Assessment of CSM–CERES–Rice as a Decision Support Tool in the Identification of High-Yielding Drought-Tolerant Upland Rice Genotypes
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Experimental Details
2.2. Drought Susceptibility Index (DSI)
2.3. Model Calibration
2.4. Model Application and Decision Support in Genotypic Identification
2.5. Statistics
3. Results
3.1. Weather Conditions and Soil Moisture Contents
3.2. Drought Susceptibility Index (DSI) and Grain Yield (GY)
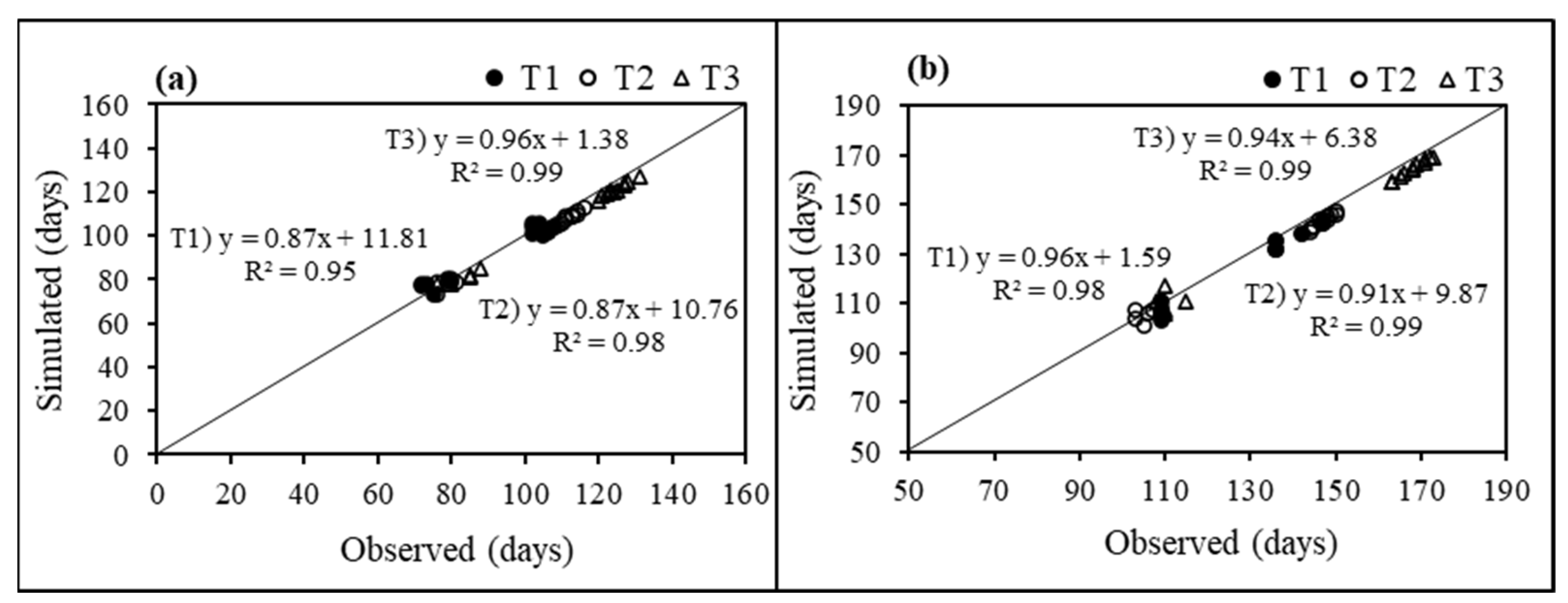
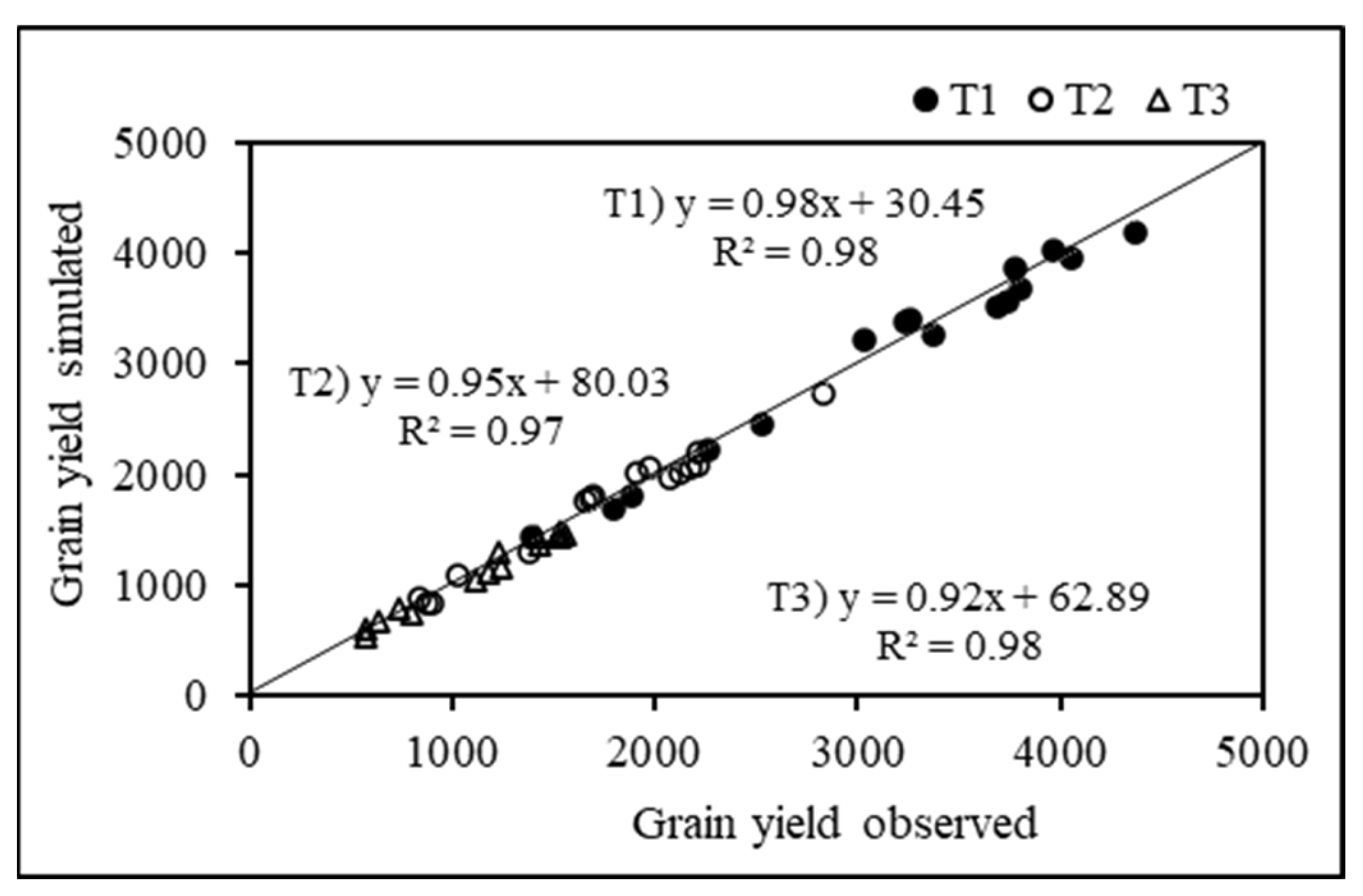
3.3. Model Calibration
3.4. Simulated Phenology and Grain Yield for Yield Assessment Experiment
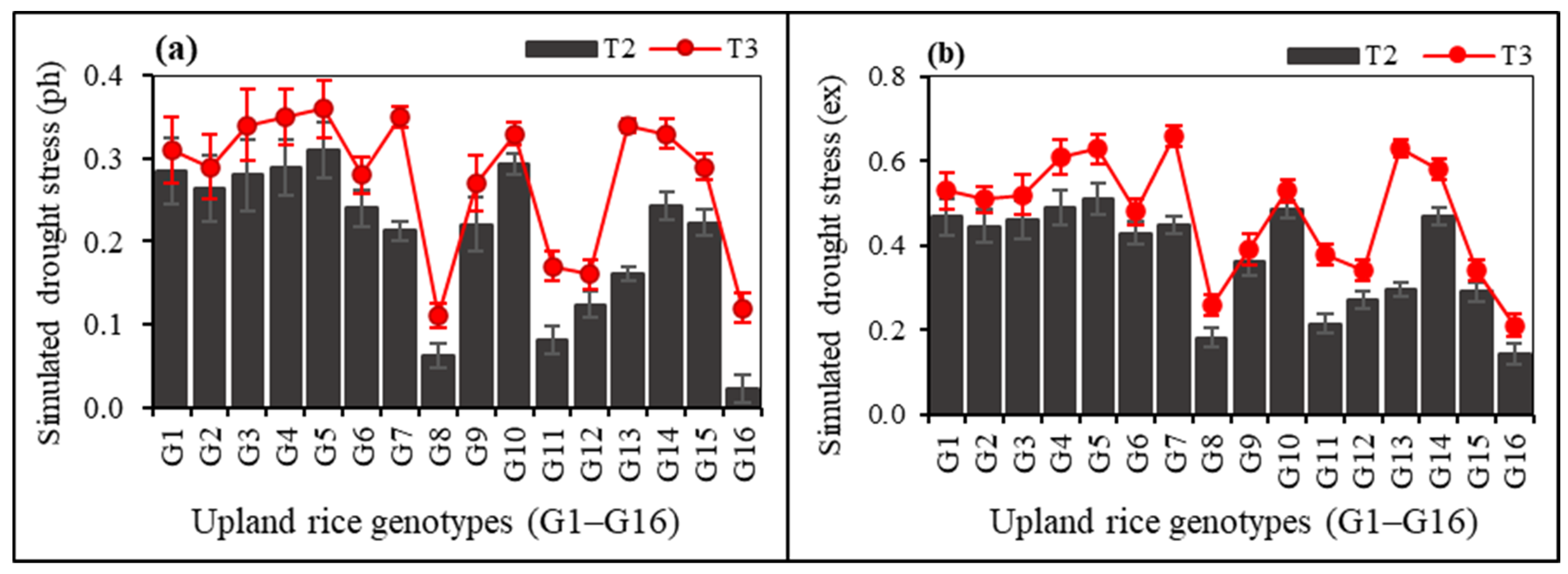
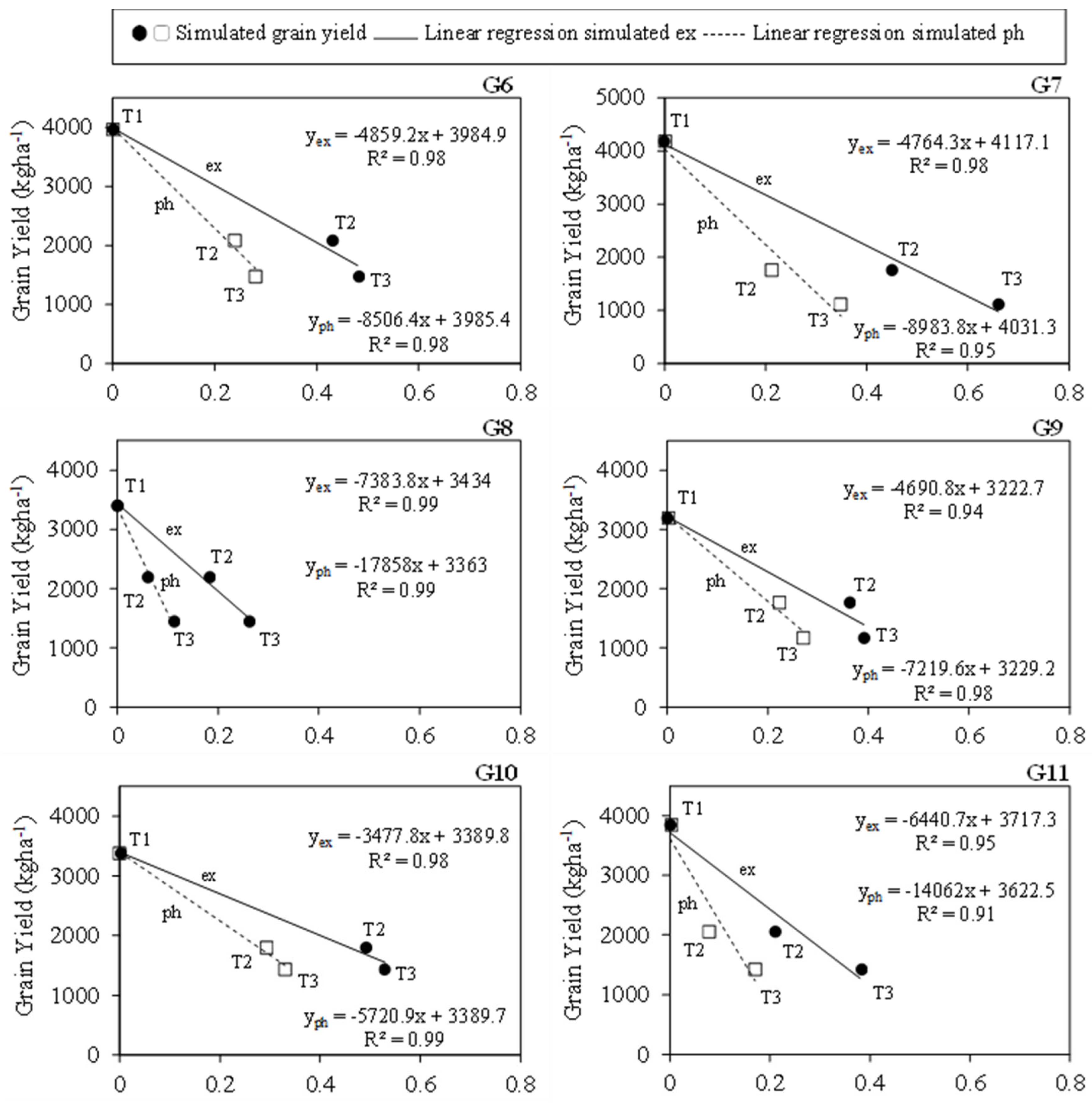
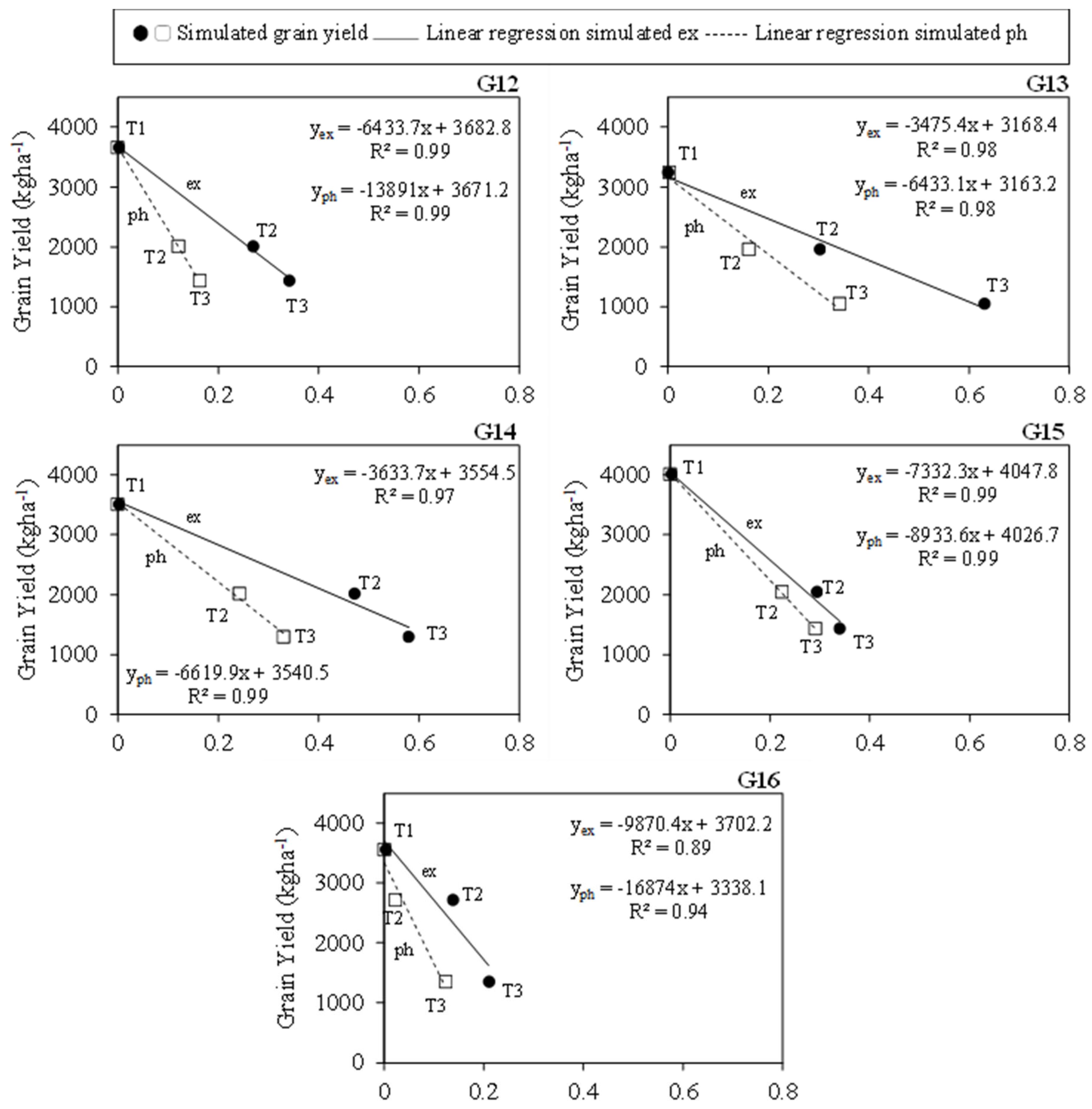
3.5. Simulated Drought Stress Indices
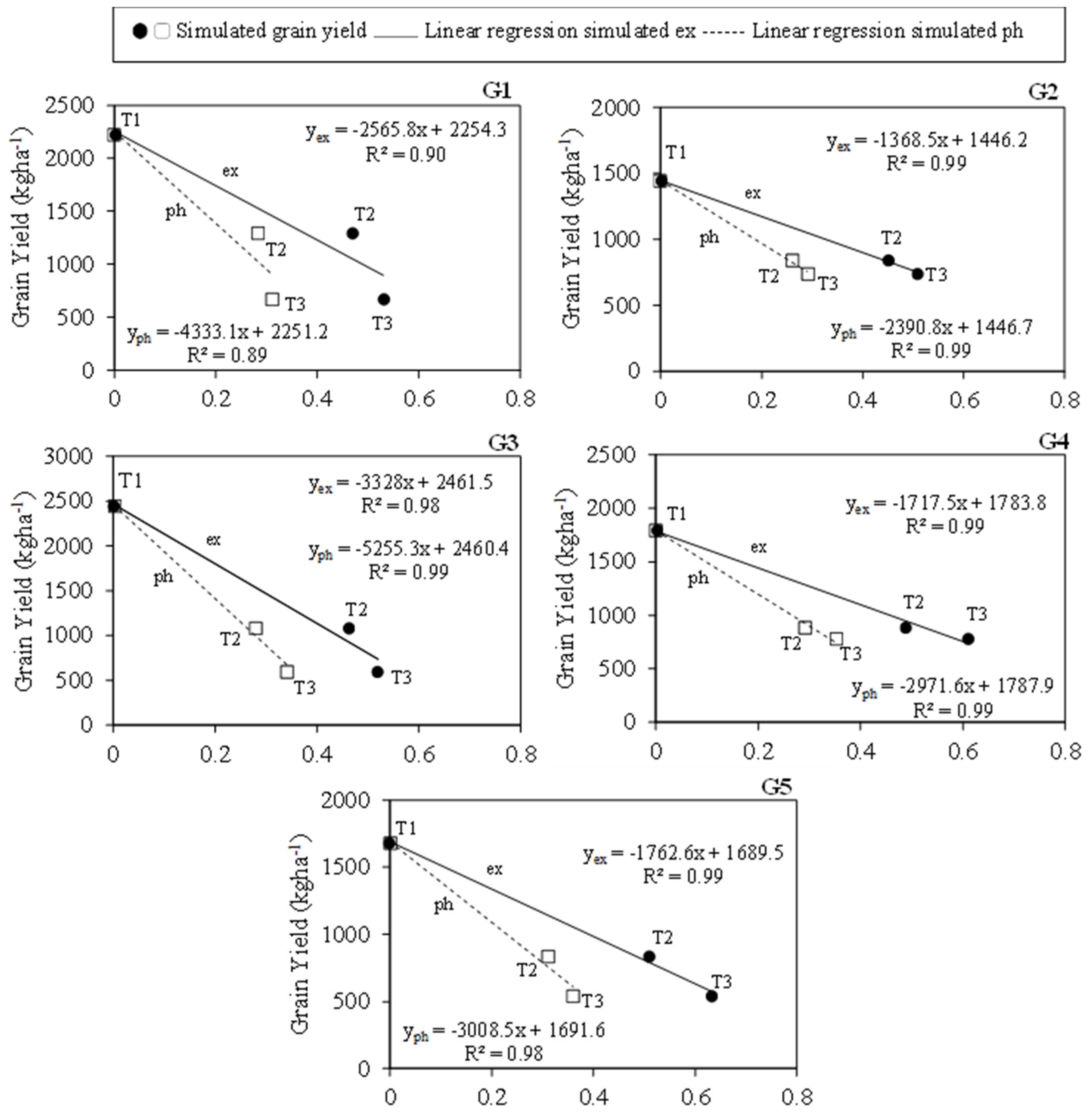
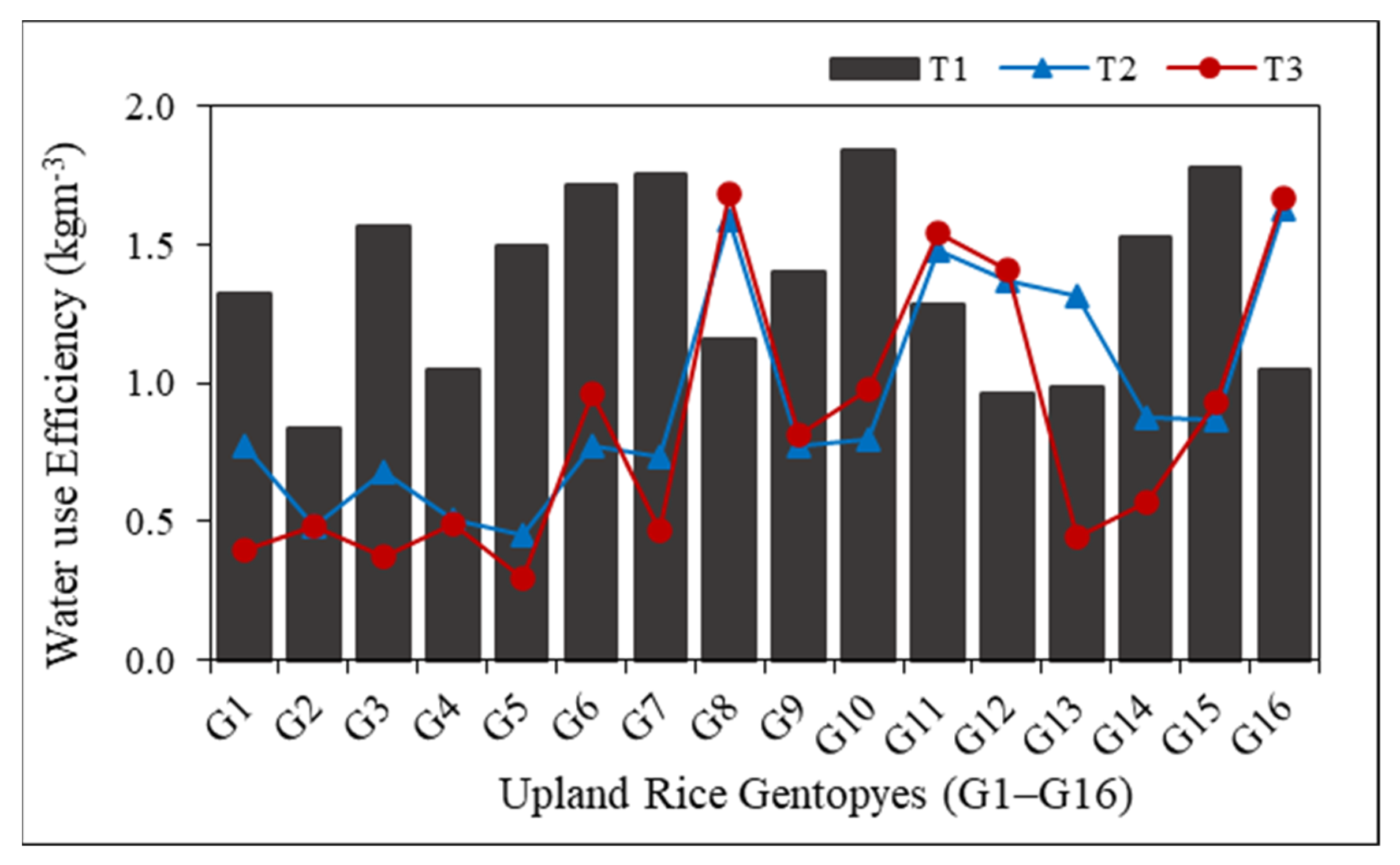
3.6. Simulated Water Use Efficiency (WUE)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Agarwal, P.; Parida, S.K.; Raghuvanshi, S.; Kapoor, S.; Khurana, P.; Khurana, J.P.; Tyagi, A.K. Rice Improvement Through Genome-Based Functional Analysis and Molecular Breeding in India. Rice 2016, 9, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Campozano, L.; Ballari, D.; Montenegro, M.; Avilés, A. Future Meteorological Droughts in Ecuador: Decreasing Trends and Associated Spatio-Temporal Features Derived From CMIP5 Models. Front. Earth Sci. 2020, 8. [Google Scholar] [CrossRef]
- Chutia, J.; Borah, S.P. Water Stress Effects on Leaf Growth and Chlorophyll Content but Not the Grain Yield in Traditional Rice (Oryza sativa Linn.) Genotypes of Assam, India II. Protein and Proline Status in Seedlings under PEG Induced Water Stress. Am. J. Plant Sci. 2012, 3, 971–980. [Google Scholar] [CrossRef]
- Kandel, B.P.; Joshi, L.P.; Sharma, S.; Adhikari, P.; Koirala, B.; Shrestha, K. Drought tolerance screening of rice genotypes in mid-hills of Nepal using various drought indices. Acta Agric. Scand. Sect. B: Soil Plant Sci. 2022, 72, 744–750. [Google Scholar] [CrossRef]
- Hussain, T.; Hussain, N.; Ahmed, M.; Nualsri, C.; Duangpan, S. Responses of lowland rice genotypes under terminal water stress and identification of drought tolerance to stabilize rice productivity in southern thailand. Plants 2021, 10, 2565. [Google Scholar] [CrossRef]
- Smartt, J.; Stalker, H.T. Speciation and Cytogenetics in Arachis; AGRIS: Rome, Italy, 1982. [Google Scholar]
- Fukai, S.; Cooper, M. Development of drought-resistant cultivars using physiomorphological traits in rice. Field Crops Res. 1995, 40, 67–86. [Google Scholar] [CrossRef]
- Singh, B.; Reddy, K.R.; Redoña, E.D.; Walker, T. Screening of Rice Cultivars for Morpho-Physiological Responses to Early-Season Soil Moisture Stress. Rice Sci. 2017, 24, 322–335. [Google Scholar] [CrossRef]
