Abstract
The yield and quality of field crops are affected by abiotic stresses such as water deficit, which can negatively impact crop growth, productivity, and quality. However, nanotechnology holds great promise for increasing crop yield, maintaining quality, and thus mitigating abiotic stresses. Therefore, the current study was conducted to examine the influences of 0, 50, and 100 mg L−1 zinc oxide (ZnO) nanoparticles and 0, 25, and 50 mg L−1 silicon dioxide (SiO2) nanoparticles on the yield and quality traits of potato plants grown under water deficit conditions (100%, 75%, and 50% ETc). Water deficit significantly reduced yield traits (average tuber weight, number of plant tubers, and tuber yield) and quality traits (tuber diameter, crude protein, and mineral content). However, it enhanced tuber dry weight, specific gravity, ascorbic acid, starch, and total soluble solids. Foliar applications of ZnO and SiO2 nanoparticles under water deficit treatments significantly enhanced yield and improved quality traits of potato plants. Moreover, significant and positive correlations were found among yield traits. Thus, it can be concluded that using ZnO NPs at 100 mg L−1 significantly improves potato productivity and quality traits by mitigating the negative effects of water deficit in arid regions.
1. Introduction
Abiotic stresses can induce major challenges to crop productivity. Abiotic stressors such as water deficit, salinity, and heat are the main causes of crop loss due to their effect on reducing the average yield of most crops by 50–70% []. Drought is one of the most influential abiotic stresses []. As the human population grows, food accessibility may become a major concern on a global scale []. Potato (Solanum tuberosum) is the third most important staple food crop after rice and wheat []. Abiotic stresses can induce major challenges to crop productivity. Potatoes are a wholesome food, contributing vitamins, starch, minerals, nutrients, protein, amino acids, and antioxidants to the energy and nutritional requirements of large populations worldwide [,,]. They are a valuable and high-yielding food crop. However, potatoes can have a significant yield loss when subjected to the water deficit. Although potato growth and yield are adversely affected by short periods of water deficit at any growth stage, the effects at tuber initiation and flowering are the strongest [,,,].
Because many potato varieties have shallow roots, they are more susceptible to water deficit than most other crops []. Water scarcity has now emerged as the most significant global limiting factor for crop production. Thus, potato growth in arid and semiarid regions is becoming increasingly difficult. Water and fertilizer have a general influence on potato productivity, and the requirements for these factors vary depending on soil type and potato variety []. Water deficit negatively affects the reproductive stage by shortening the growth cycle [] and reducing tuber size [], number, weight, and yield [,]. Furthermore, water deficit can have an impact on the quality of potato tubers []. Environmental factors along with population growth necessitate sustainable and innovative agricultural solutions to maintain food demand [,].
Nanotechnology applications in agriculture have grown in recent years []. Nanotechnology holds great promise for increasing agricultural productivity, and thus, aiding future food security. Nanoparticles are particles with at least one dimension with a diameter of less than 100 nanometers. They differ from macrosized particles in terms of physical and chemical properties, as well as a high surface area–volume ratio that allows interaction with plant cells [,]. Nanofertilizers can increase the surface area of a plant for various metabolic reactions and can increase photosynthesis rate and productivity. They can also protect plants from biotic and abiotic stresses [,,]. Furthermore, nanofertilizers are valuable for increasing nutrient efficiency and producing higher yields []. Zinc oxide (ZnO) and silicon dioxide (SiO2) play an important role in mitigating the stressful effects of abiotic stresses such as drought and salinity [,]. When zinc nanoparticles are applied, the productivity of rice (Oryza sativa L.), wheat (Triticum aestivum L), maize (Zea mays L.), pearl millet (Pennisetum glaucum L.), sugarcane (Saccharum officinarum Linn.), potato, sunflower (Helianthus annuus L.), and brassica (Brassica juncea) plants have significantly increased [,]. Similarly, the application of silicon NPs resulted in an increment in the fruit weight and productivity of tomato plants. Furthermore, it resulted in an increase in ascorbic acid and enhanced fruit size and quality [].
Therefore, the aim of the current study is to investigate the beneficial effects of the foliar application of nanoparticles (zinc oxide, or ‘ZnO-NPs’, and silicon dioxide, or ‘SiO2-NPs’) on the yield and quality traits of potatoes grown under water deficit. In the current study, it is hypothesized that the exogenous application of ZnO-NPs or SiO2-NPs can enhance the yield and quality traits of potatoes grown under water deficit conditions, especially in arid regions.
2. Materials and Methods
2.1. Experimental Location
This study was conducted at the experimental farm of the College of Food and Agriculture Sciences, King Saud University, Riyadh, Saudi Arabia, (latitude: 24°43′ N; longitude: 46°36′ E). Before cultivation, soil samples were collected from the experimental site for chemical and physical analyses (Table 1) according to the method described by Sparks and Page [].

Table 1.
Physical and chemical characteristics of the soil.
2.2. Plant Materials, Growth Conditions, and Experimental Design
Certified potato seed tubers, medium-maturity (Solanum tuberosum L. cv. Hermes) were provided by the Saudi Agricultural Development Company, Riyadh. Potato tubers with an average weight of 59–64 g were cultivated on 25 September 2021. Each subplot area was 30 m2 (6 m length × 5 m width). The distance between the two potato tubers was 40 cm, while the width of each row was 100 cm. The number of plants per each subplot was 75. Fertilization and plant protection were performed as commonly recommended []. The fertilizers were applied to the different treatment plots based on the following rate: 230 kg ha−1 N–P2O5–K2O (20–20–20), 200 kg ha−1 N–P2O5–K2O (10–10–43), 40 L ha−1 H3PO4. Air temperature and humidity during the growing season were collected from nearby weather station (Figure 1).
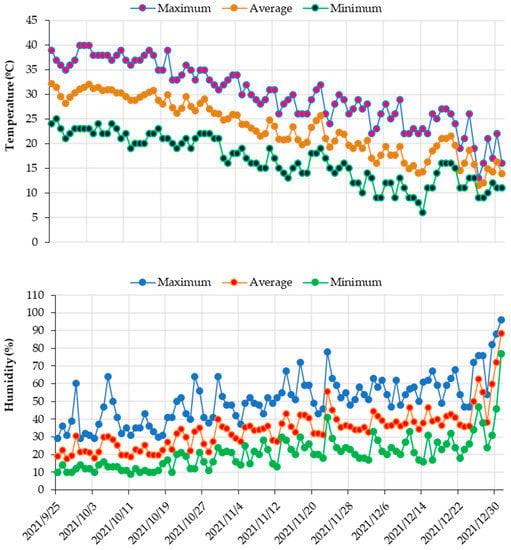
Figure 1.
Average daily air temperature and relative humidity during the field experiment.
The experimental design was a randomized complete block (RCBD) in a split-plot system with three replicates. The water deficit treatments (100, 75, and 50% ETc) were randomly allocated in the main plots, while the foliar applications of ZnO NPs (50 and 100 mg L−1) and SiO2 NPs (25 and 50 mg L−1) were randomly placed in subplots.
