Bioengineering of Canopy Photosynthesis in Rice for Securing Global Food Security: A Critical Review
Abstract
:1. Introduction
2. Approaches for Improving Rice Productivity
2.1. Improving Radiation Use Efficiency
2.2. Improving Canopy Photosynthesis
2.3. Improving Light Distribution and Reducing Shading Losses
2.4. Improving Canopy and Panicle Architecture
2.5. Increasing Calvin Cycle Efficiency in Rice Leaf
2.6. Introduction of Cyanobacterial CO2-Concentrating Mechanisms into Chloroplasts
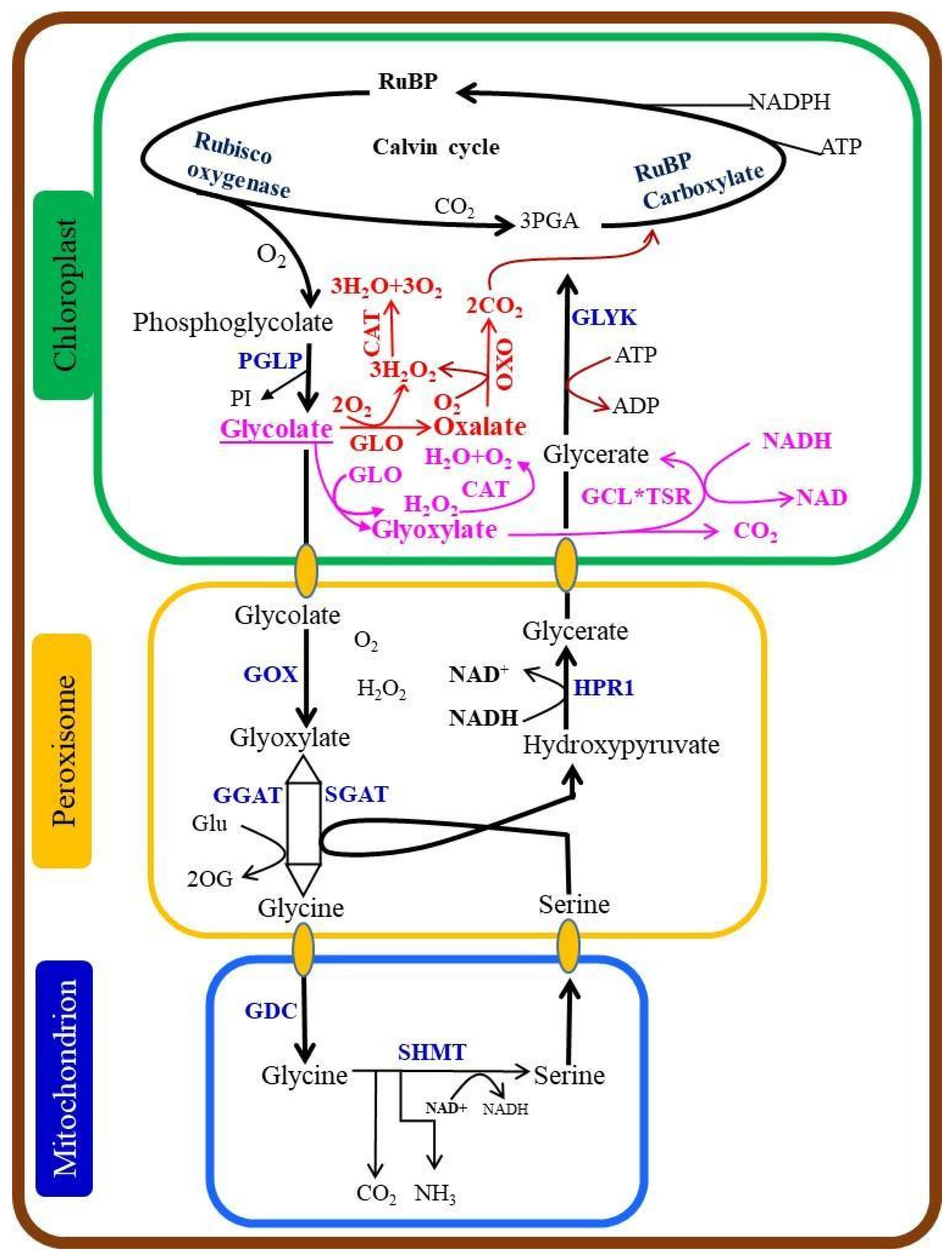
2.7. Pathway Editing for Minimization of Photorespiration
2.8. Scavenging of Photorespiratory CO2
2.9. Optimization of the Photorespiratory Enzymes and Photorespiratory Bypasses
2.10. Conversion of C3 Photosynthetic Pathway into C4 in Rice
2.11. Balancing Source and Sink
2.12. QTL-Based Targeting of Genetic Loci and Genes
3. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Available online: https://www.fao.org/ (accessed on 1 December 2022).
- Do Amaral, M.N.; Arge, L.W.; Benitez, L.C.; Danielowski, R.; da Silveira Silveira, S.F.; da Rosa Farias, D.; Deuner, S.; de Oliveira, A.C.; Braga, E.J.; da Maia, L.C. Differential expression of photosynthesis-related genes and quantification of gas exchange in rice plants under abiotic stress. Acta Physiol. Plant. 2016, 38, 153. [Google Scholar] [CrossRef]
- Menguer, P.K.; Sperotto, R.A.; Ricachenevsky, F.K. A walk on the wild side: Oryza species as source for rice abiotic stress tolerance. Genet. Mol. Biol. 2017, 40, 238–252. [Google Scholar] [CrossRef]
- Chang, T.G.; Zhao, H.; Wang, N.; Song, Q.F.; Xiao, Y.; Qu, M.; Zhu, X.G. A three-dimensional canopy photosynthesis model in rice with a complete description of the canopy architecture, leaf physiology, and mechanical properties. J. Exp. Bot. 2019, 70, 2479–2490. [Google Scholar] [CrossRef] [PubMed]
- Ikawa, H.; Chen, C.P.; Sikma, M.; Yoshimoto, M.; Sakai, H.; Tokida, T.; Usui, Y.; Nakamura, H.; Ono, K.; Maruyama, A.; et al. Increasing canopy photosynthesis in rice can be achieved without a large increase in water use—A model based on free-air CO2 enrichment. Glob. Chang. Biol. 2018, 24, 1321–1341. [Google Scholar] [CrossRef]
- Mohapatra, T.; Robin, S.; Sarla, N.; Sheshashayee, M.; Singh, A.K.; Singh, K.; Sharma, R.P. EMS induced mutants of upland rice variety Nagina22: Generation and characterization. Proc. Indian Natl. Sci. Acad. USA 2014, 80, 163–172. [Google Scholar] [CrossRef]
- Khush, G.S. Strategies for increasing the yield potential of cereals: Case of rice as an example. Plant Breed. 2013, 132, 433–436. [Google Scholar] [CrossRef]
- Iñiguez, C.; Aguiló-Nicolau, P.; Galmés, J. Improving photosynthesis through the enhancement of Rubisco carboxylation capacity. Biochem. Soc. Trans. 2021, 49, 2007–2019. [Google Scholar] [CrossRef]
- Li, J.; Xie, R.Z.; Wang, K.R.; Hou, P.; Ming, B.; Zhang, G.Q.; Liu, G.Z.; Wu, M.; Yang, Z.S.; Li, S.K. Response of canopy structure, light interception and grain yield to plant density in maize. J. Agric. Sci. 2018, 156, 785–794. [Google Scholar] [CrossRef]
- Ambavaram, M.M.; Basu, S.; Krishnan, A.; Ramegowda, V.; Batlang, U.; Rahman, L.; Baisakh, N.; Pereira, A. Coordinated regulation of photosynthesis in rice increases yield and tolerance to environmental stress. Nat. Commun. 2014, 5, 5302. [Google Scholar] [CrossRef]
- Simkin, A.J.; Kapoor, L.; Doss, C.; Hofmann, T.A.; Lawson, T.; Ramamoorthy, S. Therole of photosynthesis related pigments in light harvesting, photoprotection and enhancement of photosynthetic yield in planta. Photosynth. Res. 2022, 152, 23–42. [Google Scholar] [CrossRef]
- Yamori, W. Improving photosynthesis to increase food and fuel production by biotechnological strategies in crops. J. Plant Biochem. Physiol. 2013, 1, 3. [Google Scholar]
- Theeuwen, T.P.; Logie, L.L.; Harbinson, J.; Aarts, M.G. Genetics as a key to improving crop photosynthesis. J. Exp. Bot. 2022, 73, 3122–3137. [Google Scholar] [CrossRef] [PubMed]
- Verma, V.; Ramamoorthy, R.; Kohli, A.; Kumar, P.P. Rice research to break yield barriers. Cosmos 2015, 11, 37–54. [Google Scholar] [CrossRef]
- Benavente, E.; Giménez, E. Modern approaches for the genetic improvement of rice, wheat and maize for abiotic constraints-related traits: A comparative overview. Agronomy 2021, 11, 376. [Google Scholar] [CrossRef]
- Ort, D.R.; Merchant, S.S.; Alric, J.; Barkan, A.; Blankenship, R.E.; Bock, R.; Croce, R.; Hanson, M.R.; Hibberd, J.M.; Long, S.P.; et al. Redesigning photosynthesis to sustainably meet global food and bioenergy demand. Proc. Natl. Acad. Sci. USA 2015, 112, 8529–8536. [Google Scholar] [CrossRef]
- Pazhamala, L.T.; Kudapa, H.; Weckwerth, W.; Millar, A.H.; Varshney, R.K. Systems biology for crop improvement. Plant Genome 2021, 14, e20098. [Google Scholar] [CrossRef]
- Stanley, C.; Mojiri, A.; Rosengarten, G. Spectral light management for solar energy conversion systems. Nanophotonics 2016, 5, 161–179. [Google Scholar] [CrossRef]
- Yin, X.; Struik, P.C. Constraints to the potential efficiency of converting solar radiation into phytoenergy in annual crops: From leaf biochemistry to canopy physiology and crop ecology. J. Exp. Bot. 2015, 66, 6535–6549. [Google Scholar] [CrossRef]
- Monteith, J.L. Climate and the efficiency of crop production in Britain. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1977, 281, 277–294. [Google Scholar] [CrossRef]
- Liu, K.; Yang, R.; Deng, J.; Huang, L.; Wei, Z.; Ma, G.; Tian, X.; Zhang, Y. High radiation use efficiency improves yield in the recently developed elite hybrid rice Y-liangyou 900. Field Crop. Res. 2020, 253, 107804. [Google Scholar] [CrossRef]
- Zhang, H.; Mittal, N.; Leamy, L.J.; Barazani, O.; Song, B.H. Back into the wild–Apply untapped genetic diversity of wild relatives for crop improvement. Evol. Appl. 2017, 10, 5–24. [Google Scholar] [CrossRef] [PubMed]
- Sadras, V.O.; Villalobos, F.J.; Fereres, E. Radiation interception, radiation use efficiency and crop productivity. In Principles of Agronomy for Sustainable Agriculture; Springer: New York, NY, USA, 2016; pp. 169–188. [Google Scholar] [CrossRef]
- Hatfield, J.L. Radiation use efficiency: Evaluation of cropping and management systems. Agron. J. 2014, 106, 1820–1827. [Google Scholar] [CrossRef]
