Current Agronomic Practices, Harvest & Post-Harvest Processing of Soybeans (Glycine max)—A Review †
Abstract
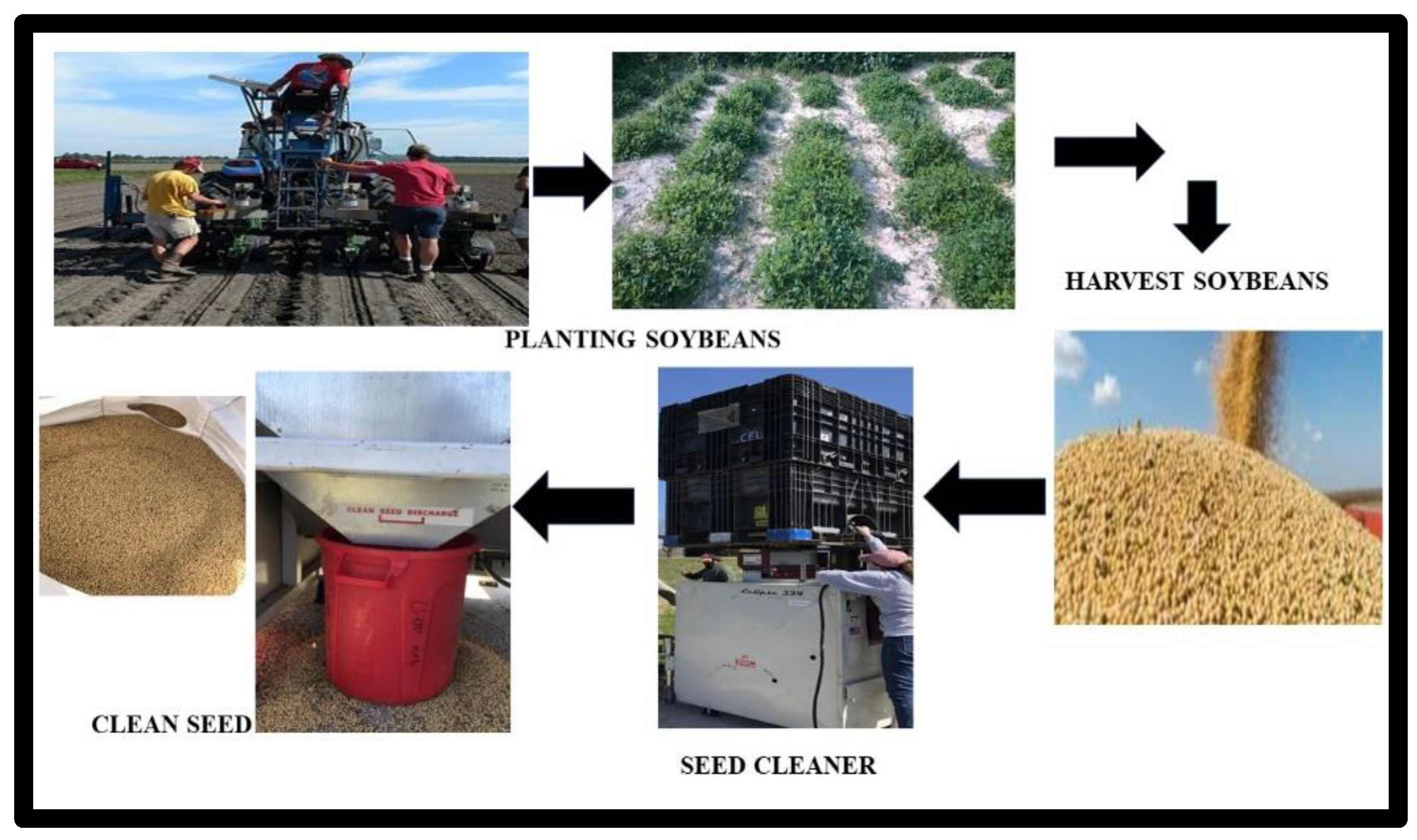
:1. Introduction and Agronomic Practices
2. Soybean Harvest and Post-Harvest
3. Soybean Processing

| Parameters | Conventional-Oleic * | High-Oleic * |
|---|---|---|
| Crude protein (%) | 36.6 | 38.15 |
| Crude fat (%) | 17.94 | 16.38 |
| Gross energy (kcal/kg) | 5238 | 5236 |
| Palmitic acid (%) | 10.5 | 6.92 |
| Stearic acid (%) | 2.89 | 0.58 |
| Oleic acid (%) | 19.5 | 81.53 |
| Linoleic acid (%) | 51.56 | 4.76 |
4. Limitations/Anti-Nutritional Factors of Soybeans/Meal and Processing Methods
| Parameters | Soybean Meal Variety | |||
|---|---|---|---|---|
| EECO | FFHO | FFCO | SECO | |
| Crude protein (%) | 43.83 | 39.86 | 39.56 | 45.74 |
| Crude fat (%) | 7.12 | 15.53 | 17.30 | 4.75 |
| Crude fiber (%) | 7.20 | 7.90 | 6.90 | 5.50 |
| Crude ash (%) | 6.02 | 5.10 | 5.39 | 6.16 |
| Moisture content (%) | 5.58 | 7.83 | 5.43 | 10.00 |
| Urease | 0.06 | 0.27 | 0.29 | 0.07 |
| Trypsin inhibitor (mg/g) | 7.64 | 6.92 | 7.99 | 2.40 |
| Gross energy (kcal/kg) | 4598 | 4890 | 4863 | 4105 |
| Palmitic acid (%) | 11.27 | 7.74 | 11.14 | 14.07 |
| Stearic acid (%) | 3.68 | 3.11 | 3.55 | 3.67 |