2.3. Irrigation Levels and Nanoparticles (NPs)
Irrigation water was provided via a drip irrigation system. Based on crop evapotranspiration (Etc), three irrigating levels (100, 75, and 50% ETc) were applied.
The quantity of irrigation water required (ETc) for potatoes was calculated following the FAO Penman Monteith method [] using the crop coefficient (Kc) values as follows:
where ETc is the crop evapotranspiration (mm day−1), ETo is the reference evapotranspiration (mm day−1), and Kc is the crop coefficient. The crop coefficient at the initial growth stage (Kc ini) was 0.50, while during the midseason stage (Kc mid) it was 1.15, and at the end of the potato growth stage (Kc end) it was 0.75. Irrigation treatments were applied at 35 days after planting (DAP). The total amounts of consumptive water for the 100, 75, and 50% ETc treatments were 5892, 4419, and 2946 m3 ha−1, respectively. The irrigation water quality had a pH of 8.11 and a sodium adsorption ratio (SAR) of 1.52, as well as an EC of 0.92 dS m−1.
ZnO NPs and SiO2 NPs were prepared in concentrations of 50 and 100 and 25 and 50 mg L−1, respectively, with double-distilled water. Ultrasounds of the suspensions were performed for 30 min to increase the dispersion of the NPs. Applications of an exogenous spray of nanoparticles were applied at 45 and 65 DAP using a handheld aerosol-propelled sprayer. TEM images of ZnO and SiO2 NPs are shown in Figure 2A and Figure 2B, respectively. The surface image of the ZnO NPs showed a smooth, semispherical-to-hexagonal wurtzite shape with a few nonspherical monoclinic particles, while the surface image of the SiO2 NPs showed nonsmooth and nearly spherical shapes with small sizes [].
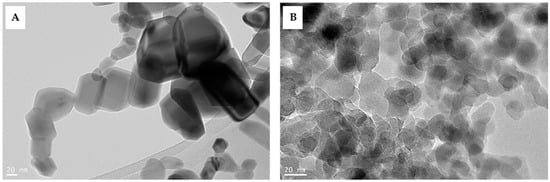
Figure 2.
TEM images of ZnO NPs (A) and SiO2 NPs (B).
2.4. Measurements
2.4.1. Tuber Yield and Traits
At harvest time (110 DAP), fresh potato tuber samples were collected from three replicates in each plot. Data of average tuber weight (g), tuber number per plant−1, tuber yield per plant−1, and tuber yield per m2 were collected.
2.4.2. Tuber Physical Traits
A random sample of fresh potato tubers from each experimental unit (subplot) was selected for analyzing tuber physical traits. The average tuber diameter was measured using a digital caliper. Tuber dry weight was estimated in an air oven at 70 °C until the weight became constant (48–72 h). Accordingly, the dry weight was calculated with regard to fresh weight []. To determine specific gravity, potato tubers were randomly selected from each plot. Then, tubers were cleaned and weighed in both air and water, according to Gautam et al. []:
2.4.3. Chemical Quality Analysis of Tubers
Tuber samples from all treatments were collected at harvest to determine their chemical quality traits. Ascorbic acid was measured using a titration method with 2, 6-dichlorophenol indophenol (DCIPh) solution and was expressed in milligrams per 100 g of fresh weight []. Total soluble solids (TSS) percentages were measured in a sample of ripe potato tubers with drops of filtered juice using a portable handheld digital refractometer (Model: PR.101, Palette, ATAGO, Japan) []. The protein content of the tubers was calculated from the total nitrogen content in 2 g from each tuber sample dried following Kjeldahl’s method []. Crude protein was determined using the following equation:
The starch content in the tubers was determined according to the method described in Sit et al. []. Analyses of tuber contents of P, K, Ca, Mg, Fe, and Zn were assessed using dry, fine-ground potato tuber samples. The percentage of phosphorus content was measured calorimetrically according to Jackson []. The percentage of potassium content was determined using a flame photometer (Model 1382, ESICO, Haryana, India). Ca, Mg, Fe, and Zn were measured using inductively coupled plasma optical emission spectrometry (Optima 4300 DV, ICP-OES, PerkinElmer, Waltham, MA, USA).
2.4.4. Data Analysis
Data of the different traits were subjected to analysis of variance (ANOVA). The significance of the differences between different treatments was tested using Duncan’s multiple range test at p ≤ 0.05 with Co-State version 6.003 (CoHort, USA). Principal component analysis (PCA) and Pearson’s correlation were performed using XLSTAT (Version 2016).
3. Results and Discussion
3.1. Yield Traits
All the studied yield traits (the number of tubers, tuber fresh weight, tuber yield per plant, and tuber yield per m2) were significantly reduced under water deficit treatments (i.e., 75% and 50% ETc) (Table 2). The decreases in all yield traits were the highest when potato plants were exposed to 50% ETc. However, foliar application of 50 or 100 mg L−1 ZnO-NPs and 25 or 50 mg L−1 SiO2-NPs significantly enhanced all of the above-mentioned yield traits under water deficit treatments in comparison with those obtained from un-stressed plants. The improvements in the yield traits were the highest in plants grown with 100% ETc, followed by those grown with 75% ETc (Table 2). In the current study, the application of 100 mg L−1 ZnO-NPs enhanced yield traits of potato grown under water deficit treatments, followed by 50 mg L−1 of ZnO-NPs and 50 mg L−1 SiO2-NPs, respectively. Fresh tuber yield is determined by the dry matter allocation of tubers and tuber water content, with water content contributing up to 80% of fresh tuber mass depending on the cultivar []. As a result, water scarcity has a significant impact on fresh tuber mass [,]. In this respect, the number of tubers was decreased when plants were exposed to water deficit throughout the growing season []. Similarly, a single, short-term early stress event had an inhibitory effect on the number of tubers produced by a plant []. A recent study [] found that water deficit resulted in a reduction in the fresh tuber weights of 103 commercial potato cultivars. Consequently, the water deficit can lead to substantial reductions in plant production and yield traits. In the current study, using foliar applications of ZnO-NPs and SiO2-NPs resulted in an improvement for the yield traits of potatoes grown under water deficit treatments. This could be due to the fact that the application of ZnO-NPs can protect leaf surfaces from harmful sun rays by blocking UV radiation []. Under water deficit conditions, it is likely that of ZnO-NPs may significantly increase melatonin levels because they can act as free radical scavengers in response to stressor effects, which relieves the drought-induced impairment of chloroplasts and mitochondria []. Zn raises tryptophan levels in plant tissues, which are closely linked with the biosynthesis of indol-3-acetic acid and melatonin. Therefore, ZnO-NPs have a role in enhancing cell division and biomass production [,]. Haliloglu et al. [] reported that applying zinc oxide (ZnO) to Solanum lycopersicum altered cytosine methylation, which resulted in reducing the genotoxic effects caused by abiotic stresses. When NPs are applied into plants, they can interact with cellular machinery for instance chloroplasts. NPs such as SiO2 can enhance photosynthesis by gaining adequate light-harvesting chlorophyll–protein complexes, particularly when plants are grown under water deficit. Silica is important element for plant nutrition and its growth, as its deficiency can cause a negative impact on plant growth and its productivity [].