- Koester, R.P.; Skoneczka, J.A.; Cary, T.R.; Diers, B.W.; Ainsworth, E.A. Historical gains in soybean (Glycine max Merr.) seed yield are driven by linear increases in light interception, energy conversion, and partitioning efficiencies. J. Exp. Bot. 2014, 65, 3311–3321. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.T.; Occhialini, A.; Andralojc, P.J.; Parry, M.A.; Hanson, M.R. A faster Rubisco with potential to increase photosynthesis in crops. Nature 2014, 513, 547–550. [Google Scholar] [CrossRef] [PubMed]
- Slattery, R.A.; Walker, B.J.; Weber, A.P.; Ort, D.R. The impacts of fluctuating light on crop performance. Plant Physiol. 2018, 176, 990–1003. [Google Scholar] [CrossRef]
- Surendran, U.; Raja, P.; Jayakumar, M.; Subramoniam, S.R. Use of efficient water saving techniques for production of rice in India under climate change scenario: A critical review. J. Clean. Prod. 2021, 309, 127272. [Google Scholar] [CrossRef]
- Punia, H.; Tokas, J.; Malik, A.; Rani, A.; Gupta, P.; Kumari, A.; Mor, V.S.; Bhuker, A.; Kumar, S. Solar radiation and nitrogen use efficiency for sustainable agriculture. In Resources Use Efficiency in Agriculture; Springer: Singapore, 2020; pp. 177–212. [Google Scholar] [CrossRef]
- Parry, M.A.; Reynolds, M.; Salvucci, M.E.; Raines, C.; Andralojc, P.J.; Zhu, X.G.; Price, G.D.; Condon, A.G.; Furbank, R.T. Raising yield potential of wheat. II. Increasing photosynthetic capacity and efficiency. J. Exp. Bot. 2011, 62, 453–467. [Google Scholar] [CrossRef]
- Pan, Y.; Gao, S.; Xie, K.; Lu, Z.; Meng, X.; Wang, S.; Lu, J.; Guo, S. Higher radiation use efficiency produces greater biomass before heading and grain yield in super hybrid rice. Agronomy 2020, 10, 209. [Google Scholar] [CrossRef]
- Huang, M.; Zou, Y.B.; Jiang, P.; Xia, B.; Ibrahim, M.; Ao, H.J. Relationship between grain yield and yield components in super hybrid rice. Agric. Sci. China 2011, 10, 1537–1544. [Google Scholar] [CrossRef]
- Zhou, K.; Deng, X.; Yao, X.; Tian, Y.; Cao, W.; Zhu, Y.; Ustin, S.L.; Cheng, T. Assessing the spectral properties of sunlit and shaded components in rice canopies with near-ground imaging spectroscopy data. Sensors 2017, 17, 578. [Google Scholar] [CrossRef]
- Wang, B.; Smith, S.M.; Li, J. Genetic regulation of shoot architecture. Annu. Rev. Plant Biol. 2018, 69, 437–468. [Google Scholar] [CrossRef] [PubMed]
- Feller, U.; Anders, I.; Mae, T. Rubiscolytics: Fate of Rubisco after its enzymatic function in a cell is terminated. J. Exp. Bot. 2008, 59, 1615–1624. [Google Scholar] [CrossRef] [PubMed]
- Song, Q.; Chu, C.; Parry, M.A.; Zhu, X.G. Genetics-based dynamic systems model of canopy photosynthesis: The key to improve light and resource use efficiencies for crops. Food Energy Secur. 2016, 5, 18–25. [Google Scholar] [CrossRef]
- Wang, D.; Fahad, S.; Saud, S.; Kamran, M.; Khan, A.; Khan, M.N.; Hammad, H.M.; Nasim, W. Morphological acclimation to agronomic manipulation in leaf dispersion and orientation to promote “Ideotype” breeding: Evidence from 3D visual modeling of “super” rice (Oryza sativa L.). Plant Physiol. Biochem. 2019, 135, 499–510. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.; Chang, T.; Song, Q.; Wu, J.; Luo, Y.; Chen, X.; Zhu, X.-G.; Deng, Q. Architectural and physiological features to gain high yield in an elite rice line YLY1. Rice 2020, 13, 60. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Chen, T.; Feng, B.; Zhang, C.; Peng, S.; Zhang, X.; Fu, G.; Tao, L. Non-photochemical quenching plays a key role in light acclimation of rice plants differing in leaf color. Front. Plant Sci. 2017, 7, 1968. [Google Scholar] [CrossRef]
- Wei, H.-H.; Yang, Y.-L.; Shao, X.-Y.; Shi, T.-Y.; Meng, T.-Y.; Lu, Y.; Tao, Y.; Li, X.-Y.; Ding, E.-H.; Chen, Y.-L.; et al. Higher leaf area through leaf width and lower leaf angle were the primary morphological traits for yield advantage of japonica/indica hybrids. J. Integr. Agric. 2020, 19, 483–494. [Google Scholar] [CrossRef]
- Yamori, W.; Kusumi, K.; Iba, K.; Terashima, I. Increased stomatal conductance induces rapid changes to photosynthetic rate in response to naturally fluctuating light conditions in rice. Plant Cell Environ. 2020, 43, 1230–1240. [Google Scholar] [CrossRef]
- Acevedo-Siaca, L.G.; Coe, R.; Wang, Y.; Kromdijk, J.; Quick, W.P.; Long, S.P. Variation in photosynthetic induction between rice accessions and its potential for improving productivity. New Phytol. 2020, 227, 1097–1108. [Google Scholar] [CrossRef] [Green Version]
- Hasan, M.M.; Rahman, M.A.; Skalicky, M.; Alabdallah, N.M.; Waseem, M.; Jahan, M.S.; Ahammed, G.J.; El-Mogy, M.M.; El-Yazied, A.A.; Ibrahim, M.F.M.; et al. Ozone Induced Stomatal Regulations, MAPK and Phytohormone Signaling in Plants. Int. J. Mol. Sci. 2021, 22, 6304. [Google Scholar] [CrossRef]
- Song, Q.; Zhang, G.; Zhu, X.G. Optimal crop canopy architecture to maximise canopy photosynthetic CO2 uptake under elevated CO2 a theoretical study using a mechanistic model of canopy photosynthesis. Funct. Plant Biol. 2013, 40, 108–124. [Google Scholar] [CrossRef] [PubMed]
- Graefe, J.; Yu, W.; Körner, O. A photosynthetic light acclimation model accounting for the effects of leaf age, chlorophyll content, and intra-leaf radiation transfer. Front. Plant Sci. 2022, 13, 889709. [Google Scholar] [CrossRef] [PubMed]
- Charbonnier, F.; Roupsard, O.; Le-Maire, G.; Guillemot, J.; Casanoves, F.; Lacointe, A.; Vaast, P.; Allinne, C.; Audebert, L.; Cambou, A.; et al. Increased light-use efficiency sustains net primary productivity of shaded coffee plants in agroforestry system. Plant Cell Environ. 2017, 40, 1592–1608. [Google Scholar] [CrossRef] [PubMed]
- Emmel, C.; D’Odorico, P.; Revill, A.; Hörtnagl, L.; Ammann, C.; Buchmann, N.; Eugster, W. Canopy photosynthesis of six major arable crops is enhanced under diffuse light due to canopy architecture. Glob. Chang. Biol. 2020, 26, 5164–5177. [Google Scholar] [CrossRef]
- Hua, S.; Cao, B.; Zheng, B.; Li, B.; Sun, C. Quantitative evaluation of influence of PROSTRATE GROWTH 1 gene on rice canopy structure based on three-dimensional structure model. Field Crop. Res. 2016, 194, 65–74. [Google Scholar] [CrossRef]
- Lacasa, J.; Hefley, T.J.; Otegui, M.E.; Ciampitti, I.A. A practical guide to estimating the light extinction coefficient with nonlinear models—A case study on maize. Plant Methods 2021, 17, 60. [Google Scholar] [CrossRef]
- Li, W.; Fang, H.; Wei, S.; Weiss, M.; Baret, F. Critical analysis of methods to estimate the fraction of absorbed or intercepted photosynthetically active radiation from ground measurements: Application to rice crops. Agric. For. Meteorol. 2021, 297, 108273. [Google Scholar] [CrossRef]