| Oleic acid (%) | 19.72 | 71.67 | 18.04 | 14.25 |
| Linoleic acid (%) | 53.21 | 11.03 | 55.20 | 55.87 |
| Formulated metabolizable energy (kcal/kg) | 2927 | 2927 | 2927 | 2927 |
| Parameters | TRT1 | TRT2 | TRT3 | TRT4 | SEM | p-Value |
|---|---|---|---|---|---|---|
| DC, Fat | 0.841 | 0.842 | 0.749 | 0.711 | 0.067 | 0.44 |
| DC, Protein | 0.695 | 0.724 | 0.677 | 0.725 | 0.048 | 0.869 |
| AMEn (kcal/kg) | 2671 | 2764 | 2804 | 2745 | 51 | 0.356 |
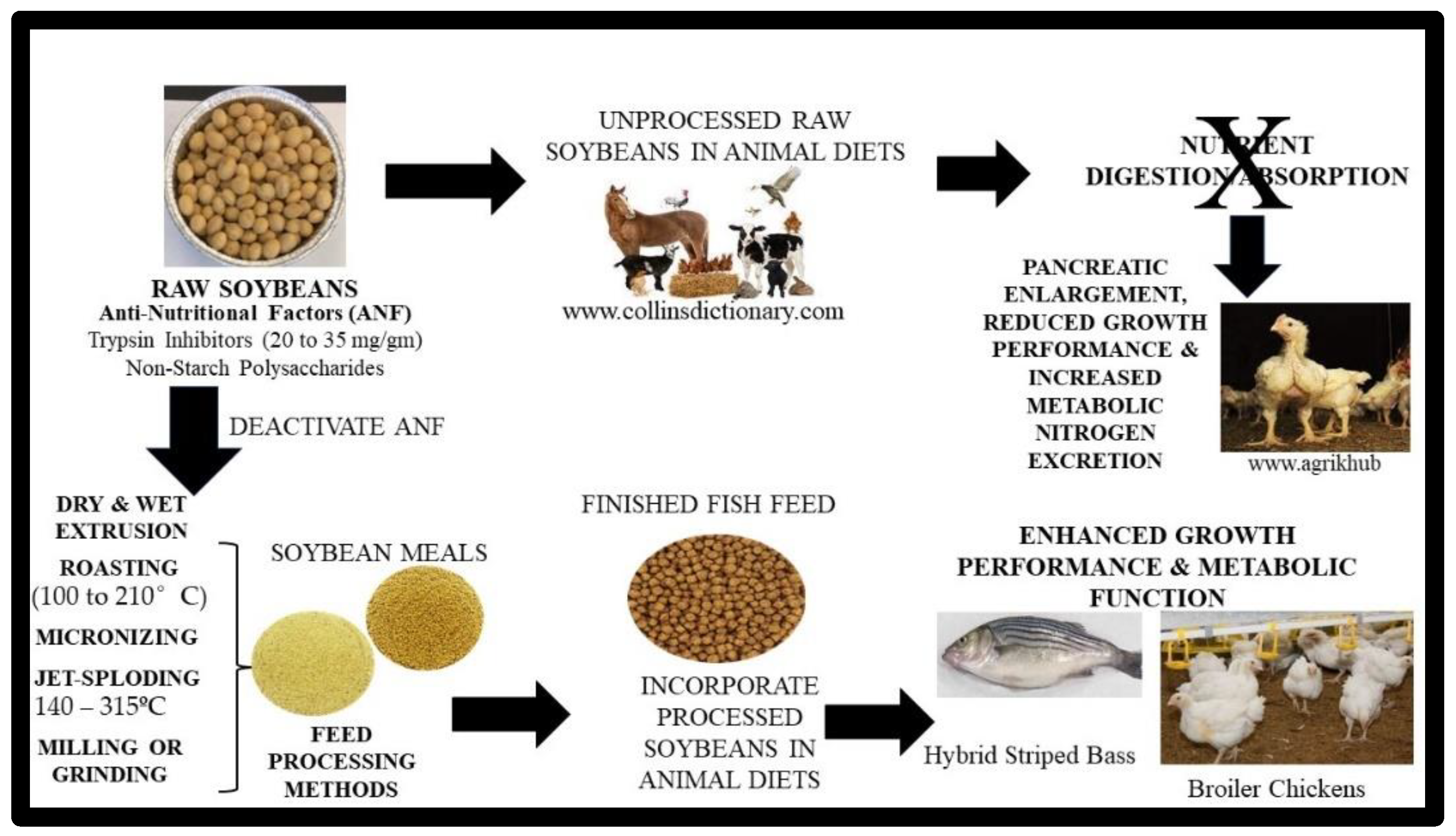
5. Soybean Meal Anti-Nutritional Quality Control Methods
| Heat (°C) | BWG (g) | FCR (kg/kg) | UA (Δ pH) | |
|---|---|---|---|---|
| 1 Lab 1 | 1 Lab 2 | |||
| 115 | 92.2 bc | 1.953 bc | 2.189 a | 1.876 b |
| 125 | 105.1 b | 1.735 c | 0.433 a | 0.239 c |
| 135 | 135.5 a | 1.350 a | 0.080 c | 0.069 c |
| 145 | 138.6 a | 1.335 a | 0.026 c | 0.044 c |
| 165 | 85.3 c | 1.899 c | 0.028 c | 0.035 c |
| Temperature of Extrusion (°C) | Degree of FFSB Processing | UA (Δ pH) |
|---|---|---|
| <135 | Under–processed | >0.20 |
| 135–145 | Adequately processed | 0.05–0.20 |
| >145 | Over–processed | <0.05 |
| Indices | Extruded Soybean Meal Processing Temperatures | |||||||
|---|---|---|---|---|---|---|---|---|
| Target Range | Raw Soybeans | 135 °C | 145 °C | 155 °C | 160 °C | 165 °C | 170 °C | |
