Endophytic Bacteria in Ricinus communis L.: Diversity of Bacterial Community, Plant−Growth Promoting Traits of the Isolates and Its Effect on Cu and Cd Speciation in Soil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Illumina High−Throughput Sequencing of Endophytic Bacterial Community of the Castor
2.3. Isolation of Endophytic Bacteria
2.4. Plant Growth−Promoting Properties of the Isolates
2.5. The Tolerance of the Isolates to Cu and Cd
2.6. Molecular Identification of the Isolates
2.7. Cu Mobilization Assay
2.8. Impact of the Endophytic Bacteria on Heavy Metal Speciation and Available Phosphate in Soil
3. Results
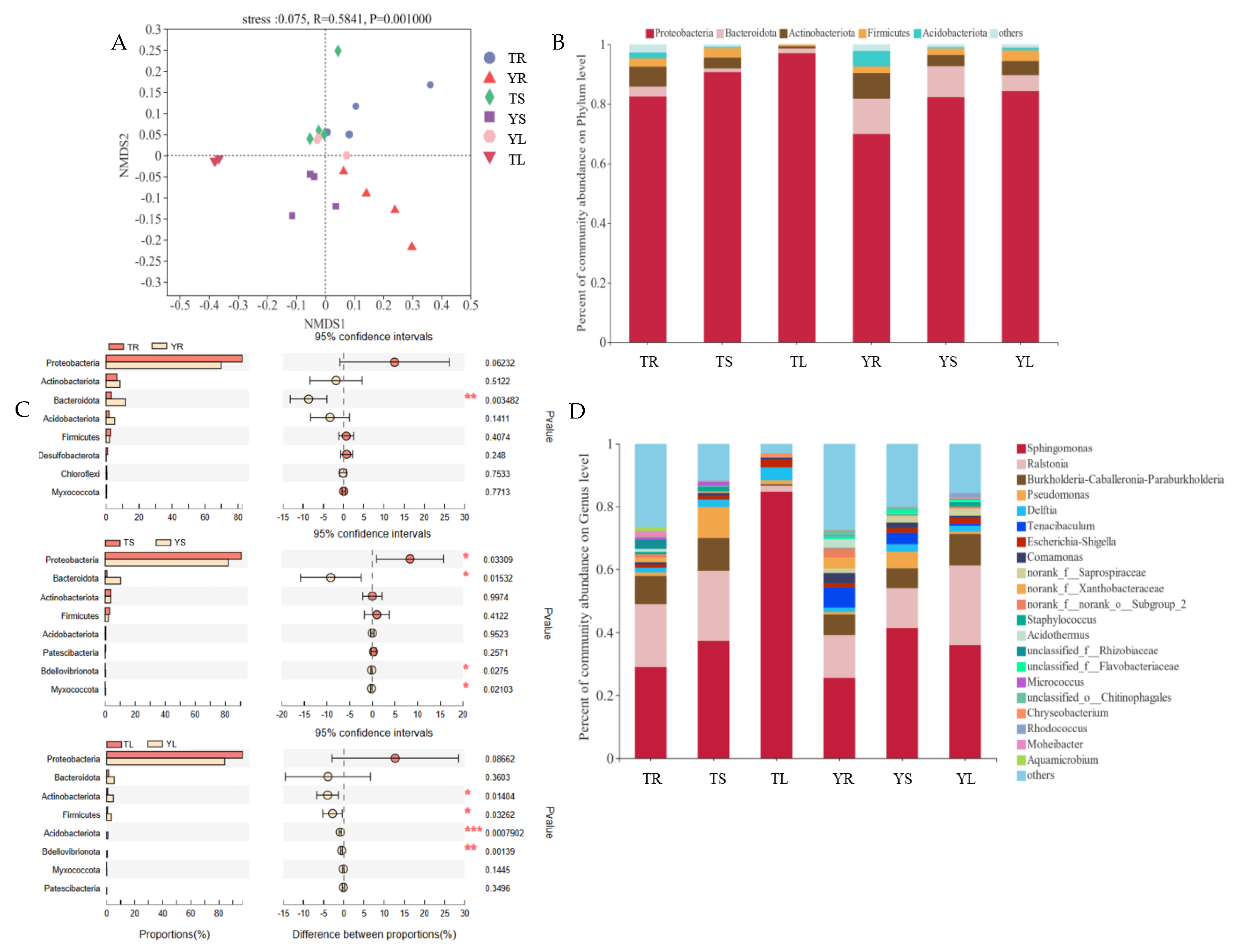
3.1. The Composition and Diversity of Endophytic Bacteria in Ricinus communis L.
3.2. Isolation and Plant Growth−Promoting Characteristics of Endophytic Bacteria
3.3. Characterization of Endophytic Bacterial Isolates
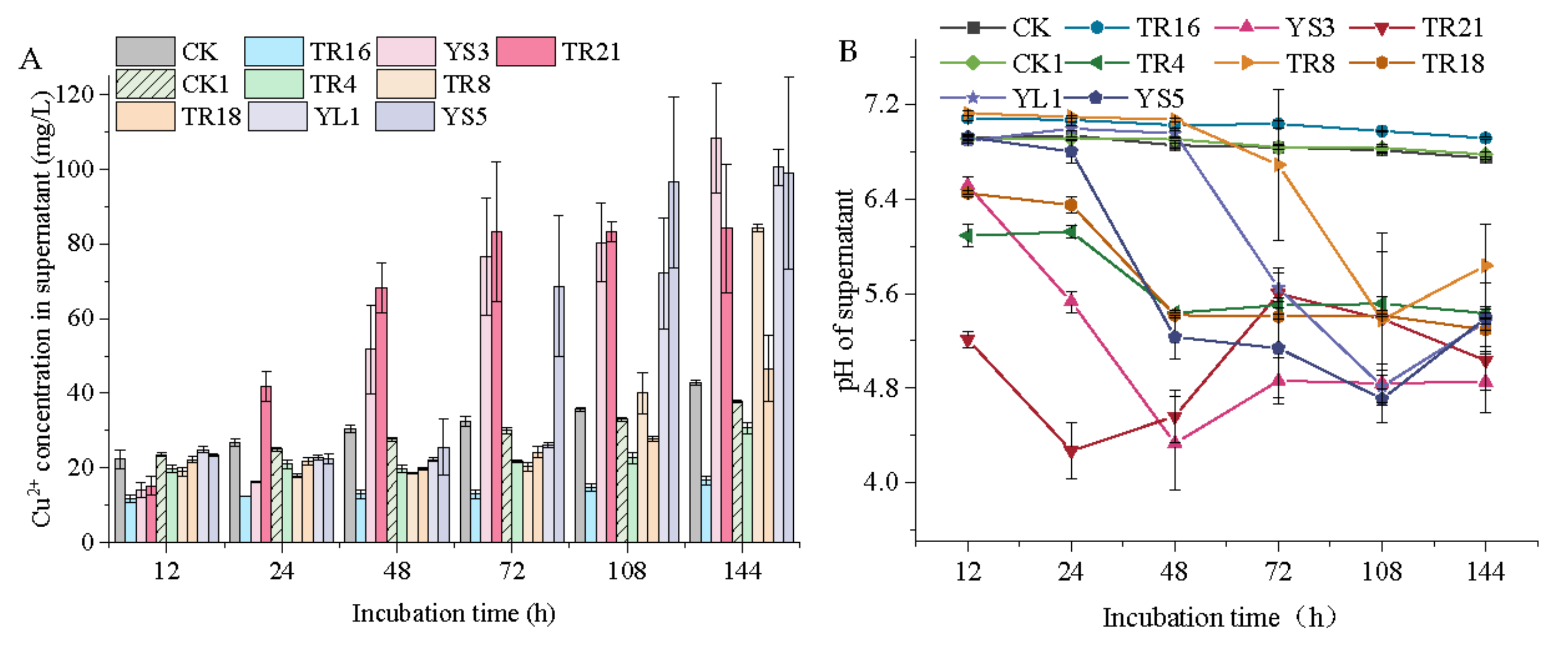
3.4. The Mobilization of Cu by Endophytic Bacteria
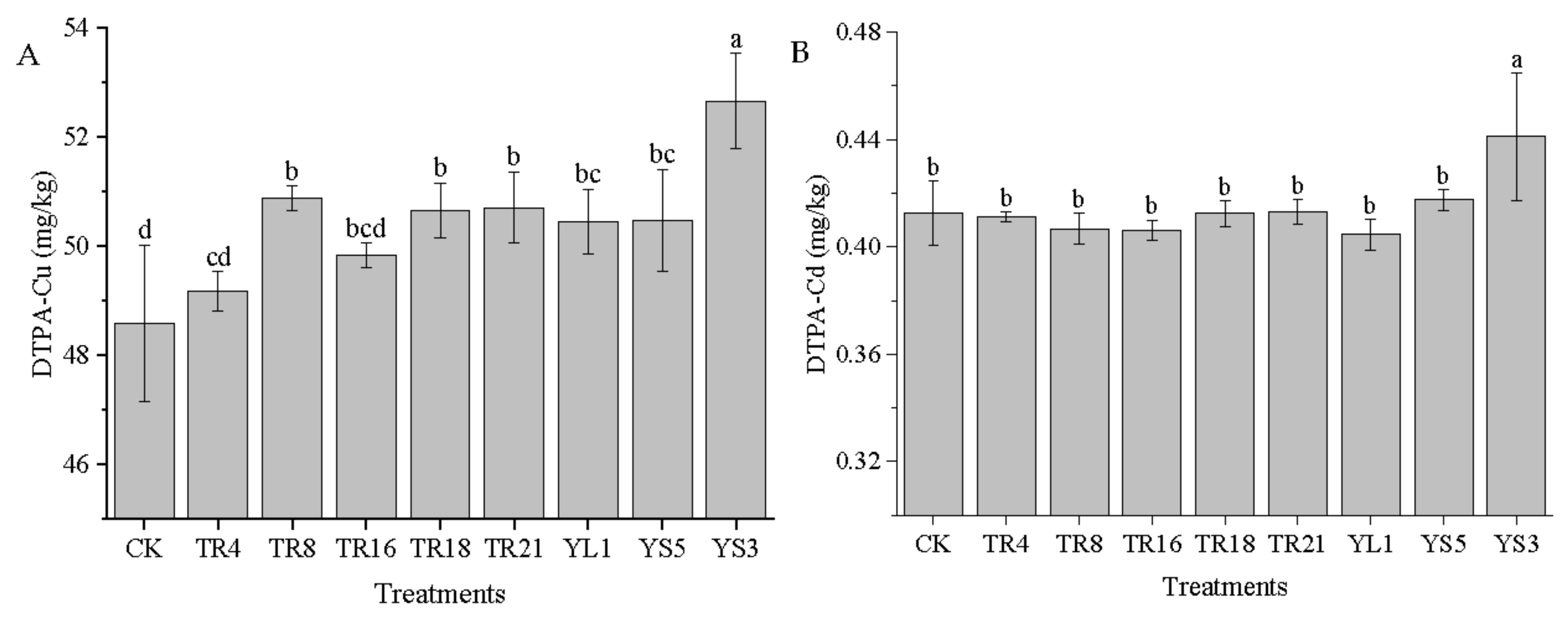
3.5. The Inoculation of Endophytic Bacteria on DTPA−Cu/Cd and Speciation in Soil
3.6. The Inoculation of Endophytic Bacteria on Soil Available Phosphate
4. Discussion
4.1. Differences in Diversity of Endophytic Bacterial Community of Castor Grown on Two Sites
4.2. Cu Mobilization of Eight Endophytic Bacteria Isolates in Medium
4.3. Effect of the Eight Endophytic Isolates on Soil Cu and Cd Speciation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Afzal, M.; Khan, Q.M.; Sessitsch, A. Endophytic bacteria: Prospects and applications for the phytoremediation of organic pollutants. Chemosphere 2014, 117, 232–242. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Rajkumar, M.; Zhang, C.; Freitas, H. Beneficial role of bacterial endophytes in heavy metal phytoremediation. J. Environ. Manag. 2016, 174, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.; He, H.D.; Xiao, L.; Zhong, T.; Liu, H.; Li, S.B.; Deng, P.Y.; Ye, Z.H.; Jing, Y.X. Enhancement of Cd phytoextraction by two Amaranthus species with endophytic Rahnella sp. JN27. Chemosphere 2014, 103, 99–104. [Google Scholar] [CrossRef]
- Han, Y.L.; Wang, R.; Yang, Z.R.; Zhan, Y.H.; Ma, Y.; Ping, S.Z.; Zhang, L.W.; Lin, M.; Yan, Y.L. 1−Aminocyclopropane−1−Carboxylate deaminase from Pseudomonas stutzeri A1501 facilitates the growth of rice in the presence of salt or heavy metals. J. Microbiol. Biotechnol. 2015, 25, 1119–1128. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Ge, C.F.; Xu, S.A.; Wu, Y.J.; Sahito, Z.A.; Ma, L.Y.; Pan, F.S.; Zhou, Q.Y.; Huang, L.K.; Feng, Y.; et al. The endophytic bacterium Sphingomonas SaMR12 alleviates Cd stress in oilseed rape through regulation of the GSH−AsA cycle and antioxidative enzymes. BMC Plant Biol. 2020, 20, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masmoudi, F.; Tounsi, S.; Dunlap, C.A.; Trigui, M. Endophytic halotolerant Bacillus velezensis FMH2 alleviates salt stress on tomato plants by improving plant growth and altering physiological and antioxidant responses. Plant Physiol. Biochem. 2021, 165, 217–227. [Google Scholar] [CrossRef]
- Robinson, R.J.; Fraaije, B.A.; Clark, I.M.; Jackson, R.W.; Hirsch, P.R.; Mauchline, T.H. Endophytic bacterial community composition in wheat (Triticum aestivum) is determined by plant tissue type, developmental stage and soil nutrient availability. Plant Soil 2016, 405, 381–396. [Google Scholar] [CrossRef]
- Ma, Y.; Weisenhorn, P.; Guo, X.; Wang, D.; Yang, T.; Shi, Y.; Zhang, H.; Chu, H. Effect of long−term fertilization on bacterial communities in wheat endosphere. Pedosphere 2021, 31, 538–548. [Google Scholar] [CrossRef]
- Wang, Q.; Pan, F.S.; Xu, X.M.; Rafiq, M.T.; Yang, X.E.; Chen, B.; Feng, Y. Cadmium level and soil type played a selective role in the endophytic bacterial community of hyperaccumulator Sedum alfredii Hance. Chemosphere 2021, 263, 127986. [Google Scholar]
- Ruiz Olivares, A.; Carrillo−Gonzalez, R.; Gonzalez−Chavez, M.D.C.A.; Soto Hernandez, R.M. Potential of castor bean (Ricinus communis L.) for phytoremediation of mine tailings and oil production. J. Environ. Manag. 2013, 114, 316–323. [Google Scholar] [CrossRef]
- Andreazza, R.; Bortolon, L.; Pieniz, S.; Camargo, F.A.O. Use of high−yielding bioenergy plant castor bean (Ricinus communis L.) as a potential phytoremediator for copper−contaminated soils. Pedosphere 2013, 23, 651–661. [Google Scholar] [CrossRef]
- Kang, W.; Bao, J.G.; Zheng, J.; Hu, H.Q.; Du, J.K. Distribution and chemical forms of copper in the root cells of castor seedlings and their tolerance to copper phytotoxicity in hydroponic culture. Environ. Sci. Pollut. Res. Int. 2015, 22, 7726–7734. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.M.; Islam, E.; Irem, S.; Akhtar, K.; Ashraf, M.Y.; Iqbal, J.; Liu, D. Pb−induced phytotoxicity in para grass (Brachiaria mutica) and Castorbean (Ricinus communis L.): Antioxidant and ultrastructural studies. Chemosphere 2018, 200, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Xiong, P.P.; He, C.Q.; Oh, K.; Chen, X.P.; Liang, X.; Liu, X.Y.; Cheng, X.; Wu, C.L.; Shi, Z.C. Medicago sativa L. enhances the phytoextraction of cadmium and zinc by Ricinus communis L. on contaminated land in situ. Ecol. Eng. 2018, 116, 61–66. [Google Scholar] [CrossRef]
