The Effect of Fertilization on the Structure of the Aboveground Biomass of Several Cultivars of Jerusalem Artichoke (Helianthus tuberosus L.)
Abstract
:1. Introduction
2. Materials and Methods
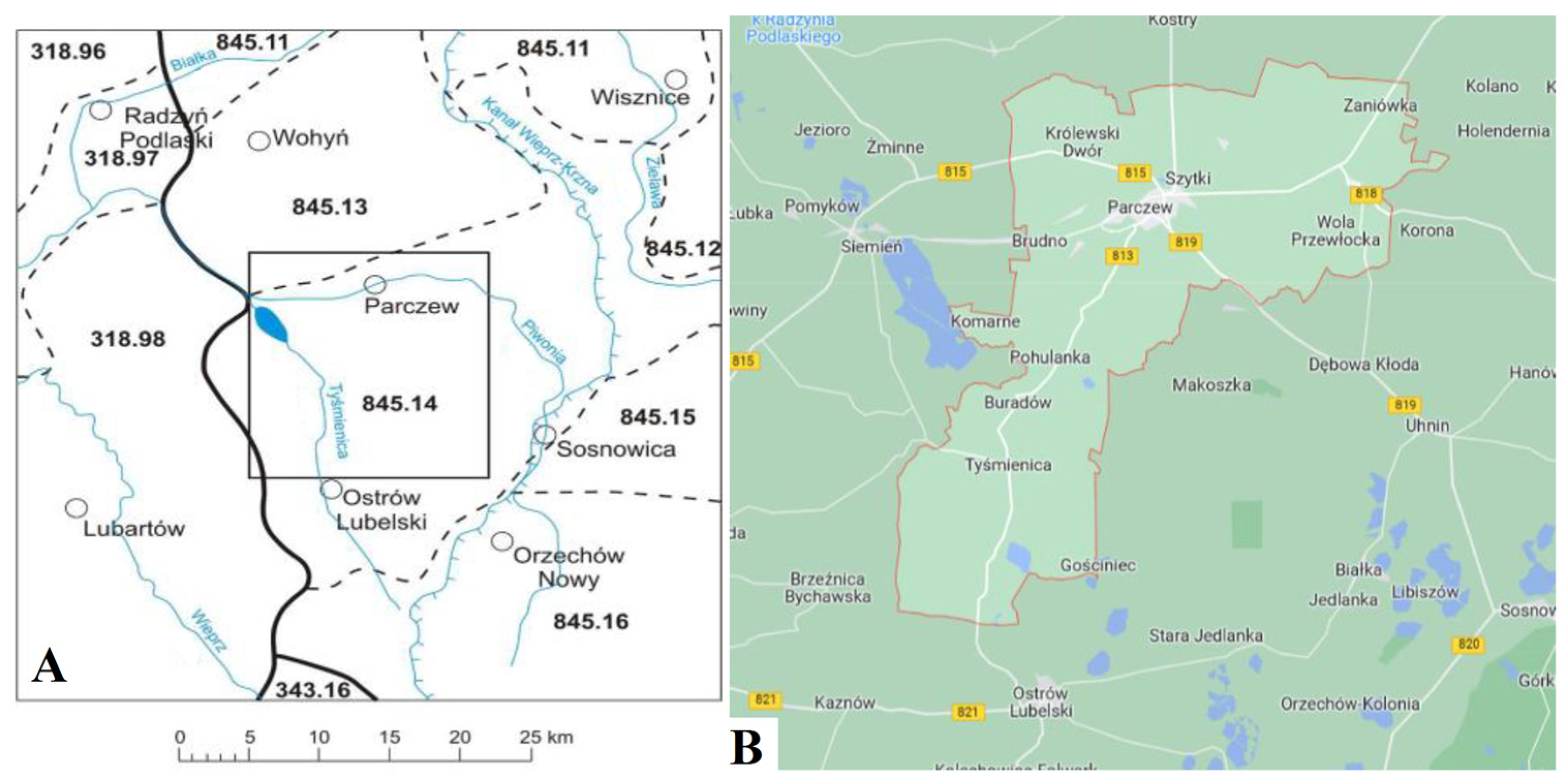
2.1. Location of the Experiment
2.2. Experimental Design
2.3. Characteristics of Cultivars
2.4. Soil Sampling
2.5. Conducting a Field Experiment
2.6. Collection of Plant Samples
2.7. Determination of the Physical and Chemical Properties of the Soil
2.8. Soil Conditions
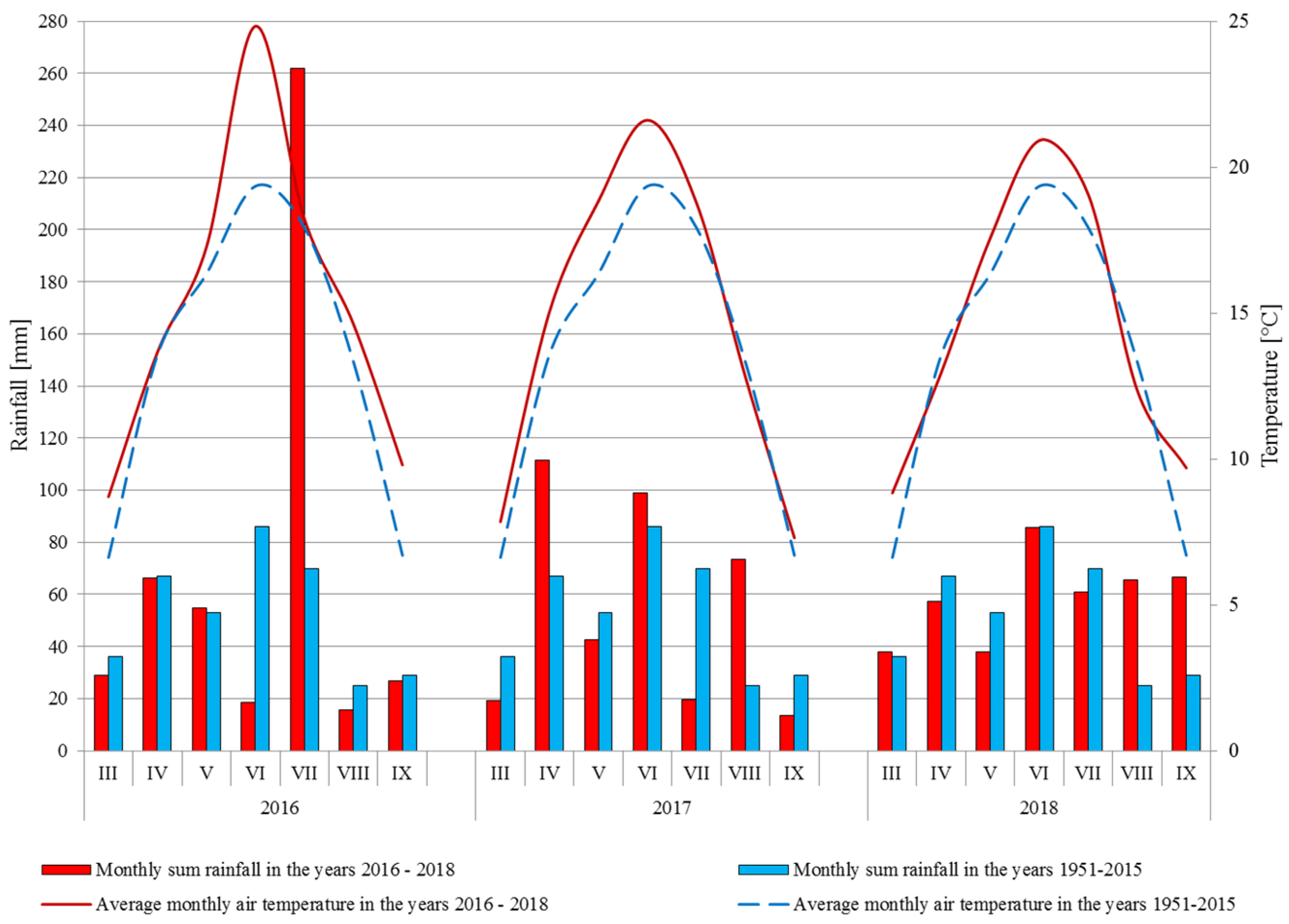
2.9. Meteorological Conditions
2.10. Statistical Calculations
3. Results
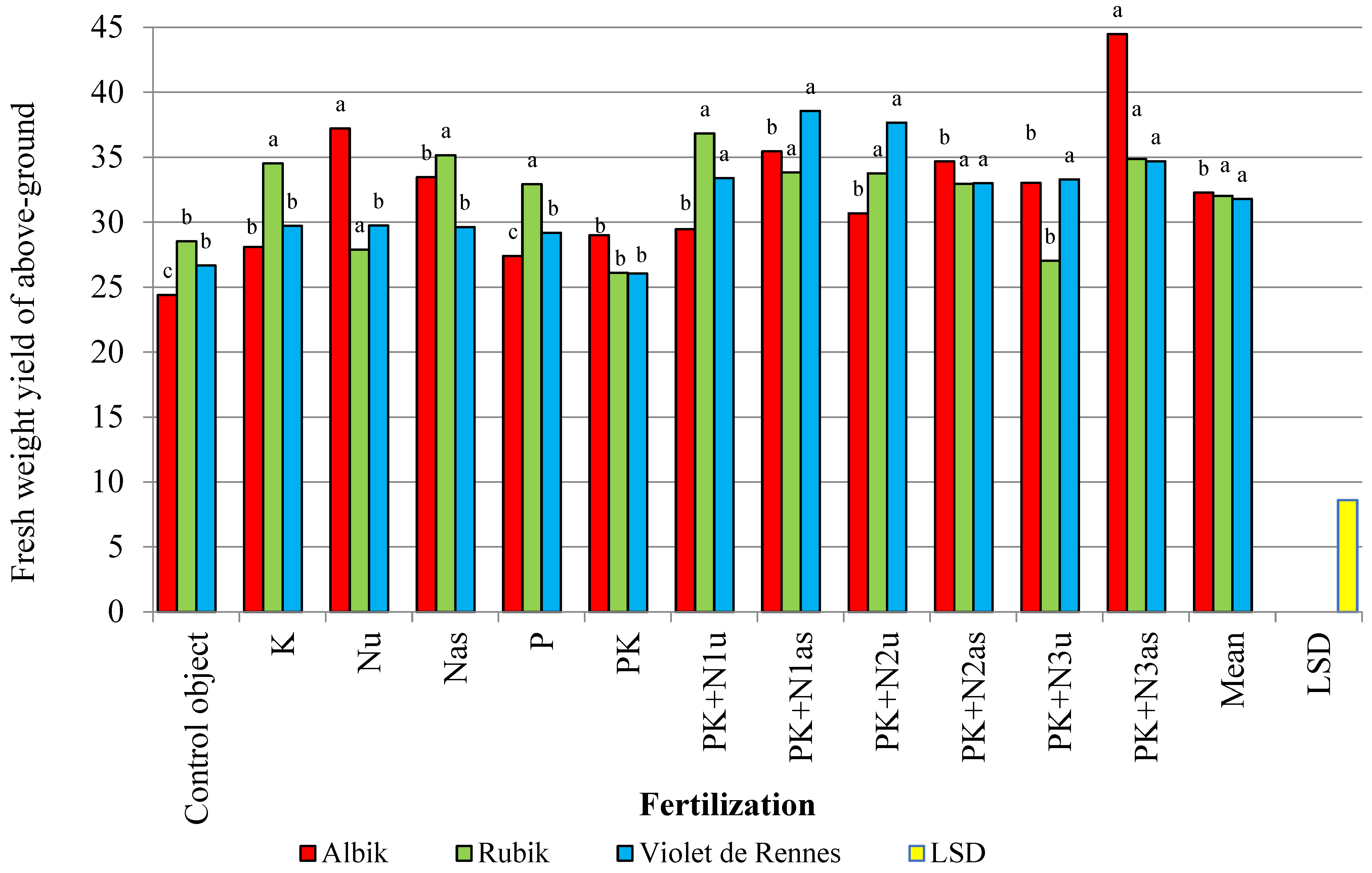
3.1. Aboveground Biomass Yield
3.2. The Structure of the Yield of the Aboveground Parts of Plants
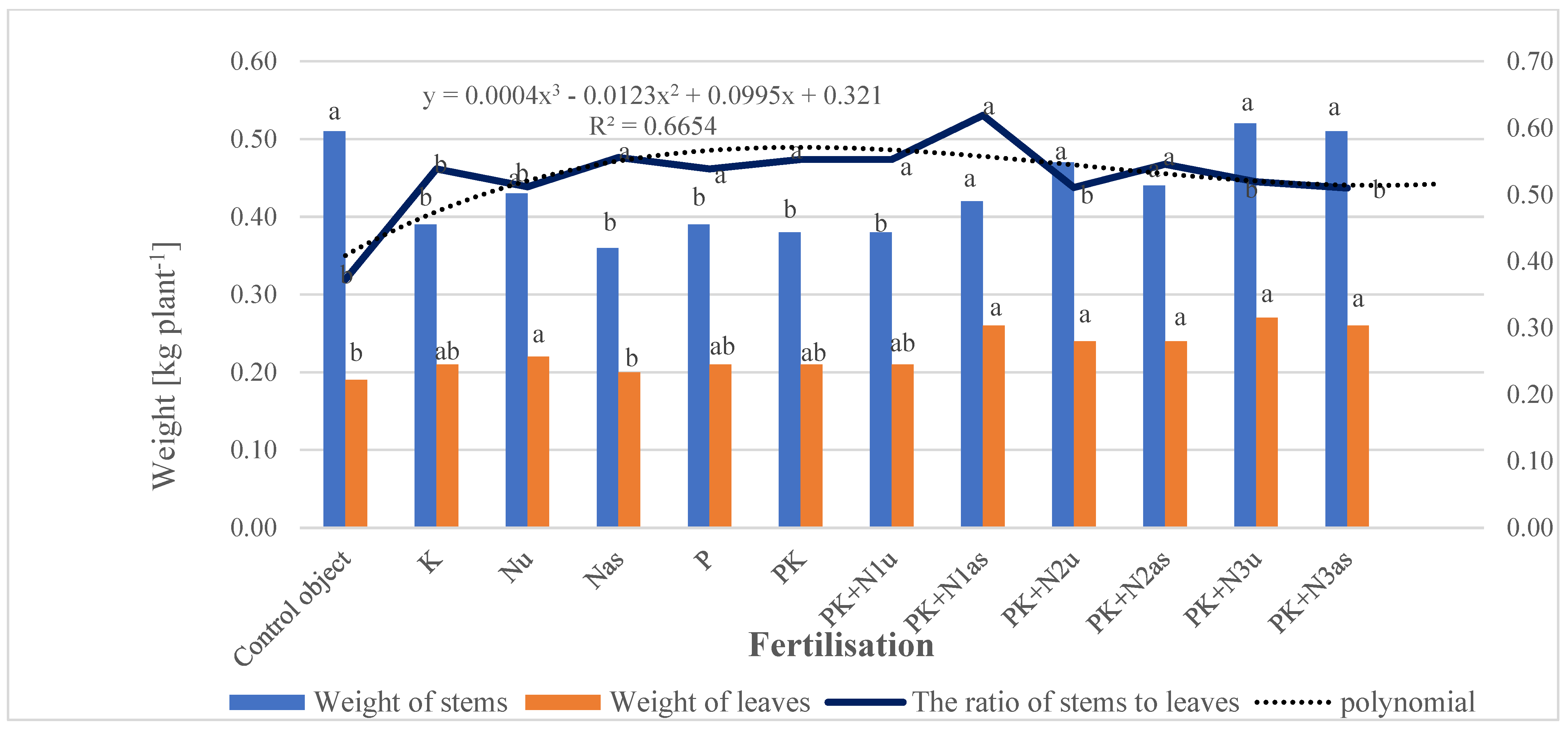
3.2.1. Mass of Aboveground Parts
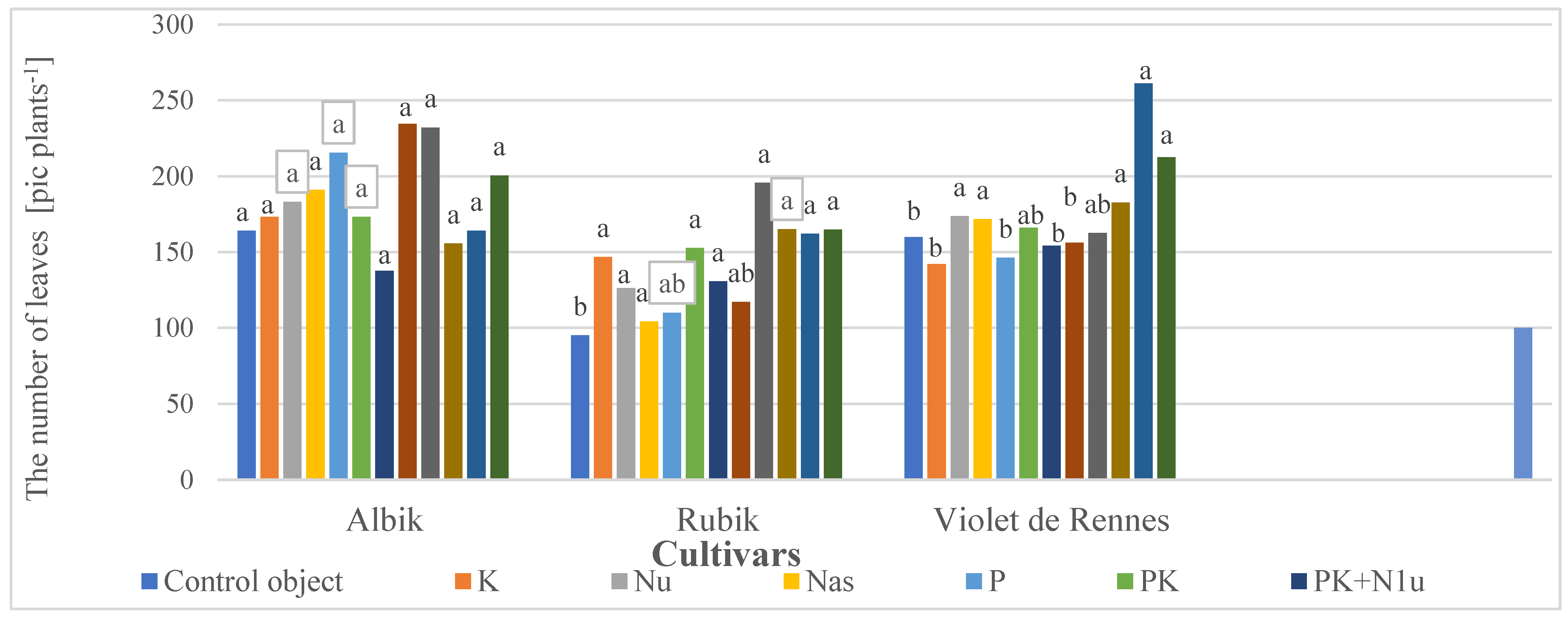
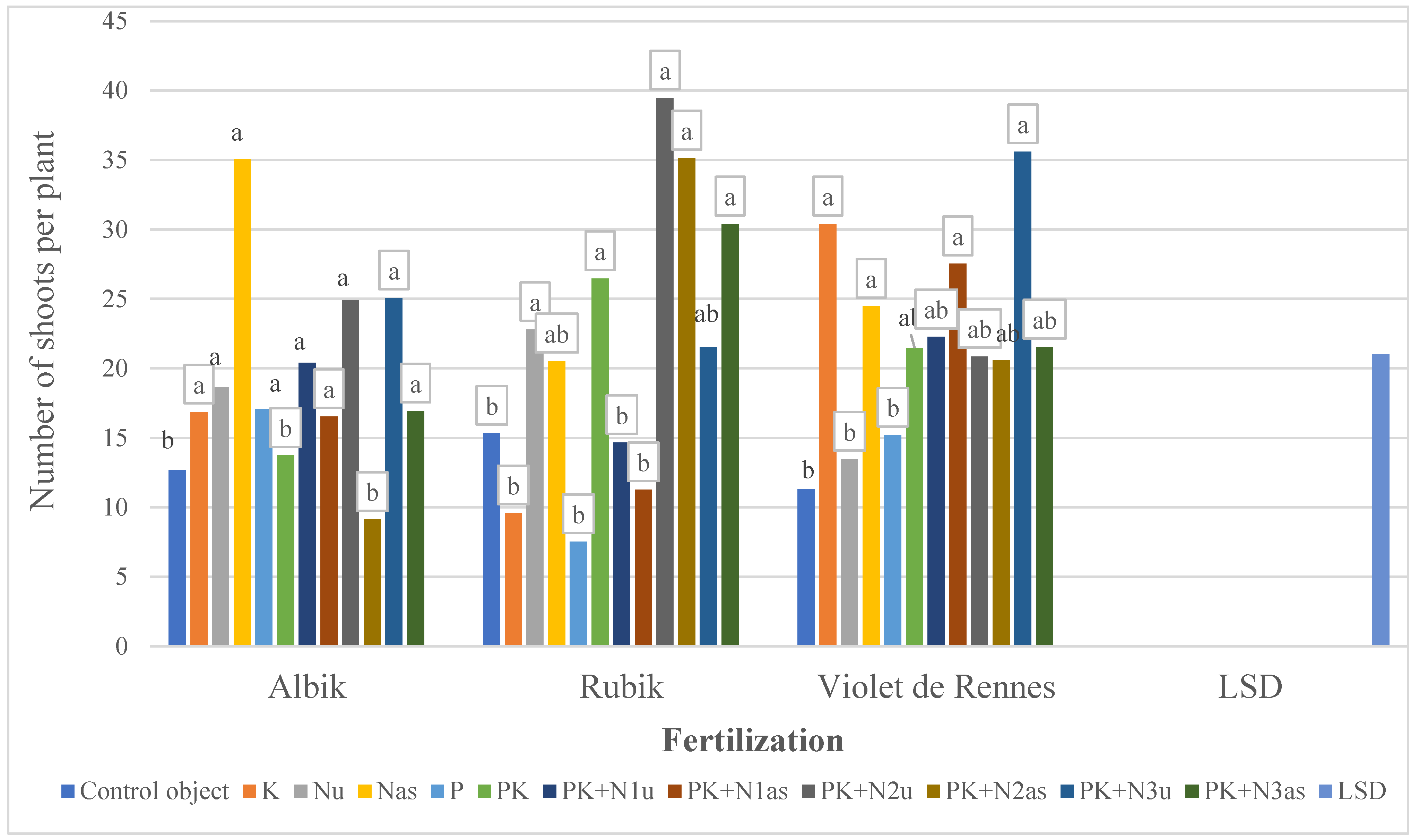
3.2.2. Number of Stems and Leaves in the Aboveground Biomass
3.3. Descriptive Statistics of Yield and of Structure of Aboveground Biomass of JA
4. Discussion
4.1. Effect of Fertilization on Aboveground Biomass Yield
4.1.1. Effect of Nitrogen Fertilization on Yield
4.1.2. Potassium Fertilization
4.1.3. Fertilization with Phosphorus
4.2. Impact of Mineral Fertilization on the Aboveground Biomass Structure
4.3. Influence of Cultivars on Yield and Aboveground Biomass Structure
4.4. Reaction of Cultivars to Mineral Fertilization
5. Conclusions and Prospects for the Future
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| JA | Jerusalem artichoke |
| C:N | Carbon to Nitrogen ratio |
| N | Nitrogen |
| N-NH4 | Ammonium Nitrogen |
| N-NO3 | Nitrate |
| NUE | Nitrogen-use efficiency |
| PK | Phosphorus–potassium fertilization |
References
- Register.arimr.gov.pl. Available online: https://rejestrupraw.arimr.gov.pl/# (accessed on 20 November 2022). (In Polish)
- Sawicka, B. Słonecznik bulwiasty (Helianthus tuberosus L.). Biologia, hodowla, znaczenie użytkowe; Uniwersytet Przyrodniczy w Lublinie: Lublin, Poland, 2016; p. 241. ISBN 978-83-72-59-251-2. (In Polish) [Google Scholar]
- Krochmal-Marczak, B.; Sawicka, B.; Barbaś, P. Chapter 4. Utility meaning of Jerusalem artichoke. In Jerusalem Artichoke Food Science and Technology, 1st ed.; Helianthus Tuberosus; Sawicka, B., Krochmal-Marczak, B., Eds.; Springer Nature: Singapore, 2022; pp. 91–138. ISBN 978-981-19-0804-0. [Google Scholar] [CrossRef]
