Abstract
Leaf nitrogen (N), phosphorus (P), and potassium (K) stoichiometry can reflect plant strategies of nutrient allocation, which play key roles in ensuring food security and maintaining nutrient balance in the agroecosystem. Arbuscular mycorrhizal fungi (AMF) inoculation is an effective and green management measure affecting nutrient uptake and utilization strategies, especially in the agroecosystem. However, the interplay between AMF and leaf nutrient stoichiometry that is important for sustainable agriculture remain underexplored. Therefore, the efficacy of AMF in improving leaf nutrients of host plants in agricultural ecosystems were tested with meta-analysis by 1932 pairs of observations in research publications from 1995 to 2022. Overall analysis showed that AMF inoculation increases leaf N, P, and K by 8.75%, 24.61%, and 13.54%, respectively. Moreover, leaf P: K increased by 11.74% by AMF inocula, but leaf N: P and N: K of host plants decreased by 15.38% and 5.52%, respectively. Furthermore, the AMF effect on leaf nutrient stoichiometry was significantly regulated by species, life cycle, and growth habits of host plants. The prominent efficacy of AMF was higher for leaf P in fruit (30.06%), perennial (30.19%), and woody plants (31.6%) than other groups. Moreover, AMF effects on leaf N: P: K stoichiometry of inoculated crops varied depending on the identity of AMF. The Glomeraceae (especially Rhizophagus genera) increased more leaf P content than other AMF families. Thus, the leaf nutrient of host plants significantly increased by AMF inocula, especially leaf P content in the agroecosystem. The effect of AMF on leaf N: P: K stoichiometry was related to plant species, plant life cycle, plant growth habits, and the identity of AMF. These findings highlight the response of AMF to the strategies of nutrient in host plants and provide a theoretical and applicable way for better crop yield and sustainable agriculture.
1. Introduction
Nitrogen (N), phosphorus (P), and potassium (K) play a critical role in controlling crop growth and yield, which are generally considered to be the limiting elements to the agroecosystem [1,2]. Plant nutrient stoichiometry refers to the relative proportion of chemical elements in the biomass, which are important indicators of nutrient cycling and ecological functions [3,4]. In addition, plant nutrient stoichiometry can be used to assess nutrient limitation in agroecosystems [5,6]. Previous studies have reported that plant nutrient stoichiometric dynamics and characteristics can be used to predict nutrient limitations of host crop for production or quality [7,8,9]. Yuan and Chen [10] found that plant P has a weaker response compared with N because of the limitation of P in soil, but plant N: P and N: K ratios increased due to K remaining relatively constant. The leaf is the primary photosynthetic organ of crops, and its stoichiometry reflects nutrient requirements for plant growth [11,12]. Therefore, leaf stoichiometry has attracted increasing attention recently [13,14,15]. Previous researches have explored the leaf nutrient stoichiometry dynamics in the forest, grassland, and other terrestrial ecosystem at a global scale [16,17,18]. Generally, the results of numerous studies demonstrated that leaf stoichiometry could be very sensitive to nutrient availability [10,19], which also serves as an effective indicator of crop nutrient limitation [20,21]. For example, the limiting element of plant growth is N if leaf N: P ratio less than 14; the limiting element of plant growth is P if leaf N: P more than 16; and the limiting elements are both N and P if leaf N: P between 14 and 16 [21,22]. The leaf nutrient stoichiometry could be a vital tool to reveal nutrient limitation for crop yield and quality [22]. Thus, it is critical to explore plant leaf stoichiometry to understand nutrient cycling and limitation in agroecosystems.
There are many management practices to increase crop yield and quality in the agroecosystem, such as different tillage systems, water management, and fertilizer application [23,24,25]. Biological fertilizer application, especially arbuscular mycorrhizal fungi (AMF) inoculation, has aroused wide attention due to its potential in developing environment-friendly and sustainable agriculture [26]. AMF is a group of soil microorganisms that associates with the roots of 92% of plant species [27,28]. AMF inoculation is a useful management practice in sustainable agriculture to enhance grain yield [29]. Additionally, AMF inoculation also improved grain quality significantly [30,31]. Improving biomass and nutrient uptake of plants are two main ways for AMF to promote the productivity and growth of host plants [32,33,34]. Generally, AMF allocates more nutrients to the host plants by expanding the absorption area of roots with their hyphae [35,36]. Previous studies confirmed that AMF could stimulate the nutrient acquisition of host plants, especially N, P, and K [37,38,39]. Wu et al. [40] demonstrated that mycorrhizal inoculation significantly increased leaf P and K content in fruit. Therefore, AMF inoculation is recommended as an effective agricultural management strategy not only for its contribution to crop production but also for its key influence on functions of agroecosystem, such as plant nutrient cycle, and on soil microbial structure [30]. However, the responses of AMF on host plants were different, which is mainly related to AMF species, the types of mycorrhizal inocula, and other environmental conditions [41,42,43]. In Rocha’s study [44], plants inoculated with mix AMF had better growth than those symbiosis with single AMF. Crossay et al. [43] also pointed out that plant inoculation with mix AMF from different families had higher biomass and nutrient availability than those inoculated with single species. It has also been reported that the functions of AMF colonization are more beneficial in mixed AMF for the host plant than in single AMF because the effect of different AMF species could complement each other [41,45]. On the contrary, there are a few researchers have suggested that it did not always present the positive effect of AMF on host plants to enhance mycorrhizal diversity [46,47]. Furthermore, the effect of mycorrhizal fungi on crop nutrient resorption also depends on AMF species [41,48]. For example, Wang et al. [49] found that there were significant differences in the P acquisition efficiency of host plants inoculated with different AMF species. Pandey and Grag [50] also studied pigeon pea and found higher biomass and mycorrhizal colonization in plants inoculated with Rhizophagus irregularis than in those with Funneliformis mossseae under salty stress. Therefore, the responses of leaf nutrient to AMF were varied with different variables including plant species, AMF inocula, AMF species, and experimental conditions. However, it lacks a comprehensive understanding of the relationship between AMF and leaf nutrient stoichiometry in agroecosystem.
Leaf nutrient stoichiometry, as a factor of nutrient limitation, is often coupled tightly with AMF in crops. To estimate the vital role of AMF for leaf nutrients and its stoichiometry in the agroecosystem, we need accurate quantitative research. The mechanism with which AMF influences the leaf nutrient has been tested in some crops including maize, wheat, pepper, tomato, and so on [51,52,53,54]. However, there is a lack of quantitative estimation of moderator variables on leaf nutrient responses to crops inoculated with AMF in the agroecosystem. Here, we conducted a global meta-analysis on the effect of AMF on the leaf nutrient stoichiometry of crops. Therefore, the purpose of our study is to answer the following questions: (1) what is the overall and specific effect of AMF on leaf nutrients and its stoichiometry in the agroecosystem, and (2) what factors do the AMF effect on leaf nutrients and its stoichiometry depend on?