- He, C.Q.; Zhao, Y.P.; Wang, F.F.; Oh, K.; Zhao, Z.Z.; Wu, C.L.; Zhang, X.Y.; Chen, X.P.; Liu, X.Y. Phytoremediation of soil heavy metals (Cd and Zn) by castor seedlings: Tolerance, accumulation and subcellular distribution. Chemosphere 2020, 252, 126471. [Google Scholar] [CrossRef] [PubMed]
- Nie, F.H. New comprehensions of Hyperaccumulator. Ecol. Environ. 2005, 14, 136–138. (In Chinese) [Google Scholar]
- Bhargava, A.; Carmona, F.F.; Bhargava, M.; Srivastava, S. Approaches for enhanced phytoextraction of heavy metals. J. Environ. Manag. 2012, 105, 103–120. [Google Scholar] [CrossRef]
- Bauddh, K.; Singh, R.P. Cadmium tolerance and its phytoremediation by two oil yielding plants Ricinus communis (L.) and Brassica juncea (L.) from the contaminated soil. Int. J. Phytoremediat. 2012, 14, 772–785. [Google Scholar] [CrossRef]
- Wood, J.L.; Tang, C.; Franks, A.E. Microbial associated plant growth and heavy metal accumulation to improve phytoextraction of contaminated soils. Soil Biol. Biochem. 2016, 103, 131–137. [Google Scholar] [CrossRef] [Green Version]
- Ashraf, M.A.; Hussain, I.; Rasheed, R.; Iqbal, M.; Riaz, M.; Arif, M.S. Advances in microbe−assisted reclamation of heavy metal contaminated soils over the last decade: A review. J. Environ. Manag. 2017, 198, 132–143. [Google Scholar] [CrossRef]
- Franco−Franklin, V.; Moreno−Riascos, S.; Ghneim−Herrera, T. Are endophytic bacteria an option for increasing heavy metal tolerance of plants? a meta−analysis of the effect size. Front. Environ. Sci. 2021, 8, 603668. [Google Scholar] [CrossRef]
- Bulgarelli, D.; Rott, M.; Schlaeppi, K.; van Themaat, E.V.L.; Ahmadinejad, N.; Assenza, F.; Rauf, P.; Huettel, B.; Reinhardt, R.; Schmelzer, E.; et al. Revealing structure and assembly cues for Arabidopsis root−inhabiting bacterial microbiota. Nature 2012, 488, 91–95. [Google Scholar] [CrossRef]
- Lundberg, D.S.; Lebeis, S.L.; Paredes, S.H.; Yourstone, S.; Gehring, J.; Malfatti, S.; Tremblay, J.; Engelbrektson, A.; Kunin, V.; del Rio, T.G.; et al. Defining the core Arabidopsis thaliana root microbiome. Nature 2012, 488, 86–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bulgarelli, D.; Garrido−Oter, R.; Muench, P.C.; Weiman, A.; Droege, J.; Pan, Y.; McHardy, A.C.; Schulze−Lefert, P. Structure and function of the bacterial root microbiota in wild and domesticated Barley. Cell Host Microbe 2015, 17, 392–403. [Google Scholar] [CrossRef] [Green Version]