- San, N.S.; Suzuki, K.; Soda, K.; Adachi, S.; Kasahara, H.; Yamamoto, T.; Ikka, T.; Kondo, K.; Yamanouchi, U.; Sugimoto, K.; et al. Semi-dwarf 1 (sd1) gene enhances light penetration into the canopy through regulating leaf inclination angle in rice. Field Crop. Res. 2020, 246, 107694. [Google Scholar] [CrossRef]
- Manoj, K.N.; Umesh, M.R.; Ramesh, Y.M.; Anand, S.R.; Angadi, S. Dry matter production and radiation use efficiency of pulses grown under different light conditions. Bangladesh J. Bot. 2019, 48, 9–15. [Google Scholar] [CrossRef]
- Syam’un, E.; Musa, Y.; Sadimantara, G.R.; Leomo, S.; Rakian, T.C. Shading effect on generative characters of upland red rice of Southeast Sulawesi, Indonesia. IOP Conf. Ser. Earth Environ. Sci. 2018, 157, 012017. [Google Scholar] [CrossRef]
- Li, Q.; Deng, F.; Chen, H.; Zeng, Y.; Li, B.; Zhong, X.; Wang, L.; Zhou, W.; Chen, Y.; Ren, W. Shading decreases rice yield by impeding grain-filling progress after heading. Agron. J. 2020, 112, 4018–4030. [Google Scholar] [CrossRef]
- Wang, Q.; Su, Q.; Nian, J.; Zhang, J.; Guo, M.; Dong, G.; Hu, J.; Wang, R.; Wei, C.; Li, G.; et al. The Ghd7 transcription factor represses ARE1 expression to enhance nitrogen utilization and grain yield in rice. Mol. Plant 2021, 14, 1012–1023. [Google Scholar] [CrossRef] [PubMed]
- Panigrahy, M.; Ranga, A.; Das, J.; Panigrahi, K.C.S. Shade tolerance in Swarnaprabha rice is associated with higher rate of panicle emergence and positively regulated by genes of ethylene and cytokinin pathway. Sci. Rep. 2019, 9, 6817. [Google Scholar] [CrossRef]
- Sekhar, S.; Das, S.; Panda, D.; Mohanty, S.; Mishra, B.; Kumar, A.; Navadagi, D.B.; Sah, R.P.; Pradhan, S.K.; Samantaray, S.; et al. Identification of microRNAs that provide a low light stress tolerance-mediated signaling pathway during vegetative growth in rice. Plants 2022, 11, 2558. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Wang, Y.; Yu, Y.; Duan, J.; Liao, Z.; Xiong, G. Degradation of MONOCULM 1 by APC/CTAD1 regulates rice tillering. Nat. Commun. 2012, 3, 750. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; Wang, D.; Dong, H.; Gu, S.; Cheng, Z.; Gong, J.; Qin, R.; Jiang, L.; Li, G.; Wang, J.L.; et al. Rice APC/CTE controls tillering by mediating the degradation of MONOCULM 1. Nat. Commun. 2012, 3, 752. [Google Scholar] [CrossRef]
- Tabuchi, H.; Zhang, Y.; Hattori, S.; Omae, M.; Shimizu-Sato, S.; Oikawa, T.; Qian, Q.; Nishimura, M.; Kitano, H.; Xie, H.; et al. LAX PANICLE2 of rice encodes a novel nuclear protein and regulates the formation of axillary meristems. Plant Cell 2011, 23, 3276–3287. [Google Scholar] [CrossRef]
- Yang, X.; Wang, J.; Dai, Z.; Zhao, X.; Miao, X.; Shi, Z. miR156f integrates panicle architecture through genetic modulation of branch number and pedicel length pathways. Rice 2019, 12, 40. [Google Scholar] [CrossRef]
- Xiao, Y.; Liu, D.; Zhang, G.; Tong, H.; Chu, C. Brassinosteroids regulate OFP1, a DLT interacting protein, to modulate plant architecture and grain morphology in rice. Front. Plant Sci. 2017, 8, 1698. [Google Scholar] [CrossRef] [Green Version]
- Huang, D.; Wang, S.; Zhang, B.; Shang-Guan, K.; Shi, Y.; Zhang, D.; Liu, X.; Wu, K.; Xu, Z.; Fu, X.; et al. A gibberellin-mediated DELLA-NAC signaling cascade regulates cellulose synthesis in rice. Plant Cell 2015, 27, 1681–1696. [Google Scholar] [CrossRef]
- Schmitz, A.J.; Folsom, J.J.; Jikamaru, Y.; Ronald, P.; Walia, H. SUB 1 A-mediated submergence tolerance response in rice involves differential regulation of the brassinosteroid pathway. New Phytol. 2013, 198, 1060–1070. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Li, T.; Xu, J.; Wang, J.; Wang, L.; Zou, W.; Zeng, D.; Zhu, L.; Chen, G.; Hu, J.; et al. Leaf width gene LW5/D1 affects plant architecture and yield in rice by regulating nitrogen utilization efficiency. Plant Physiol. Biochem. 2020, 157, 359–369. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Feng, Z.M.; Zhou, C.L.; Ren, Y.K.; Mou, C.L.; Wu, T.; Yang, C.Y.; Liu, S.J.; Jiang, L.; Wan, J.M. Brassinosteroid (BR) biosynthetic gene lhdd10 controls late heading and plant height in rice (Oryza sativa L.). Plant Cell Rep. 2016, 35, 357–368. [Google Scholar] [CrossRef] [PubMed]
- Tong, H.; Liu, L.; Jin, Y.; Du, L.; Yin, Y.; Qian, Q.; Zhu, L.; Chu, C. DWARF AND LOW-TILLERING acts as a direct downstream target of a GSK3/SHAGGY-like kinase to mediate brassinosteroid responses in rice. Plant Cell 2012, 24, 2562–2577. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Liu, X.; Wang, M.; Xie, L.; Wu, Z.; Yu, J.; Wang, Y.; Zhang, Z.; Jia, Y.; Liu, Q. The miR528-D3 Module Regulates Plant Height in Rice by Modulating the Gibberellin and Abscisic Acid Metabolisms. Rice 2022, 15, 27. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Lin, Q.; Zhu, L.; Ren, Y.; Zhou, K.; Shabek, N.; Wu, F.; Mao, H.; Dong, W.; Gan, L.; et al. D14-SCFD3-dependent degradation of D53 regulates strigolactone signalling. Nature 2013, 504, 406–410. [Google Scholar] [CrossRef]
- Ying, J.Z.; Chen, Y.Y.; Zhang, H.W. Functional characterization of genes/QTLs for increasing rice yield potential. In Rice-Germplasm, Genetics and Improvement; IntechOpen: Rijeka, Croatia, 2014. [Google Scholar] [CrossRef]
- Wang, Y.P.; Tang, S.Q.; Chen, H.Z.; Wu, Z.F.; Zhang, H.; Duan, E.C.; Shi, Q.H.; Wu, Z.M. Identification and molecular mapping of indica high-tillering dwarf mutant htd4, a mild phenotype allelic mutant of D14 in rice (Oryza sativa L.). Plant Biol. 2017, 19, 851–858. [Google Scholar] [CrossRef]
- Jiang, L.; Liu, X.; Xiong, G.; Liu, H.; Chen, F.; Wang, L.; Meng, X.; Liu, G.; Yu, H.; Yuan, Y.; et al. DWARF 53 acts as a repressor of strigolactone signalling in rice. Nature 2013, 504, 401–405. [Google Scholar] [CrossRef] [Green Version]
- Priyadarshi, R.; Arremsetty, H.P.S.; Singh, A.K.; Khandekar, D.; Ulaganathan, K.; Shenoy, V. Molecular stacking of wide compatibility gene, S5 n and elongated uppermost internode (eui) gene into IR 58025B, an elite maintainer line of rice. J. Plant Biochem. Biotechnol. 2017, 26, 425–435. [Google Scholar] [CrossRef]
- Liao, Z.; Yu, H.; Duan, J.; Yuan, K.; Yu, C.; Meng, X.; Kou, L.; Chen, M.; Jing, Y.; Liu, G.; et al. SLR1 inhibits MOC1 degradation to coordinate tiller number and plant height in rice. Nat. Commun. 2019, 10, 2738. [Google Scholar]
- Koumoto, T.; Shimada, H.; Kusano, H.; She, K.C.; Iwamoto, M.; Takano, M. Rice monoculm mutation moc2, which inhibits outgrowth of the second tillers, is ascribed to lack of a fructose-1,6-bisphosphatase. Plant Biotechnol. 2013, 30, 47–56. [Google Scholar] [CrossRef]