Table 2.
Influence of foliar nanoparticles (NPs) on yield traits of potato grown under different water deficit conditions (irrigation levels).
Previous studies showed that nanoparticles can mitigate photosynthetic pigment deterioration and regulate stomatal conductance under water deficit conditions, resulting in high photosynthesis rates and increased crop dry matter and yield. They also can protect plants from various biotic and abiotic stresses [,,,]. High photosynthetic activity, which results from high Rubisco enzyme activity, was linked with ZnO-NPs []. On the other hand, silicon nanoparticles (SiO2 NPs) can protect the cell wall during water deficit conditions by reducing the cell wall permeability of leaves, resulting in low lipid peroxidation []. Under water deficit conditions, silicic acid is polymerized and converted to silica gel concentrated in the surfaces of shoots, which can act as a double layer, causing a remarkable decrease in water loss through leaf transpiration [,]. Silicon NPs are widely used in agriculture and play an important role in plant stress tolerance []. According to a previous study, exogenous silica application can improve the photosynthesis process due to the increased chlorophyll content and the decreased transpiration rate; consequently, such application can improve the production and quality in stressed plants []. For instance, the application of ZnO-NPs and SiO2 resulted in a positive effect on tomatoes such as increasing fruit weight per plant and fruit yield per ha [,].
3.2. Quality of Tubers
3.2.1. Physical Traits
Tuber diameter was significantly diminished under the highest water deficit level (WD-3) (50% ETc) compared to the WD-1 and WD-2 treatments (100 and 75% ETc, respectively) (Table 3). However, the application of ZnO-NPs (i.e., 50 and 100 mg L−1) and SiO2-NPs (i.e., 25 and 50 mg L−1) improved tuber diameter under all the irrigation treatment levels compared to the control plants (without ZnO-NPs or SiO2-NPs). Tuber diameter was the highest in plants grown in plots treated with 100% ETc, followed by those obtained at 75% ETc. Among all the foliar NP treatments, ZnO-NPs at 100 mg L−1 resulted in the highest tuber diameter in the current study. The tuber initiation stage was the period when tuber size was negatively affected by water deficit []. Therefore, using nanoparticles for plants grown under abiotic stress can improve the physical properties of potato tubers, including tuber diameter [].

Table 3.
Influence of foliar nanoparticles (NPs) on physical traits of potato grown under different water deficit conditions (irrigation levels).
In the current study, other physical traits of the potato tubers such as tuber dry weight and specific gravity were increased with the reduction in the irrigation levels from WD-1 to WD-2 and then to WD-3 (Table 3). In this respect, Steyn et al. [] suggested that some potato cultivars can produce relatively higher tuber dry matter under water deficit, regardless of their performances under well-irrigated conditions. On the other hand, the tuber dry weight (TDW) of potatoes is considered to be primarily composed of starch, with trace amounts of sugars, fiber, protein, and ash. It typically ranges from 16 to 28% depending on crop development stages, and peaks near the end of crop growth []. Water deficit in potatoes tends to improve chip quality due to a higher percentage of TDW, making the chips suitable for industry [,]. Moreover, the specific gravity of potato tubers tended to decrease as the water amount increased []. A similar tendency was observed by Abd-Elrahman et al. [], who reported that the highest specific gravity was obtained from tubers of potato plants irrigated with 50% irrigation requirements compared to those irrigated with 100% or 75% of irrigation requirements. However, the application of NPs can increase the surface area of the plant that can be used for various metabolic reactions, resulting in a high photosynthesis rate, and consequently high crop dry matter [,,].
3.2.2. Nutraceutical and Mineral Content in Potato Tubers
Nutraceutical Content
Ascorbic acid (ASA), total soluble solids (TSS), starch, and crude protein were significantly affected by water deficit treatments. They were the highest in potato plants grown under WD-3 treatment (50% ETc), followed by those grown under WD-2 at 75% ETc. On the contrary, the lowest values of those traits were recorded in potato plants grown under WD-1 at 100% ETc (Table 4). Previous studies [,] have shown that water deficit can promote chemical contents for components such as vitamin C, total soluble solids, and starch, as well as increased quality traits in potato tubers. The highest values of crude protein were recorded in tubers of potato grown under the WD-1 treatment at 100% ETc, followed by that at 75% ETc, while the lowest protein was obtained from tubers of potato grown under WD-3 treatment (Table 4). These results confirm the findings of Elhani et al. [], who reported that the protein content of tubers gradually decreased with reduced amounts of irrigation needed for potato plants.

Table 4.
Influence of foliar nanoparticles (NPs) on quality attributes: ascorbic acid (AsA), total soluble solids (TSS), starch, and crude protein (CP) of potato tubers grown under water deficit conditions (irrigation levels).
However, the applications of ZnO (50 and 100 mg L−1) and SiO2-NPs (25 and 50 mg L−1) significantly and positively affected the chemical traits of potato tubers (Table 4). The maximum contents of ASA, TSS, and starch were recorded in plants treated with the foliar application of 100 mg L−1 ZnO-NPs, followed by 50 mg L−1 of ZnO-NPs and 50 mg L−1 of SiO2-NPs, respectively, when grown under 50% ETc, followed by 75% ETc. On the contrary, the highest protein contents were observed from tubers of potato treated with ZnO-NPs at 100 mg L−1 and grown under WD-1 at 100% ETc and/or WD-2 at 75% ETc, without significant differences between these treatments. In this concern, previous studies have shown significant increases in starch, protein, and ascorbic acid in potatoes fertilized with nanofertilizers compared to those untreated with NPs [,]. For instance, the application of B, Si, Zn, and zeolite nanoparticles was used to mitigate abiotic stress in potato plants, and resulted in significant increases in tuber yield and its quality []. Furthermore, when zinc nanoparticles were applied to peanut plants (Arachis hypogaea L.), the crude protein and soluble carbohydrate contents were increased []. On the other hand, the application of SiO2 NPs can increase ascorbic acid and enhance the fruit size and quality of tomatoes grown under abiotic stress [].
Mineral Contents
The macronutrients (K+, Ca2+, P, and Mg2+) and micronutrients (Zn2+ and Fe2+) of potato tubers significantly decreased under different water deficit treatments (WD-3) at 50% ETc, while the highest values of these nutrients were obtained from those grown under WD-1 treatment (100% ETc), followed by WD-2 (75% ETc) (Table 5 and Table 6). Soil water deficit reduced nutrient diffusion around the roots, as well as nutrient uptake, due to decreases in active transport, transpiration flux, and membrane permeability []. Furthermore, water deficit affected tuber mineral contents by acting on the mineral composition of different tissues, and as a consequence, on the redistribution of minerals within the plants []. These results are in agreement with the findings of Semida et al. [], who found that mineral contents were recorded at lower concentrations in eggplant samples grown under water deficit conditions.