- Antonkiewicz, J.; Kołodziej, B.; Bielińska, E.J.; Witkowicz, R.; Tabor, S. Wykorzystanie karczocha jerozolimskiego do ekstrakcji metali ciężkich z komunalnych osadów ściekowych. Zmieniona gleba. Polish J. Środowisko. Stadnina 2018, 27, 513–527. [Google Scholar]
- Manokhina, A.A.; Dorokhov, A.S.; Kobozeva, T.P.; Fomina, T.N.; Starovoitova, O.A. Varietal Characteristics of Jerusalem Artichoke as a High Nutritional Value Crop for Herbivorous Animal Husbandry. Appl. Sci. 2022, 12, 4507. [Google Scholar] [CrossRef]
- Samal, L.; Chaturvedi, V.B.; Pattanaik, A.K. Effects of dietary supplementation with Jerusalem artichoke (Helianthus tuberosus L.) tubers on growth performance, nutrient digestibility as well as activity and composition of large intestinal microbiota in rats. J. Anim. Feed Sci. 2017, 26, 50–58. [Google Scholar] [CrossRef]
- Dascălu, T.; Munteanu, N.; Hamburdă, S.B.; Avasiloaiei, D.I. Results Regarding biological particularities study of Jerusalem Artichoke (Helianthus tuberosus L.), Lucrări Ştiinţifice, s. Horticultură 2013, 56, 167–172. [Google Scholar]
- Liava, V.; Karkanis, A.; Danalatos, N.; Tsiropoulos, N. Cultivation Practices, Adaptability, and Phytochemical Composition of Jerusalem artichoke (Helianthus tuberosus L.): A Weed with Economic Value. Agronomy 2021, 11, 914. [Google Scholar] [CrossRef]
- Dias, N.S.; Ferreira, J.F.S.; Liu, X.; Suarez, D.L. Jerusalem artichoke (Helianthus tuberosus L.) maintains high inulin, tuber yield, and antioxidant capacity under moderately-saline irrigation waters. Ind. Crops Prod. 2016, 94, 1009–1024. [Google Scholar] [CrossRef]
- Breton, C.; Breton, S.D.; Kiru, A.; Bervillé, N.Y. Anushkevich. Breeding of Jerusalem artichoke with the desired traits for different directions of use: Retrospective, approaches, and prospects. Agric. Biol. 2017, 52, 940–951. [Google Scholar]
- Boroń, F. Wymagania biotopowe w rozmnażaniu wegetatywnym topinamburu (Helianthus tuberosus L.) w warunkach uprawy wazonowej. Acta Juvenum 2021, 6, 21–27. (In Polish) [Google Scholar]
- Miller, A.J.; Cramer, M.D. Root nitrogen acquisition and assimilation. Plant Soil 2004, 274, 1–36. [Google Scholar] [CrossRef]
- Sawicka, B. Effectiveness of Nutrient Utilization for Food Security, Sustainable Development and Immunity. In Zero Hunger. Encyclopedia of UN Sustainable Development Goals; Leal Filho, W., Azul, A., Brandli, L., Özuyar, P., Wall, T., Eds.; Springer: Cham, Switzerland, 2020. [Google Scholar]