- Sheng, X.F.; Xia, J.J.; Jiang, C.Y.; He, L.Y.; Qian, M. Characterization of heavy metal−resistant endophytic bacteria from rape (Brassica napus) roots and their potential in promoting the growth and lead accumulation of rape. Environ. Pollut. 2008, 156, 1164–1170. [Google Scholar] [CrossRef] [PubMed]
- Schwyn, B.; Neilands, J.B. Universal chemical assay for the detection and determination of siderophores. Anal. Biochem. 1987, 160, 47–56. [Google Scholar] [CrossRef]
- Penrose, D.M.; Glick, B.R. Methods for isolating and characterizing ACC deaminase−containing plant growth−promoting rhizobacteria. Physiol. Plant 2003, 118, 10–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Behera, B.C.; Yadav, H.; Singh, S.K.; Sethi, B.K.; Mishra, R.R.; Kumari, S.; Thatoi, H. Alkaline phosphatase activity of a phosphate solubilizing Alcaligenes faecalis, isolated from Mangrove soil. Biotechnol. Res. Innov. 2017, 1, 101–111. [Google Scholar] [CrossRef]
- Yoon, S.H.; Ha, S.M.; Kwon, S.; Lim, J.; Chun, J. Introducing EzBioCloud: A taxonomically united database of 16S rRNA gene sequences and whole−genome assemblies. Int. J. Syst. Evol. Microbiol. 2017, 67, 1613–1617. [Google Scholar] [CrossRef]
- Rauret, G.; López−Sánchez, J.F.; Sahuquillo, A.; Rubio, R.; Davidson, C.; Ure, A.; Quevauviller, P. Improvement of the BCR three step sequential extraction procedure prior to the certification of new sediment and soil reference materials. J. Environ. Monit. 1999, 1, 57–61. [Google Scholar] [CrossRef]
- Compant, S.; Clément, C.; Sessitsch, A. Plant growth−promoting bacteria in the rhizo− and endosphere of plants: Their role, colonization, mechanisms involved and prospects for utilization. Soil Biol. Biochem. 2010, 42, 669–678. [Google Scholar] [CrossRef] [Green Version]
- Deng, S.Q.; Ke, T.; Li, L.T.; Cai, S.W.; Zhou, Y.Y.; Liu, Y.; Guo, L.M.; Chen, L.Z.; Zhang, D.Y. Impacts of environmental factors on the whole microbial communities in the rhizosphere of a metal−tolerant plant: Elsholtzia haichowensis Sun. Environ. Pollut. 2018, 237, 1088–1097. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.L.; Xu, Z.H.; Xie, J.Y.; Hesselberg−Thomsen, V.; Tan, T.M.; Zheng, D.Y.; Strube, M.L.; Dragos, A.; Shen, Q.R.; Zhang, R.F.; et al. Bacillus velezensis stimulates resident rhizosphere Pseudomonas stutzeri for plant health through metabolic interactions. ISME J. 2022, 16, 774–787. [Google Scholar] [CrossRef] [PubMed]
- Durand, A.; Leglize, P.; Lopez, S.; Sterckeman, T.; Benizri, E. Noccaea caerulescens seed endosphere: A habitat for an endophytic bacterial community preserved through generations and protected from soil influence. Plant Soil 2022, 472, 257–278. [Google Scholar] [CrossRef]