2. Materials and Methods
2.1. Establishment of Database
We searched for papers in Web of Science database (1950–2022) using search terms ‘leaf N AND leaf P AND leaf C AND arbuscular AND crop’ on 15 June 2022. A total of 120 publications presented in Web of Science. All the articles included in our research had to satisfy the following criteria to ensure the accuracy of results: (1) the articles had to be original research articles, (2) report leaf N, P, K and their stoichiometry data, with at least 1 pair of data (an AMF inoculated treatment and a control treatment), (3) contain replicated controlled treatments, and (4) have multiple observations at different times that were considered to be independent. Based on the above criteria, 1932 pairs of observations from 41 articles that were published between 1995 and 2022 were collected (Supplementary Data Sheets). Information on leaf nutrients and their stoichiometry, replications, and other related observations were extracted from 41 articles. The free digitizing software (GetData GRAPH DIGITIZER v.2.20) was used to extract data from graphs.
A total of seven groups of indicators related to AMF and leaf nutrients were classified for detailed investigation with the methods described in Zhang et al. and Hoeksema et al. [55,56]. Numerous studies found that the AMF effect on crop growth indicators varied according to different experimental conditions. To define the research type for which our results apply, we classified the experimental conditions into field and lab according to the methods in Zhang et al. [55]. Moreover, as is well known, the AMF effect differs according to crop types. To evaluate how crop types influence the effect size of AMF, we classified the data into crop groups (grain, fruits and others), crop life cycle (annual and perennial), and crop growth habits (herb and woody) according to the methods in Chandrasekaran [57]. Among the moderators related to microbes, the influence of AMF inocula were tested. AMF inocula had two levels, including single and mix. The single level means that only one AMF species was inoculated with the host plant during the experiment. The mix level means that at least two or more AMF species were inoculated with the host plant during the experiment. According to all the data we collected, the Glomerales account for over 85%. To examine how AMF families and genera affect the effect size of AMF, the most commonly used AMF inocula in agroecosystem were chosen. AMF families had two levels, including Glomeraceae and Claroideoglomeraceae. AMF genus were classified into Funneliformis, Glomus, and Rhizophaguss. The classification of AMF was obtained on the website (http://www.speciesfungorum.org/ (accessed on 30 June 2022)). Ultimately, we contributed the Dataset of AMF and Leaf N: P: K stoichiometry in Agroecosystem in Supplementary Data Sheets.
2.2. The Meta-Analysis of Effect Size
The natural logarithm-transformed response ratio (ln R) was used as effect size to examine how AMF affected leaf nutrient and their stoichiometry in the agroecosystem. The effect size relates to the mean of the AMF inoculated treatment compared to the control (non-AMF inoculation) from the same article with the same method. The effect size in an individual observation (Dij) was calculated using the following equation:
where Dij is the effect size in an individual observation, Xt is the average value in AMF inoculation treatment, and Xn indicates the the average value of the control treatment (non-AMF inoculation).
Dij = Ln R = ln (Xt/Xc)
We used the weighting method to caculate the overall weighted mean effect size followed by Hoeksema et al. [56]. The heterogeneity was assessed in the effect size by performing I2 statistics [58]. We used a random effect model to estimate the overall effect of AMF on leaf N, P, K, and their stoichiotry with the rma.uni() function in R (version 3.4.1, R Core Team, 2017). All meta-analyses were conducted with ‘METAFOR’ package in R [59].
2.3. Statistical Analysis
To facilitate understanding, the significance of results were presented by effect size and confidence intervals (CIs) which is similar to previous meta-analyses [55,57]. AMF significantly affected observed moderator variables when the 95% CIs did not overlap with 0. If the 95% CIs do not overlap with 0, the effect size is significant at p < 0.05. In addition, the difference presented significantly among groups when the 95% CIs of each group did not overlap. To explore the relationship among leaf N, leaf P, and leaf K under different AMF genus, linear regression analysis was performed using SPSS 17.0. The significant difference among different AMF genus was performed based on p-values (p < 0.05).
3. Results
3.1. AMF Effects on Leaf Nutrients and Their Stoichiometry
Across all observations, AMF significantly increased leaf N content (8.8%, CI = 4.3–13.2%, p < 0.05), leaf P content (24.6%, CI = 19.5–29.7%, p < 0.05), and leaf K content (13.5%, CI = 8.8–18.3%, p < 0.05) in the agroecosystem (Figure 1). Moreover, the effect size of leaf P is significantly higher than leaf N. For the distribution of every effect size of leaf N, the positive, neutral, and negative effect size accounted for 64.0%, 16.2%, and 19.8 %, respectively. For leaf P, the effect size changed from −40.5% to 257.9% with 76.5% positive effect size. The distribution of each effect size of leaf K is positive (60.1%), neutral (29.7%), and negative (10.2%).
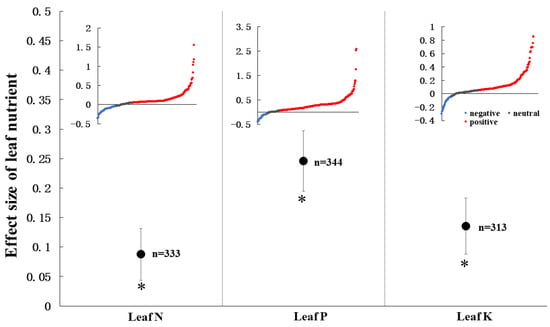
Figure 1.
AMF effect on leaf nutrients. Every effect is presented by weighted means (95% CIs). The red, blue, and grey points show the effect size of positive, negative, and neutral. ‘*’ represents p < 0.05. ‘n’ represents trial numbers.
AMF also affected leaf nutrient stoichiometry (Figure 2). Overall, the leaf N: P and N: K ratios decreased 15.4% (CI = −20.6–10.2%, p < 0.05) and 5.6% (CI = 6.2–17.3%, p < 0.05), respectively. The effect size of leaf N: P was significant lower than N: K. The leaf P: K effect size of AMF increased 11.7% (CI = −10.0–1.1%, p < 0.05). The distribution of effect size of leaf nutrient stoichiometry presented a difference. The leaf N: P included positive, negative, and neutral effect size with 22.2%, 58.4%, and 19.4%, respectively. The leaf N: K effect size ranged from -75.3% to 81.1% with a 36.0% negative effect size. However, the distribution of the effect size of leaf P: K included positive (55.9%), neutral (19.5%), and negative (24.6%).

Figure 2.
AMF effect on leaf nutrients stoichiometry. Every effect is presented by weighted means (95% CIs). The red, blue, and grey points show the effect size of positive, negative, and neutral. ‘*’ represents p < 0.05. ‘n’ represents trial numbers.
The AMF effect on leaf nutrients depended on crop species and crop growth habits (Figure 3). AMF increased leaf N in other crops, but did not affect leaf N in grain and fruit. AMF increased leaf P and K in grain, fruit, and other crops. However, there were no significant differences among grain, fruit, and other crops in leaf N and P.

Figure 3.
AMF effect on leaf nutrients under different variables. Every effect is presented by weighted means (95% CIs). The red and grey points show the effect size of positive and neutral, respectively. ‘*’ represents p < 0.05.