- Hussain, N.; Ahmed, M.; Duangpan, S.; Hussain, T.; Taweekun, J. Potential impacts of water stress on rice biomass composition and feedstock availability for bioenergy production. Sustainability 2021, 13, 10449. [Google Scholar] [CrossRef]
- IRRI. Standard Evaluation System for Rice, 5th ed.International Rice Research Institute: Los Banos, PI, USA, 2014. [Google Scholar]
- Mansour, E.; Desoky, E.S.M.; Ali, M.M.A.; Abdul-Hamid, M.I.; Ullah, H.; Attia, A.; Datta, A. Identifying drought-tolerant genotypes of faba bean and their agro-physiological responses to different water regimes in an arid Mediterranean environment. Agric. Water Manag. 2021, 247, 106754. [Google Scholar] [CrossRef]
- Wasae, A. Evaluation of Drought Stress Tolerance Based on Selection Indices in Haricot Bean Varieties Exposed to Stress at Different Growth Stages. Int. J. Agron. 2021, 2021, 6617874. [Google Scholar] [CrossRef]
- Ahmad, A.; Aslam, Z.; Naz, M.; Hussain, S.; Javed, T.; Aslam, S.; Raza, A.; Ali, H.M.; Siddiqui, M.H.; Salem, M.Z.M.; et al. Exogenous salicylic acid-induced drought stress tolerance in wheat (Triticum aestivum L.) grown under hydroponic culture. PLoS ONE 2021, 16, 1–17. [Google Scholar] [CrossRef]
- Fischer, R.A.; Maurer, R. Drought resistance in spring wheat cultivars. I. Grain yield responses. Aust. J. Agric. Res. 1978, 29, 897–912. [Google Scholar] [CrossRef]
- Fernandez, G.C. Effective selection criteria for assessing plant stress tolerance. In Proceedings of the International Symposium on Adaptation of Vegetables and other Food Crops in Temperature and Water Stress, Shanhua, Taiwan, 13–16 August 1992; pp. 257–270. [Google Scholar]
- Schneider, K.A.; Rosales-serna, R.; Ibarra-perez, F.; Cazares-enriquez, B.; Acosta-gallegos, J.A.; Ramirez-vallejo, P.; Wassimi, N.; Kelly, J.D. Improving common bean performance under drought stress. Crop Sci. 1997, 37, 43–50. [Google Scholar] [CrossRef]
- Hossain, A.B.S.; Sears, R.G.; Cox, T.S.; Paulsen, G.M. Desiccation tolerance and its relationship to assimilate partitioning in winter wheat. Crop Sci. 1990, 30, 622–627. [Google Scholar] [CrossRef]
- Bouslama, M.; Schapaugh, W.T. Stress tolerance in soybeans. I. Evaluation of three screening techniques for heat and drought tolerance. Crop Sci. 1984, 24, 933–937. [Google Scholar] [CrossRef]
- Rosielle, A.A.; Hamblin, J. Theoretical aspects of selection for yield in stress and non-stress environments. Crop Sci. 1981, 21, 943–946. [Google Scholar] [CrossRef]
- Arif, A.; Parveen, N.; Waheed, M.Q.; Atif, R.M.; Waqar, I.; Shah, T.M. A Comparative Study for Assessing the Drought-Tolerance of Chickpea Under Varying Natural Growth Environments. Front. Plant Sci. 2021, 11. [Google Scholar] [CrossRef]