- Abbasian, A.; Ahmadi, A.; Abbasi, A.R.; Darvishi, B. Effect of different concentrations of phosphorus and calcium on potato tuber production. J. Plant Nutr. 2018, 41, 1765–1777. [Google Scholar] [CrossRef]
- Wierzbowska, J.; Cwalina-Ambroziak, B.; Bogucka, B. The Effect of Nitrogen Fertilization on Yield and Macronutrient Concentrations in Three Cultivars of Jerusalem artichoke (Helianthus tuberosus L.). Agronomy 2021, 11, 2161. [Google Scholar] [CrossRef]
- Bogucka, B.; Pszczółkowska, A.; Okorski, A.; Jankowski, K. The Effects of Potassium Fertilization and Irrigation on the Yield and Health Status of Jerusalem Artichoke (Helianthus tuberosus L.). Agronomy 2021, 11, 234. [Google Scholar] [CrossRef]
- Peltovuori, T. Phosphorus in Agricultural Soils of Finland—Characterization of Reserves and Retention in Mineral Soil Profiles. Ph.D. Thesis, Department of Applied Chemistry and Microbiology, University of Helsinki, Helsinki, Finland, 2006. [Google Scholar]
- Sawicka, B.; Kalembasa, D. Variation of macroelements content in tubers of Helianthus tuberosus L. at different levels of nitrogen fertilization. Acta Sci. Pol. Agric. 2008, 7, 67–82. [Google Scholar]
- Grzebisz, W. Pierwiastki w środowisku–Fosfor. J. Elem. 2003, 8, 160. (In Polish) [Google Scholar]
- Lemanowicz, J.; Koper, J. Zawartość wybranych form fosforu w glebie i kończynie łąkowej oraz aktywność fosfataz glebowych na tle zróżnicowanego nawożenia mineralnego i organicznego. Woda Sr. Obsz. Wiej. 2009, 9, 119–139. (In Polish) [Google Scholar]
- WRB. World Reference Base for Soil Resources. International Soil Classification System for Naming Soils and Creating Legends for Soil Maps; World Soil Resources Reports; Food and Agriculture Organization of the United Nations Vaile delle Terme di Caracalla: Rome, Italy, 2014; Volume 106, p. 192. [Google Scholar]
- Ghoneim, I.M. Effect of harvesting dates and potassium fertilization levels on vegetative growth, tuber yield and quality of Jerusalem artichoke. J. Agric. Env. Sci. Alex. Univ. Egypt 2005, 4, 37–63. [Google Scholar]
- Sowiński, J.; Szydełko-Rabska, E.; Kulczycki, G. Impact of different forms of nitrogen fertilizers on the content and uptake of microelements from sorghum. J. Elem. 2014, 19, 567–576. [Google Scholar] [CrossRef]
- Gworek, B.; Łabętowicz, J.; Kijeńska, M.; Tokarz, L.; Barański, A. Nitrogen transformations from nitrogen fertilizers in soils of central and eastern Europe in changing climatic conditions. Soil Sci. Annu. 2021, 72, 132440. [Google Scholar]