- Lu, Z.; Shao, G.; Xiong, J.; Jiao, Y.; Wang, J.; Liu, G.; Meng, X.; Liang, Y.; Xiong, G.; Wang, Y.; et al. MONOCULM 3, an ortholog of WUSCHEL in rice, is required for tiller bud formation. J. Genet. Genom. 2015, 42, 71–78. [Google Scholar] [CrossRef]
- Yeh, S.Y.; Chen, H.W.; Ng, C.Y.; Lin, C.Y.; Tseng, T.H.; Li, W.H.; Ku, M.S. Down-regulation of cytokinin oxidase 2 expression increases tiller number and improves rice yield. Rice 2015, 8, 36. [Google Scholar] [CrossRef]
- Lu, K.; Wu, B.; Wang, J.; Zhu, W.; Nie, H.; Qian, J.; Huang, W.; Fang, Z. Blocking amino acid transporter OsAAP 3 improves grain yield by promoting outgrowth buds and increasing tiller number in rice. Plant Biotechnol. J. 2018, 16, 1710–1722. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.; Tanaka, T.; Nakamura, H.; Ichikawa, H.; Kobayashi, K.; Yaeno, T.; Yamaoka, N.; Shimomoto, K.; Takayama, K.; Nishina, H.; et al. Overexpression of a rice heme activator protein gene (Os HAP 2E) confers resistance to pathogens, salinity and drought, and increases photosynthesis and tiller number. Plant Biotechnol. J. 2015, 13, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Hilscher, J.; Stoger, E.; Christou, P.; Zhu, C. Modification of cereal plant architecture by genome editing to improve yields. Plant Cell Rep. 2021, 40, 953–978. [Google Scholar] [CrossRef]
- Bai, S.; Smith, S.M.; Li, J. Rice plant architecture: Molecular basis and application in breeding. In Rice Genomics, Genetics and Breeding; Springer: Berlin/Heidelberg, Germany, 2018; pp. 129–154. [Google Scholar] [CrossRef]
- Zhang, Z.; Sun, X.; Ma, X.; Xu, B.; Zhao, Y.; Ma, Z.; Li, G.; Khan, N.U.; Pan, Y.; Liang, Y.; et al. GNP6, a novel allele of MOC1, regulates panicle and tiller development in rice. Crop J. 2021, 9, 57–67. [Google Scholar] [CrossRef]
- Duan, E.; Wang, Y.; Li, X.; Lin, Q.; Zhang, T.; Wang, Y.; Zhou, C.; Zhang, H.; Jiang, L.; Wang, J.; et al. OsSHI1 regulates plant architecture through modulating the transcriptional activity of IPA1 in rice. Plant Cell 2019, 31, 1026–1042. [Google Scholar] [CrossRef] [Green Version]
- Lu, H.; Dai, Z.; Li, L.; Wang, J.; Miao, X.; Shi, Z. OsRAMOSA2 shapes panicle architecture through regulating pedicel length. Front. Plant Sci. 2017, 8, 1538. [Google Scholar] [CrossRef]
- Ding, F.; Wang, M.; Zhang, S.; Ai, X. Changes in SBPase activity influence photosynthetic capacity, growth, and tolerance to chilling stress in transgenic tomato plants. Sci. Rep. 2016, 6, 32741. [Google Scholar] [CrossRef]
- Makino, A. Photosynthesis, grain yield, and nitrogen utilization in rice and wheat. Plant physiol. 2011, 155, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Salesse-Smith, C.E.; Sharwood, R.E.; Busch, F.A.; Kromdijk, J.; Bardal, V.; Stern, D.B. Overexpression of Rubisco subunits with RAF1 increases Rubisco content in maize. Nat. Plants 2018, 4, 802–810. [Google Scholar] [CrossRef] [PubMed]
- Ermakova, M.; Heyno, E.; Woodford, R.; Massey, B.; Birke, H.; von-Caemmerer, S. Enhanced abundance and activity of the chloroplast ATP synthase in rice through the overexpression of the AtpD subunit. J. Exp. Bot. 2022, 73, 6891–6901. [Google Scholar] [CrossRef]
- Driever, S.M.; Simkin, A.J.; Alotaibi, S.; Fisk, S.J.; Madgwick, P.J.; Sparks, C.A.; Jones, H.D.; Lawson, T.; Parry, M.A.J.; Raines, C.A. Increased SBPase activity improves photosynthesis and grain yield in wheat grown in greenhouse conditions. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2017, 372, 20160384. [Google Scholar] [CrossRef]
- Simkin, A.J.; McAusland, L.; Headland, L.R.; Lawson, T.; Raines, C.A. Multigene manipulation of photosynthetic carbon assimilation increases CO2 fixation and biomass yield in tobacco. J. Exp. Bot. 2015, 66, 4075–4090. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Li, N.; Huang, R.; Chen, C.; Guo, J.; Yang, X.; Zhang, X.; Sun, C.; Deng, X.; Wang, P.A. single nucleotide substitution at the 3′-end of SBPase gene involved in Calvin cycle severely affects plant growth and grain yield in rice. BMC Plant Biol. 2020, 20, 345. [Google Scholar] [CrossRef]
- Kale, R.S.; Sallans, L.; Frankel, L.K.; Bricker, T.M. High-Resolution Tandem Mass Spectrometry Indicates Rubisco Activase is Associated with PS I-LHC I-LHC II Membranes. bioRxiv 2022. [Google Scholar] [CrossRef]
- Yamori, W.; Masumoto, C.; Fukayama, H.; Makino, A. Rubisco activase is a key regulator of non-steady-state photosynthesis at any leaf temperature and, to a lesser extent, of steady-state photosynthesis at high temperature. Plant J. 2012, 71, 871–880. [Google Scholar] [CrossRef]
- Masumoto, C.; Fukayama, H.; Hatanaka, T.; Uchida, N. Photosynthetic characteristics of antisense transgenic rice expressing reduced levels of Rubisco activase. Plant Prod. Sci. 2012, 15, 174–182. [Google Scholar] [CrossRef]
- Suganami, M.; Suzuki, Y.; Tazoe, Y.; Yamori, W.; Makino, A. Co-overproducing Rubisco and Rubisco activase enhances photosynthesis in the optimal temperature range in rice. Plant Physiol. 2021, 185, 108–119. [Google Scholar] [CrossRef]
- Zhang, C.; Zhong, X.; Lin, D.; Wu, K.; Wu, Z.; Zhang, Z.; Peng, X. Grain Quality Affected by Introducing Photorespiratory Bypasses into Rice. Agronomy 2022, 12, 566. [Google Scholar] [CrossRef]
- Wei, S.; Li, X.; Lu, Z.; Zhang, H.; Ye, X.; Zhou, Y.; Li, J.; Yan, Y.; Pei, H.; Duan, F.; et al. A transcriptional regulator that boosts grain yields and shortens the growth duration of rice. Science 2022, 377, eabi8455. [Google Scholar] [CrossRef]
- Price, G.D.; Pengelly, J.J.; Forster, B.; Du, J.; Whitney, S.M.; von-Caemmerer, S.; Badger, M.R.; Howitt, S.M.; Evans, J.R. The cyanobacterial CCM as a source of genes for improving photosynthetic CO2 fixation in crop species. J. Exp. Bot. 2013, 64, 753–768. [Google Scholar] [CrossRef] [PubMed]
- Price, G.D.; Howitt, S.M. Towards turbocharged photosynthesis. Nature 2014, 513, 497–498. [Google Scholar] [CrossRef]
- Yin, X.; Struik, P.C. Can increased leaf photosynthesis be converted into higher crop mass production? A simulation study for rice using the crop model GECROS. J. Exp. Bot. 2017, 68, 2345–2360. [Google Scholar] [CrossRef]
- Orr, D.J.; Worrall, D.; Lin, M.T.; Carmo-Silva, E.; Hanson, M.R.; Parry, M.A. Hybrid cyanobacterial-tobacco rubisco supports autotrophic growth and procarboxysomal aggregation. Plant Physiol. 2020, 182, 807–818. [Google Scholar] [CrossRef]