Moreover, the effect of AMF on leaf N: P: K stoichiometry was dependent on crop species, crop life cycle, and crop growth habits (Figure 4). Considering the leaf N: P: K stoichiometry, the effect of AMF on leaf N: P and N: K were negative in fruit, but the effect of AMF on leaf N: P and N: K were neutral in grain and other crop. AMF significant increased leaf P: K in fruit, but did not influence P: K in grain and other crop. AMF significantly decreased leaf N: P and increased P: K in perennial plants. For the annual plant, the effect of AMF on leaf N: P and P: K were neutral, but the effect of AMF on leaf N: K was negative. AMF significantly decreased leaf N: P ratio and increased P: K, but did not affect N: K in woody plants.

Figure 4.
AMF effect on leaf nutrients stoichiometry under different variables. Every effect is presented by weighted means (95% CIs). The red, blue, and grey points shows the effect size of positive, negative, and neutral, respectively. ‘*’ represents p < 0.05.
3.2. AMF Identity Effects on Leaf Nutrients and Their Stoichiometry
The types of AMF inocula did not affect leaf N, P and, K (Figure 5). Both single AMF inoculation and mix AMF inoculation had increased leaf N, P, and K content. However, there was no significant difference between single and mix AMF inocula in leaf N, P, and K content.
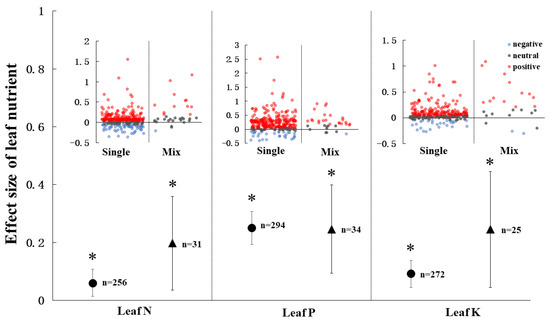
Figure 5.
AMF effect on leaf nutrients under single and mix inocula. Every effect is presented by weighted means (95% CIs). The red, blue, and grey points means the effect size of positive, negative, and neutral, respectively. ‘*’ represents p < 0.05. ‘n’ represents trial numbers.
The leaf N: P: K stoichiometry depended on AMF inocula (Figure 6). The effect size of leaf N: P: K were neutral in plant associated with mix AMF. The plant inoculation with single AMF significantly increased leaf P: K by 5.8% (CI = 10.7–22.2%, p < 0.05), decreased leaf N: P by 18.1% (CI = −23.7–12.4%, p < 0.05), and had no effect in leaf N: K.

Figure 6.
AMF effect on leaf nutrients stoichiometry under single and mix inocula. Every effect is presented by weighted means (95% CIs). The red, blue, and grey points shows the effect size of positive, negative, and neutral, respectively. ‘*’ represents p < 0.05. ‘n’ represents trial numbers.
The AMF effect on leaf N, P, K and their stoichiometry also depended on the AMF families (Figure 7 and Figure 8). The Glomeraceae increased crop leaf N, P and, K by 4.9% (CI = 0.6–10.4%, p < 0.05), 6.0% (CI = 19.7–31.7%, p < 0.05), and 4.8% (CI = 2.8–12.4%, p < 0.05), respectively. On the other hand, the Claroideoglomeraceae had no influence on leaf N, P, and K content. For the leaf nutrient stoichiometry, the leaf N: P ratio had a negative effect size with -0.19 (CI = −0.26–−0.13, p < 0.05) and the leaf P: K ratio had a positive effect size with 0.19 (CI = 0.13–0.25, p < 0.05) in plant association with Glomeraceae. The effect of Glomeraceae on leaf N: K was neutral. However, the plant inoculation with Claroideoglomeraceae had no effect in leaf N: P: K stoichiometry.
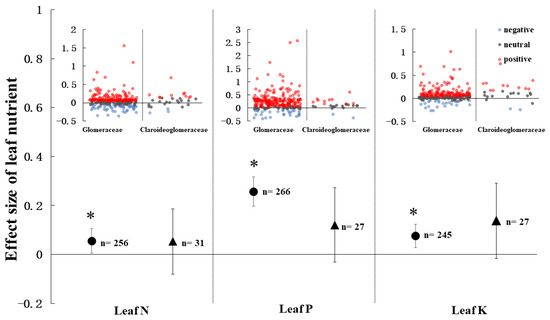
Figure 7.
AMF effect on leaf nutrients under different AMF families. Every effect is presented by weighted means (95% CIs). The red, blue, and grey points show the effect size of positive, negative, and neutral, respectively. ‘*’ represents p < 0.05. ‘n’ represents trial numbers.

Figure 8.
AMF effect on leaf nutrients stoichiometry under different AMF families. Every effect is presented by weighted means (95% CIs). The red, blue, and grey points show the effect size of positive, negative, and neutral, respectively. ‘*’ represents p < 0.05. ‘n’ represents trial numbers.
Furthermore, the Glomeraceae influence on leaf N, P, K, and their stoichiometry was analyzed. The results in Figure 9 show that the AMF effect on leaf N, P, and K was dependent on AMF genera. The effect size of Funneliformis on crop leaf N was positive with 0.11 (CI = −0.002–0.148, p < 0.05) The Funneliformis and Rhizophagus increased leaf P and K content by 40.1% (CI = 31.2–49%, p < 0.05) and 14.1% (CI = 6.0–19.9%, p < 0.05). In contrast, there was no influence on leaf N, P, and K in Glomus. Considering leaf N: P: K stoichiometry, AMF genera varied leaf P: K and N: P (Figure 10). The Rhizophagus significantly decreased N: P by 36.0% (CI = −44.0–27.9%, p < 0.05) and increased P: K by 32.2% (CI = 22.9–41.5%, p < 0.05). The Funneliformis and Glomus had no influence on leaf N: P: K stoichiometry.

Figure 9.
AMF effect on leaf nutrients under under different AMF genera from Glomeraceae. Every effect was presented by weighted means (95% CIs). The red and grey points show the effect size of positive and neutral, respectively. ‘*’ represents p < 0.05.

Figure 10.
AMF effect on leaf nutrients stoichiometry under different AMF genera from Glomeraceae. Every effect was presented by weighted means (95% CIs). The red, blue, and grey points show the effect size of positive, negative, and neutral, respectively. ‘*’ represents p < 0.05.
The effect size of leaf N, P, and K showed different relationship in different fungi genera (Figure 11). The effect size of leaf P increased significantly as the leaf N effect size increased in Funneliformis (p < 0.01), whereas the effect size of leaf P did not vary with the effect size of leaf N in Rhizophagus (p < 0.05). The leaf K effect size increased remarkably as leaf N was enhanced in both Funneliformis and Rhizophagus fungi (p < 0.01). The effect size of leaf K in Funneliformis increased the most with the increase of the effect size of leaf P (p < 0.01), followed by Rhizophagus.