- Ata-Ul-Karim, S.T.; Begum, H.; Lopena, V.; Borromeo, T.; Virk, P.; Hernandez, J.E.; Gregorio, G.B.; Collard, B.C.Y.; Kato, Y. Genotypic variation of yield-related traits in an irrigated rice breeding program for tropical Asia. Crop Environ. 2022, 1, 173–181. [Google Scholar] [CrossRef]
- Boote, K.J.; Jones, J.W.; Pickering, N.B. Potential uses and limitations of crop models. Agron. J. 1996, 88, 704–716. [Google Scholar] [CrossRef]
- Hoogenboom, G. Contribution of agrometeorology to the simulation of crop production and its applications. Agric. For. Meteorol. 2000, 103, 137–157. [Google Scholar] [CrossRef]
- Park, S.; Ahn, J.; Kim, D.; Sitthikone, M. Climate risk management for the rainfed rice yield in Lao PDR using APCC MME seasonal forecasts. Agric. Water Manag. 2022, 274, 107976. [Google Scholar] [CrossRef]
- Kumar, P.; Ines, A.V.M.; Han, E.; Cruz, R.; Prasad, P.V.V. A comparison of multiple calibration and ensembling methods for estimating genetic coefficients of CERES-Rice to simulate phenology and yields. Field Crops Res. 2022, 284, 108560. [Google Scholar] [CrossRef]
- Li, S.; Fleisher, D.; Timlin, D.; Reddy, R.V.; Wang, Z.; McClung, A. Evaluation of Different Crop Models for Simulating Rice Development and Yield in the U.S. Mississippi Delta. Agronomy 2020, 10, 1905. [Google Scholar] [CrossRef]
- Putto, C.; Patanothai, A.; Jogloy, S.; Pannangpetch, K.; Boote, K.J.; Hoogenboom, G. Determination of efficient test sites for evaluation of peanut breeding lines using the CSM-CROPGRO-peanut model. Field Crops Res. 2009, 110, 272–281. [Google Scholar] [CrossRef]
- Nicolas, F.; Migliaccio, K.W.; Hoogenboom, G.; Rathinasabapathi, B.R.; Eisenstadt, W.R. Assessing the potential impact of climate change on rice yield in the Artibonite valley of Haiti unsing the CSM-CERES-Rice model. Am. Soc. Agric. Biol. Eng. 2020, 63, 1385–1400. [Google Scholar] [CrossRef]
- Ansari, A.; Lin, Y.-P.; Lur, H.-S. Evaluating and Adapting Climate Change Impacts on Rice Production in Indonesia: A Case Study of the Keduang Subwatershed, Central Java. Environments 2021, 8, 117. [Google Scholar] [CrossRef]
- Alejo, L.A. Assessing the impacts of climate change on aerobic rice production using the DSSAT-CERES-Rice model Lanie A. Alejo. J. Water Clim. Chang. 2021, 12, 696–708. [Google Scholar] [CrossRef]
- Tojo Soler, C.M.; Suleiman, A.; Anothai, J.; Flitcroft, I.; Hoogenboom, G. Scheduling irrigation with a dynamic crop growth model and determining the relation between simulated drought stress and yield for peanut. Irrig. Sci. 2013, 31, 889–901. [Google Scholar]
- Hussain, T.; Anothai, J.; Nualsri, C.; Soonsuwon, W. Application of CSM-CERES-Rice in scheduling irrigation and simulating effect of drought stress on upland rice yield. Indian J. Agric. Res. 2018, 52, 140–145. [Google Scholar] [CrossRef]