- Zhou, Y.; Wei, Y.L.; Zhao, Z.J.; Li, J.S.; Li, H.M.; Yang, P.Z.; Tian, S.Z.; Ryder, M.; Toh, R.; Yang, H.T.; et al. Microbial communities along the soil−root continuum are determined by root anatomical boundaries, soil properties, and root exudation. Soil Biol. Biochem. 2022, 171, 108721. [Google Scholar] [CrossRef]
- Huang, J.; Zhu, X.Y.; Lu, J.; Sun, Y.; Zhao, X.Q. Effects of different land use types on microbial community diversity in the shizishan mining area. Environ. Sci. 2019, 40, 5551–5560. (In Chinese) [Google Scholar]
- Stefanowicz, A.M.; Kapusta, P.; Szarek−Łukaszewska, G.; Grodzińska, K.; Niklińska, M.; Vogt, R.D. Soil fertility and plant diversity enhance microbial performance in metal−polluted soils. Sci. Total Environ. 2012, 439, 211–219. [Google Scholar] [CrossRef]
- Sun, A.Q.; Jiao, X.Y.; Chen, Q.L.; Wu, A.L.; Zheng, Y.; Lin, Y.X.; He, J.Z.; Hu, H.W. Microbial communities in crop phyllosphere and root endosphere are more resistant than soil microbiota to fertilization. Soil Biol. Biochem. 2021, 153, 108113. [Google Scholar] [CrossRef]
- Sun, W.H.; Xiong, Z.L.; Li, W.; Soares, M.A.; White, J.F., Jr.; Li, H.Y. Bacterial communities of three plant species from Pb−Zn contaminated sites and plant−growth promotional benefits of endophytic Microbacterium sp. (strain BXGe71). J. Hazard. Mater. 2019, 370, 225–231. [Google Scholar] [CrossRef]
- Lv, X.; Wang, Q.K.; Zhang, X.Y.; Hao, J.J.; Li, L.; Chen, W.; Li, H.K.; Wang, Y.H.; Ma, C.P.; Wang, J.L.; et al. Community structure and associated networks of endophytic bacteria in pea roots throughout plant life cycle. Plant Soil 2021, 468, 225–238. [Google Scholar] [CrossRef]
- Glick, B.R.; Cheng, Z.Y.; Czarny, J.; Jin, D. New perspectives and approaches in plant growth−promoting rhizobacteria research. Eur. J. Plant Pathol. 2007, 119, 329–339. [Google Scholar] [CrossRef]
- Grobelak, A.; Kokot, P.; Hutchison, D.; Grosser, A.; Grosser, A. Plant growth−promoting rhizobacteria as an alternative to mineral fertilizers in assisted bioremediation−sustainable land and waste management. J. Environ. Manag. 2018, 227, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P. Efficiency of bacteria and bacterial assisted phytoremediation of heavy metals: An update. Bioresour. Technol. 2021, 328, 124835. [Google Scholar] [CrossRef]
- Sarwar, N.; Imran, M.; Shaheen, M.R.; Ishaque, W.; Kamran, M.A.; Matloob, A.; Rehim, A.; Hussain, S. Phytoremediation strategies for soils contaminated with heavy metals: Modifications and future perspectives. Chemosphere 2017, 171, 710–721. [Google Scholar] [CrossRef]