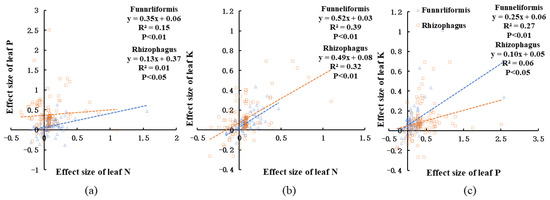
Figure 11.
The relationship between effect size of leaf N and leaf P in Funneliformis and Rhizophagus (a), the relationship between effect size of leaf N and leaf K in Funneliformis and Rhizophagus (b), and between effect size of leaf P and leaf K in Funneliformis and Rhizophagus (c). The blue triangle and blue line mean Funneliformis, and the orange rectangle and orange line mean Rhizophagus.
4. Discussion
In this study, the AMF effects on leaf nutrients have been explored in the agriculture ecosystem. The significance of this research is to provide support for improving agriculture productivity, the guidance of nutrient management, and the understanding of biogeochemical cycles through a study on leaf nutrient stoichiometry of AMF inoculation crops in the agroecosystem. The changes in leaf N, P, K, and their stoichiometry in response to AMF present differences by various variables. Our results confirmed those of previous studies that AMF inoculation promote leaf N, P, and K content of host plants [60,61]. On one hand, AMF is good at increasing photosynthesis and further improving the absorption of mineral nutrients [62], which could explain why the leaf of plant inoculation with AMF had higher N and P contents than non-inoculated plants. On the other hand, AMF also could modify root morphology and structure and osmotic adjustment through the extensive hyphal network, and eventually increase plant nutrient absorption from soil [63,64]. Shi et al. [60] showed that AMF significantly enhanced leaf N and P in legume species. Wang et al. [65] also confirmed that AMF inoculation enhanced nutrient contents in leaves, such as N, P, and K. Moreover, the N: P: K stoichiometry of plant leaf is widely treated as an estimation of limiting nutrient to productivity [6,11,66]. In our research, AMF inoculation increased P: K ratios and decreased N: P and N: K ratio (Figure 2). This result indicates that the leaf P had the strongest response out of the other two elements. It may be caused by the following reasons. Firstly, the host plants inoculation with AMF is better at increasing P absorption efficiency than N and K, which is confirmed in our research and numerous studies (Figure 1) [67,68]. Secondly, the plants inoculation with AMF grow faster than non-inoculated plants, which need more P-rich RNA to promote protein synthesis [5].
There was no variability in leaf nutrient and their stoichiometry under different experimental conditions (field vs. lab). In Lekberg and Koide’s research [69], the beneficial response of AMF to plants was stronger in the lab than that in the field. However, in our study the experimental condition was an unimportant factor to the AMF effect on host plants, which is consistent with Hoeksema et al. [56]. This result indicated that there was no difference between lab experiment and field experiment in leaf N: P: K stoichiometry. Among the plant group, all the crops showed the positive effect for P and K uptake in leaf across all studies. On the other hand, only other crops showed a significant positive effect on leaf N of host plant. Thus, this study emphasizes that there were variations in leaf nutrients of AMF inoculation between inter-species and intra-species, which was illustrated in Chandrasekaran’s study [57]. Furthermore, the leaf nutrient in fruit and grain crop had a weaker response than them in other crops, especially leaf N and K. For the fruit, the effect of AMF on N: P and N: K were negative and the effect size on P: K was positive, which indicated that AMF inoculation were the most effective strategy to enhance available P acquisition of host fruit plants [70]. The main reason is that the phosphate transporters of host plants have been reported to be required for both P transfer and AMF symbiosis [70]. Many previous studies also concluded that the main effect of the AMF inoculation is an improved P status in fruit [71,72]. Our results also showed the difference on leaf nutrient stoichiometry of host plant in the life cycle (annual vs. perennial) and growth habits (herb vs. woody). Comparing with other plant functional groups, perennial plants and woody plants had a positive effect on leaf P: K and a negative effect on leaf N: P (Figure 4), which demonstrated the facilitation of P acquisition through inoculation with AMF in perennial plants or woody plants [73]. In other words, AMF improve productivity by enhancing biomass, which is related to increased nutrient availability, especially P absorption [74,75].
The AMF inocula (single vs. mix) may affect the leaf nutrient stoichiometry of host plant (Figure 5 and Figure 6). Leaf nutrient had higher effect size in mix inoculation than single inoculation, which is opposite to Chandrasekaran [57]. The main reason was the small data of leaf nutrient in mix inoculation AMF in our database. AMF effects in leaf N: P: K stoichiometry of inoculated crops varied depending on the identity of AMF. Glomeraceae and Claroideoglomeraceae, which are domain families in AMF, varied in their effect on leaf nutrient and their stoichiometry, which is consisted with Smilauer et al. [76]. Glomeraceae showed positive effect size on leaf N, P, and K of the host plant, but Claroideoglomeraceae showed no effect on leaf N, P, and K (Figure 7). These results may illustrate functional differences among AMF families [77,78,79]. Chagnon et al. [80] also made a conclusion that that the abundance of extraradical hyphae were bigger in Glomeraceae than in other families. Moreover, the capacity of root colonization in Glomeraceae was faster than in other AMF families [78,81], which results in cope nutrient content and soil nutrient cycling [82]. Therefore, it is indicated that the Glomeraceae strategy was regarded as a way to improve nutrient uptake from the soil in agroecosystem because of extensive root colonization and randomly connect hyphae with mycelium [80,82]. The pattern of N: P: K stoichiometry in Figure 8 also supports the point of Helgason et al. [83] who concluded that Glomeraceae improve P uptake and enhance the growth of the host plant in temperate deciduous forest.
In further analysis, different AMF genera responded differently to leaf nutrient and their stoichiometry. The leaf N, P, and K had a positive effect in Funneliformis but there was no variation in effect size of leaf nutrient (Figure 9). On one hand, there was a relation between leaf N, P, and K in Funneliformis (Figure 11). On the other hand, the Funneliformis, as the most dominant and common genus in agroecosystem, could increase nutrient absorption in the host plant because they can produce more spores in a short time than other genera [84]. Bainard et al. [85] also make a conclusion that Funneliformis can improve the nutrient uptake due to high colonization and soil nutrient availability. The Rhizophagus had no effect on leaf N. Nevertheless, the contribution of Rhizophagus to leaf P was the higher than leaf K, which was also confirmed in N: P and P: K ratios (Figure 10). Hao et al. [86] pointed out that plant inoculation with Rhizophagus significantly enhanced P contents. Moreover, our results indicated that there was no relation between leaf N and P in the Rhizophagus (Figure 11). However, the Glomus did not contribute greatly to leaf nutrient and their stoichiometry in agroecosystem, which is in contrast to Hontoria et al. [87]. It may be due to the small number of observations in Glomus in our dataset. Therefore, the mechanisms of symbiosis with AMF might vary with different AMF genera and, finally, affect AMF function [88]. Our results may be helpful to screen the functional genes.