- Jones, J.W.; Hoogenboom, G.; Porter, C.H.; Boote, K.J.; Batchelor, W.D.; Hunt, L.A.; Wilkens, P.W.; Singh, U.; Gijsman, A.J.; Ritchie, J.T. The DSSAT cropping system model. Eur. J. Agron. 2003, 18, 235–265. [Google Scholar] [CrossRef]
- Sun, Q.; Zhao, Y.; Zhang, Y.; Che, X.; Yang, Z.; Song, Y.; Zheng, X. Improvement of the CERES-Rice model using controlled experiments and a Meta-analysis. Theor. Appl. Clim. 2020, 141, 1271–1284. [Google Scholar] [CrossRef]
- Nasir, I.R.; Rasul, F. Climate change impacts and adaptations for fine, coarse, and hybrid rice using CERES-Rice. Environ. Sci. Pollut. Res. 2020, 27, 9454–9464. [Google Scholar] [CrossRef]
- Ritchie, J.T. Soil water balance and plant water stress. In Understanding Options for Agricultural Production; Springer: Berlin/Heidelberg, Germany, 1998; pp. 41–54. [Google Scholar]
- Priestley, C.H.B.; Taylor, R.J. On the assessment of surface heat flux and evaporation using large-scale parameters. Mon. Weather. Rev. 1972, 100, 81–92. [Google Scholar] [CrossRef]
- Allen, R.G.; Pereira, L.S.; Raes, D.; Smith, M. Crop evapotranspiration-Guidelines for computing crop water requirements-FAO Irrigation and drainage paper 56. Fao Rome 1998, 300, D05109. [Google Scholar]
- Boote, K.J.; Sau, F.; Hoogenboom, G.; Jones, J.W. Experience with water balance, evapotranspiration, and predictions of water stress effects in the CROPGRO model. Response Crops Ltd. Water Underst. Model. Water Stress Eff. Plant Growth Process 2008, 1, 59–103. [Google Scholar]
- Sau, F.; Boote, K.J.; McNair Bostick, W.; Jones, J.W.; Ines Minguez, M. Testing and improving evapotranspiration and soil water balance of the DSSAT crop models. Agron. J. 2004, 96, 1243–1257. [Google Scholar] [CrossRef]
- Ali, M.F.; Ali, U.; Bilal, S.; Zulfiqar, U.; Sohail, S.; Hussain, T. Response of sorghum and millet to poultry and farmyard manure–based biochar treatments. Arab. J. Geosci. 2022, 15, 1592. [Google Scholar] [CrossRef]
- Wu, B.; Lin, X.; Ali, M.F.; Wang, D. Development of an irrigation regime for winter wheat to save water resources by avoiding irrigation at anthesis stage. J. Agron. Crop Sci. 2022, 209, 188–203. [Google Scholar] [CrossRef]
- Hunt, L.A.; Boote, K.J. Data for model operation, calibration, and evaluation. In Understanding Options for Agricultural Production; Springer: Berlin/Heidelberg, Germany, 1998; pp. 9–39. [Google Scholar]
- Boote, K.J. Concepts for calibrating crop growth models. DSSAT Version 1999, 3, 179–199. [Google Scholar]
- Liu, H.L.; Yang, J.Y.; Drury, C.F.a.; Reynolds, W.D.; Tan, C.S.; Bai, Y.L.; He, P.; Jin, J.; Hoogenboom, G. Using the DSSAT-CERES-Maize model to simulate crop yield and nitrogen cycling in fields under long-term continuous maize production. Nutr. Cycl. Agroecosystems 2011, 89, 313–328. [Google Scholar] [CrossRef]