- Pengelly, J.J.; Förster, B.; von-Caemmerer, S.; Badger, M.R.; Price, G.D.; Whitney, S.M. Transplastomic integration of a cyanobacterial bicarbonate transporter into tobacco chloroplasts. J. Exp. Bot. 2014, 65, 3071–3080. [Google Scholar] [CrossRef]
- Bellasio, C.; Burgess, S.J.; Griffiths, H.; Hibberd, J.M. A high throughput gas exchange screen for determining rates of photorespiration or regulation of C4 activity. J. Exp. Bot. 2014, 65, 3769–3779. [Google Scholar] [CrossRef] [Green Version]
- Timm, S.; Florian, A.; Wittmiß, M.; Jahnke, K.; Hagemann, M.; Fernie, A.R.; Bauwe, H. Serine acts as a metabolic signal for the transcriptional control of photorespiration-related genes in Arabidopsis. Plant Physiol. 2013, 162, 379–389. [Google Scholar] [CrossRef] [PubMed]
- Aliyev, J.A. Photosynthesis, photorespiration and productivity of wheat and soybean genotypes. Physiol. Plant. 2012, 145, 369–383. [Google Scholar] [CrossRef]
- Chakraborty, K.; Swain, P.; Baig, M.J.; Kumar, A.; Basak, N.; Hanjagi, P.S.; Awaji, S. Physiological and Biochemical Perspectives of Rice Activities, Achievements and Aspirations; ICAR-NRRI: Cuttack, India, 2019; pp. 139–173. [Google Scholar]
- Shim, S.-H.; Lee, S.-K.; Lee, D.-W.; Brilhaus, D.; Wu, G.; Ko, S.; Lee, C.-H.; Weber, A.P.; Jeon, J.-S. Loss of function of rice plastidicglycolate/glycerate translocator 1 impairs photorespiration and plant growth. Front. Plant Sci. 2020, 10, 1726. [Google Scholar] [CrossRef] [PubMed]
- Peterhansel, C.; Krause, K.; Braun, H.P.; Espie, G.S.; Fernie, A.R.; Hanson, D.T.; Keech, O.; Maurino, V.G.; Mielewczik, M.; Sage, R.F. Engineering photorespiration: Current state and future possibilities. Plant Biol. 2013, 15, 754–758. [Google Scholar] [CrossRef] [PubMed]
- Peterhansel, C.; Blume, C.; Offermann, S. Photorespiratory bypasses: How can they work? J. Exp. Bot. 2013, 64, 709–715. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Karki, S.; Coe, R.A.; Bagha, S.; Khoshravesh, R.; Balahadia, C.P.; Sagun, J.V.; Tapia, R.; Israel, W.K.; Montecillo, F.; et al. Targeted knockdown of GDCH in rice leads to a photorespiratory-deficient phenotype useful as a building block for C4 rice. Plant Cell Physiol. 2016, 57, 919–932. [Google Scholar] [CrossRef]
- Driever, S.M.; Lawson, T.; Andralojc, P.J.; Raines, C.A.; Parry, M.A. Natural variation in photosynthetic capacity, growth, and yield in 64 field-grown wheat genotypes. J. Exp. Bot. 2014, 65, 4959–4973. [Google Scholar] [CrossRef]
- Betti, M.; Bauwe, H.; Busch, F.A.; Fernie, A.R.; Keech, O.; Levey, M.; Ort, D.R.; Parry, M.A.; Sage, R.; Timm, S.; et al. Manipulating photorespiration to increase plant productivity: Recent advances and perspectives for crop improvement. J. Exp. Bot. 2016, 67, 2977–2988. [Google Scholar] [CrossRef]
- Hagemann, M.; Bauwe, H. Photorespiration and the potential to improve photosynthesis. Curr. Opin. Chem. Biol. 2016, 35, 109–116. [Google Scholar] [CrossRef]
- Aleku, G.A.; Roberts, G.W.; Titchiner, G.R.; Leys, D. Synthetic Enzyme-Catalyzed CO2 Fixation Reactions. ChemSusChem 2021, 14, 1781–1804. [Google Scholar] [CrossRef]
- South, P.F.; Cavanagh, A.P.; Lopez-Calcagno, P.E.; Raines, C.A.; Ort, D.R. Optimizing photorespiration for improved crop productivity. J. Integr. Plant Biol. 2018, 60, 1217–1230. [Google Scholar] [CrossRef]
- Sage, R.F.; Khoshravesh, R. Passive CO2 concentration in higher plants. Curr. Opin. Plant Biol. 2016, 31, 58–65. [Google Scholar] [CrossRef]
- Haworth, M.; Marino, G.; Loreto, F.; Centritto, M. The evolution of diffusive and biochemical capacities for photosynthesis was predominantly shaped by [CO2] with a smaller contribution from [O2]. Sci. Total Environ. 2022, 840, 156606. [Google Scholar] [CrossRef]
- Pärnik, T.; Keerberg, O. Advanced radiogasometric method for the determination of the rates of photorespiratory and respiratory decarboxylations of primary and stored photosynthates under steady-state photosynthesis. Physiol. Plant. 2007, 129, 34–44. [Google Scholar] [CrossRef]
- Sage, T.L.; Sage, R.F. The functional anatomy of rice leaves: Implications for refixation of photorespiratory CO2 and efforts to engineer C4 photosynthesis into rice. Plant Cell Physiol. 2009, 50, 756–772. [Google Scholar] [CrossRef] [PubMed]
- Hatakeyama, Y.; Ueno, O. Intracellular position of mitochondria and chloroplasts in bundle sheath and mesophyll cells of C3 grasses in relation to photorespiratory CO2 loss. Plant Prod. Sci. 2016, 19, 540–551. [Google Scholar] [CrossRef]
- Bykova, N.V.; Møller, I.M.; Gardeström, P.; Igamberdiev, A.U. The function of glycine decarboxylase complex is optimized to maintain high photorespiratory flux via buffering of its reaction products. Mitochondrion 2014, 19, 357–364. [Google Scholar] [CrossRef]
- Sunil, B.; Saini, D.; Bapatla, R.B.; Aswani, V.; Raghavendra, A.S. Photorespiration is complemented by cyclic electron flow and the alternative oxidase pathway to optimize photosynthesis and protect against abiotic stress. Photosynth. Res. 2019, 139, 67–79. [Google Scholar] [CrossRef]
- Zhang, Z.; Liang, X.; Lu, L.; Xu, Z.; Huang, J.; He, H.; Peng, X. Two glyoxylate reductase isoforms are functionally redundant but required under high photorespiration conditions in rice. BMC Plant Biol. 2020, 20, 357. [Google Scholar] [CrossRef]
- Umnajkitikorn, K.; Sade, N.; Rubio-Wilhelmi, M.D.M.; Gilbert, M.E.; Blumwald, E. Silencing of OsCV (chloroplast vesiculation) maintained photorespiration and N assimilation in rice plants grown under elevated CO2. Plant Cell Environ. 2020, 43, 920–933. [Google Scholar] [CrossRef]
- Shen, B.-R.; Wang, L.; Lin, X.-L.; Yao, Z.; Xu, H.-W.; Zhu, C.-H.; Teng, H.-Y.; Cui, L.-L.; Liu, E.-E.; Zhang, J.-J.; et al. Engineering a new chloroplastic photorespiratory bypass to increase photosynthetic efficiency and productivity in rice. Mol. Plant 2019, 12, 199–214. [Google Scholar] [CrossRef]
- Wang, Y.; Pang, Y.; Chen, K.; Zhai, L.; Shen, C.; Wang, S.; Xu, J. Genetic bases of source-, sink-, and yield-related traits revealed by genome-wide association study in Xian rice. Crop J. 2020, 8, 119–131. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, J.; Zhai, L.; Liang, C.; Chen, K.; Xu, J. Identify QTLs and candidate genes underlying source-, sink-, and grain yield-related traits in rice by integrated analysis of bi-parental and natural populations. PLoS ONE 2020, 15, e0237774. [Google Scholar] [CrossRef]