5. Conclusions
AMF inoculation significantly increased the leaf nutrient, especially P content in the agroecosystem. Moreover, AMF increased P: K ratio and decreased N: P and N: K ratio, which further explains how AMF mainly improved P uptake of the host plant. Our study also concluded that AMF effects in leaf N: P: K stoichiometry of inoculated crop plants varied depending on plant species, plant life cycle, plant growth habits, and the identity of AMF. However, there are limitations in this study. The data we collected did not include all the AMF families because of the restricted publications. Our study will certainly be helpful for better agricultural production, plant nutrient cycle, and sustainable agriculture. Our results offer a new understanding about the relationship between leaf nutrient stoichiometry and AMF in agroecosystem and enriched the AMF functional diversity theory.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agronomy13020358/s1, Supplementary File, The Dataset of AMF and Leaf N: P: K stoichiometry in Agroecosystem.
Author Contributions
Conceptualization, S.W. and Z.S.; methodology, S.W. and Z.S.; software, Z.S. and J.G.; validation, S.W. and Z.S.; investigation, Z.S.; resources, S.W., Z.S. and M.H.; data curation, Z.S.; writing—original draft preparation, S.W.; writing—review and editing, S.W. and Z.S.; visualization, S.W. and Z.S.; supervision, Z.S. and Y.L.; project administration, Z.S.; funding acquisition, Z.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by NSFC (32171620, 31670499), Natural Science Foundation of Henan Province (402421011901), Student Research Training Program of Henan University of Science and Technology (2022461).
Data Availability Statement
All the data supporting the findings of this study are included in this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bihari, B.; Singh, Y.K.; Shambhavi, S.; Mandal, J.; Kumar, S.; Kumar, R. Nutrient use efficiency indices of N, P, and K under rice-wheat cropping system in LTFE after 34th crop cycle. J. Plant Nutr. 2021, 45, 123–140. [Google Scholar] [CrossRef]
- Zuluaga, D.L.; Sonnante, G. The Use of Nitrogen and Its Regulation in Cereals: Structural Genes, Transcription Factors, and the Role of miRNAs. Plants 2019, 8, 294. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Yan, Q.L.; Yuan, J.F.; Li, R.; Lu, X.T.; Liu, S.L.; Zhu, J.J. Temporal effects of thinning on the leaf C:N: P stoichiometry of regenerated broad leaved trees in larch plantations. Forests 2020, 11, 54. [Google Scholar] [CrossRef]
- Hessen, D.O.; Elser, J.J.; Sterner, R.W.; Urabe, J. Ecological stoichiometry: An elementary approach using basic principles. Limnol. Oceanogr. 2013, 58, 2219–2236. [Google Scholar] [CrossRef]
- Sterner, R.W.; Elser, J.J. Ecological Stoichiometry: The Biology of Elements from Molecules to the Biosphere; Princeton University Press: Princeton, NJ, USA, 2002; pp. 225–226. [Google Scholar]
- Elser, J.J.; Bracken, M.E.S.; Cleland, E.E.; Gruner, D.S.; Harpole, W.S.; Hillebrand, H.; Ngai, J.T.; Seabloom, E.W.; Shurin, J.B.; Smith, J.E. Global analysis of nitrogen and phosphorus limitation of primary producers in freshwater, marine and terrestrial ecosystems. Ecol. Lett. 2007, 10, 1135–1142. [Google Scholar] [CrossRef] [PubMed]
- Arba, M.; Falisse, A.; Choukr-Allah, R.; Sindic, M. Effects of nitrogen and phosphorus fertilization on fruit yield and quality of cactus pear Opuntia ficus-indica (L. ) Mill. Fruits 2017, 72, 212–220. [Google Scholar] [CrossRef]
- Thamrin, M.; Susanto, S.; Susila, A.D.; Suta, D.A. Correlation between nitrogen, phosphorus and potassium leaf nutrient with fruit production of Pummelo citrus (Citrus maxima). Asian J. Appl. Sci. 2014, 7, 129–139. [Google Scholar] [CrossRef]
- Olivos, A.; Johnson, S.; Qin, X.Q.; Carlos, C.H. Fruit phosphorous and nitrogen deficiencies affect ‘Grand Pearl’ nectarine flesh browning. Hortscience 2012, 47, 391–394. [Google Scholar] [CrossRef]
- Yuan, Z.Y.; Chen, H.Y.H. Negative effects of fertilization on plant nutrient resorption. Ecology 2015, 96, 373–380. [Google Scholar] [CrossRef]
- Güsewell, S. N:P ratios in terrestrial plants: Variation and functional significance. New Phytol. 2004, 164, 243–266. [Google Scholar] [CrossRef]
- Wright, I.J.; Reich, P.B.; Westoby, M.; Ackerly, D.D.; Baruch, Z.; Bongers, F.; Cavender-Bares, J.; Chapin, T.; Cornelissen, J.H.C.; Diemer, M.; et al. The worldwide leaf economics spectrum. Nature 2004, 428, 821–827. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Stagg, C.L.; Cai, Y.; Lü, X.; Wang, X.; Shen, R.; Lan, Z. Scaling responses of leaf nutrient stoichiometry to the lakeshore flooding duration gradient across different organizational levels. Sci. Total. Environ. 2020, 740, 139740. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Liu, B.; You, Y.; Li, W.; Liu, M.; Shang, H.; He, J.-S. Solar radiation regulates the leaf nitrogen and phosphorus stoichiometry across alpine meadows of the Tibetan Plateau. Agric. For. Meteorol. 2019, 271, 92–101. [Google Scholar] [CrossRef]
- Yang, D.; Song, L.; Jin, G. The soil C:N:P stoichiometry is more sensitive than the leaf C:N:P stoichiometry to nitrogen addition: A four-year nitrogen addition experiment in a Pinus koraiensis plantation. Plant Soil 2019, 442, 183–198. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, M.; Wen, Y.; Tong, R.; Wang, G.; Wu, Q.; Li, Y.; Wu, T. The effects of stand age on leaf N:P cannot be neglected: A global synthesis. For. Ecol. Manag. 2022, 518, 120294. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, B.; Yue, Z.; Zeng, F.; Li, X.; Li, L. Effects of short-term nitrogen and phosphorus addition on leaf stoichiometry of a dominant alpine grass. Peerj 2021, 9, e12611. [Google Scholar] [CrossRef]