- Duangpan, S.; Tongchu, Y.; Hussain, T.; Eksomtramage, T.; Onthong, J. Beneficial Effects of Silicon Fertilizer on Growth and Physiological Responses in Oil Palm. Agronomy 2022, 12, 413. [Google Scholar] [CrossRef]
- Hussain, T.; Hussain, N.; Tahir, M.; Raina, A.; Ikram, S.; Maqbool, S.; Ali, M.F.; Duangpan, S. Impacts of Drought Stress on Water Use Efficiency and Grain Productivity of Rice and Utilization of Genotypic Variability to Combat Climate Change. Agronomy 2022, 12, 2518. [Google Scholar] [CrossRef]
- Loague, K.; Green, R.E. Statistical and graphical methods for evaluating solute transport models: Overview and application. J. Contam. Hydrol. 1991, 7, 51–73. [Google Scholar] [CrossRef]
- Willmott, C.J.; Ackleson, S.G.; Davis, R.E.; Feddema, J.J.; Klink, K.M.; Legates, D.R.; O’donnell, J.; Rowe, C.M. Statistics for the evaluation and comparison of models. J. Geophys. Res. Ocean. 1985, 90, 8995–9005. [Google Scholar] [CrossRef]
- Balbaa, M.G.; Osman, H.T.; Kandil, E.E.; Javed, T.; Lamlom, S.F.; Ali, H.M.; Kalaji, H.M.; Wróbel, J.; Telesiñski, A.; Brysiewicz, A.; et al. Determination of morpho-physiological and yield traits of maize inbred lines (Zea mays L.) under optimal and drought stress conditions. Front. Plant Sci. 2022, 13, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, M.K.; Hasan, M.A.; Bahadur, M.M.; Islam, M.R.; Hakim, M.A.; Iqbal, M.A.; Javed, T.; Raza, A.; Shabbir, R.; Sorour, S.; et al. Evaluation of drought tolerance of some wheat (Triticum aestivum L.) genotypes through phenology, growth, and physiological indices. Agronomy 2021, 11, 1792. [Google Scholar] [CrossRef]
- Singhal, R.K.; Fahad, S.; Kumar, P.; Choyal, P.; Javed, T.; Jinger, D.; Singh, P.; Saha, D.; Md, P.; Bose, B.; et al. Beneficial elements: New Players in improving nutrient use efficiency and abiotic stress tolerance. Plant Growth Regul. 2022, 1–29. [Google Scholar] [CrossRef]
- Shabbir, R.; Javed, T.; Hussain, S.; Ahmar, S.; Naz, M.; Zafar, H.; Pandey, S.; Chauhan, J.; Siddiqui, M.H.; Pinghua, C. Calcium homeostasis and potential roles to combat environmental stresses in plants. South Afr. J. Bot. 2022, 148, 683–693. [Google Scholar] [CrossRef]
- Ali, A.; Altaf, M.T.; Nadeem, M.A.; SHAH, A.N.; Azeem, H.; Baloch, F.S.; Karaköy, T.; Hussain, T.; Duangpan, S.; AASIM, M. Recent Advancement in OMICS approaches to enhance abiotic stress tolerance in Legumes. Front. Plant Sci. 2022, 13, 3222. [Google Scholar] [CrossRef]
- Haldhar, S.M.; Kumar, R.; Corrado, G.; Berwal, M.K.; Gora, J.S.; Thaochan, N.; Samadia, D.K.; Hussain, T.; Rouphael, Y. A Field Screening of a Pomegranate (Punica granatum) Ex-Situ Germplasm Collection for Resistance against the False Spider Mite (Tenuipalpus punicae). Agriculture 2022, 12, 1686. [Google Scholar] [CrossRef]