- Mahmud, K.; Panday, D.; Mergoum, A.; Missaoui, A. Nitrogen Losses and Potential Mitigation Strategies for a Sustainable Agroecosystem. Sustainability 2021, 13, 2400. [Google Scholar] [CrossRef]
- Gacek, E. (Ed.) Lista odmian roślin rolniczych; Pod red.; COBORU: Słupia Wielka, Poland, 1998. (In Polish) [Google Scholar]
- ISO 18400-205; Soil Quality-Sampling-Part 205: Guidelines for the Procedure for Testing Natural, Almost Natural, and Cultivated Areas. ISO: Geneva, Switzerland, 2018. Available online: https://www.iso.org/obp/ui/#iso:std:iso:18400:-205:ed-1:v1:en (accessed on 31 August 2022).
- Kliszcz, A. Phenological growth stages and BBCH-identification keys of Jerusalem artichoke (Helianthus tuberosus L.). Ann. Univ. Paedagog. Crac. Stud. Nat. 2021, 6, 203–225. [Google Scholar] [CrossRef]
- Anonymous. Procedura pobierania prób roślinnych. 2022. Available online: https://www.nexbio.pl/assets/upload/files/Analiza%20patogen%C3%B3w%20ro%C5%9Blin/%23Procedura%20pobierania%20pro%CC%81b%20ros%CC%81linnych.pdf (accessed on 31 August 2022). (In Polish).
- Egner, H.; Riehm, H.; Domingo, W.R. Untersuchungen über die chemische Boden Analyse als Grundlage für die Beurteilung des nahrstoffzustandes der Boden. II. Chemische extraktions-methoden zu Phosphor–and kalimbestimmung kungle. Lantbrukshoegsk. Ann. 1960, 26, 204–209. [Google Scholar]
- ISO 10390; Soil Quality–pH Determination. ISO: Geneva, Switzerland, 2005. Available online: https://www.iso.org/standard/40879.html (accessed on 31 August 2022).
- ISO 10694; Soil Quality–Determination of Organic and Total Carbon after Dry Combustion (Elemental Analysis). ISO: Geneva, Switzerland, 1995. Available online: https://www.iso.org/obp/ui/#iso:std:iso:10694:ed-1:v1:en (accessed on 30 September 2022).
- AOAC. Official Methods of Analysis of AOAC International, 18th ed.; Association of Analytical Communities: Gaithersburg, MD, USA, 2005. [Google Scholar]
- AOCS. Official Methods and Recommended Practices of the AOCS, 7th ed.; 2nd Printing; AOCS: Urbana, IL, USA, 2017; ISBN 978-1-630670-60-3. Available online: https://myaccount.aocs.org/PersonifyEbusiness/Store/Product-Details/productId/70978091 (accessed on 30 September 2022).
- Skowera, B. Zmiany warunków hydrotermicznych na obszarze Polski (1971−2010). Fragm. Agron. 2014, 31, 74–87. (In Polish) [Google Scholar]
- SAS, I.I. SAS/STAT®9.2. Users Guide; SAS Institute: Cary, NC, USA, 2008. [Google Scholar]