- Rangan, P.; Wankhede, D.; Subramani, R.; Chinnusamy, V.; Pathania, P.; Bartwal, A.; Singh, K. Upregulation of Chloroplastic Pyruvate Dehydrogenase Genes in Rice Leaf Would Potentially Drive the in Planta Photorespiratory Bypass for Higher Biomass. Research Square Preprint. 2021. Available online: https://www.researchsquare.com/article/rs-562750/v1 (accessed on 1 December 2022). [CrossRef]
- Muthusamy, S.K.; Lenka, S.K.; Katiyar, A.; Chinnusamy, V.; Singh, A.K.; Bansal, K.C. Genome-Wide Identification and Analysis of Biotic and Abiotic Stress Regulation of C4 Photosynthetic Pathway Genes in Rice. Appl. Biochem. Biotechnol. 2019, 187, 221–238. [Google Scholar] [CrossRef] [PubMed]
- Giuliani, R.; Karki, S.; Covshoff, S.; Lin, H.C.; Coe, R.A.; Koteyeva, N.K.; Evans, M.A.; Quick, W.P.; von Caemmerer, S.; Furbank, R.T.; et al. Transgenic maize phosphoenolpyruvate carboxylase alters leaf–atmosphere CO2 and 13CO2 exchanges in Oryza sativa. Photosynth. Res. 2019, 142, 153–167. [Google Scholar] [CrossRef] [PubMed]
- Lowder, L.G.; Zhou, J.; Zhang, Y.; Malzahn, A.; Zhong, Z.; Hsieh, T.-F.; Voytas, D.F.; Zhang, Y.; Qi, Y. Robust transcriptional activation in plants using multiplexed CRISPR-Act2.0 and mTALE-Act systems. Mol. Plant 2018, 11, 245–256. [Google Scholar] [CrossRef]
- Wang, S.; Tholen, D.; Zh, X.G. C4 photosynthesis in C3 rice: A theoretical analysis of biochemical and anatomical factors. Plant Cell Environ. 2017, 40, 80–94. [Google Scholar] [CrossRef]
- Zhao, H.; Wang, Y.; Lyu, M.J.A.; Zhu, X.G. Two major metabolic factors for an efficient NADP-malic enzyme type C4 photosynthesis. Plant Physiol. 2022, 189, 84–98. [Google Scholar] [CrossRef]
- Behera, D.; Swain, A.; Karmakar, S.; Dash, M.; Swain, P.; Baig, M.J.; Molla, K.A. Overexpression of Setaria italica phosphoenolpyruvate carboxylase gene in rice positively impacts photosynthesis and agronomic traits. Plant Physiol. Biochem. 2023, 194, 169–181. [Google Scholar] [CrossRef]
- Lin, H.; Arrivault, S.; Coe, R.A.; Karki, S.; Covshoff, S.; Bagunu, E.; Lunn, J.E.; Stitt, M.; Furbank, R.T.; Hibberd, J.M.; et al. A partial C4 photosynthetic biochemical pathway in rice. Front. Plant Sci. 2020, 11, 564463. [Google Scholar] [CrossRef]
- Ermakova, M.; Arrivault, S.; Giuliani, R.; Danila, F.; Alonso-Cantabrana, H.; Vlad, D.; Ishihara, H.; Feil, R.; Guenther, M.; Borghi, G.L.; et al. Installation of C4 photosynthetic pathway enzymes in rice using a single construct. Plant Biotechnol. J. 2021, 19, 575–588. [Google Scholar] [CrossRef]
- Wang, L.-M.; Shen, B.-R.; Li, B.-D.; Zhang, C.-L.; Lin, M.; Tong, P.-P.; Cui, L.-L.; Zhang, Z.-S.; Peng, X.-X. A synthetic photorespiratory shortcut enhances photosynthesis to boost biomass and grain yield in rice. Mol. Plant 2020, 13, 1802–1815. [Google Scholar] [CrossRef]
- Yagioka, A.; Hayashi, S.; Kimiwada, K.; Kondo, M. Sink production and grain-filling ability of a new high-yielding rice variety, Kitagenki. Field Crop. Res. 2021, 260, 107991. [Google Scholar] [CrossRef]
- Wei, H.; Zhang, H.; Blumwald, E.; Li, H.; Cheng, J.; Dai, Q.; Huo, Z.; Xu, K.; Guo, B. Different characteristics of high yield formation between inbred japonica super rice and inter-sub-specific hybrid super rice. Field Crop. Res. 2016, 198, 179–187. [Google Scholar] [CrossRef]
- Won, P.L.; Kanno, N.; Banayo, N.P.; Bueno, C.S.; Cruz, P.S.; Kato, Y. Source–sink relationships in short-duration and hybrid rice cultivars in tropical Asia. Field Crop. Res. 2022, 282, 108485. [Google Scholar] [CrossRef]
- Shrestha, S.; Asch, F.; Dusserre, J.; Ramanantsoanirina, A.; Brueck, H. Climate effects on yield components as affected by genotypic responses to variable environmental conditions in upland rice systems at different altitudes. Field Crop. Res. 2012, 134, 216–228. [Google Scholar] [CrossRef]
- Fujita, D.; Trijatmiko, K.R.; Tagle, A.G.; Sapasap, M.V.; Koide, Y.; Sasaki, K.; Tsakirpaloglou, N.; Gannaban, R.B.; Nishimura, T.; Yanagihara, S.; et al. NAL1 allele from a rice landrace greatly increases yield in modern indica cultivars. Proc. Natl. Acad. Sci. USA 2013, 110, 20431–20436. [Google Scholar] [CrossRef]
- Takai, T.; Adachi, S.; Taguchi-Shiobara, F.; Sanoh-Arai, Y.; Iwasawa, N.; Yoshinaga, S.; Hirose, S.; Taniguchi, Y.; Yamanouchi, U.; Wu, J.; et al. A natural variant of NAL1, selected in high-yield rice breeding programs, pleiotropically increases photosynthesis rate. Sci. Rep. 2013, 3, 2149. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.; Lee, S.; Kim, T.H.; Lee, J.H.; Park, J.; Lee, J.; Lee, J.Y.; Cho, L.H.; Choi, J.Y.; Lee, W.; et al. Natural variations at the Stay-Green gene promoter control lifespan and yield in rice cultivars. Nat. Commun. 2020, 11, 2819. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, Y.; Mi, X.F.; Shan, J.X.; Li, X.M.; Xu, J.L.; Lin, H.X. The QTL GNP1 encodes GA20ox1, which increases grain number and yield by increasing cytokinin activity in rice panicle meristems. PLoS Genet. 2016, 12, e1006386. [Google Scholar] [CrossRef]
- Tu, B.; Tao, Z.; Wang, S.; Zhou, L.; Zheng, L.; Zhang, C.; Li, X.; Zhang, X.; Yin, J.; Zhu, X.; et al. Loss of Gn1a/OsCKX2 confers heavy-panicle rice with excellent lodging resistance. J. Integr. Plant Biol. 2022, 64, 23–38. [Google Scholar] [CrossRef]
- Li, S.; Zhao, B.; Yuan, D.; Duan, M.; Qian, Q.; Tang, L.; Wang, B.; Liu, X.; Zhang, J.; Wang, J.; et al. Rice zinc finger protein DST enhances grain production through controlling Gn1a/OsCKX2 expression. Proc. Natl. Acad. Sci. USA 2013, 110, 3167–3172. [Google Scholar] [CrossRef]
- Lin, L.; Du, M.; Li, S.; Sun, C.; Wu, F.; Deng, L.; Chen, Q.; Li, C. Mediator complex subunit MED25 physically interacts with DST to regulate spikelet number in rice. J. Integr. Plant Biol. 2022, 64, 871–883. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.-H.; Li, S.Y.; Wang, L.; Ye, W.J.; Zeng, D.L.; Rao, Y.-C.; Peng, Y.-L.; Hu, J.; Yang, Y.-L.; Xu, J.; et al. LSCHL4 from japonica cultivar, which is allelic to NAL1, increases yield of indica super rice 93-11. Mol. Plant 2014, 7, 1350–1364. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, X.; Zhong, X.; Chang, S.; Qian, Q.; Zhang, Y.; Zhu, X. Partially functional NARROW LEAF1 balances leaf photosynthesis and plant architecture for greater rice yield. Plant Physiol. 2022, 189, 772–789. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Shi, Y.; Zhang, X.; Xu, X.; Wu, J.L. Characterization of a novel rice dynamic Narrow-Rolled Leaf mutant with deficiencies in aromatic amino acids. Int. J. Mol. Sci. 2020, 21, 1521. [Google Scholar] [CrossRef]