- Tessier, A.; Campbell, P.G.C.; Bisson, M. Sequential extraction procedure for the speciation of particulate trace metals. Anal. Chem. 1979, 51, 844–851. [Google Scholar] [CrossRef]
- Yang, P.; Zhou, X.F.; Wang, L.L.; Li, Q.S.; Zhou, T.; Chen, Y.K.; Zhao, Z.Y.; He, B.Y. Effect of phosphate−solubilizing bacteria on the mobility of insoluble cadmium and metabolic analysis. Int. J. Environ. Res. Public Health 2018, 15, 1330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, W.L.; Wang, J.F.; Lv, Y.; Dong, H.J.; Wang, L.L.; He, T.; Li, Q.S. Improving cadmium mobilization by phosphate−solubilizing bacteria via regulating organic acids metabolism with potassium. Chemosphere 2020, 244, 125475. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Luo, S.L.; Li, X.J.; Wan, Y.; Chen, J.L.; Liu, C.B. Interaction of Cd−hyperaccumulator Solanum nigrum L. and functional endophyte Pseudomonas sp. Lk9 on soil heavy metals uptake. Soil Biol. Biochem. 2014, 68, 300–308. [Google Scholar] [CrossRef]
- Wang, Q.; Ma, L.Y.; Zhou, Q.Y.; Chen, B.; Zhang, X.C.; Wu, Y.J.; Pan, F.S.; Huang, L.K.; Yang, X.E.; Feng, Y. Inoculation of plant growth promoting bacteria from hyperaccumulator facilitated non−host root development and provided promising agents for elevated phytoremediation efficiency. Chemosphere 2019, 234, 769–776. [Google Scholar] [CrossRef]
- Wu, B.; He, T.; Wang, Z.; Qiao, S.; Wang, Y.; Xu, F.; Xu, H. Insight into the mechanisms of plant growth promoting strain SNB6 on enhancing the phytoextraction in cadmium contaminated soil. J. Hazard. Mater. 2020, 385, 121587. [Google Scholar] [CrossRef]
- Liu, C.J.; Lin, H.; Dong, Y.B.; Li, B. Increase of P and Cd bioavailability in the rhizosphere by endophytes promoted phytoremediation efficiency of Phytolacca acinosa. J. Hazard. Mater. 2022, 431, 128546. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Lin, H.; Dong, Y.B.; Li, B.; He, Y.H. Effects of endophytes inoculation on rhizosphere and endosphere microecology of Indian mustard (Brassica juncea) grown in vanadium−contaminated soil and its enhancement on phytoremediation. Chemosphere 2020, 240, 124891. [Google Scholar] [CrossRef] [PubMed]


| Sampling Sites | Cu mg/kg | Cd mg/kg | pH | Available P mg/kg | OM (g/kg) | NH4+−N mg/kg | NO3−−N mg/kg |
|---|---|---|---|---|---|---|---|
| Tonglushan | 693.8 | 4.3 | 8.10 | 4.4 | 7.0 | 3.8 | 3.4 |
| Gangxia | 833.0 | 9.5 | 7.76 | 22.4 | 39.5 | 5.8 | 59.2 |
| Sources | Samples | Sequences | OTUs | Simpson | Chao1 | Coverage (%) |
|---|---|---|---|---|---|---|
| Tonglushan castor | TR | 36,116 | 783 | 0.14 | 361.0 | 99.9 |
| TS | 45,662 | 505 | 0.23 | 264.7 | 99.8 | |
| TL | 48,579 | 110 | 0.71 | 71.9 | 99.8 | |