- Koronacki, J. Statistics, for Students of Technical and Natural Sciences; WNT: Warsaw, Poland, 2009; p. 491. ISBN 83-204-2994-3. [Google Scholar]
- SPSS Statistics, version 28.0.1. IBM: Armonk, NY, USA. Available online: https://www.ibm.com/uk-en/products/spss-statistics. (accessed on 31 August 2022).
- Ciereszko, I. Czy można usprawnić pobieranie fosforanów przez rośliny? Kosmos 2005, 4, 394–400. (In Polish) [Google Scholar]
- Matei, G.; Valda, V.; Pistacia, S.; Pânzaru, R.L.; Popa, D. Potential of Jerusalem artichoke (Helianthus tuberosus L.) as a biomass crop. Scientific Papers. Series A. Agronomy 2020, 63, 387–393. [Google Scholar]
- Mansour, F.; Abd El-Rahman, L. An Attempt to Reduce the Use of Potassium Fertilization in Jerusalem Artichoke Plants in Clay Soil. J. Soil Sci. Agric. Eng. 2021, 12, 603–610. [Google Scholar] [CrossRef]
- Sawicka, B.; Danilčenko, H.; Jariene, E.; Skiba, D.; Rachoń, L.; Barbaś, P.; Pszczółkowski, P. Nutritional Value of Jerusalem Artichoke Tubers (Helianthus tuberosus L.) Grown in Organic System under Lithuanian and Polish Conditions. Agriculture 2021, 11, 440. [Google Scholar] [CrossRef]
- Izsáki, Z.; Kádi, G.N. Biomass Accumulation and Nutrient Uptake of Jerusalem Artichoke (Helianthus tuberosus L.). Am. J. Plant Sci. 2013, 4, 1629–1640. [Google Scholar] [CrossRef] [Green Version]
- Epie, K.E.; Santanen, A.; Mäkelä, P.S.A.; Stoddard, F.L. Fertilizer and intercropped legumes as nitrogen source for Jerusalem artichoke (Helianthus tuberosus L.) tops for bioenergy. Agric. Food Sci. 2018, 27, 199–205. [Google Scholar] [CrossRef]
- Grześkowiak, A. Nawożenie mineralne w bezpłużnych technologiach uprawy roli. Nasza Rola 2007, 8, 18–19. (In Polish) [Google Scholar]
- Żołnowski, C. Badania nad Zmiennością Plonu i Cechami Jakości Ziemniaka Stołowego (Solanum tuberosum L.) Uprawianego Przy Zróżnicowanym Poziomie Nawożenia Mineralnego; Uniwersytet Warmińsko-Mazurski, Rozprawy i Monografie: Olsztyn, Poland, 2013; Volume 191, p. 259. ISBN 978-83-7299-832-3. (In Polish) [Google Scholar]
- Sawicka, B. Efekt stosowania herbicydów w uprawie Helianthus tuberosus L. Cz. II. Plon i struktura nadziemnych części roślin. Biul. IHAR 2002, 223/224, 397–414. (in Polish). [Google Scholar]
- USDA. National Nutrient Database for Standard Reference, Legacy Release. 2018. Available online: https://data.nal.usda.gov/dataset/usda-national-nutrient-database-standard-reference-legacy-release (accessed on 28 November 2022).