Table 5.
Influence of foliar nanoparticles (NPs) on potassium, calcium, and phosphorus percentage in potato tubers grown under different water deficit conditions (irrigation levels).

Table 6.
Influence of foliar nanoparticles (NPs) on magnesium, iron, and zinc in potato tubers grown under different water deficit conditions (irrigation levels).
The contents of macronutrients and micronutrients correlated with the exogenous application of ZnO and SiO2-NPs (Table 5 and Table 6), where the highest values were recorded under high concentrations (100 mg L−1 of ZnO-NPs, followed by 50 mg L−1 of ZnO-NPs and 50 mg L−1 SiO2-NPs). Nanoparticles have a beneficial effect on nutrient uptake as well. In other studies, certain NPs, such as ZnO-NPs and silica NPs, were shown to improve nutritional efficiency by increasing calcium, potassium, and magnesium absorption []. In addition to improving membrane stability and plant water status, exogenous ZnO-NPs improved potato nutritional status, allowing plants to mitigate the effects of water deficit on growth and productivity. On the other hand, silicon can improve the availability and accumulation of some macronutrients in plants such as K and Ca, as well as micronutrients, such as Fe []. Furthermore, Uresti et al. [] reported that using ZnO-NPs can improve the mineral contents (P, K, Ca, Mg, and Zn) of bell pepper fruits. In addition, previous research has shown that ZnO-NPs can boost macro- and micronutrient levels in pinto beans [] and sorghum []. Alsaeedi et al. [] reported an improvement in the growth and productivity of Cucumis sativus due to increased N and K uptake under water deficit and salt conditions when SiO2 NPs was applied. However, the impact of NPs on nutritional parameters is governed by their physical and chemical properties [].
3.3. Attributes Interrelationship
Associations among the evaluated yield and quality traits of potato tubers were estimated based on a principal components analysis (PCA, Figure 3) and Pearson’s correlation analysis (Figure 4). Considering the differences in the yield and quality traits of potato plants treated with ZnO and SiO2 nanoparticles under water deficit conditions, 17 indices were assimilated using two-dimensional principal component analysis (PCA) with XLSTAT software (2016). PCA was additionally used to integrate the results of the yield and quality traits. The principal components (F1 and F2) provided 93.52% of the total variance in the dataset. The contribution rates of F1 and F2 were 64.92% and 28.60% of the variance in the dataset, respectively.
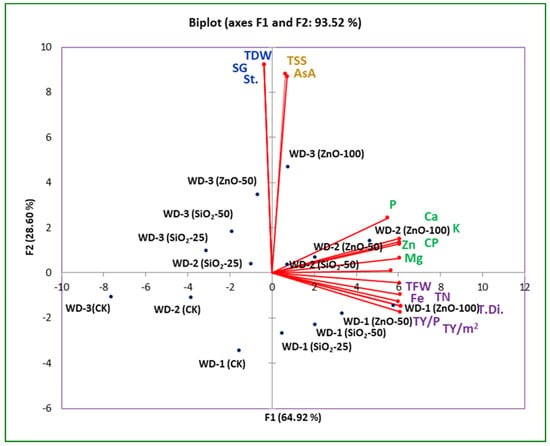
Figure 3.
Biplot of the first two principal components for the yield parameters and quality (physical and chemical) traits of potato tubers. The yield parameters included tuber number (TN), tuber fresh weight (TFW), tuber yield per plant (TY/p), and tuber yield per m2 (TY/m2). The physical parameters comprised tuber diameter (TDi.), tuber dry weight (TDW), and specific gravity (SG). The chemical attributes include ascorbic acid (ASA), total soluble solids (TSS), crude protein (CP), starch (St.), and minerals (Ca2+, K+, P, Mg2+, Fe2+, and Zn2+). CK (control), WD-1 (100% ETc), WD-2 (75% ETc), and WD-3 (50% ETc).
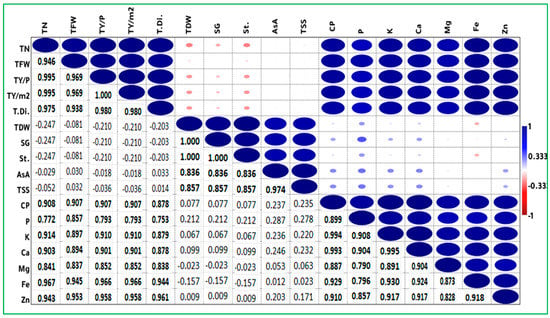
Figure 4.
Correlation analysis between different traits of potato plants grown under water deficit and treated with ZnO and SiO2 nanoparticles. TN: tuber number; TFW: tuber fresh weight; TY/p: tuber yield per plant; TY/m2: tuber yield per m2; T.Di: tuber diameter; TDW: tuber dry weight; SG: specific gravity; ASA: ascorbic acid; TSS: total soluble solids; CP: crude protein; St.: starch. Minerals: P, K+, Ca2+, Mg2+, Fe2+, and Zn2+. Values in bold are different from 0 with a significance level alpha = 0.05. Color and size changes represent the degree of correlation. The larger the circular area and the darker the color, the stronger the correlation, and vice versa. Red color represents negative correlations and blue color represents positive correlations.
The PCA analysis showed that yield traits (number of tubers, tuber fresh weight, yield per plant, and yield per m2) were correlated with each other, while quality traits (P, K+, Ca2+, Mg2+, Fe2+, Zn2+, CP, and tuber diameter) were correlated with each other. F2 substantially demonstrated that tuber dry weight (TDW) had the strongest correlation with specific gravity (SG) and starch content in the tubers. The application of ZnO-NPs at 100 mg L−1 showed clear and positive correlations with most yield and quality traits.
Pearson correlation coefficients were calculated to determine the positive and negative correlations between the yield and quality traits of potato plants grown under water deficit and treated with nanoparticles (ZnO and SiO2). The significant correlations (bold numbers) and insignificant relationships (nonbolded numbers) are presented in Figure 4. Tuber yields were significantly and positively correlated with the yield traits (number of tubers, tuber fresh weight, and yield per plant), as well as with some quality traits (P, K+, Ca2+, Mg2+, Fe2+, Zn+2, CP, and tuber diameter). These findings confirm that the applications of nanoparticles (ZnO and SiO2) were effective in alleviating water deficit effects by improving photosynthesis, water use efficiency, and essential nutrient uptake. The results also suggest that changes resulting from the ZnO and SiO2-NP applications could lead to improvements in plant drought tolerance. In addition, tuber dry weight (TDW) showed a highly positive association with specific gravity (SG) and starch content (r = 1.00 ***). These results are in agreement with those reported by Elfnesh et al. [], who demonstrated that the dry matter content of potato tubers was positively and highly significantly correlated with specific gravity (r = 0.99 **). The dry mass of tubers influences potato quality []. The specific gravity of tubers is an important indicator of potato tuber quality and a determinant for harvest quality, as it indicates the dry matter content of tubers []. Consequently, high specific gravity has a positive role in the processing quality of tubers. As a rule, high specific gravity means high dry matter content and a high recovery percentage of chips, which makes them appropriate for use in industry [,].