| Gangxia castor | YR | 52,468 | 1124 | 0.09 | 654.8 | 99.6 |
| YS | 55,584 | 1061 | 0.19 | 678.2 | 99.4 | |
| YL | 63,409 | 902 | 0.20 | 443.6 | 99.5 |
| Strain | PGP Traits | Heavy Metal Tolerance | ||||||
|---|---|---|---|---|---|---|---|---|
| IAA (mg/L) | Siderophores Production ((λ0 − λ)/λ0) | ACCD μmol α− KA/(h·mg) | Phosphate Solubilization | Nitrogen Fixation | Cu2+ (mg/L) | Cd2+ (mg/L) | ||
| Soluble P Concentration (mg/L) | pH in Supernatant | |||||||
| TR1 | 51.8 ± 1.5 | 0.159 | − | − | − | + | 120 | 10 |
| TR2 | 49.4 ± 0.2 | 0.264 | 73.7 ± 2.9 | − | − | + | 600 | 25 |
| TR3 | 119.1 ± 15.4 | 0.245 | 138.1 ± 42.2 | 141.5 ± 8.0 | 5.0 ± 0.1 | + | 600 | 150 |
| TR4 | 93.4 ± 19.4 | 0.232 | 107.4 ± 43.8 | 314.7 ± 71.2 | 4.7 ± 0.4 | + | 600 | 150 |
| TR5 | 46.6 ± 12.3 | 0.141 | 38.6 ± 1.8 | 492.9 ± 14.4 | 4.3 ± 0.1 | + | 500 | 10 |
| TR6 | 140.1 ± 9.5 | 0.098 | 27.6 ± 2.5 | − | − | + | 400 | 50 |
| TR7 | 4.0 ± 0.0 | 0.161 | − | 416.2 ± 10.0 | 4.6 ± 0.1 | + | 500 | 10 |
| TR8 | 63.5 ± 7.8 | 0.109 | 71.4 ± 7.6 | 190.7 ± 5.1 | 4.9 ± 0.0 | + | 600 | 100 |
| TR10 | 73.1 ± 11.9 | 0.215 | − | 479.4 ± 26.2 | 4.5 ± 0.0 | + | 600 | 100 |
| TR11 | 43.4 ± 0.1 | − | 35.9 ± 0.1 | 483.1 ± 23.5 | 4.4 ± 0.1 | + | 600 | 25 |
| TR12 | 7.1 ± 0.0 | 0.289 | 109.6 ± 41.7 | 115.5 ± 29.0 | 5.0 ± 0.1 | + | 600 | 150 |
| TR13 | 40.0 ± 1.2 | 0.130 | − | − | − | + | 500 | 25 |
| TR14 | 32.6 ± 1.3 | 0.223 | 22.6 ± 2.5 | − | − | + | 300 | 10 |
| TR15 | 13.0 ± 1.0 | 0.309 | 143.8 ± 6.1 | 130.1 ± 5.0 | 5.1 ± 0.0 | + | 600 | 150 |
| TR16 | 6.6 ± 1.3 | 0.358 | 22.0 ± 2.0 | 495.5 ± 10.3 | 4.5 ± 0.0 | + | 1100 | 100 |
| TR18 | 25.7 ± 1.6 | 0.291 | 47.4 ± 1.4 | 127.4 ± 7.3 | 5.0 ± 0.0 | + | 600 | 150 |
| TR20 | 29.6 ± 2.6 | 0.241 | 37.9 ± 1.6 | − | − | + | 500 | 10 |
| TR21 | 20.9 ± 5.2 | − | 233.9 ± 30.6 | 87.2 ± 4.1 | 5.0 ± 0.0 | + | 500 | 100 |
| TR23 | 53.5 ± 9.0 | 0.339 | 50.2 ± 2.8 | 450.6 ± 7.4 | 4.4 ± 0.0 | + | 500 | 10 |
| TS1 | 36.4 ± 1.4 | 0.236 | 23.8 ± 1.3 | − | − | + | 120 | − |
| TS2 | 10.8 ± 0.7 | 0.092 | − | − | − | + | 120 | 25 |
| TS8 | 11.9 ± 0.1 | 0.582 | 28.7 ± 0.4 | − | − | + | 40 | 25 |
| TL1 | 6.8 ± 0.0 | 0.111 | 33.8 ± 0.2 | − | − | + | 120 | 10 |
| TL2 | 5.6 ± 0.0 | 0.282 | − | − | − | − | 120 | 50 |
| TR24 | 13.4 ± 0.5 | 0.250 | − | 467.9 ± 40.9 | 4.5 ± 0.1 | + | 120 | 50 |
| YR1 | 50.3 ± 6.8 | 0.302 | 18.6 ± 0.4 | − | − | + | 120 | − |
| YR2 | 28.5 ± 6.6 | 0.320 | 43.9 ± 3.6 | − | − | + | 600 | 10 |
| YL1 | 8.1 ± 0.0 | 0.268 | − | 445.3 ± 2.1 | 4.5 ± 0.1 | + | 120 | 50 |
| YL2 | 6.3 ± 0.0 | 0.241 | − | 85.6 ± 30.9 | 5.1 ± 0.2 | + | 80 | 50 |