- Chołuj, D.; Podlaski, S.; Wiśniewski, G.; Szmalec, J. Kompleksowa ocena biologicznej przydatności 7 gatunków roślin wykorzystywanych na cele energetyczne. Stud. I Rap. IUNG-PIB 2008, 11, 81–99. (In Polish) [Google Scholar]
- Sawicka, B.; Michałek, W. Evaluation and Productivity of Helianthus tuberosus L. in the Conditions of Central-East Poland. EJPAU 2005, 8, 42. Available online: http://www.ejpau.media.pl/volume8/issue3/art-42.html. (accessed on 26 August 2022).
- Kantar, M.; Betts, K.; Hulke, B.E.; Stupar, R.M.; Wyse, D. Breaking tuber dormancy in Helianthus tuberosus L. and interspecific hybrids of Helianthus annuus L. × Helianthus tuberosus. J. Exp. Bot. 2013, 64, 421–431. [Google Scholar] [CrossRef] [Green Version]
- Lakić, Ž.; Balalaika, I.; Nožinić, M. Genetic variability for yield and yield components in Jerusalem artichoke (Helianthus tuberosus L.). Genetica 2018, 50, 45–57. [Google Scholar]
- El-Zohiri, S.S.M.; Youssef, M.E.A. Response of Jerusalem artichoke to cut off irrigation before harvest and fertilization with Ca, Mg and B. J. Product. Dev. 2015, 20, 61–81. [Google Scholar] [CrossRef]
- Kays, S.J.; Nottingham, S.F. Biology and Chemistry of Jerusalem Artichoke (Helianthus tuberosus L.); CRC Press Taylor & Francis Group: Broken Sound Parkway, NW, USA, 2008. [Google Scholar]
- Kays, S.J.; Kultur, F. Genetic Variation in Jerusalem Artichoke (Helianthus tuberosus L.) Flowering Date and Duration. Hort Science 2005, 40, 1675–1678. [Google Scholar] [CrossRef] [Green Version]
- Žaldarienė, S. Chemical Composition of Different Genotypes of Organic Jerusalem Artichoke (Helianthus tuberosus L.) along the Ontogenesis Cycle. Master’s Thesis, Aleksandras Stulginskis University, Akademija, Lithuania, 2017; p. 178. [Google Scholar]
- Monti, E.; Reggiani, C.; Franchi, M.V.; Toniolo, L.; Sandri, M.; Armani, A.; Zampieri, S.; Giacomello, E.; Sarto, F.; Sirago, G.; et al. Neuromuscular junction instability and altered intracellular calcium handling as early determinants of force loss during unloading in humans. J. Physiol. 2021, 599, 3037–3061. [Google Scholar] [CrossRef]
- Suturin, A.I.; Suturin, V.I. Artichoke-biotech crop XXI century. In Proceedings of the International Conference in Moskov, Moskow, Russia, 24–25 November 2011; pp. 24–25. (In Russian). [Google Scholar]
- Żyromski, A.; Szulczewski, W.; Biniak-Pieróg, M.; Okrasińska, H. Zastosowanie modelu wsmt do oceny ewapotranspiracji miskanta i topinamburu. Woda-Sr. Obsz. Wiej./Water-Environ. Rural. Areas 2012, 12, 401–409. (In Polish) [Google Scholar]
- Sawicka, B.; Michałek, W.; Pszczółkowski, P. The relationship of potato tubers chemical composition with selected physiological indicators. Zemdirb. Agric. 2015, 102, 41–50. [Google Scholar] [CrossRef] [Green Version]
- Cosgrove, D.R.; Oelke, E.A.; Doll, J.D.; Davis, D.W.; Undersander, D.J.; Oplinger, E.S. Jerusalem artichoke. In Alternative Field Crops Manual [online]; University of Wisconsin: Madison, WI, USA, 2011. [Google Scholar]
- Kocurek, M.; Pilarski, J. Implication of stem structures for photosynthetic functions in select herbaceous plants. Pol. J. Environ. Stud. 2012, 21, 1687–1696. [Google Scholar]
- McLaurin, W.J.; Somda, Z.C.; Kays, S.J. Jerusalem artichoke growth, development, and field storage. I. Numerical assessment of plant development and dry matter acquisition and allocation. J. Plant Nutr. 1999, 22, 1303–1313. [Google Scholar] [CrossRef]
- Leontiev, N.V.; Dubar, D.A.; Lugin, V.G.; Feskova, A.E.V.; Ignatovets, O.S.; Titok, V.V. Biological potential of Jerusalem artichoke as a feedstock for food and pharmaceutical industries. Chemistry, organic substances Technology and Biotechnology. Proc. BSTU 2014, 4, 206–209. [Google Scholar]
- Szpunar-Krok, E.; Bobrecka-Jamro, D.; Grochowska, S.; Buczek, J. Yield of the aboveground parts and tubers of Jerusalem artichoke (Helianthus tuberosus L.) depending on plant density. Acta Sci. Pol. Agric. 2016, 15, 69–78. [Google Scholar]
- Encheva, J.; Christov, M.; Ivanov, P. Characterization of Interspecific Hybrids between Cultivated Sunflower H. annuus L. (cv. Albena) and Wild Species Helianthus tuberosus. Helia 2003, 26, 43–50. [Google Scholar] [CrossRef] [Green Version]
- Abdalla, N.; Taha, H.; Arafa, N.; Ragab, M.; El-Miniawy, S.; Kovács, S.; Tóth, I.; El-Ramady, H.; El-Nady, M. Anatomical Studies on Three Jerusalem Artichoke (Helianthus tuberosus L.) Cultivars Grown in Hungary. J. Sus. Agric. Sci. 2022, 48, 101–109. [Google Scholar] [CrossRef]
- Swanton, C.J. Ecological aspects of growth and development of Jerusalem artichoke (Helianthus tuberosus L.). Ph.D. Thesis, University Western Ontario, London, ON, Canada, 1986; p. 181. [Google Scholar]
- Góral, S. Wartość użytkowa topinamburu (Helianthus tuberosus L.). Zesz. Probl. Postępów Nauk. Rol. 2000, 468, 17–30. (In Polish) [Google Scholar]
- Coetzee, P.E.; Ceronio, G.M.; Preez, C.C.D. Effect of phosphorus and nitrogen sources on essential nutrient concentration and uptake by maize (Zeal mays L.) during early growth and development. South Afr. J. Plant Soil 2017, 34, 55–64. [Google Scholar] [CrossRef]
- Benzaazoua, M.; Marion, P.; Liouville-Bourgeois, L.; Joussemet, R.; Houot, R.; Franco, A.; Pinto, A. Mineralogical distribution of some minor and trace elements during a laboratory flotation processing of Neves-Corvo ore (Portugal). Int. J. Miner. Process. 2002, 66, 163–181. [Google Scholar] [CrossRef]
- Sawicka, B. Energy value of Jerusalem artichoke (Helianthus tuberosus L.) as a source of biomass. Zesz. Nauk. UP Wrocław Rol. 2010, 97, 245–256. (In Polish) [Google Scholar]
- Zelenkov, N.; Romanova, N.G. Jerusalem artichoke: Agrobiological portrait and prospects for innovative use. Available online: https://www.topinambour.ru/information/170914195656.html (accessed on 20 November 2022).