- Hu, Y.; Liu, X.; He, N.; Pan, X.; Long, S.; Li, W.; Zhang, M.; Cui, L. Global patterns in leaf stoichiometry across coastal wetlands. Glob. Ecol. Biogeogr. 2021, 30, 852–869. [Google Scholar] [CrossRef]
- Yuan, Z.Y.; Chen, H.Y.H. Decoupling of nitrogen and phosphorus in terrestrial plants associated with global changes. Nat. Clim. Chang. 2015, 5, 465–469. [Google Scholar] [CrossRef]
- Shi, L.; Li, Q.; Fu, X.; Kou, L.; Dai, X.; Wang, H. Foliar, root and rhizospheric soil C:N:P stoichiometries of overstory and understory species in subtropical plantations. Catena 2020, 198, 105020. [Google Scholar] [CrossRef]
- Li, X.; Bi, Y.; Du, S.; Wang, Y.; Chen, G.; Christie, P. Response of Ecological Stoichiometry and Stoichiometric Homeostasis in the Plant-Litter-Soil System to Re-Vegetation Type in Arid Mining Subsidence Areas. J. Arid. Environ. 2021, 184, 104298. [Google Scholar]
- Su, H.; Wu, Y.; Xia, W.; Yang, L.; Chen, J.; Han, W.; Fang, J.; Xie, P. Stoichiometric mechanisms of regime shifts in freshwater ecosystem. Water Res. 2018, 149, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Bakhshandeh, S.; Corneo, P.E.; Mariotte, P.; Kertesz, M.A.; Dijkstra, F.A. Effect of crop rotation on mycorrhizal colonization and wheat yield under different fertilizer treatments. Agric. Ecosyst. Environ. 2017, 247, 130–136. [Google Scholar] [CrossRef]
- Kikuta, M.; Makihara, D.; Arita, N.; Miyazaki, A.; Yamamoto, Y. Growth and yield responses of upland NERICAs to variable water management under field conditions. Plant Prod. Sci. 2016, 20, 36–46. [Google Scholar] [CrossRef]
- Sadras, V.; Rodriguez, D. Modelling the nitrogen-driven trade-off between nitrogen utilisation efficiency and water use efficiency of wheat in eastern Australia. Field Crop. Res. 2010, 118, 297–305. [Google Scholar] [CrossRef]
- Ali, A.; Ghani, M.I.; Ding, H.; Fan, Y.; Cheng, Z.; Iqbal, M. Co-Amended Synergistic Interactions between Arbuscular Mycorrhizal Fungi and the Organic Substrate-Induced Cucumber Yield and Fruit Quality Associated with the Regulation of the AM-Fungal Community Structure under Anthropogenic Cultivated Soil. Int. J. Mol. Sci. 2019, 20, 1539. [Google Scholar] [CrossRef]
- Abd_Allah, E.F.; Hashem, A.; Alqarawi, A.A.; Bahkali, A.H.; Alwhibi, M.S. Enhancing growth performance and systemic acquired resistance of medicinal plant Sesbania sesban (L.) Merr using arbuscular mycorrhizal fungi under salt stress. Saudi J. Biol. Sci. 2015, 22, 274–283. [Google Scholar] [CrossRef]
- Bonfante, P. The future has roots in the past: The ideas and scientists that shaped mycorrhizal research. New Phytol. 2018, 220, 982–995. [Google Scholar] [CrossRef]
- Zhang, D.-J.; Yang, Y.-J.; Liu, C.-Y.; Zhang, F.; Hu, W.; Gong, S.-B.; Wu, Q.-S. Auxin modulates root-hair growth through its signaling pathway in citrus. Sci. Hortic. 2018, 236, 73–78. [Google Scholar] [CrossRef]
- Rillig, M.C.; Aguilar-Trigueros, C.A.; Camenzind, T.; Cavagnaro, T.R.; Degrune, F.; Hohmann, P.; Lammel, D.R.; Mansour, I.; Roy, J.; van der Heijden, M.G.A.; et al. Why farmers should manage the arbuscular mycorrhizal symbiosis. New Phytol. 2019, 222, 1171–1175. [Google Scholar] [CrossRef]
- Ryan, M.H.; Graham, J.H. Little evidence that farmers should consider abundance or diversity of arbuscular mycorrhizal fungi when managing crops. New Phytol. 2018, 220, 1092–1107. [Google Scholar] [CrossRef]
- Karagiannidis, N.; Bletsos, F.; Stavropoulos, N. Effect of Verticillium wilt (Verticillium dahliae Kleb.) and mycorrhiza (Glomus mosseae) on root colonization, growth and nutrient uptake in tomato and eggplant seedlings. Sci. Hortic. 2002, 94, 145–156. [Google Scholar] [CrossRef]
- Evelin, H.; Kapoor, R.; Giri, B. Arbuscular mycorrhizal fungi in alleviation of salt stress: A review. Ann. Bot. 2009, 104, 1263–1280. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Shi, Z.; Chen, X.; Gao, J.; Wang, X. Arbuscular mycorrhizal fungi increase crop yields by improving biomass under rainfed condition: A meta-analysis. Peerj 2022, 10, e12861. [Google Scholar] [CrossRef] [PubMed]
- Wright, S.F.; Franke-Snyder, M.; Morton, J.B.; Upadhyaya, A. Time-course study and partial characterization of a protein on hyphae of arbuscular mycorrhizal fungi during active colonization of roots. Plant Soil 1996, 181, 193–203. [Google Scholar] [CrossRef]
- He, S.; Long, M.; He, X.; Guo, L.; Yang, J.; Yang, P.; Hu, T. Arbuscular mycorrhizal fungi and water availability affect biomass and C:N:P ecological stoichiometry in alfalfa (Medicago sativa L.) during regrowth. Acta Physiol. Plant. 2017, 39, 199. [Google Scholar] [CrossRef]
- Smith, L.T.; Allaith, A.A.; Smith, G.M. Nutrient Transport in Mycorrhizas: Structure, Physiology and Consequences for Efficiency of the Symbiosis. Plant Soil 1994, 161, 103–113. [Google Scholar] [CrossRef]
- Cozzolino, V.; Pigna, M.; Di Meo, V.; Caporale, A.; Violante, A. Effects of arbuscular mycorrhizal inoculation and phosphorus supply on the growth of Lactuca sativa L. and arsenic and phosphorus availability in an arsenic polluted soil under non-sterile conditions. Appl. Soil Ecol. 2010, 45, 262–268. [Google Scholar] [CrossRef]
- Giovannetti, M.; Tolosano, M.; Volpe, V.; Kopriva, S.; Bonfante, P. Identification and Functional Characterization of a Sulfate Transporter Induced by Both Sulfur Starvation and Mycorrhiza Formation in Lotus Japonicus. New Phytol. 2015, 204, 609–619. [Google Scholar] [CrossRef]
- Wu, Q.-S.; He, J.-D.; Srivastava, A.K.; Zhang, F.; Zou, Y.-N. Development of propagation technique of indigenous AMF and their inoculation response in citrus. Indian J. Agric. Sci. 2019, 89, 130–134. [Google Scholar] [CrossRef]