| Dry matter (%) | --- | 90.19 | 94.68 | 95.07 | 95.60 | 95.75 | 96.15 | 96.43 |
| Crude protein (%) | --- | 37.59 | 40.57 | 41.74 | 41.59 | 41.59 | 43.85 | 45.23 |
| Lysine (%) | --- | 2.45 | 2.65 | 2.54 | 2.61 | 2.61 | 2.65 | 2.71 |
| PDI (%) | 30–35 | --- | 40.27 | 36.05 | 33.47 | 33.47 | 28.61 | 26.47 |
| KOH protein solubility (%) | <73 | 77.07 | 79.09 | 73.50 | 74.57 | 74.57 | 68.29 | 57.04 |
| Urease index (U) | 0.05–0.3 | 2.09 | 0.08 | 0.04 | 0.02 | 0.02 | 0.03 | 0.04 |
| Trypsin inhibitor | 1–3.5 | 2.44 | 3.76 | 3.91 | 3.65 | 3.52 | 2.26 | 0.04 |
| Lys:CP ratio | >6 | 6.50 | 6.53 | 6.07 | 6.26 | 6.43 | 6.04 | 5.99 |
| Process of FFSM | AME (Kcal/kg) 1 | NR (%) 1 | Cresol Red Absorption (%) 2 |
|---|---|---|---|
| Wet Extrusion | 4278 | 54 | 4.60 |
| Dry Extrusion | 4159 | 59 | 4.06 |
| Micronized | 3681 | 48 | 4.00 |
| Jet-Sploded | 3513 | 61 | 3.98 |
| Toasted | 3728 | 57 | 3.81 |
| Raw | 3227 | 30 | 2.50 |
6. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Pettigrew, J.E.; Soltwedel, K.T.; Miguel, J.C.; Palacios, M.F. Fact Sheet—Soybean Use for Swine. Soybean Meal Information Centre. Available online: http://www.soymeal.org/FactSheets/SwineSoybeanUse.pdf (accessed on 21 January 2021).