| Years | Contents of the Granulometric Fractions (%) | Soil Classification | ||
|---|---|---|---|---|
| Sand 2.0–0.05 | Silt 0.05–0.002 | Loam <0.002 | ||
| 2016 | 67.12 a* | 29.89 b | 2.99 a | Sandy loam |
| 2017 | 67.93 a | 30.09 b | 2.98 a | Sandy loam |
| 2018 | 66.33 b | 30.86 a | 2.81 a | Sandy loam |
| Average | 66.79 | 30.28 | 2.93 | |
| Years | Humus (g kg−1) | Content of Assimilable Macroelements (mg·kg−1 of DM of soil) | Content of Assimilable Forms of Microelements (mg·kg−1 of DM of soil) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| P | K | Mg | B | Mn | Cu | Zn | Fe | ||
| 2016 | 1.86 b | 105.0 b | 168.0 a | 34.1 b | 0.57 | 155.0 a | 1.62 a | 7.90 b | 658 b |
| 2017 | 1.62 b | 140.1 a | 75.0 b | 17.0 c | 0.49 | 140.0 b | 1.11 b | 7.66 b | 630 b |
| 2018 | 2.15 a | 97.0 b | 152.1 a | 52.0 a | 0.61 | 160.0 a | 1.68 a | 9.04 a | 767 a |
| Average | 1.88 | 114.0 | 132.0 | 34.0 | 0.56 | 151.7 | 1.47 | 8.20 | 685 |
| Years | C (g kg−1) | Total N (g kg−1) | C:N Ratio | N-NO3−1 (mg kg−1) | N-NO4+ (mg kg−1) | pHKCl | pHH20 |
|---|---|---|---|---|---|---|---|
| 2016 | 10.75 b* | 1.05 b | 10.24 b | 25.01 ab | 31.10 b | 6.22 b | 6.35 b |
| 2017 | 10.98 b | 1.07 b | 10.26 b | 24.91 b | 31.98 a | 6.77 a | 6.94 a |
| 2018 | 11.97 a | 1.11 a | 10.78 a | 26.08 a | 32.94 a | 5.97 b | 6.09 b |
| Mean | 11.23 | 1.08 | 10.43 | 25.33 | 32.01 | 6.32 | 6.46 |
| Year | Month | Sum of Rainfall (mm) | Air Temperature (°C) | Hydrothermal Coefficient of Sielianinov * | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Decade of Month | Month | Decade of Month | Mean | |||||||
| 1 | 2 | 3 | 1 | 2 | 3 | |||||
| 2016 | April (IV) | 26.5 | 2.4 | 0.0 | 28.9 | 6.1 | 7.6 | 12.4 | 8.7 | 1.11 |
| May (V) | 5.2 | 17.5 | 43.4 | 66.1 | 13.1 | 14.6 | 14.5 | 14.1 | 1.52 | |
| June (VI) | 29.1 | 0.0 | 25.6 | 54.7 | 11.9 | 18.1 | 21.8 | 17.3 | 1.06 | |
| July (VII) | 0.0 | 16.1 | 2.5 | 18.6 | 22.4 | 21.4 | 26.6 | 23.4 | 0.27 | |
| August (VIII) | 86.5 | 121.9 | 53.6 | 262.0 | 19.7 | 18.9 | 17.7 | 18.8 | 4.70 | |
| September (IX) | 14.9 | 0.8 | 0.0 | 15.7 | 16.0 | 14.5 | 13.5 | 14.6 | 0.36 | |
| October (X) | 19.2 | 0.2 | 7.4 | 26.8 | 12.9 | 7.1 | 10.2 | 10.1 | 0.89 | |
| Total | 472.8 | 1.47 | ||||||||
| 2017 | April (IV) | 13.8 | 5.4 | 0.0 | 19.2 | 5.5 | 8.9 | 9.2 | 7.9 | 0.82 |
| May (V) | 23.0 | 25.4 | 62.9 | 111.3 | 9.6 | 15.3 | 22.2 | 15.7 | 2.34 | |
| June (VI) | 23.9 | 9.2 | 9.6 | 42.7 | 18.3 | 20.5 | 17.7 | 18.8 | 0.76 | |
| July (VII) | 54.2 | 19.3 | 25.5 | 99.0 | 17.7 | 21.4 | 21.5 | 20.2 | 1.65 | |
| August (VIII) | 12.9 | 6.8 | 0.0 | 19.7 | 18.2 | 19.7 | 20.2 | 19.4 | 0.35 | |
| September (IX) | 57.1 | 3.8 | 12.6 | 73.5 | 14.2 | 11.7 | 12.6 | 12.8 | 1.91 | |
| October (X) | 5.3 | 6.3 | 2.0 | 13.6 | 9.2 | 5.9 | 7.4 | 7.5 | 0.61 | |
| Total | 379.0 | 1.23 | ||||||||
| 2018 | April (IV) | 13.4 | 22.7 | 1.8 | 37.9 | 7.9 | 9.0 | 95.7 | 8.8.3 | 1.43 |
| May (V) | 31.8 | 16.0 | 9.3 | 57.1 | 11.8 | 13.7 | 14.7 | 13.4 | 1.45 | |
| June (VI) | 3.4 | 19.9 | 14.7 | 38.0 | 17.6 | 16.7 | 18.4 | 17.6 | 0.72 | |
| July (VII) | 36.7 | 18.8 | 30.1 | 85.6 | 18.0 | 19.5 | 20.9 | 19.4 | 1.47 | |
| August (VIII) | 24.4 | 16.1 | 20.4 | 60.9 | 19.5 | 20.8 | 18.6 | 19.6 | 1.03 | |
| September (IX) | 4.6 | 32.8 | 28.1 | 65.5 | 18.4 | 8.7 | 9.9 | 12.3 | 1.77 | |
| October (X) | 30.6 | 21.6 | 14.4 | 66.6 | 10.2 | 10.0 | 9.7 | 10.0 | 2.25 | |
| Total | 411.6 | 1.36 | ||||||||
| Experimental Factors | Years | Mean | |||
|---|---|---|---|---|---|
| 2016 | 2017 | 2018 | |||
| Cultivars | ‘Albik’ | 30.69 b*** | 28.01 b | 40.30 a | 33.00 a |
| ‘Rubik’ | 34.17 a | 26.86 b | 36.04 a | 32.35 a | |
| ‘Violet de Rennes’ | 31.26 b | 27.06 b | 38.47 a | 32.27 a | |
| LSDp0.05 | 5.07 | ns ** | |||
| Fertilization * | Control object | 26.65 a | 23.37 a | 30.57 a | 26.53 b |
| K | 30.64 a | 25.49 a | 36.21 a | 30.78 b | |
| Nu | 30.22 a | 27.56 a | 37.08 a | 31.62 a | |
| Nas | 31.87 a | 28.22 a | 38.16 a | 32.75 a | |
| P | 31.35 a | 26.39 a | 31.77 a | 29.84 b | |
| PK | 23.63 a | 24.57 a | 32.94 a | 27.05 b | |
| PK+N1u | 32.31 a | 27.49 a | 39.90 a | 33.23 a | |
| PK+N1as | 37.06 a | 24.74 a | 46.05 a | 35.95 a | |
| PK+N2u | 36.25 a | 29.71 a | 36.16 a | 34.04 a | |
| PK+N2as | 34.64 a | 26.78 a | 39.81 a | 33.55 a | |