4. Conclusions
The present study concluded that the negative effects of water deficit on productivity and quality traits of potatoes could be mitigated through the exogenous application of ZnO-NPs or SiO2-NPs. The exogenous application of 100 mg ZnO-NPs L−1 resulted in the highest yield and its components as well as the best quality traits of potato when grown under different water-deficit treatments. In addition, it showed clear and positive correlations with most of the yield and quality traits evaluated for potato plants exposed to water deficit conditions. Nonetheless, additional studies are required to investigate the effects of different levels of different NPs and their combinations on potato productivity and quality to ensure the safety of nanotreated potato plants for food usage.
Author Contributions
Conceptualization, M.F.S. and A.A.A.; methodology, W.A.A.-S. and A.A.I.; software, M.F.S. and W.A.A.-S.; formal analysis, M.F.S., W.A.A.-S. and A.A.A.; investigation, M.F.S., W.A.A.-S. and A.A.A.; resources, M.F.S. and A.A.A.; data curation, M.F.S. and A.A.A.; writing—original draft preparation, M.F.S., W.A.A.-S. and A.A.A.; writing—review and editing, M.F.S., W.A.A.-S., A.A.I., M.S. and A.A.A.; supervision, M.F.S. and A.A.A.; project administration, M.F.S. and A.A.A.; funding acquisition, M.F.S. All authors have read and agreed to the published version of the manuscript.
Funding
The authors extend their appreciation to the Deputyship for Research & Innovation, Ministry of Education in Saudi Arabia for funding this research work through the project no. (IFKSURG-2-64).
Data Availability Statement
All data are presented within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Francini, A.; Sebastiani, L. Abiotic stress effects on performance of horticultural crops. Horticulturae 2019, 5, 67. [Google Scholar] [CrossRef]
- Seleiman, M.F.; Al-Suhaibani, N.; Ali, N.; Akmal, M.; Alotaibi, M.; Refay, Y.; Dindaroglu, T.; Abdul-Wajid, H.H.; Battaglia, M.L. Drought Stress Impacts on Plants and Different Approaches to Alleviate Its Adverse Effects. Plants 2021, 10, 259. [Google Scholar] [CrossRef]
- Seleiman, M.F.; Selim, S.; Alhammad, B.A.; Alharbi, B.M.; Juliatti, F.C. Will novel coronavirus (COVID-19) pandemic impact agriculture, food security and animal sectors? Biosci. J. 2020, 36, 1315–1326. [Google Scholar] [CrossRef]
- Faostat. Available online: http://www.fao.org/faostat/en/#data/QC/visualize (accessed on 10 December 2022).
- Lal, M.K.; Tiwari, R.K.; Kumar, A.; Dey, A.; Kumar, R.; Kumar, D.; Jaiswal, A.; Changan, S.S.; Raigond, P.; Dutt, S. Mechanistic Concept of Physiological, Biochemical, and Molecular Responses of the Potato Crop to Heat and Drought Stress. Plants 2022, 11, 2857. [Google Scholar] [CrossRef] [PubMed]
- Mukhtar, T.; Rehman, S.u.; Smith, D.; Sultan, T.; Seleiman, M.F.; Alsadon, A.A.; Amna; Ali, S.; Chaudhary, H.J.; Solieman, T.H.I.; et al. Mitigation of Heat Stress in Solanum lycopersicum L. by ACC-deaminase and Exopolysaccharide Producing Bacillus cereus: Effects on Biochemical Profiling. Sustainability 2020, 12, 2159. [Google Scholar] [CrossRef]
- Mwakidoshi, E.R.; Gitari, H.I.; Muindi, E.M.; Wamukota, A.W.; Seleiman, M.F.; Maitra, S. Smallholder farmers’ knowledge of the use of bioslurry as a soil fertility amendment for potato production in Kenya. Land Degrad. Dev. 2023. [Google Scholar] [CrossRef]
- Anithakumari, A.; Nataraja, K.N.; Visser, R.G.; van der Linden, C.G. Genetic dissection of drought tolerance and recovery potential by quantitative trait locus mapping of a diploid potato population. Mol. Breed. 2012, 30, 1413–1429. [Google Scholar] [CrossRef] [PubMed]
- Lahlou, O.; Ouattar, S.; Ledent, J.-F. The effect of drought and cultivar on growth parameters, yield and yield components of potato. Agronomie 2003, 23, 257–268. [Google Scholar] [CrossRef]
- Schafleitner, R.; Rosales, R.O.G.; Gaudin, A.; Aliaga, C.A.A.; Martinez, G.N.; Marca, L.R.T.; Bolivar, L.A.; Delgado, F.M.; Simon, R.; Bonierbale, M. Capturing candidate drought tolerance traits in two native Andean potato clones by transcription profiling of field grown plants under water stress. Plant Physiol. Biochem. 2007, 45, 673–690. [Google Scholar] [CrossRef]
- Stark, J.; Love, S.; King, B.; Marshall, J.; Bohl, W.; Salaiz, T. Potato cultivar response to seasonal drought patterns. Am. J. Potato Res. 2013, 90, 207–216. [Google Scholar] [CrossRef]
- Wagg, C.; Hann, S.; Kupriyanovich, Y.; Li, S. Timing of short period water stress determines potato plant growth, yield and tuber quality. Agric. Water Manag. 2021, 247, 106731. [Google Scholar] [CrossRef]
- Xing, Y.; Zhang, T.; Jiang, W.; Li, P.; Shi, P.; Xu, G.; Cheng, S.; Cheng, Y.; Fan, Z.; Wang, X. Effects of irrigation and fertilization on different potato varieties growth, yield and resources use efficiency in the Northwest China. Agric. Water Manag. 2022, 261, 107351. [Google Scholar] [CrossRef]
- Kumar, S.; Asrey, R.; Mandal, G. Effect of Differential Irrigation Regimes on Potato (Solanum tuberosum) Yield and Post-Harvest Post-Harvest Attributes; Indian Council of Agricutural Research: New Delhi, India, 2011. [Google Scholar]
- Schafleitner, R.; Gutierrez, R.; Legay, S.; Evers, D.; Bonierbale, M. Drought stress tolerance traits of potato. In Proceedings of the 15th International Symposium of the International Society for Tropical Root Crops (ISTRC), Lima, Peru, 2–7 November 2009. [Google Scholar]