- Soya Tech. Soy Facts. Available online: http://www.soyatech.com/soy_facts.htm (accessed on 14 January 2021).
- United Soybean Board. What Are Soybeans Used For? 2020. Available online: https://www.unitedsoybean.org/article/what-are-soybeans-used-for (accessed on 14 January 2021).
- WorldAtlas. Top Soybean Producing US States. Available online: https://www.worldatlas.com/articles/top-soybean-producing-us-states.html (accessed on 17 January 2021).
- Erickson, D.R. Practical Handbook of Soybean Processing and Utilization; AOAC Press: St. Louis, MO, USA, 2015; p. 39. ISBN 978-0935315639. [Google Scholar]
- Shea, Z.; Singer, W.M.; Zhang, B. Soybean Production, Versatility, and Improvement. Available online: https://www.intechopen.com/chapters/71498 (accessed on 21 November 2022).
- Bender, F.R.; Nagamatsu, S.T.; Delamuta, J.R.M.; Ribeiro, R.A.; Nogueira, M.A.; Hungria, M. Genetic variation in symbiotic islands of natural variant strains of soybean Bradyrhizobium japonicum and Bradyrhizobium diazoefficiens differing in competitiveness and in the efficiency of nitrogen fixation. Microb. Genom. 2022, 8, 000795. [Google Scholar] [CrossRef]
- Flynn, R.; Idowu, J. Nitrogen Fixation by Legumes. Available online: https://pubs.nmsu.edu/_a/A129/ (accessed on 14 January 2023).
- Hock, S.M.; Knezevic, S.Z.; Martin, A.R.; Lindquist, J.L. Soybean row spacing and weed emergence time influence weed competitiveness and competitive indices. Weed Sci. 2006, 54, 38–46. [Google Scholar] [CrossRef] [Green Version]
- Neupane, K.; Baysal-Gurel, F. Automatic Identification and Monitoring of Plant Diseases Using Unmanned Aerial Vehicles: A Review. Remote Sens. 2021, 13, 3841. [Google Scholar] [CrossRef]
- Xiaoming, Z.; Qiong, L. A brief introduction of main diseases and insect pests in soybean production in the global top five soybean producing countries. Plant Dis. Pests. 2018, 9, 17–21. [Google Scholar]
- Vivian, R.; Reis, A.; Kálnay, P.A.; Vargas, L.; Camara Ferreira, A.C.; Mariani, F. Weed Management in Soybean—Issues and Practices. Available online: https://www.intechopen.com/chapters/42592 (accessed on 21 November 2022).
- Devos, Y.; Cougnon, M.; Vergucht, S.; Bulcke, R.; Haesaert, G.; Steurbaut, W.; Reheul, D. Environmental impact of herbicide regimes used with genetically modified herbicide-resistant maize. Transgenic. Res. 2008, 17, 1059–1077. [Google Scholar] [CrossRef] [PubMed]
- Parry, M.E. Monsanto—The Launch of Roundup Ready Soybeans. Darden Case No. UVA-M-0619. 1996. Available online: https://doi.org/10.2139/ssrn.910079 (accessed on 30 November 2022).