- Yoshida, S. Effects of temperature on growth of the rice plant (oryza sativa l.) in a controlled environment. Soil Sci. Plant Nutr. 1973, 19, 299–310. [Google Scholar] [CrossRef]
- Buddhaboon, C.; Jintrawet, A.; Hoogenboom, G. Effects of planting date and variety on flooded rice production in the deepwater area of Thailand. Field Crops Res. 2011, 124, 270–277. [Google Scholar] [CrossRef]
- Aslam, M.A.; Ahmed, M.; Hassan, F.-U.; Afzal, O.; Mehmood, M.Z.; Qadir, G.; Asif, M.; Komal, S.; Hussain, T. Impact of temperature fluctuations on plant morphological and physiological traits. In Building Climate Resilience in Agriculture; Springer: Cham, Switzerland, 2022; pp. 25–52. [Google Scholar]
- Davatgar, N.; Neishabouri, M.R.; Sepaskhah, A.R.; Soltani, A. Physiological and morphological responses of rice (Oryza sativa L.) to varying water stress management strategies. Int. J. Plant Prod. 2009, 3, 19–32. [Google Scholar] [CrossRef]
- Saikumar, S.; Varma, C.M.K.; Saiharini, A.; Kalmeshwer, G.P.; Nagendra, K.; Lavanya, K.; Ayyappa, D. Grain yield responses to varied level of moisture stress at reproductive stage in an interspecific population derived from Swarna/O. glaberrima introgression line. NJAS Wagening. J. Life Sci. 2016, 78, 111–122. [Google Scholar] [CrossRef]
- Pantuwan, G.; Fukai, S.; Cooper, M.; Rajatasereekul, S.; O’Toole, J.C. Yield response of rice (Oryza sativa L.) genotypes to drought under rainfed lowland 3. Plant factors contributing to drought resistance. Field Crops Res. 2002, 73, 181–200. [Google Scholar] [CrossRef]
- Zhao, D.L.; Atlin, G.N.; Amante, M.; Cruz, M.T.S.; Kumar, A. Developing aerobic rice cultivars for water-short irrigated and drought-prone rainfed areas in the tropics. Crop Sci. 2010, 50, 2268–2276. [Google Scholar] [CrossRef]
- Kumar, A.; Verulkar, S.; Dixit, S.; Chauhan, B.; Bernier, J.; Venuprasad, R.; Zhao, D.; Shrivastava, M.N. Yield and yield-attributing traits of rice (Oryza sativa L.) under lowland drought and suitability of early vigor as a selection criterion. Field Crops Res. 2009, 114, 99–107. [Google Scholar] [CrossRef]
- Torres, R.O.; McNally, K.L.; Cruz, C.V.; Serraj, R.; Henry, A. Screening of rice Genebank germplasm for yield and selection of new drought tolerance donors. Field Crops Res. 2013, 147, 12–22. [Google Scholar] [CrossRef]
- Bruckner, P.L.; Frohberg, R.C. Stress Tolerance and Adaptation in Spring Wheat 1. Crop Sci. 1987, 27, 31–36. [Google Scholar] [CrossRef]
- Clarke, J.M.; Townley-Smith, F.; McCaig, T.N.; Green, D.G. Growth analysis of spring wheat cultivars of varying drought resistance. Crop Sci. 1984, 24, 537–541. [Google Scholar] [CrossRef]
- Clarke, J.M.; DePauw, R.M.; Townley-Smith, T.F. Evaluation of methods for quantification of drought tolerance in wheat. Crop Sci. 1992, 32, 723–728. [Google Scholar] [CrossRef]