- Jansa, J.; Smith, F.; Smith, S.E. Are There Benefits of Simultaneous Root Colonization by Different Arbuscular Mycorrhizal Fungi? New Phytol. 2008, 177, 779–789. [Google Scholar] [CrossRef]
- da Silva, I.R.; da Silva, D.; de Souza, F.; Oehl, F.; Maia, L.C. Changes in Arbuscular Mycorrhizal Fungal Communities Along a River Delta Island in Northeastern Brazil. Acta Oecologica-Int. J. Ecol. 2017, 79, 798–817. [Google Scholar] [CrossRef]
- Crossay, T.; Majorel, C.; Redecker, D.; Gensous, S.; Medevielle, V.; Durrieu, G.; Cavaloc, Y.; Amir, H. Is a Mixture of Arbuscular Mycorrhizal Fungi Better for Plant Growth Than Single-Species Inoculants? Mycorrhiza 2019, 29, 325–339. [Google Scholar] [CrossRef] [PubMed]
- Rocha, I.; Duarte, I.; Ma, Y.; Souza-Alonso, P.; Látr, A.; Vosátka, M.; Freitas, H.; Rui, S. Oliveira. Seed Coating with Arbuscular Mycorrhizal Fungi for Improved Field Production of Chickpea. Agronomy 2019, 9, 471. [Google Scholar] [CrossRef]
- Koch, A.M.; Antunes, P.M.; Klironomos, J.N. Diversity Effects on Productivity Are Stronger within than between Trophic Groups in the Arbuscular Mycorrhizal Symbiosis. PLoS ONE 2012, 7, e36950. [Google Scholar] [CrossRef] [PubMed]
- Janoušková, M.; Seddas, P.; Mrnka, L.; Van Tuinen, D.; Dvořáčková, A.; Tollot, M.; Gianinazzi-Pearson, V.; Vosátka, M.; Gollotte, A. Development and activity of Glomus intraradices as affected by co-existence with Glomus claroideum in one root system. Mycorrhiza 2009, 19, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Tavasolee, A.; Aliasgharzad, N.; SalehiJouzani, G.; Mardi, M.; Asgharzadeh, A. Interactive Effects of Arbuscular Mycorrhizal Fungi and Rhizobial Strains on Chickpea Growth and Nutrient Content in Plant. Afr. J. Biotechnol. 2011, 10, 7585–7591. [Google Scholar]
- Sharma, D.; Kayang, H. Effects of arbuscular mycorrhizal fungi (amf) on Camellia sinensis (L.) o. kuntze under greenhouse conditions. J. Exp. Biol. Agric. Sci. 2017, 5, 235–241. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, S.; Bücking, H. Arbuscular mycorrhizal growth responses are fungal specific but do not differ between soybean genotypes with different phosphate efficiency. Ann. Bot. 2016, 118, 11–21. [Google Scholar] [CrossRef]
- Pandey, R.; Garg, N. High effectiveness of Rhizophagus irregularis is linked to superior modulation of antioxidant defence mechanisms in Cajanus cajan (L.) Millsp. genotypes grown under salinity stress. Mycorrhiza 2017, 27, 669–682. [Google Scholar] [CrossRef]
- Di Martino, C.; Palumbo, G.; Vitullo, D.; Di Santo, P.; Fuggi, A. Regulation of mycorrhiza development in durum wheat by P fertilization: Effect on plant nitrogen metabolism. J. Plant Nutr. Soil Sci. 2018, 181, 429–440. [Google Scholar] [CrossRef]
- Ma, J.; Wang, W.; Yang, J.; Qin, S.; Yang, Y.; Sun, C.; Pei, G.; Zeeshan, M.; Liao, H.; Liu, L.; et al. Mycorrhizal symbiosis promotes the nutrient content accumulation and affects the root exudates in maize. BMC Plant Biol. 2022, 22, 64. [Google Scholar] [CrossRef]
- Gómez-Bellot, M.J.; Lorente, B.; Nortes, P.; Ortuño, M.; Sánchez-Blanco, M.; Alarcón, J.J. Effect of Mixed Substrate with Different Mycorrhizal Fungi Concentrations on the Physiological and Productive Response of Three Varieties of Tomato. Sci. Hortic. 2021, 283, 110040. [Google Scholar] [CrossRef]
- Kaya, C.; Ashraf, M.; Sonmez, O.; Aydemir, S.; Tuna, A.L.; Cullu, M.A. The influence of arbuscular mycorrhizal colonisation on key growth parameters and fruit yield of pepper plants grown at high salinity. Sci. Hortic. 2009, 121, 1–6. [Google Scholar] [CrossRef]
- Zhang, S.; Lehmann, A.; Zheng, W.; You, Z.; Rillig, M.C. Arbuscular mycorrhizal fungi increase grain yields: A meta-analysis. New Phytol. 2018, 222, 543–555. [Google Scholar] [CrossRef]
- Hoeksema, J.D.; Chaudhary, V.B.; Gehring, C.A.; Johnson, N.C.; Karst, J.; Koide, R.; Pringle, A.; Zabinski, C.; Bever, J.D.; Moore, J.C.; et al. A meta-analysis of context-dependency in plant response to inoculation with mycorrhizal fungi. Ecol. Lett. 2010, 13, 394–407. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekaran, M. Arbuscular Mycorrhizal Fungi Mediated Enhanced Biomass, Root Morphological Traits and Nutrient Uptake under Drought Stress: A Meta-Analysis. J. Fungi 2022, 8, 660. [Google Scholar] [CrossRef]
- Cooper, H.; Hedges, L.V.; Valentine, J.C. The Handbook of Research Synthesis and Meta-Analysis; Russell Sage Foundation: New York, NY, USA, 2009. [Google Scholar]
- Viechtbauer, W. Conducting Meta-Analyses in R with the Metafor Package. J. Stat. Softw. 2010, 36, 1–48. [Google Scholar] [CrossRef]
- Shi, Z.Y.; Xu, S.X.; Yang, M.; Zhang, M.G.; Lu, S.C.; Chang, H.Q.; Wang, X.G.; Chen, X.N. Leaf Nitrogen and Phosphorus Stoichiometry are Closely Linked with Mycorrhizal Type Traits of Legume Species. Legum. Res.-Int. J. 2020, 44, 81–87. [Google Scholar] [CrossRef]
- Rubio-Sanz, L.; Jaizme-Vega, M.C. Mycorrhization of Moringa oleifera Improves Growth and Nutrient Accumulation in Leaves. J. Plant Nutr. 2022, 45, 1765–1773. [Google Scholar] [CrossRef]
- Smith, S.E.; Read, D.J. The Symbionts Forming Arbuscular Mycorrhizas. In In Mycorrhizal Symbiosis; Smith, E.S., Read, D.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2008; pp. 13–41. [Google Scholar]
- Berta, G.; Trotta, A.; Fusconi, A.; Hooker, J.E.; Munro, M.; Atkinson, D.; Giovannetti, M.; Morini, S.; Fortuna, P.; Tisserant, B.; et al. Arbuscular mycorrhizal induced changes to plant growth and root system morphology in Prunus cerasifera. Tree Physiol. 1995, 15, 281–293. [Google Scholar] [CrossRef]
- Lozano, J.M.R.; Azcón, R. Symbiotic efficiency and infectivity of an autochthonous arbuscular mycorrhizal Glomus sp. from saline soils and Glomus deserticola under salinity. Mycorrhiza 2000, 10, 137–143. [Google Scholar] [CrossRef]