- Batool, T.; Ali, S.; Seleiman, M.F.; Naveed, N.H.; Ali, A.; Ahmed, K.; Abid, M.; Rizwan, M.; Shahid, M.R.; Alotaibi, M. Plant growth promoting rhizobacteria alleviates drought stress in potato in response to suppressive oxidative stress and antioxidant enzymes activities. Sci. Rep. 2020, 10, 16975. [Google Scholar] [CrossRef] [PubMed]
- Nasir, M.W.; Toth, Z. Effect of Drought Stress on Potato Production: A Review. Agronomy 2022, 12, 635. [Google Scholar] [CrossRef]
- Eid, M.A.; Abdel-Salam, A.A.; Salem, H.M.; Mahrous, S.E.; Seleiman, M.F.; Alsadon, A.A.; Solieman, T.H.; Ibrahim, A.A. Interaction effects of nitrogen source and irrigation regime on tuber quality, yield, and water Use efficiency of Solanum tuberosum L. Plants 2020, 9, 110. [Google Scholar] [CrossRef]
- Aliche, E.B.; Oortwijn, M.; Theeuwen, T.P.; Bachem, C.W.; Visser, R.G.; van der Linden, C.G. Drought response in field grown potatoes and the interactions between canopy growth and yield. Agric. Water Manag. 2018, 206, 20–30. [Google Scholar] [CrossRef]
- Guleria, G.; Thakur, S.; Shandilya, M.; Sharma, S.; Thakur, S.; Kalia, S. Nanotechnology for sustainable agro-food systems: The need and role of nanoparticles in protecting plants and improving crop productivity. Plant Physiol. Biochem. 2023, 194, 533–549. [Google Scholar] [CrossRef]
- Seleiman, M.F.; Almutairi, K.F.; Alotaibi, M.; Shami, A.; Alhammad, B.A.; Battaglia, M.L. Nano-fertilization as an emerging fertilization technique: Why can modern agriculture benefit from its use? Plants 2021, 10, 2. [Google Scholar] [CrossRef]
- Fayez, K.; El-Deeb, B.; Mostafa, N. Toxicity of biosynthetic silver nanoparticles on the growth, cell ultrastructure and physiological activities of barley plant. Acta Physiol. Plant. 2017, 39, 155. [Google Scholar] [CrossRef]
- Badawy, S.A.; Zayed, B.A.; Bassiouni, S.M.A.; Mahdi, A.H.A.; Majrashi, A.; Ali, E.F.; Seleiman, M.F. Influence of Nano Silicon and Nano Selenium on Root Characters, Growth, Ion Selectivity, Yield, and Yield Components of Rice (Oryza sativa L.) under Salinity Conditions. Plants 2021, 10, 1657. [Google Scholar] [CrossRef]
- Singh, M.D. Nano-fertilizers is a new way to increase nutrients use efficiency in crop production. Int. J. Agric. Sci. ISSN 2017, 9, 0975–3710. [Google Scholar]
- Elshayb, O.M.; Nada, A.M.; Sadek, A.H.; Ismail, S.H.; Shami, A.; Alharbi, B.M.; Alhammad, B.A.; Seleiman, M.F. The Integrative Effects of Biochar and ZnO Nanoparticles for Enhancing Rice Productivity and Water Use Efficiency under Irrigation Deficit Conditions. Plants 2022, 11, 1416. [Google Scholar] [CrossRef] [PubMed]
- Abobatta, W.F. Nanotechnology application in agriculture. Acta Sci. Agric. 2018, 2, 99–102. [Google Scholar]
- Elshayb, O.M.; Nada, A.M.; Farroh, K.Y.; AL-Huqail, A.A.; Aljabri, M.; Binothman, N.; Seleiman, M.F. Utilizing Urea–Chitosan Nanohybrid for Minimizing Synthetic Urea Application and Maximizing Oryza sativa L. Productivity and N Uptake. Agriculture 2022, 12, 944. [Google Scholar] [CrossRef]
- Hafez, E.M.; Osman, H.S.; Gowayed, S.M.; Okasha, S.A.; Omara, A.E.-D.; Sami, R.; Abd El-Monem, A.M.; Abd El-Razek, U.A. Minimizing the adversely impacts of water deficit and soil salinity on maize growth and productivity in response to the application of plant growth-promoting rhizobacteria and silica nanoparticles. Agronomy 2021, 11, 676. [Google Scholar] [CrossRef]
- Faizan, M.; Hayat, S.; Pichtel, J. Effects of zinc oxide nanoparticles on crop plants: A perspective analysis. In Sustainable Agriculture Reviews 41; Springer: Berlin/Heidelberg, Germany, 2020; pp. 83–99. [Google Scholar]
- Moghaddasi, S.; Fotovat, A.; Khoshgoftarmanesh, A.H.; Karimzadeh, F.; Khazaei, H.R.; Khorassani, R. Bioavailability of coated and uncoated ZnO nanoparticles to cucumber in soil with or without organic matter. Ecotoxicol. Environ. Saf. 2017, 144, 543–551. [Google Scholar] [CrossRef]
- Monreal, C.; De Rosa, M.; Mallubhotla, S.; Bindraban, P.; Dimkpa, C. Nanotechnologies for increasing the crop use efficiency of fertilizer-micronutrients. Biol. Fertil. Soils 2016, 52, 423–437. [Google Scholar] [CrossRef]
- Pinedo-Guerrero, Z.H.; Cadenas-Pliego, G.; Ortega-Ortiz, H.; González-Morales, S.; Benavides-Mendoza, A.; Valdés-Reyna, J.; Juárez-Maldonado, A. Form of silica improves yield, fruit quality and antioxidant defense system of tomato plants under salt stress. Agriculture 2020, 10, 367. [Google Scholar] [CrossRef]
- Sparks, D.L.; Page, A.L.; Helmke, P.A.; Loeppert, R.H. Methods of Soil Analysis, Part 3: Chemical Methods; John Wiley & Sons: Hoboken, NJ, USA, 2020; Volume 14. [Google Scholar]
- Maynard, D.N.; Hochmuth, G.J. Knott’s Handbook for Vegetable Growers; John Wiley & Sons: Hoboken, NJ, USA, 2006. [Google Scholar]
- Salokhe, V.; Babel, M.; Tantau, H. Water requirement of drip irrigated tomatoes grown in greenhouse in tropical environment. Agric. Water Manag. 2005, 71, 225–242. [Google Scholar]
- Al-Selwey, W.A.; Alsadon, A.A.; Ibrahim, A.A.; Labis, J.P.; Seleiman, M.F. Effects of Zinc Oxide and Silicon Dioxide Nanoparticles on Physiological, Yield, and Water Use Efficiency Traits of Potato Grown under Water Deficit. Plants 2023, 12, 218. [Google Scholar] [CrossRef]
- Steyn, J.; Kagabo, D.; Annandale, J. Potato growth and yield responses to irrigation regimes in contrasting seasons of a subtropical region. In Proceedings of the 8th African Crop Science Society Conference, El-Minia, Egypt, 27–31 October 2007; pp. 1647–1651. [Google Scholar]