| YL3 | 4.9 ± 0.4 | 0.220 | 20.1 ± 3 | − | − | + | 120 | − |
| YL4 | 38.3 ± 2.1 | − | 22.5 ± 0.4 | − | − | + | 120 | 10 |
| YL5 | 22.0 ± 1.2 | − | − | − | − | + | 120 | − |
| YL6 | 13.8 ± 0.0 | 0.130 | − | − | − | + | 120 | 50 |
| YL7 | 6.2 ± 0.0 | − | 29.2 ± 0.0 | − | − | + | 120 | 25 |
| YS1 | 33.1 ± 1.9 | 0.344 | − | 183.3 ± 12.2 | 4.9 ± 0.0 | + | 40 | 200 |
| YS2 | 54.0 ± 3.1 | 0.347 | 19.3 ± 2.2 | − | − | + | 120 | 10 |
| YS3 | 124.3 ± 3.4 | 0.306 | 21.5 ± 4.7 | − | − | + | 500 | 200 |
| YS4 | 26.9 ± 6.4 | 0.344 | 41.5 ± 0.4 | − | − | + | 200 | 10 |
| YS5 | 10.9 ±0.0 | 0.232 | − | 469.8 ± 9.2 | 4.7 ± 0.0 | + | 120 | 25 |
| YS6 | 4.0 ± 0.0 | − | − | − | − | − | 120 | 25 |
| YS7 | 15.3 ± 6.0 | 0.327 | 32.9 ± 2.9 | − | − | + | 120 | − |
| YS8 | 18.1 ± 1.2 | 0.232 | 11.6 ± 3.7 | − | − | + | 300 | 10 |
| YS9 | 9.0 ± 0.0 | 0.261 | 18.8 ± 0.5 | − | − | + | 120 | 25 |
| YS10 | 42.3 ± 1.4 | 0.300 | 32.5 ± 11.1 | − | − | + | 120 | 10 |
| Strains | Accession Number | Closest Relative Strain | Similarity (%) | Genus |
|---|---|---|---|---|
| TR4 | OP310027 | Enterobacter kobei (CP017181) | 99.8 | Enterobacter |
| TR8 | OP310028 | Buttiauxella agrestis (JMPI01000079) | 99.3 | Buttiauxella |
| TR16 | OP310029 | Acinetobacter pittii (APQP01000001) | 99.0 | Acinetobacter |
| TR18 | OP310030 | Enterobacter kobei (CP017181) | 99.8 | Enterobacter |
| TR21 | OP310031 | Enterobacter huaxiensis (FYBI01000003) | 99.3 | Enterobacter |
| YL1 | OP310032 | Bacillus altitudinis (ASJC01000029) | 99.2 | Bacillus |
| YS3 | OP310033 | Proteus myxofaciens (LXEN01000172) | 98.5 | Proteus |
| YS5 | OP310034 | Metabacillus idriensis (AY904033) | 99.8 | Metabacillus |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Q.; Fu, Q.; Zhu, J.; Sun, Y.; He, H.; Hu, H. Endophytic Bacteria in Ricinus communis L.: Diversity of Bacterial Community, Plant−Growth Promoting Traits of the Isolates and Its Effect on Cu and Cd Speciation in Soil. Agronomy 2023, 13, 333. https://doi.org/10.3390/agronomy13020333
Li Q, Fu Q, Zhu J, Sun Y, He H, Hu H. Endophytic Bacteria in Ricinus communis L.: Diversity of Bacterial Community, Plant−Growth Promoting Traits of the Isolates and Its Effect on Cu and Cd Speciation in Soil. Agronomy. 2023; 13(2):333. https://doi.org/10.3390/agronomy13020333
Chicago/Turabian StyleLi, Qian, Qingling Fu, Jun Zhu, Yuxin Sun, Huan He, and Hongqing Hu. 2023. "Endophytic Bacteria in Ricinus communis L.: Diversity of Bacterial Community, Plant−Growth Promoting Traits of the Isolates and Its Effect on Cu and Cd Speciation in Soil" Agronomy 13, no. 2: 333. https://doi.org/10.3390/agronomy13020333