- Cheyglinted, S.; Ranamukhaarachchi, S.L.; Singh, G. Assessment of the CERES-Rice model for rice production in the Central Plain of Thailand. J. Agric. Sci. 2001, 137, 289–298. [Google Scholar] [CrossRef]
- Vilayvong, S.; Banterng, P.; Patanothai, A.; Pannangpetch, K. CSM-CERES-Rice model to determine management strategies for lowland rice production. Sci. Agric. 2015, 72, 229–236. [Google Scholar] [CrossRef]
- Anothai, J.; Soler, C.M.T.; Green, A.; Trout, T.J.; Hoogenboom, G. Evaluation of two evapotranspiration approaches simulated with the CSM-CERES-Maize model under different irrigation strategies and the impact on maize growth, development and soil moisture content for semi-arid conditions. Agric. For. Meteorol. 2013, 176, 64–76. [Google Scholar] [CrossRef]
- Verulkar, S.B.; Mandal, N.P.; Dwivedi, J.L.; Singh, B.N.; Sinha, P.K.; Mahato, R.N.; Dongre, P.; Singh, O.N.; Bose, L.K.; Swain, P.; et al. Breeding resilient and productive genotypes adapted to drought-prone rainfed ecosystem of India. Field Crops Res. 2010, 117, 197–208. [Google Scholar] [CrossRef]
- Pantuwan, G.; Fukai, S.; Cooper, M.; Rajatasereekul, S.; O’Toole, J.C.; Basnayake, J. Yield response of rice (Oryza sativa L.) genotypes to drought under rainfed lowlands 4. Vegetative stage screening in the dry season. Field Crop. Res. 2004, 89, 281–297. [Google Scholar] [CrossRef]
- Hu, Y.C.; Shao, H.B.; Chu, L.Y.; Gang, W. Relationship between water use efficiency (WUE) and production of different wheat genotypes at soil water deficit. Colloids Surf. B Biointerfaces 2006, 53, 271–277. [Google Scholar] [CrossRef]
- Blum, A. Drought resistance, water-use efficiency, and yield potential—Are they compatible, dissonant, or mutually exclusive? Aust. J. Agric. Res. 2005, 56, 1159–1168. [Google Scholar] [CrossRef]
- Monclus, R.; Dreyer, E.; Villar, M.; Delmotte, F.M.; Delay, D.; Petit, J.M.; Barbaroux, C.; Le Thiec, D.; Bréchet, C.; Brignolas, F. Impact of drought on productivity and water use efficiency in 29 genotypes of Populus deltoides x Populus nigra. New Phytol. 2006, 169, 765–777. [Google Scholar] [CrossRef]
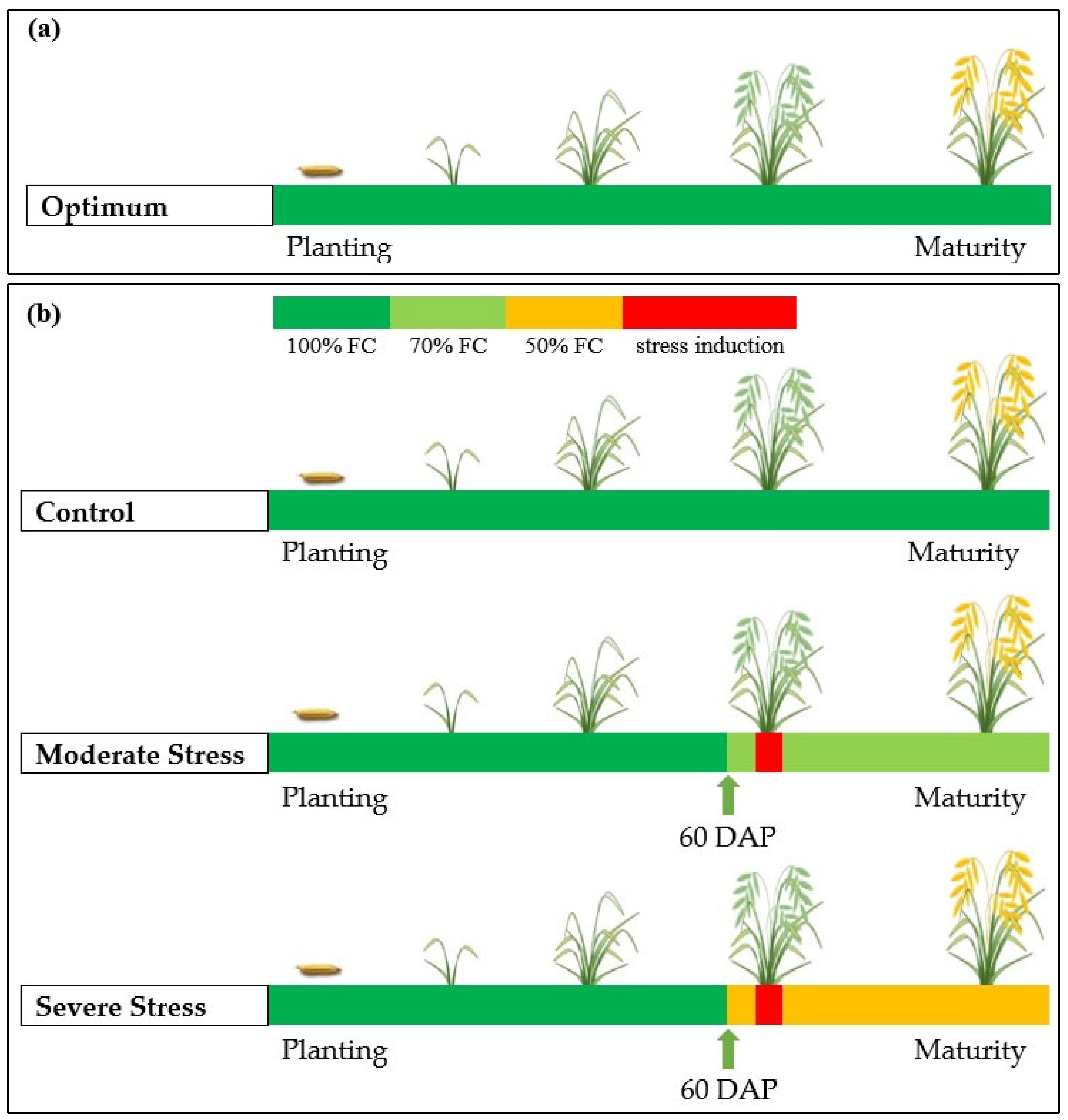
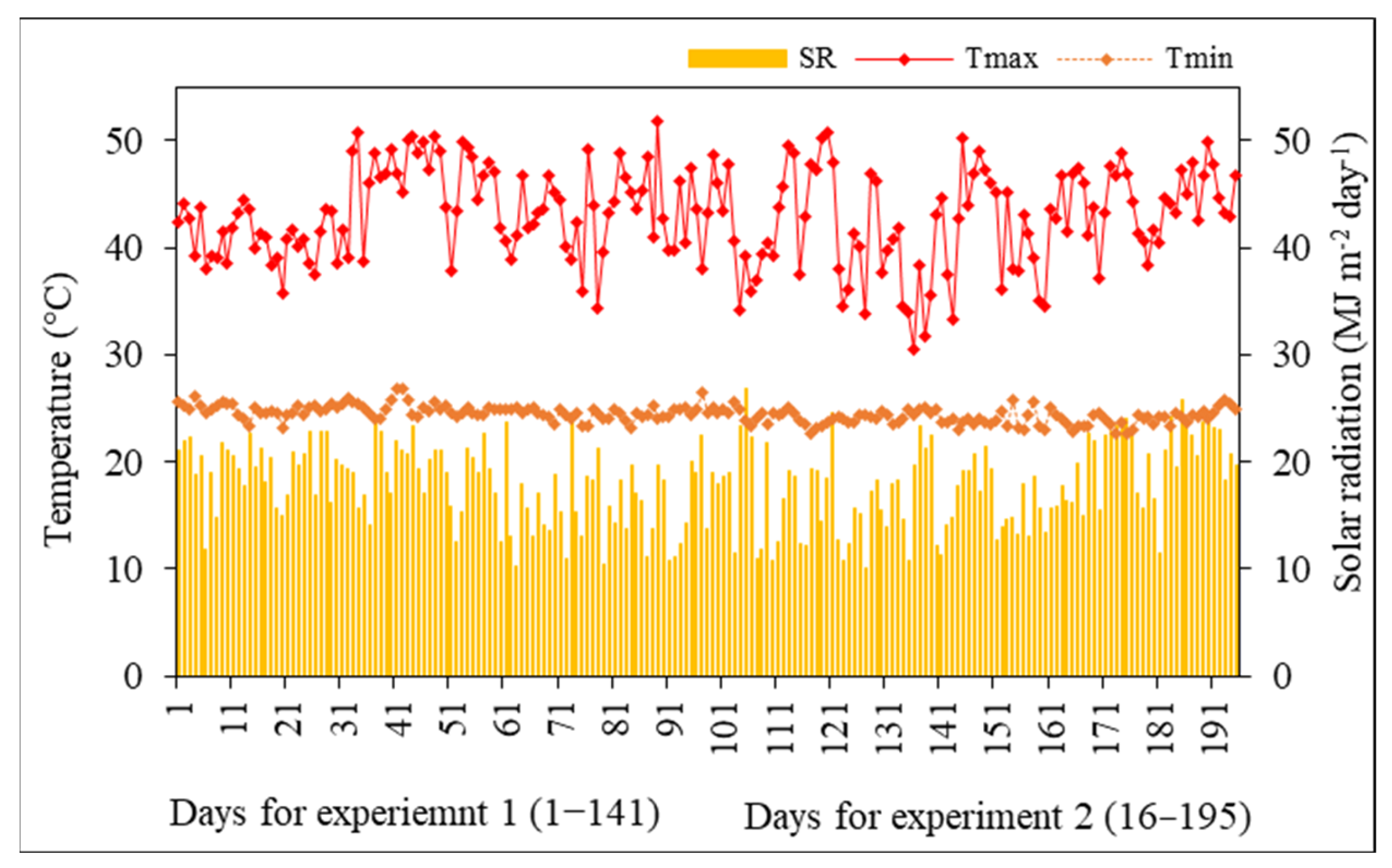
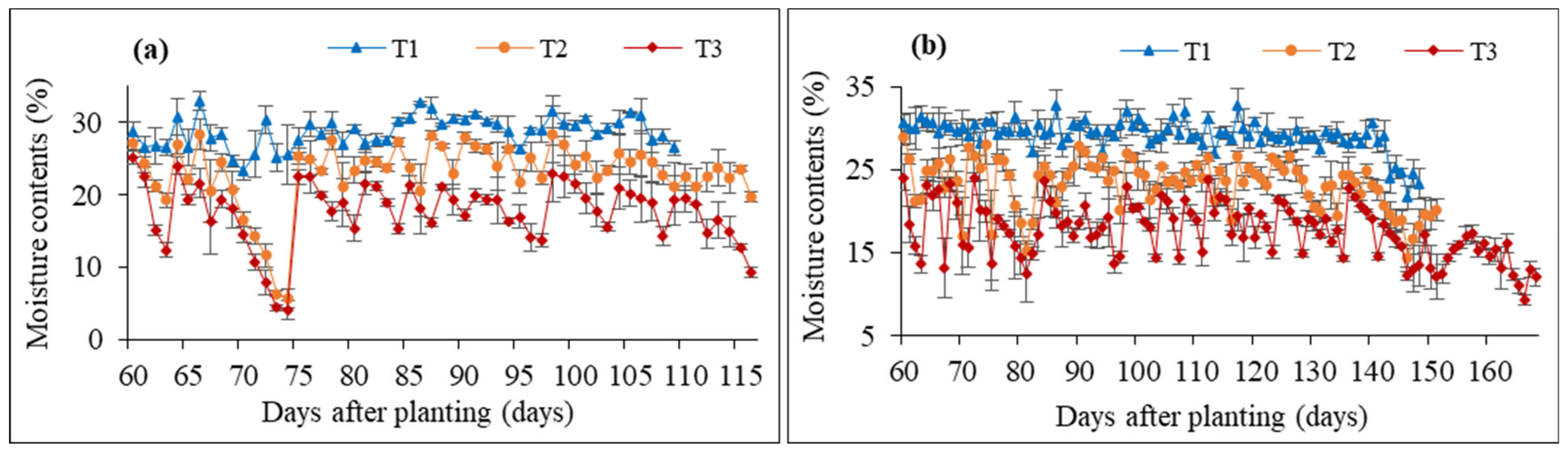
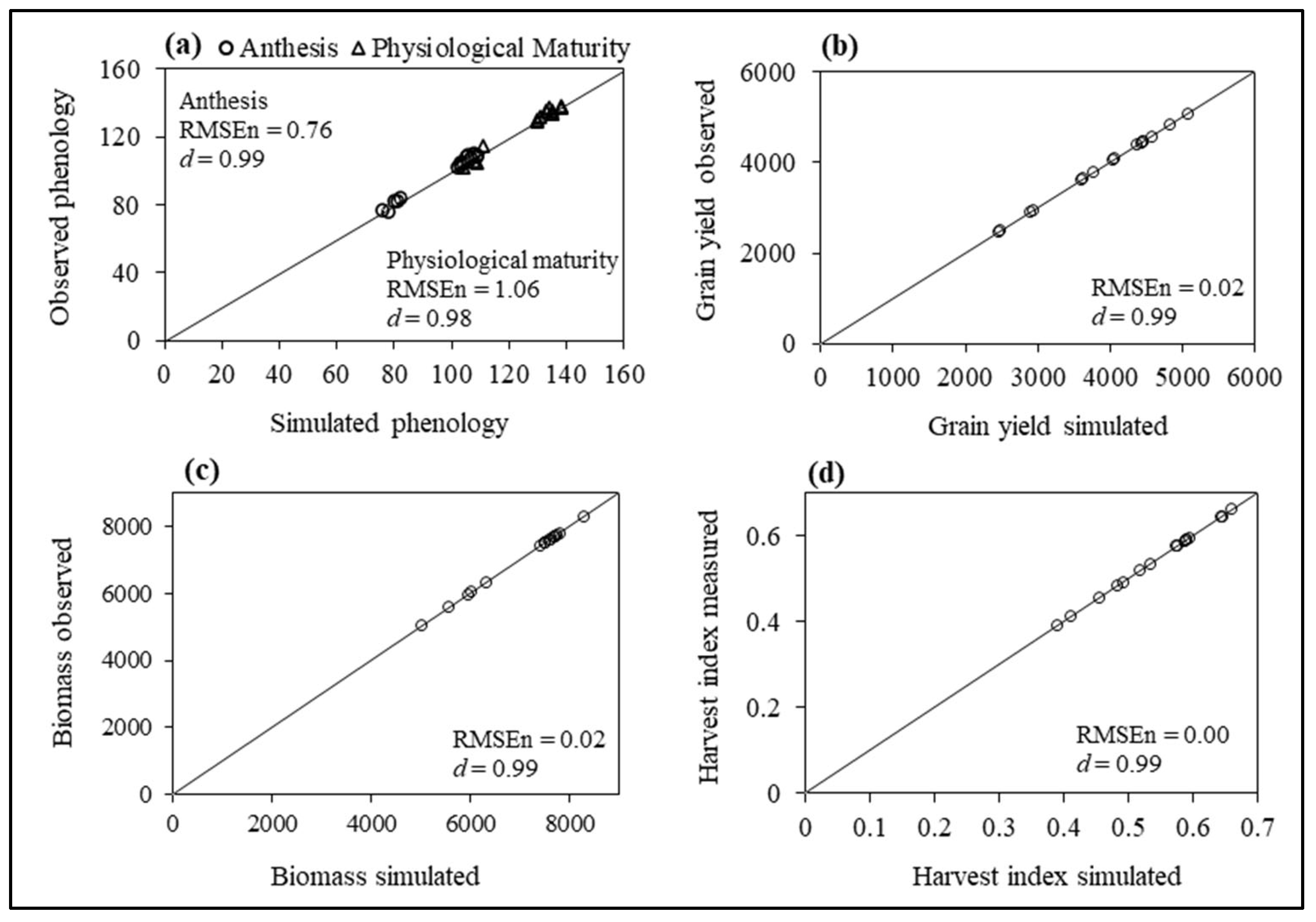
| Genotype | Control (100% FC) | Moderate Stress (70% FC) | Severe Stress (50% FC) | |||||
|---|---|---|---|---|---|---|---|---|
| GY ± SE | RYW | GY ± SE | DSI | RYS | GY ± SE | DSI | RYS | |
| G1 | 2267 ± 277 | 0.52 | 1387 ± 207 | 0.86 | 0.49 | 633 ± 179 | 1.14 | 0.41 |
| G2 | 1393 ± 79 | 0.32 | 900 ± 140 | 0.79 | 0.32 | 793 ± 41 | 0.68 | 0.51 |
| G3 | 2533 ± 419 | 0.58 | 1020 ± 130 | 1.33 | 0.36 | 567 ± 133 | 1.23 | 0.36 |
| G4 | 1880 ± 122 | 0.43 | 833 ± 29 | 1.24 | 0.29 | 740 ± 0 | 0.96 | 0.48 |
| G5 | 1800 ± 313 | 0.41 | 880 ± 64 | 1.13 | 0.31 | 573 ± 97 | 1.08 | 0.37 |