- Gautam, I.P.; Sharma, M.D.; Khatri, B.B. Yield, Storability and Processing Quality Of Potato: Yield, Storage and Quality Parameters (Solanum tuberosum L.); LAP LAMBERT Academic Publishing: Saarbruecken, Germany, 2016. [Google Scholar]
- AOAC. Official Methods of Analysis, 20th ed.; AOAC: Washington, DC, USA, 2005. [Google Scholar]
- Pace, B.; Cefola, M. Innovative preservation technology for the fresh fruit and vegetables. Foods 2021, 10, 719. [Google Scholar] [CrossRef] [PubMed]
- AOAC. Official Methods of Analysis of AOAC International; Horwitz, W., Latimer, G., Eds.; AOAC: Gaithersburg, MD, USA, 2016. [Google Scholar]
- Sit, N.; Misra, S.; Deka, S.C. Physicochemical, functional, textural and colour characteristics of starches isolated from four taro cultivars of North-E ast India. Starch Stärke 2013, 65, 1011–1021. [Google Scholar] [CrossRef]
- Jackson, M. Soil Chemical Analysis; Prentice-Hall of India Pvt. Ltd.: New Delhi, India, 1967; p. 498. [Google Scholar]
- Navarre, D.A.; Goyer, A.; Shakya, R. Nutritional value of potatoes: Vitamin, phytonutrient, and mineral content. In Advances in Potato Chemistry and Technology; Elsevier: Amsterdam, The Netherlands, 2009; pp. 395–424. [Google Scholar]
- Obidiegwu, J.E.; Bryan, G.J.; Jones, H.G.; Prashar, A. Coping with drought: Stress and adaptive responses in potato and perspectives for improvement. Front. Plant Sci. 2015, 6, 542. [Google Scholar] [CrossRef]
- Carli, C.; Yuldashev, F.; Khalikov, D.; Condori, B.; Mares, V.; Monneveux, P. Effect of different irrigation regimes on yield, water use efficiency and quality of potato (Solanum tuberosum L.) in the lowlands of Tashkent, Uzbekistan: A field and modeling perspective. Field Crops Res. 2014, 163, 90–99. [Google Scholar] [CrossRef]
- Elemike, E.E.; Uzoh, I.M.; Onwudiwe, D.C.; Babalola, O.O. The role of nanotechnology in the fortification of plant nutrients and improvement of crop production. Appl. Sci. 2019, 9, 499. [Google Scholar] [CrossRef]
- Sun, L.; Song, F.; Zhu, X.; Liu, S.; Liu, F.; Wang, Y.; Li, X. Nano-ZnO alleviates drought stress via modulating the plant water use and carbohydrate metabolism in maize. Arch. Agron. Soil Sci. 2021, 67, 245–259. [Google Scholar] [CrossRef]
- Tognetti, V.B.; Mühlenbock, P.; Van Breusegem, F. Stress homeostasis–the redox and auxin perspective. Plant Cell Environ. 2012, 35, 321–333. [Google Scholar] [CrossRef]
- Tan, D.X.; Hardeland, R.; Back, K.; Manchester, L.C.; Alatorre-Jimenez, M.A.; Reiter, R.J. On the significance of an alternate pathway of melatonin synthesis via 5-methoxytryptamine: Comparisons across species. J. Pineal Res. 2016, 61, 27–40. [Google Scholar] [CrossRef]
- Haliloglu, K.; Hosseinpour, A.; Cinisli, K.T.; Ozturk, H.I.; Ozkan, G.; Pour-Aboughadareh, A.; Poczai, P. Investigation of the protective roles of zinc oxide nanoparticles and plant growth promoting bacteria on DNA damage and methylation in tomato (Solanum lycopersicum L.) under salinity stress. Hortic. Environ. Biotechnol. 2020, 10, 521. [Google Scholar] [CrossRef]
- Yu, O.Y.; Harper, M.; Hoepfl, M.; Domermuth, D. Characterization of biochar and its effects on the water holding capacity of loamy sand soil: Comparison of hemlock biochar and switchblade grass biochar characteristics. Environ. Prog. Sustain. Energy 2017, 36, 1474–1479. [Google Scholar] [CrossRef]
- Foroutan, L.; Solouki, M.; Abdossi, V.; Fakheri, B.A. The effects of zinc oxide nanoparticles on enzymatic and osmoprotectant alternations in different Moringa peregrina populations under drought stress. Int. J. Basic Sci. Med. 2018, 3, 178–187. [Google Scholar] [CrossRef]
- Zhu, Z.; Wei, G.; Li, J.; Qian, Q.; Yu, J. Silicon alleviates salt stress and increases antioxidant enzymes activity in leaves of salt-stressed cucumber (Cucumis sativus L.). Plant Sci. 2004, 167, 527–533. [Google Scholar] [CrossRef]
- Sangster, A.; Hodson, M.; Tubb, H. Silicon deposition in higher plants. In Studies in Plant Science; Elsevier: Amsterdam, The Netherlands, 2001; Volume 8, pp. 85–113. [Google Scholar]
- Maghsoudi, K.; Emam, Y.; Pessarakli, M. Effect of silicon on photosynthetic gas exchange, photosynthetic pigments, cell membrane stability and relative water content of different wheat cultivars under drought stress conditions. J. Plant Nutr. 2016, 39, 1001–1015. [Google Scholar] [CrossRef]
- Alsaeedi, A.; El-Ramady, H.; Alshaal, T.; El-Garawany, M.; Elhawat, N.; Al-Otaibi, A. Silica nanoparticles boost growth and productivity of cucumber under water deficit and salinity stresses by balancing nutrients uptake. Plant Physiol. Biochem. 2019, 139, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, I.; Akhtar, M.S. Use of nanoparticles in alleviating salt stress. In Salt Stress, Microbes, and Plant Interactions: Causes and Solution; Springer: Berlin/Heidelberg, Germany, 2019; pp. 199–215. [Google Scholar]
- Kanwal, A.; Sharma, I.; Bala, A.; Upadhyay, S.K.; Singh, R. Agricultural Application of Synthesized ZnS Nanoparticles for the Development of Tomato Crop. Lett. Appl. NanoBioSci. 2022, 12, 1–9. [Google Scholar]
- Hassan, A.; Sarkar, A.; Ali, M.; Karim, N. Effect of deficit irrigation at different growth stages on the yield of potato. Pak. J. Biol. Sci. 2002, 5, 128–134. [Google Scholar] [CrossRef]
- Mahmoud, A.W.M.; Samy, M.M.; Sany, H.; Eid, R.R.; Rashad, H.M.; Abdeldaym, E.A. Nanopotassium, nanosilicon, and biochar applications improve potato salt tolerance by modulating photosynthesis, water status, and biochemical constituents. Sustainability 2022, 14, 723. [Google Scholar] [CrossRef]