- Wang, F.; Tang, Z.; Wang, Y.; Fu, J.; Yang, W.; Wang, S.; Wang, Y.; Bai, T.; Huang, Z.; Yin, H.; et al. Leaf Mutant 7 Encoding Heat Shock Protein OsHSP40 Regulates Leaf Size in Rice. Int. J. Mol. Sci. 2022, 23, 4446. [Google Scholar] [CrossRef]
- Guo, T.; Wang, D.; Fang, J.; Zhao, J.; Yuan, S.; Xiao, L.; Li, X. Mutations in the rice OsCHR4 gene, encoding a CHD3 family chromatin remodeler, induce narrow and rolled leaves with increased cuticular wax. Int. J. Mol. Sci. 2019, 20, 2567. [Google Scholar] [CrossRef]
- Qiao, J.; Zhang, Y.; Han, S.; Chang, S.; Gao, Z.; Qi, Y.; Qian, Q. OsARF4 regulates leaf inclination via auxin and brassinosteroid pathways in rice. Front. Plant Sci. 2022, 13, 979033. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, S.; Xu, Y.; Yu, C.; Shen, C.; Qian, Q.; Geisler, M.; Jiang, D.A.; Qi, Y. The auxin response factor, OsARF19, controls rice leaf angles through positively regulating OsGH3-5 and OsBRI1. Plant Cell Environ. 2015, 38, 638–654. [Google Scholar] [CrossRef]
- Li, Y.; Li, J.; Chen, Z.; Wei, Y.; Qi, Y.; Wu, C. OsmiR167a-targeted auxin response factors modulate tiller angle via fine-tuning auxin distribution in rice. Plant Biotechnol. J. 2020, 18, 2015–2026. [Google Scholar] [CrossRef]
- Yang, S.; Fang, G.; Zhang, A.; Ruan, B.; Jiang, H.; Ding, S.; Liu, C.; Zhang, Y.; Jaha, N.; Hu, P.; et al. Rice EARLY SENESCENCE 2, encoding an inositol polyphosphate kinase, is involved in leaf senescence. BMC Plant Biol. 2020, 20, 393. [Google Scholar] [CrossRef]
- Xu, Y.; Sechet, J.; Wu, Y.; Fu, Y.; Zhu, L.; Li, J.; Zhang, Y.; Gineau, E.; Gaertner, C.; Zhou, J.; et al. Rice sucrose partitioning mediated by a putative pectin methyltransferase and homogalacturonan methylesterification. Plant Physiol. 2017, 174, 1595–1608. [Google Scholar] [CrossRef] [PubMed]
- Phan, T.T.T.; Ishibashi, Y.; Miyazaki, M.; Tran, H.T.; Okamura, K.; Tanaka, S.; Nakamura, J.; Yuasa, T.; Iwaya-Inoue, M. High temperature-induced repression of the rice sucrose transporter (OsSUT1) and starch synthesis-related genes in sink and source organs at milky ripening stage causes chalky grains. J. Agron. Crop Sci. 2013, 199, 178–188. [Google Scholar] [CrossRef]
- Hu, Z.; Tang, Z.; Zhang, Y.; Niu, L.; Yang, F.; Zhang, D.; Hu, Y. Rice SUT and SWEET Transporters. Int. J. Mol. Sci. 2021, 22, 11198. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Bao, S.; Tang, Z.; Wang, X.; Yang, F.; Zhang, D.; Hu, Y. Function of sucrose transporter OsSUT5 in rice pollen development and seed setting. Sci. Agric. Sin. 2021, 55, 3369–3380. (In Chinese) [Google Scholar]
- Wu, Y.; Lee, S.K.; Yoo, Y.; Wei, J.; Kwon, S.Y.; Lee, S.W.; Jeon, J.S.; An, G. Rice Transcription Factor OsDOF11 Modulates Sugar Transport by Promoting Expression of Sucrose Transporter and SWEET Genes. Mol. Plant 2018, 11, 833–845. [Google Scholar] [CrossRef]
- Liu, D.; Xu, L.; Wang, W.; Jia, S.; Jin, S.; Gao, J. OsRRM, an RNA-Binding Protein, modulates sugar transport in rice (Oryza sativa L.). Front. Plant Sci. 2020, 11, 605276. [Google Scholar] [CrossRef]
- Jin, S.K.; Zhang, M.Q.; Leng, Y.J.; Xu, L.N.; Jia, S.W.; Wang, S.L.; Song, T.; Wang, R.A.; Yang, Q.Q.; Tao, T.; et al. OsNAC129 regulates seed development and plant growth and participates in the brassinosteroid signaling pathway. Front. Plant Sci. 2022, 13, 905148. [Google Scholar] [CrossRef]
- Xu, R.; Duan, P.; Yu, H.; Zhou, Z.; Zhang, B.; Wang, R.; Li, J.; Zhang, G.; Zhuang, S.; Lyu, J.; et al. Control of grain size and weight by the OsMKKK10-OsMKK4-OsMAPK6 signaling pathway in rice. Mol. Plant 2018, 11, 860–873. [Google Scholar] [CrossRef] [Green Version]
- Guo, T.; Chen, K.; Dong, N.Q.; Shi, C.L.; Ye, W.W.; Gao, J.P.; Shan, J.X.; Lin, H.X. Grain size and number1 negatively regulates the OsMKKK10-OsMKK4-OsMPK6 cascade to coordinate the trade-off between grain NUMBER per panicle and grain size in rice. Plant Cell 2018, 30, 871–888. [Google Scholar] [CrossRef]
- Guo, T.; Lu, Z.Q.; Shan, J.X.; Ye, W.W.; Dong, N.Q.; Lin, H.X. ERECTA1 Acts Upstream of the OsMKKK10-OsMKK4-OsMPK6 Cascade to Control Spikelet Number by Regulating Cytokinin Metabolism in Rice. Plant Cell 2020, 32, 2763–2779. [Google Scholar] [CrossRef]
- Zheng, S.; Ye, C.; Lu, J.; Liufu, J.; Lin, L.; Dong, Z.; Li, J.; Zhuang, C. Improving the rice photosynthetic efficiency and yield by editing OsHXK1 via CRISPR/Cas9 system. Int. J. Mol. Sci. 2021, 22, 9554. [Google Scholar] [CrossRef] [PubMed]
- Yun, P.; Li, Y.; Wu, B.; Zhu, Y.; Wang, K.; Li, P.; Gao, G.; Zhang, Q.; Li, X.; Li, Z.; et al. OsHXK3 encodes a hexokinase-like protein that positively regulates grain size in rice. Theor. Appl. Genet. 2022, 135, 3417–3431. [Google Scholar] [CrossRef] [PubMed]
| Sl. No | Gene | Encoded Protein | Functions and Impact on Biomass/Yield | Reference |
|---|---|---|---|---|
| 1. | SP1 | Peptide transporter family protein. | Positive regulator of panicle elongation. Mutants exhibited a short panicle phenotype in rice. | [56] |
| 2. | TAD1 | Co-activator of APC/C. | Targets MOC1 for degradation. Negatively regulates tillering and panicle number. | [58] |
| 3. | TE | Substrate-recognition and binding factor of APC/C. | Degradation of MOC1. Negatively regulates tillering and branching. | [59] |
| 4. | LAX1 | bHLH transcription factors. | Required for initiation of lateral meristem. | [60] |
| 5. | LAX2/GNP4 | Nuclear protein. | Regulates formation of axillary meristem. Positively regulates number of branches and spikelet. | [61] |
| 6. | DLT | GRAS family transcription factor. | Positively regulates tiller number, panicle length, and seed set. | [62] |
| 7. | SD1 | Gibberellin biosynthesis gene. | Positively regulates plant height. Negatively regulates yield. | [63] |
| 8. | SUB1A | Ethylene response factor. | Limits shoot elongation by modulating GA signaling. | [64] |
| 9. | D1/LW5 | G protein α subunit. | Source–sink balance, plant architecture, grain size. | [65] |
| 10. | D2, D11 | BR biosynthesis, members of cytochrome P450 family. | Promotes plant height, leaf, panicle grain morphology. | [66] |
| 11. | D61 | BR receptor kinase. | Promotes internodes and panicle elongation. | [67] |
| 12. | D3 | F box LRR protein. | Promotes bud dormancy and reduces bud activity. Regulates culm length, grain size. | [68] |
| 13. | D17, IHTD1, D10 | Strigolactone biosynthesis. | Negatively regulates axillary buds, tillering, and panicle size. | [69] |
| 14. | D27 | F-box, leucine-rich repeat (LRR). | Tiller bud outgrowth. | [70] |