| PK+N3u | 30.64 a | 27.39 a | 35.33 a | 31.12 b | |
| PK+N3as | 34.41 a | 32.06 a | 47.55 a | 38.01 a | |
| LSDp0.05 | ns | 6.76 | |||
| Mean | 32.04 b | 27.31 c | 38.27 a | 32.54 | |
| LSDp0,05 | 1.69 | ||||
| Specification | Control Object | K ** | Nu | Nas | P | PK | PK+N1u | PK+N1as | PK+N2u | PK+N2as | PK+N3u | PK+N3as |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mediana | 26.65 | 30.64 | 30.22 | 31.70 | 31.35 | 24.57 | 32.31 | 37.06 | 36.16 | 34.64 | 30.64 | 34.41 |
| Stand. Dev. | 3.60 | 5.36 | 4.91 | 5.04 | 2.99 | 5.13 | 6.26 | 10.70 | 3.75 | 6.56 | 3.99 | 8.35 |
| Skewness | 0.27 | 0.12 | 1.18 | 0.85 | −1.69 | 1.67 | 0.65 | −0.46 | −1.73 | −0.60 | 0.53 | 1.58 |
| Range | 7.20 | 10.72 | 9.52 | 9.94 | 5.38 | 9.31 | 12.41 | 21.31 | 6.54 | 13.03 | 7.94 | 15.49 |
| Minimum | 23.37 | 25.49 | 27.56 | 28.22 | 26.39 | 23.63 | 27.49 | 24.74 | 29.71 | 26.78 | 27.39 | 32.06 |
| Maximum | 30.57 | 36.21 | 37.08 | 38.16 | 31.77 | 32.94 | 39.90 | 46.05 | 36.25 | 39.81 | 35.33 | 47.55 |
| * V (%) | 13.42 | 17.42 | 15.53 | 15.43 | 10.03 | 18.95 | 18.83 | 29.76 | 11.02 | 19.44 | 12.83 | 21.96 |
| Experiment Factors | Weight of Stems | Weight of Leaves | Plant Weight | Number of Leaves | Number of Stems | |
|---|---|---|---|---|---|---|
| kg plant−1 | Pcs plant−1 | |||||
| Cultivars | ‘Albik’ ‘Rubik’ ‘Violet de Rennes’ | 0.44 b 0.36 c 0.48 a | 0.26 a 0.19 b 0.24 a | 0.70 a 0.55 b 0.73 a | 187.33 a 143.23 c 175.37 b | 19.49 c 21.76 b 23.04 a |
| LSDp0.05 | 0.03 | 0.02 | 0.05 | 8.43 | 1.07 | |
| Fertilization * | Control object | 0.52 a | 0.19 b | 0.71 a | 139.69 bc | 13.11 d |
| K | 0.39 b | 0.21 ab | 0.60 ab | 154.02 b | 18.96 c | |
| Nu | 0.43 a | 0.22 ab | 0.66 a | 161.00 b | 18.31 c | |
| Nas | 0.36 b | 0.20 b | 0.56 b | 155.64 b | 26.69 a | |
| P | 0.39 b | 0.21 ab | 0.60 ab | 157.27 b | 13.27 d | |
| PK | 0.38 b | 0.21 ab | 0.59 b | 163.98 ab | 20.56 bc | |
| PK+N1u | 0.38 b | 0.21 ab | 0.59 b | 140.93 bc | 19.11 c | |
| PK+N1as | 0.42 a | 0.26 a | 0.68 a | 169.27 ab | 18.44 c | |
| PK+N2u | 0.47 a | 0.24 a | 0.72 a | 196.82 a | 28.42 a | |
| PK+N2as | 0.44 a | 0.24 a | 0.68 a | 167.82 ab | 21.62 b | |
| PK+N3u | 0.52 a | 0.27 a | 0.79 a | 195.73 a | 27.40 a | |
| PK+N3as | 0.51 a | 0.26 a | 0.78 a | 192.58 a | 22.96 b | |
| LSDp0.05 | 0.13 | 0.07 | 0.20 | 32.2 | 4.28 | |
| Years | 2016 2017 2018 | 0.38 c 0.49 a 0.41 b | 0.27 a 0.27 a 0.16 b | 0.65 b 0.75 a 0.57 c | 179.01 a 157.82 c 169.10 b | 26.36 a 26.23 a 11.70 b |
| LSDp0.05 | 0.03 | 0.02 | 0.05 | 8.43 | 1.07 | |
| Mean | 0.43 | 0.23 | 0.66 | 168.64 | 21.43 | |
| Specifications | Plant Weight | Stem Weight | Leaf Weight | Number of Stems | Number of Leaves | Fresh Weight Yield |
|---|---|---|---|---|---|---|
| Median | 0.71 | 0.23 | 0.10 | 10.72 | 83.1 | 24.01 |
| Standard dev. | 0.07 | 0.05 | 0.02 | 2.34 | 12.15 | 12.21 |
| Kurtosis | 2.94 | 7.82 | 3.26 | 3.02 | 2.91 | 4.67 |
| Skewness | 3.64 | 6.57 | 1.46 | 1.45 | 1.54 | 2.23 |
| Coefficient of variation (%) | 9.80 | 10.35 | 9.64 | 10.92 | 7.31 | 25.41 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skiba, D.; Jariene, E.; Barbaś, P.; Krochmal-Marczak, B.; Sawicka, B. The Effect of Fertilization on the Structure of the Aboveground Biomass of Several Cultivars of Jerusalem Artichoke (Helianthus tuberosus L.). Agronomy 2023, 13, 314. https://doi.org/10.3390/agronomy13020314
Skiba D, Jariene E, Barbaś P, Krochmal-Marczak B, Sawicka B. The Effect of Fertilization on the Structure of the Aboveground Biomass of Several Cultivars of Jerusalem Artichoke (Helianthus tuberosus L.). Agronomy. 2023; 13(2):314. https://doi.org/10.3390/agronomy13020314
Chicago/Turabian StyleSkiba, Dominika, Elvyra Jariene, Piotr Barbaś, Barbara Krochmal-Marczak, and Barbara Sawicka. 2023. "The Effect of Fertilization on the Structure of the Aboveground Biomass of Several Cultivars of Jerusalem Artichoke (Helianthus tuberosus L.)" Agronomy 13, no. 2: 314. https://doi.org/10.3390/agronomy13020314
APA StyleSkiba, D., Jariene, E., Barbaś, P., Krochmal-Marczak, B., & Sawicka, B. (2023). The Effect of Fertilization on the Structure of the Aboveground Biomass of Several Cultivars of Jerusalem Artichoke (Helianthus tuberosus L.). Agronomy, 13(2), 314. https://doi.org/10.3390/agronomy13020314










