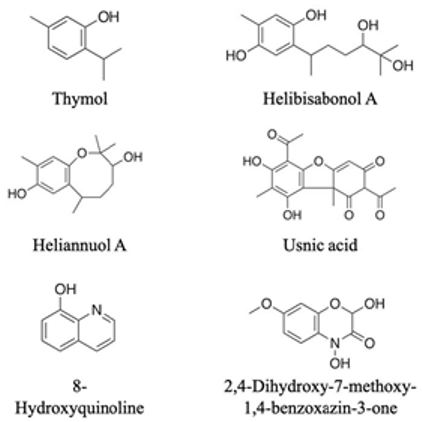 | Effect on all mitosis stages, respiration, and membrane permeability | [108,109] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Misra, D.; Dutta, W.; Jha, G.; Ray, P. Interactions and Regulatory Functions of Phenolics in Soil-Plant-Climate Nexus. Agronomy 2023, 13, 280. https://doi.org/10.3390/agronomy13020280
Misra D, Dutta W, Jha G, Ray P. Interactions and Regulatory Functions of Phenolics in Soil-Plant-Climate Nexus. Agronomy. 2023; 13(2):280. https://doi.org/10.3390/agronomy13020280
Chicago/Turabian StyleMisra, Deblina, Writuparna Dutta, Gaurav Jha, and Puja Ray. 2023. "Interactions and Regulatory Functions of Phenolics in Soil-Plant-Climate Nexus" Agronomy 13, no. 2: 280. https://doi.org/10.3390/agronomy13020280
APA StyleMisra, D., Dutta, W., Jha, G., & Ray, P. (2023). Interactions and Regulatory Functions of Phenolics in Soil-Plant-Climate Nexus. Agronomy, 13(2), 280. https://doi.org/10.3390/agronomy13020280