| 15. | D14/D88/ HTD2 | Iron-containing, esterase/lipase/thioesterase. | Negatively regulates tiller bud outgrowth. | [71] |
| 16. | D53 | Repressor protein. | Strigolactone signaling. | [72] |
| 17. | EUI | Cytochrome P450. | Deactivates the bioactive gibberellin, GA4, to control plant height. | [73] |
| 18. | MOC1 | GRAS TF (GAI, RGA and SCR). | Positively regulates tillering, panicle number, and yield. | [74] |
| 19. | MOC2 | GRAS TF (GAI, RGA and SCR). | Tiller bud outgrowth. | [75] |
| 20. | MOC3 | GRAS TF (GAI, RGA and SCR). | Axillary bud formation. | [76] |
| 21. | OsCKX2 | Cytokinin oxidase/dehydrogenase. | Promotes root growth. Reduces yield. | [77] |
| 22. | OsAAP3 | Amino acid transporter. | Negatively regulates tiller number. | [78] |
| 23. | OsHAP2E | Heme activator protein. | Increases photosynthesis and tillering. | [79] |
| Gene | Complete Name & Function | Remarks | Reference |
|---|---|---|---|
| Leaf Area | |||
| NAL1/SPIKE | NARROW LEAF1/SPIKELET NUMBER. Involved in polar Auxin Transport (Os04g52479). | Loss of function leads to narrow leaf; the functional japonica NAL1 allele confers larger panicles, leaves, and seed yield; LSCHL4 allele enhances photosynthesis; partially functional NAL1/GREEN FOR PHOTOSYNTHESIS (GPS) balances leaf photosynthesis. | [149,150] |
| TDD1 | TRYPOTOHAN DEFICIENT DWARF 1. Anthranilate synthase beta-subunit, which catalyzes the first step of the Trp biosynthesis pathway. | Loss-of function mutation led to reduced leaf width, increase in leaf angle. | [151] |
| lm7 | Leaf Mutant 7. OsHSP40 (heat shock protein). | Loss of function mutation led to reduced leaf size. | [152] |
| OsCHR4 | A CHD3 family chromatin remodeler. | Loss of function causes narrow and rolled leaves with increased cuticular wax. | [153] |
| Leaf Angle | |||
| OsARF4 | Auxin Response Factor. | OsARF4-overexpressing lines showed erect leaves. | [154] |
| OsARF19 | Auxin response factor binds to the promoter of OsGH3-5 and brassinosteroid insensitive 1 (OsBRI1) directing their expression. | Loss of function causes erect leaves. | [155] |
| OsmiR167a | It targets OsARF12, OsARF17 and OsARF25. | Control rice tiller angle. | [156] |
| Leaf Area Duration | |||
| OsSGR | STAY-GREEN. Chlorophyll-degrading Mg++-dechelatase. | Promoter variation in japonica OsSGR alleles associated with less expression. Indica genotypes introgressed with japonica OsSGR allele led to delayed senescence, enhanced photosynthesis, and, thus, higher grain yield. | [157] |
| NYC1 | NON-YELLOW COLORING 1. Short-chain dehydrogenase/reductase (SDR). | nyc1and nol (nyc1-like) mutant is stay-green and shows delayed leaf senescence. | [157] |
| Photosynthate Partitioning and Assimilation | |||
| OsQUA2 | Pectin methyltransferase. | Osqua2 mutant showed decrease in the methylesterification of Homogalacturonan in the culm-sieve element cell wall, sucrose overaccumulation in the culm, and lower yield. | [158] |
| OsSUT1, OsSUT5 | Sucrose–proton symporter SUT family members. | Mutants are impaired in seed filling and reduced yield. | [159,160,161] |
| OsDOF11 | DNA BINDING WITH ONE FINGER 11. OsDOF11 directly binds the promoter of sugar transporters. | Positive regulator of SUT (OsSUT1, OsSUT3, OsSUT4, and OsSUT5) and SWEET (OsSWEET11 and OsSWEET14) sugar transporter genes. | [162] |
| OsRRM | RNA-Binding Protein. | OsRRM binds directly to messenger RNAs encoded by sugar transporter genes and thus helps stabilize and enhance expression of sugar transporter genes; osrrm mutant is impaired in sugar partitioning, seed filling, and reduced yield. | [163] |
| OsNAC129 | NAM, ATAF1/2, and CUC2 (NAC) TF. | Negative regulator of grain size and starch biosynthesis. | [164] |
| Sink Strength (Grain Number and Size) | |||
| OsMKKK10 | Signaling cascade OsMKKK10-OsMKK4-OsMAPK6. | Loss of function osmkk10 results in small and light grains and short panicles, while constitutively active OsMKKK10 results in large and heavy grains and long panicles. OsMKK4 gain-of-function mutant (large11-1D) produces large and heavy grains. | [165] |
| GSN1 | GRAIN SIZE AND NUMBER 1. Mitogen-activated protein kinase phosphatase OsMKP1, a dual-specificity phosphatase inactivates OsMPK6 via dephosphorylation. | GSN1 is a negative regulator of the OsMKKK10-OsMKK4-OsMPK6 cascade; GSN1 negatively regulates grain size but positively regulates grain number. | [166] |
| OsER1 | OsERECTA1. Negatively regulates spikelet number per panicle | OsER1 acts upstream of the OsMKKK10-OsMKK4-OsMPK6. In er1 mutant, CKX2 was significantly downregulated; OsMPK6 phosphorylates DST which in turn activates the expression of CKX2. | [167] |
| OsDIP1 | DST-interacting protein 1 (DIP1), a Mediator subunit OsMED25, acts as an interacting coactivator of DST. | Similar to dst mutant, osmed25 mutant also exhibited enlarged panicles, with enhanced branching and spikelet number. | [148] |
| OsHKX1-10 | Rice genome encodes 10 hexokinases, which act as sugar sensor except HKX3; regulate photosynthetic gene expression. | hkx1 mutants exhibited enhanced photosynthesis and grain yield; hkx3 exhibited lower grain size, and overexpression increased grain yield in rice. | [168,169] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vishwakarma, C.; Krishna, G.K.; Kapoor, R.T.; Mathur, K.; Lal, S.K.; Saini, R.P.; Yadava, P.; Chinnusamy, V. Bioengineering of Canopy Photosynthesis in Rice for Securing Global Food Security: A Critical Review. Agronomy 2023, 13, 489. https://doi.org/10.3390/agronomy13020489
Vishwakarma C, Krishna GK, Kapoor RT, Mathur K, Lal SK, Saini RP, Yadava P, Chinnusamy V. Bioengineering of Canopy Photosynthesis in Rice for Securing Global Food Security: A Critical Review. Agronomy. 2023; 13(2):489. https://doi.org/10.3390/agronomy13020489
Chicago/Turabian StyleVishwakarma, Chandrapal, Gopinathan Kumar Krishna, Riti Thapar Kapoor, Komal Mathur, Shambhu Krishan Lal, Ravi Prakash Saini, Pranjal Yadava, and Viswanathan Chinnusamy. 2023. "Bioengineering of Canopy Photosynthesis in Rice for Securing Global Food Security: A Critical Review" Agronomy 13, no. 2: 489. https://doi.org/10.3390/agronomy13020489
APA StyleVishwakarma, C., Krishna, G. K., Kapoor, R. T., Mathur, K., Lal, S. K., Saini, R. P., Yadava, P., & Chinnusamy, V. (2023). Bioengineering of Canopy Photosynthesis in Rice for Securing Global Food Security: A Critical Review. Agronomy, 13(2), 489. https://doi.org/10.3390/agronomy13020489