- Guo, W.; Zhang, F.; Bao, A.; You, Q.; Li, Z.; Chen, J.; Chen, J.; Cheng, Y.; Zhao, W.; Shen, X.; et al. The soybean Rhg1 amino acid transporter gene alters glutamate homeostasis and jasmonic acid-induced resistance to soybean cyst nematode. Mol. Plant Pathol. 2019, 20, 270–286. [Google Scholar] [CrossRef] [Green Version]
- Baidoo, R.; Yan, G. Developing a Real-Time PCR Assay for Direct Identification and Quantification of Soybean Cyst Nematode, Heterodera glycines, in Soil and Its Discrimination from Sugar Beet Cyst Nematode, Heterodera schachtii. Plant. Dis. 2021, 105, 3848–3857. [Google Scholar] [CrossRef]
- Soybean Research and Information Network. Available online: https://soybeanresearchinfo.com/ (accessed on 1 February 2021).
- Ohio State University Extension, Sclerotinia Stem Rot (White Mold) of Soybean. Available online: https://ohioline.osu.edu/factsheet/plpath-soy-3 (accessed on 21 November 2022).
- Willis, H. Harvesting and Storing Soybeans. Available online: https://www.ecofarmingdaily.com/grow-crops/grow-soybeans/soybean-harvesting/harvesting-and-storing-soybeans/#:~:text=When%20field%20mature%2C%20seeds%2C%20pods,to%20five%20days%20after%20this (accessed on 21 November 2022).
- Michigan State University Extension. Consider Harvesting Soybeans Earlier to Manage Risk and Improve Net Income. Available online: https://www.canr.msu.edu/news/consider_harvesting_soybeans_earlier_to_reduce_risk_and_improve_income (accessed on 21 November 2022).
- Pioneer. Timing Soybean Desiccation as a Harvest Aid. Available online: https://www.pioneer.com/us/agronomy/Timing-Soybean-Desiccation-As-A-Harvest-Aid.html#:~:text=at%20%CE%B1%3D0.05.-,Desiccant%20Options,from%20application%20to%20soybean%20harvest. (accessed on 21 November 2022).
- Islas-Rubio, A.R.; Higuera-Ciapara, I. Soybeans: Post-Harvest Operations. Post-Production Operations. Food and Agriculture Organization of the United Nations. Available online: http://www.soiaefrutta.com/wp-content/uploads/2013/06/A.2.2-Estratto-FAO-Soybeans-post-harvest.pdf (accessed on 17 January 2021).
- Mannaa, M.; Kim, K.D. Effect of Temperature and Relative Humidity on Growth of Aspergillus and Penicillium spp. and Biocontrol Activity of Pseudomonas protegens AS15 against Aflatoxigenic Aspergillus flavus in Stored Rice Grains. Mycobiology 2018, 46, 287–295. [Google Scholar] [CrossRef] [Green Version]
- U.S. Department of Agriculture. Economic Research Service. Soybeans and Oil Crops. 2022. Available online: https://www.ers.usda.gov/topics/crops/soybeans-and-oil-crops/ (accessed on 16 November 2022).
- Oklahoma State University. Oil and Oilseed Processing I. Available online: https://extension.okstate.edu/fact-sheets/oil-and-oilseed-processing-i.html (accessed on 21 November 2022).
- Soybean Processing. Overview. Available online: http://foodtechinfo.com/foodpro/facility_types/311222_soybean_processing/ (accessed on 14 January 2023).
- ChEBI (Chemical Entities of Biological Interest). CHEBI:166975—Soybean Oil. Available online: https://www.ebi.ac.uk/chebi/searchId.do?chebiId=CHEBI:166975 (accessed on 2 December 2022).