- Steyn, J.; Du Plessis, H.; Fourie, P.; Hammes, P. Yield response of potato genotypes to different soil water regimes in contrasting seasons of a subtropical climate. Potato Res. 1998, 41, 239–254. [Google Scholar] [CrossRef]
- Elhani, S.; Haddadi, M.; Csákvári, E.; Zantar, S.; Hamim, A.; Villányi, V.; Douaik, A.; Bánfalvi, Z. Effects of partial root-zone drying and deficit irrigation on yield, irrigation water-use efficiency and some potato (Solanum tuberosum L.) quality traits under glasshouse conditions. Agric. Water Manag. 2019, 224, 105745. [Google Scholar] [CrossRef]
- Jensen, C.; Jacobsen, S.-E.; Andersen, M.; Nunez, N.; Andersen, S.; Rasmussen, L.; Mogensen, V. Leaf gas exchange and water relation characteristics of field quinoa (Chenopodium quinoa Willd.) during soil drying. Eur. J. Agron. 2000, 13, 11–25. [Google Scholar] [CrossRef]
- Kumar, D.; Minhas, J.; Singh, B. Abiotic stress and potato production. In The Potato: Production and Utilization in Sub-Tropics; Khurana, S.M.P., Minhas, J.S., Pandey, S.K., Eds.; Mehta Publishers: New Delhi, India, 2003. [Google Scholar]
- Yuan, B.-Z.; Nishiyama, S.; Kang, Y. Effects of different irrigation regimes on the growth and yield of drip-irrigated potato. Agric. Water Manag. 2003, 63, 153–167. [Google Scholar] [CrossRef]
- Abd-Elrahman, S.H.; Taha, N.M. Comparison between organic and mineral sources of potassium and their effects on potassium fractions in clay soil and productivity of potato plants under water stress conditions. Egypt. J. Soil Sci. 2018, 58, 193–206. [Google Scholar] [CrossRef]
- Alenazi, M.; Wahb-Allah, M.A.; Abdel-Razzak, H.S.; Ibrahim, A.A.; Alsadon, A. Water regimes and humic acid application influences potato growth, yield, tuber quality and water use efficiency. Am. J. Potato Res. 2016, 93, 463–473. [Google Scholar] [CrossRef]
- Ayas, S. The effects of different regimes on potato (Solanum tuberosum L. Hermes) yield and quality characteristics under unheated greenhouse conditions. Bulg. J. Agric. Sci. 2013, 19, 87–95. [Google Scholar]
- Al-juthery, H.; Al-taee, R.; Al-Obaidi, Z.; Ali, E.; NAl-Shami, Q. Influence of foliar application of some nano-fertilizers in growth and yield of potato under drip irrigation. J. Phys. Conf. Ser. 2019, 1294, 092024. [Google Scholar] [CrossRef]
- Mahmoud, A.W.M.; Abdeldaym, E.A.; Abdelaziz, S.M.; El-Sawy, M.B.; Mottaleb, S.A. Synergetic effects of zinc, boron, silicon, and zeolite nanoparticles on confer tolerance in potato plants subjected to salinity. Agronomy 2020, 10, 19. [Google Scholar] [CrossRef]
- Prasad, T.; Sudhakar, P.; Sreenivasulu, Y.; Latha, P.; Munaswamy, V.; Reddy, K.R.; Sreeprasad, T.; Sajanlal, P.; Pradeep, T. Effect of nanoscale zinc oxide particles on the germination, growth and yield of peanut. J. Plant Nutr. 2012, 35, 905–927. [Google Scholar] [CrossRef]
- Dimkpa, C.O.; White, J.C.; Elmer, W.H.; Gardea-Torresdey, J. Nanoparticle and ionic Zn promote nutrient loading of sorghum grain under low NPK fertilization. J. Agric. Food Chem. 2017, 65, 8552–8559. [Google Scholar] [CrossRef]
- Lefèvre, I.; Ziebel, J.; Guignard, C.; Hausman, J.F.; Gutiérrez Rosales, R.O.; Bonierbale, M.; Hoffmann, L.; Schafleitner, R.; Evers, D. Drought impacts mineral contents in Andean potato cultivars. J. Agron. Crop Sci. 2012, 198, 196–206. [Google Scholar] [CrossRef]
- Semida, W.M.; Abdelkhalik, A.; Mohamed, G.F.; Abd El-Mageed, T.A.; Abd El-Mageed, S.A.; Rady, M.M.; Ali, E.F. Foliar Application of Zinc Oxide Nanoparticles Promotes Drought Stress Tolerance in Eggplant (Solanum melongena L.). Plants 2021, 10, 421. [Google Scholar] [CrossRef]
- Singh, A.; Tiwari, S.; Pandey, J.; Lata, C.; Singh, I.K. Role of nanoparticles in crop improvement and abiotic stress management. J. Biotechnol. 2021, 337, 57–70. [Google Scholar] [CrossRef] [PubMed]
- Rea, R.S.; Islam, M.R.; Rahman, M.M.; Nath, B.; Mix, K. Growth, Nutrient Accumulation, and Drought Tolerance in Crop Plants with Silicon Application: A Review. Sustainability 2022, 14, 4525. [Google Scholar] [CrossRef]
- Uresti-Porras, J.-G.; Cabrera-De-La Fuente, M.; Benavides-Mendoza, A.; Olivares-Sáenz, E.; Cabrera, R.I.; Juárez-Maldonado, A. Effect of Graft and Nano ZnO on Nutraceutical and Mineral Content in Bell Pepper. Plants 2021, 10, 2793. [Google Scholar] [CrossRef]
- Fatollahpour Grangah, M.; Rashidi, V.; Mirshekari, B.; Khalilvand Behrouzyar, E.; Farahvash, F. Effects of nano-fertilizers on physiological and yield characteristics of pinto bean cultivars under water deficit stress. J. Plant Nutr. 2020, 43, 2898–2910. [Google Scholar] [CrossRef]
- Dimkpa, C.O.; Singh, U.; Bindraban, P.S.; Elmer, W.H.; Gardea-Torresdey, J.L.; White, J.C. Zinc oxide nanoparticles alleviate drought-induced alterations in sorghum performance, nutrient acquisition, and grain fortification. Sci. Total Environ. 2019, 688, 926–934. [Google Scholar] [CrossRef]
- Elfnesh, F.; Tekalign, T.; Solomon, W. Processing quality of improved potato (Solanum tuberosum L.) cultivars as influenced by growing environment and blanching. Afr. J. Food Sci. 2011, 5, 324–332. [Google Scholar]
- Dull, G.G.; Birth, G.S.; Leffler, R.G. Use of near infrared analysis for the nondestructive measurement of dry matter in potatoes. Am. Potato J. 1989, 66, 215–225. [Google Scholar] [CrossRef]
- Djaman, K.; Irmak, S.; Koudahe, K.; Allen, S. Irrigation management in potato (Solanum tuberosum L.) production: A review. Sustainability 2021, 13, 1504. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).