| G6 | 4053 ± 618 | 0.93 | 2213 ± 155 | 1.01 | 0.78 | 1526 ± 238 | 0.99 | 0.98 |
| G7 | 4373 ± 311 | 1.00 | 1660 ± 155 | 1.38 | 0.59 | 1180 ± 208 | 1.16 | 0.76 |
| G8 | 3260 ± 117 | 0.75 | 2213 ± 254 | 0.71 | 0.78 | 1553 ± 225 | 0.83 | 1.00 |
| G9 | 3040 ± 419 | 0.70 | 1680 ± 92 | 0.99 | 0.59 | 1240 ± 140 | 0.94 | 0.80 |
| G10 | 3233 ± 267 | 0.74 | 1700 ± 214 | 1.05 | 0.60 | 1520 ± 106 | 0.84 | 0.98 |
| G11 | 3780 ± 1024 | 0.86 | 2180 ± 20 | 0.94 | 0.77 | 1420 ± 160 | 0.99 | 0.91 |
| G12 | 3800 ± 868 | 0.87 | 2120 ± 325 | 0.98 | 0.75 | 1513 ± 208 | 0.96 | 0.97 |
| G13 | 3380 ± 577 | 0.77 | 2080 ± 356 | 0.85 | 0.73 | 1120 ± 70 | 1.06 | 0.72 |
| G14 | 3693 ± 746 | 0.84 | 1913 ± 164 | 1.07 | 0.68 | 1233 ± 394 | 1.06 | 0.79 |
| G15 | 3967 ± 1178 | 0.91 | 1973 ± 345 | 1.12 | 0.70 | 1527 ± 205 | 0.98 | 0.98 |
| G16 | 3747 ± 84 | 0.86 | 2833 ± 181 | 0.54 | 1.00 | 1433 ± 210 | 0.98 | 0.92 |
| Mean | 3137 | 0.72 | 1724 | – | 0.61 | 1161 | – | 0.75 |
| Genotypes | G1 | G2 | G3 | G4 | G5 | G6 | G7 | G8 | G9 | G10 | G11 | G12 | G13 | G14 | G15 | G16 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RMSEn (T1) | 1.02 | 2.16 | 2.03 | 2.61 | 3.82 | 1.25 | 2.5 | 2.57 | 3.11 | 2.64 | 1.08 | 1.99 | 2.15 | 2.69 | 0.92 | 2.76 |
| RMSEn (T2) | 3.67 | 3.78 | 3.51 | 3.60 | 2.95 | 3.29 | 3.58 | 0.37 | 3.33 | 3.46 | 3.02 | 2.91 | 3.11 | 3.32 | 2.43 | 2.18 |
| RMSEn (T3) | 3.65 | 3.93 | 3.05 | 3.28 | 3.02 | 1.70 | 3.13 | 3.87 | 3.31 | 3.08 | 0.61 | 2.71 | 3.40 | 3.32 | 3.25 | 2.98 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hussain, T.; Anothai, J.; Nualsri, C.; Ata-Ul-Karim, S.T.; Duangpan, S.; Hussain, N.; Ali, A. Assessment of CSM–CERES–Rice as a Decision Support Tool in the Identification of High-Yielding Drought-Tolerant Upland Rice Genotypes. Agronomy 2023, 13, 432. https://doi.org/10.3390/agronomy13020432
Hussain T, Anothai J, Nualsri C, Ata-Ul-Karim ST, Duangpan S, Hussain N, Ali A. Assessment of CSM–CERES–Rice as a Decision Support Tool in the Identification of High-Yielding Drought-Tolerant Upland Rice Genotypes. Agronomy. 2023; 13(2):432. https://doi.org/10.3390/agronomy13020432
Chicago/Turabian StyleHussain, Tajamul, Jakarat Anothai, Charassri Nualsri, Syed Tahir Ata-Ul-Karim, Saowapa Duangpan, Nurda Hussain, and Awais Ali. 2023. "Assessment of CSM–CERES–Rice as a Decision Support Tool in the Identification of High-Yielding Drought-Tolerant Upland Rice Genotypes" Agronomy 13, no. 2: 432. https://doi.org/10.3390/agronomy13020432
APA StyleHussain, T., Anothai, J., Nualsri, C., Ata-Ul-Karim, S. T., Duangpan, S., Hussain, N., & Ali, A. (2023). Assessment of CSM–CERES–Rice as a Decision Support Tool in the Identification of High-Yielding Drought-Tolerant Upland Rice Genotypes. Agronomy, 13(2), 432. https://doi.org/10.3390/agronomy13020432