- Wang, J.; Zhai, L.; Ma, J.; Zhang, J.; Wang, G.G.; Liu, X.; Zhang, S.; Song, J.; Wu, Y. Comparative physiological mechanisms of arbuscular mycorrhizal fungi in mitigating salt-induced adverse effects on leaves and roots of Zelkova serrata. Mycorrhiza 2020, 30, 341–355. [Google Scholar] [CrossRef] [PubMed]
- Cleveland, C.C.; Townsend, A.R.; Taylor, P.; Alvarez-Clare, S.; Bustamante, M.M.C.; Chuyong, G.; Dobrowski, S.Z.; Grierson, P.; Harms, K.E.; Houlton, B.Z.; et al. Relationships among net primary productivity, nutrients and climate in tropical rain forest: A pan-tropical analysis. Ecol. Lett. 2011, 14, 939–947. [Google Scholar] [CrossRef] [PubMed]
- Propster, J.R.; Johnson, N.C. Uncoupling the effects of phosphorus and precipitation on arbuscular mycorrhizas in the Serengeti. Plant Soil 2015, 388, 21–34. [Google Scholar] [CrossRef]
- Jin, J.; Tang, C.; Sale, P. The impact of elevated carbon dioxide on the phosphorus nutrition of plants: A review. Ann. Bot. 2015, 116, 987–999. [Google Scholar] [CrossRef]
- Lekberg, Y.; Koide, R.T. Is plant performance limited by abundance of arbuscular mycorrhizal fungi? A meta-analysis of studies published between 1988 and 2003. New Phytol. 2005, 168, 189–204. [Google Scholar] [CrossRef]
- Ferrol, N.; Azcón-Aguilar, C.; Pérez-Tienda, J. Review: Arbuscular mycorrhizas as key players in sustainable plant phosphorus acquisition: An overview on the mechanisms involved. Plant Sci. 2019, 280, 441–447. [Google Scholar] [CrossRef]
- Khalil, H.A.; Eissa, A.M.; El-Shazly, S.M.; Nasr, A.M.A. Improved growth of salinity-stressed citrus after inoculation with mycorrhizal fungi. Sci. Hortic. 2011, 130, 624–632. [Google Scholar] [CrossRef]
- Sharma, S.D.; Kumar, P.; Bhardwaj, S.K. Screening of AM fungi and Azotobacter chroococcum under natural, solarization, chemical sterilization and moisture conservation practices for commercial mango nursery production in north-west Himalayas. Sci. Hortic. 2011, 128, 506–514. [Google Scholar] [CrossRef]
- Johnson, N.C.; Graham, J.H. The continuum concept remains a useful framework for studying mycorrhizal functioning. Plant Soil 2012, 363, 411–419. [Google Scholar] [CrossRef]
- Chen, W.; Meng, P.P.; Feng, H.; Wang, C.Y. Effects of Arbuscular Mycorrhizal Fungi on Growth and Physiological Performance of Catalpa Bungei Camey. Under Drought Stress. Forests 2020, 11, 1117. [Google Scholar] [CrossRef]
- Wu, H.-H.; Zou, Y.-N.; Rahman, M.M.; Ni, Q.-D.; Wu, Q.-S. Mycorrhizas alter sucrose and proline metabolism in trifoliate orange exposed to drought stress. Sci. Rep. 2017, 7, 42389. [Google Scholar] [CrossRef]
- Šmilauer, P.; Šmilauerová, M.; Kotilínek, M.; Košnar, J. Foraging speed and precision of arbuscular mycorrhizal fungi under field conditions: An experimental approach. Mol. Ecol. 2020, 29, 1574–1587. [Google Scholar] [CrossRef] [PubMed]
- Hart, M.M.; Reader, R.J. Taxonomic basis for variation in the colonization strategy of arbuscular mycorrhizal fungi. New Phytol. 2002, 153, 335–344. [Google Scholar] [CrossRef]
- Hart, M.M.; Klironomos, J.N. Colonization of roots by arbuscular mycorrhizal fungi using different sources of inoculum. Mycorrhiza 2002, 12, 181–184. [Google Scholar] [CrossRef] [PubMed]
- Brundrett, M.C.; Abbott, L.K.; Jasper, D.A. Glomalean Mycorrhizal Fungi from Tropical Australia I. Comparison of the Effectiveness and Specificity of Different Isolation Procedures. Mycorrhiza 1999, 8, 305–314. [Google Scholar] [CrossRef]
- Chagnon, P.-L.; Bradley, R.L.; Maherali, H.; Klironomos, J.N. A trait-based framework to understand life history of mycorrhizal fungi. Trends Plant Sci. 2013, 18, 484–491. [Google Scholar] [CrossRef] [PubMed]
- Maherali, H.; Klironomos, J.N. Influence of Phylogeny on Fungal Community Assembly and Ecosystem Functioning. Science 2007, 316, 1746–1748. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, Q.; Koide, R.T.; Hoeksema, J.D.; Tang, J.; Bian, X.; Hu, S.; Chen, X. Taxonomic resolution is a determinant of biodiversity effects in arbuscular mycorrhizal fungal communities. J. Ecol. 2016, 105, 219–228. [Google Scholar] [CrossRef]
- Helgason, T.; Merryweather, J.W.; Denison, J.; Wilson, P.; Young, J.P.W.; Fitter, A.H. Selectivity and functional diversity in arbuscular mycorrhizas of co-occurring fungi and plants from a temperate deciduous woodland. J. Ecol. 2002, 90, 371–384. [Google Scholar] [CrossRef]
- Belay, Z.; Vestberg, M.; Assefa, F. Diversity and Abundance of Arbuscular Mycorrhizal Fungi Associated with Acacia Trees from Different Land Use Systems in Ethiopia. Afr. J. Microbiol. Res. 2013, 7, 5503–5515. [Google Scholar]
- Bainard, L.D.; Bainard, J.D.; Hamel, C.; Gan, Y. Spatial and temporal structuring of arbuscular mycorrhizal communities is differentially influenced by abiotic factors and host crop in a semi-arid prairie agroecosystem. FEMS Microbiol. Ecol. 2014, 88, 333–344. [Google Scholar] [CrossRef] [PubMed]
- Hao, Z.; Xie, W.; Jiang, X.; Wu, Z.; Zhang, X.; Chen, B. Arbuscular Mycorrhizal Fungus Improves Rhizobium–Glycyrrhiza Seedling Symbiosis under Drought Stress. Agronomy 2019, 9, 572. [Google Scholar] [CrossRef]
- Hontoria, C.; García-González, I.; Quemada, M.; Roldán, A.; Alguacil, M. The cover crop determines the AMF community composition in soil and in roots of maize after a ten-year continuous crop rotation. Sci. Total. Environ. 2019, 660, 913–922. [Google Scholar] [CrossRef] [PubMed]
- Dodd, J.C.; Boddington, C.L.; Rodríguez, A.; Gonzalez-Chavez, C.; Mansur, I. Mycelium of Arbuscular Mycorrhizal fungi (AMF) from different genera: Form, function and detection. Plant Soil 2000, 226, 131–151. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).