- Clemente, T.E.; Cahoon, E.B. Soybean oil: Genetic approaches for modification of functionality and total content. Plant Physiol. 2009, 151, 1030–1040. [Google Scholar] [CrossRef] [Green Version]
- Carlsson, A.S. Plant oils as feedstock alternatives to petroleum—A short survey of potential oil crop platforms. Biochimie 2009, 91, 665–670. [Google Scholar] [CrossRef]
- Clarke, E.; Wiseman, J. Effects of extrusion conditions on trypsin inhibitor activity of full fat soybeans and subsequent effects on their nutritional value for young broilers. Br. Poult. Sci. 2007, 48, 703–712. [Google Scholar] [CrossRef] [PubMed]
- Zanferari, F.; Vendramini, T.H.A.; Rentas, M.F.; Gardinal, R.; Calomeni, G.D.; Mesquita, L.G.; Takiya, C.S.; Rennó, F.P. Effects of chitosan and whole raw soybeans on ruminal fermentation and bacterial populations, and milk fatty acid profile in dairy cows. J. Dairy Sci. 2018, 101, 10939–10952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barletta, R.V.; Gandra, J.R.; Freitas Junior, J.E.; Verdurico, L.C.; Mingoti, R.D.; Bettero, V.P.; Benevento, B.C.; Vilela, F.G.; Rennó, F.P. High levels of whole raw soya beans in dairy cow diets: Digestibility and animal performance. J. Anim. Physiol. Anim. Nutr. 2016, 100, 1179–1190. [Google Scholar] [CrossRef] [PubMed]
- Weld, K.A.; Armentano, L.E. Feeding high oleic acid soybeans in place of conventional soybeans increases milk fat concentration. J. Dairy Sci. 2018, 101, 9768–9776. [Google Scholar] [CrossRef] [Green Version]
- Wiseman, J. Full Fat Soya, Oils and Fats in Poultry Nutrition; Am. Soybean Assoc.: Brussels, Belgium, 1994. [Google Scholar]
- Reddy, V.; Bhosale, D.T. Feed Sources. In Handbook of Poultry Nutrition, 1st ed.; American Soybean Association: New Delhi, India, 2004; ISBN 9788181890689. [Google Scholar]
- Patino, D.B. Physicochemical Characterization of Extruded Fish Feeds Having Soybean Meals of Varying Fatty Acid Profiles and its Utilization on Growth Performance and Body Composition of Domesticated Juvenile Striped Bass (Morone saxatilis). Master’s Thesis, North Carolina State University, Raleigh, NC, USA, 2022. Available online: https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&ved=2ahUKEwjP6pLUydv7AhX1FlkFHQKcC-IQFnoECBQQAQ&url=https%3A%2F%2Frepository.lib.ncsu.edu%2Fbitstream%2Fhandle%2F1840.20%2F39548%2Fetd.pdf%3Fsequence%3D1&usg=AOvVaw3iXA4xoRXXIZnKHzSoh43s (accessed on 2 December 2022).
- Maharjan, P.; Rahimi, A.; Vu, T.; Harding, K.; Malheiros, R.; Dean, L.; Anderson, K.; Toomer, O.T. Full Fat High Oleic Acid SBM in Layer Hen Diets Improved Monounsaturated Fatty Acid Profile in Adipogenic Tissue and in Egg. 2022. Available online: https://www.soymeal.org/soy-meal-articles/full-fat-high-oleic-acid-sbm-in-layer-hen-diets-improved-monounsaturated-fatty-acid-profile-in-adipogenic-tissue-and-in-egg/ (accessed on 2 December 2022).
- Davies, S.J. Digestibility Characteristics of Selected Feed Ingredients for Developing Bespoke Diets for Nile Tilapia Culture in Europe and North America. J. World. Aquac. Soc. 2011, 42, 388–396. [Google Scholar] [CrossRef]
- Ravindran, V.; Abdollahi, M.; Bootwalla, S. Nutrient analysis, apparent metabolizable energy and ileal amino acid digestibility of full fat Soybean for broilers. Anim. Feed Sci. Technol. 2014, 197, 233–240. [Google Scholar] [CrossRef]
- Karr-Lilienthal, L.; Kadzere, C.; Grieshop, C.; Fahey, J.R. Chemical and nutritional properties of soybean carbohydrates as related to nonruminants: A review. Livest. Prod. Sci. 2005, 97, 1–12. [Google Scholar] [CrossRef]
- Dei, H.K. Soybean as a feed ingredient for livestock and poultry. In Recent Trends for Enhancing the Diversity and Quality of Soybean Products; Krezhova, D., Ed.; IntechOpen: Rijeka, Croatia, 2011; pp. 215–216. [Google Scholar]
- Choct, M.; Li-Dersjant, Y.; Mcleish, J.; Peisker, M. Soy oligosaccharides and soluble non-starch polysaccharides: A review of digestion, nutritive and anti-nutritive effects in pigs and poultry. Australas. J. Anim. Sci. 2010, 23, 1386–1398. [Google Scholar] [CrossRef]
- Takács, K.; Szabó, E.E.; Nagy, A.; Cserhalmi, Z.; Falusi, J.; Gelencsér, E. The effect of radiofrequency heat treatment on trypsin inhibitor activity and in vitro digestibility of soybean varieties (Glycine max. (L.) Merr.). J. Food Sci. Technol. 2022, 59, 4436–4445. [Google Scholar] [CrossRef]
- Erdaw, M.M.; Perez-Maldonado, R.A.; Iji, P.A. Physiological and health-related response of broiler chickens fed diets containing raw, full-fat soya bean meal supplemented with microbial protease. J. Anim. Physiol. Anim. Nutr. 2018, 102, 533–544. [Google Scholar] [CrossRef]
- Erdaw, M.M.; Wu, S.; Iji, P.A. Growth and physiological responses of broiler chickens to diets containing raw, full-fat soybean and supplemented with a high-impact microbial protease. Australas. J. Anim. Sci. 2017, 30, 1303–1313. [Google Scholar] [CrossRef] [PubMed]
- Omni Tech International. The Potential Impact of Rising Petrochemical Prices on Soy Use for Industrial Applications. A Survey of Recent Chemical Price Trends. Available online: https://soynewuses.org/wp-content/uploads/MOS_PriceTrendUpdate2010.pdf (accessed on 1 December 2020).
- Napolitano, G.; Ye, Y.; Cruz-Hernandez, C. Chemical Characterization of a High-Oleic Soybean Oil. J. Am. Oil Chem. Soc. 2018, 95, 583–589. [Google Scholar] [CrossRef]
- Bueno, R.D.; Borges, L.L.; God, P.I.V.; Piovesan, N.D.; Teixeira, A.L.; Cruz, C.D.; Barros, E.G. Quantification of anti-nutritional factors and their correlations with protein and oil in soybeans. An. Acad. Bras. Cienc. 2018, 90, 205–217. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.W.; Hsu, C.K.; Yang, Y.F. Effect of thermal treatments on anti-nutritional factors and antioxidant capabilities in yellow soybeans and green-cotyledon small black soybeans. J. Sci. Food Agric. 2014, 94, 1794–1801. [Google Scholar] [CrossRef] [PubMed]
- Aderibigbe, A.S.; Cowieson, A.J.; Ajuwon, K.M.; Adeola, O. Contribution of purified soybean trypsin inhibitor and exogenous protease to endogenous amino acid losses and mineral digestibility. Poult. Sci. 2021, 100, 101486. [Google Scholar] [CrossRef] [PubMed]
- Waldroup, P.W.; Smith, K. Soybean Use–Poultry. Available online: https://www.soymeal.org/wp-content/uploads/2018/04/soybean_use_poultry.pdf (accessed on 28 November 2022).
- Bernard, J.K. Encyclopedia of Dairy Science, 3rd ed.; Elsevier: Tifton, GA, USA, 2022; pp. 614–619. [Google Scholar]
- Newkirk, R. Soybean: Feed Industry Guide. Available online: https://www.scribd.com/document/459744575/2010-Soybean-Feed-Industry-Guide-pdf (accessed on 2 December 2022).
- Moure, A.; Sineiro, J.; Dominguez, H.; Parajo, J.C. Functionality of oilseed protein products: A review. Food Res. Int. 2006, 39, 945–963. [Google Scholar] [CrossRef]
- AgriOrbit. Processing Full-Fat Soya Beans for Animal Consumption. 2020. Available online: https://www.agriorbit.com/processing-full-fat-soya-beans-animal-consumption/ (accessed on 16 January 2021).
- Albin, D. High Temperature Short Time Extrusion Cooking Prevents Protein Quality. Available online: https://www.insta-pro.com/en/blog/nutritionandtechnologies/high-temperature-short-time-extrusion-cooking-preserves-protein-quality/ (accessed on 21 January 2021).
- Riaz, M. Extruding Full Fat Soy for Maximum Quality. All about Feed. Available online: https://www.allaboutfeed.net/Nutrition/Raw-Materials/2007/12/Extruding-full-fat-soy-for-maximum-quality-AAF011248W/ (accessed on 21 January 2021).
- Ramos, M. Why Extrude Soybeans. Insta-Pro International. Available online: https://www.insta-pro.com/en/blog/nutritionandtechnologies/why-extrude-soybeans/ (accessed on 21 January 2021).
- Said, N. New Soybean Processing Technology Boosts Nutrition. Available online: https://www.feedstrategy.com/animal-feed-manufacturing/new-soybean-processing-technology-boosts-nutrition/ (accessed on 31 January 2021).
- Palić, D.V.; Lević, J.D.; Sredanović, S.A.; Đuragić, O.M. Quality control of full-fat Soybean using urease activity: Critical assessment of the method. Acta Period. Technol. 2008, 39, 47–53. [Google Scholar] [CrossRef]
- Mahesh, M.S.; Puri, P.M.; Tripathi, S.P. Potassium Hydroxide Solubility Test to Determine Protein Quality of Soybean Meal. Indian J. Anim. Nutr. 2017, 34, 118–120. [Google Scholar] [CrossRef]
- Patino, D.; Joseph, M. Ideal Extruder Temperature to Produce Best Full Fat Soybean Meal. Feed and Additive Magazine, International Magazine for Animal Feed & Additives Industry. 2022, pp. 70–73. Available online: https://www.feedandadditive.com/ideal-extruder-temperature-to-produce-best-full-fat-soybean-meal/ (accessed on 19 January 2023).
- Swick, C.A. Full Fat Soybean Meal Handbook, 3rd ed.; Northern Crops Institute: Fargo, ND, USA, 2020; Volume 1, pp. 1–80. [Google Scholar]
- Ruiz, N.; Parsons, C. Protein solubility in KOH is not correlated to poultry digestible lysine in full-fat soybeans. In Proceedings of the Poultry Science Association 104th Annual Meeting, 94(E-Supplement 1), Abstract 218, Louisville, KY, USA, 27–30 July 2015. [Google Scholar]
- Modika, K.Y. Evaluation and Standardization of Laboratory Methods Used for Determining the Degree of Soya Processing. Master of Science Thesis, University of Pretoria, Hatfield, Pretoria, South Africa, 2011. Available online: http://hdl.handle.net/2263/25919 (accessed on 30 January 2023).
- Lázaro, R.; Gonzalo, G.; Mateos, L.M.A.; Piquer, J. Whole Soybeans in Diets for Poultry. Available online: https://www.researchgate.net/profile/Gonzalo_Mateos/publication/268013616_Whole_soybeans_in_diets_for_poultry/links/55f19fe708ae199d47c3a01e/Whole-soybeans-in-diets-for-poultry.pdf (accessed on 17 January 2023).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toomer, O.T.; Oviedo, E.O.; Ali, M.; Patino, D.; Joseph, M.; Frinsko, M.; Vu, T.; Maharjan, P.; Fallen, B.; Mian, R. Current Agronomic Practices, Harvest & Post-Harvest Processing of Soybeans (Glycine max)—A Review. Agronomy 2023, 13, 427. https://doi.org/10.3390/agronomy13020427
Toomer OT, Oviedo EO, Ali M, Patino D, Joseph M, Frinsko M, Vu T, Maharjan P, Fallen B, Mian R. Current Agronomic Practices, Harvest & Post-Harvest Processing of Soybeans (Glycine max)—A Review. Agronomy. 2023; 13(2):427. https://doi.org/10.3390/agronomy13020427
Chicago/Turabian StyleToomer, Ondulla T., Edgar O. Oviedo, Muhammad Ali, Danny Patino, Michael Joseph, Mike Frinsko, Thien Vu, Pramir Maharjan, Ben Fallen, and Rouf Mian. 2023. "Current Agronomic Practices, Harvest & Post-Harvest Processing of Soybeans (Glycine max)—A Review" Agronomy 13, no. 2: 427. https://doi.org/10.3390/agronomy13020427





