Agro-Industrial Compost in Soilless Cultivation Modulates the Vitamin C Content and Phytochemical Markers of Plant Stress in Rocket Salad (Diplotaxis tenuifolia (L.) DC.)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Growing Conditions
2.2. Growth Analysis
2.3. Phytochemical Analyses
2.3.1. Glucosinolates and (Poly)phenols Extraction
2.3.2. HPLC-DAD-ESI–MSn Qualitative and Quantitative Analysis of Glucosinolates and Phenolic Compounds
2.3.3. Vitamin C and Antioxidants Content
2.4. Statistical Analysis
3. Results and Discussion
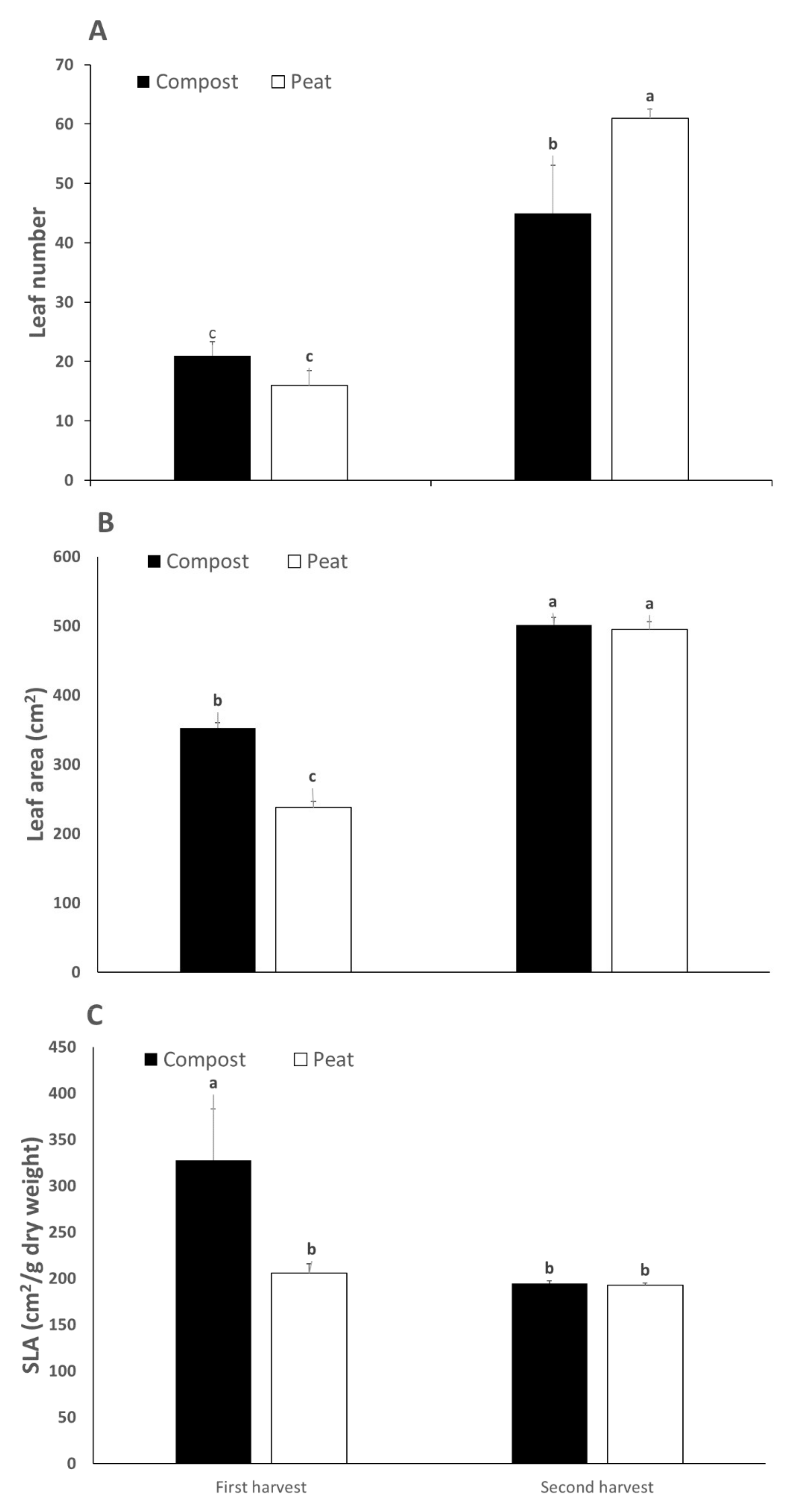
3.1. Yield and Growth Parameters
3.2. Quantitative Phytochemical Profile of Rocket Salad
3.2.1. Glucosinolates and (Poly)phenolic Profile of Rocket Salad Leaf
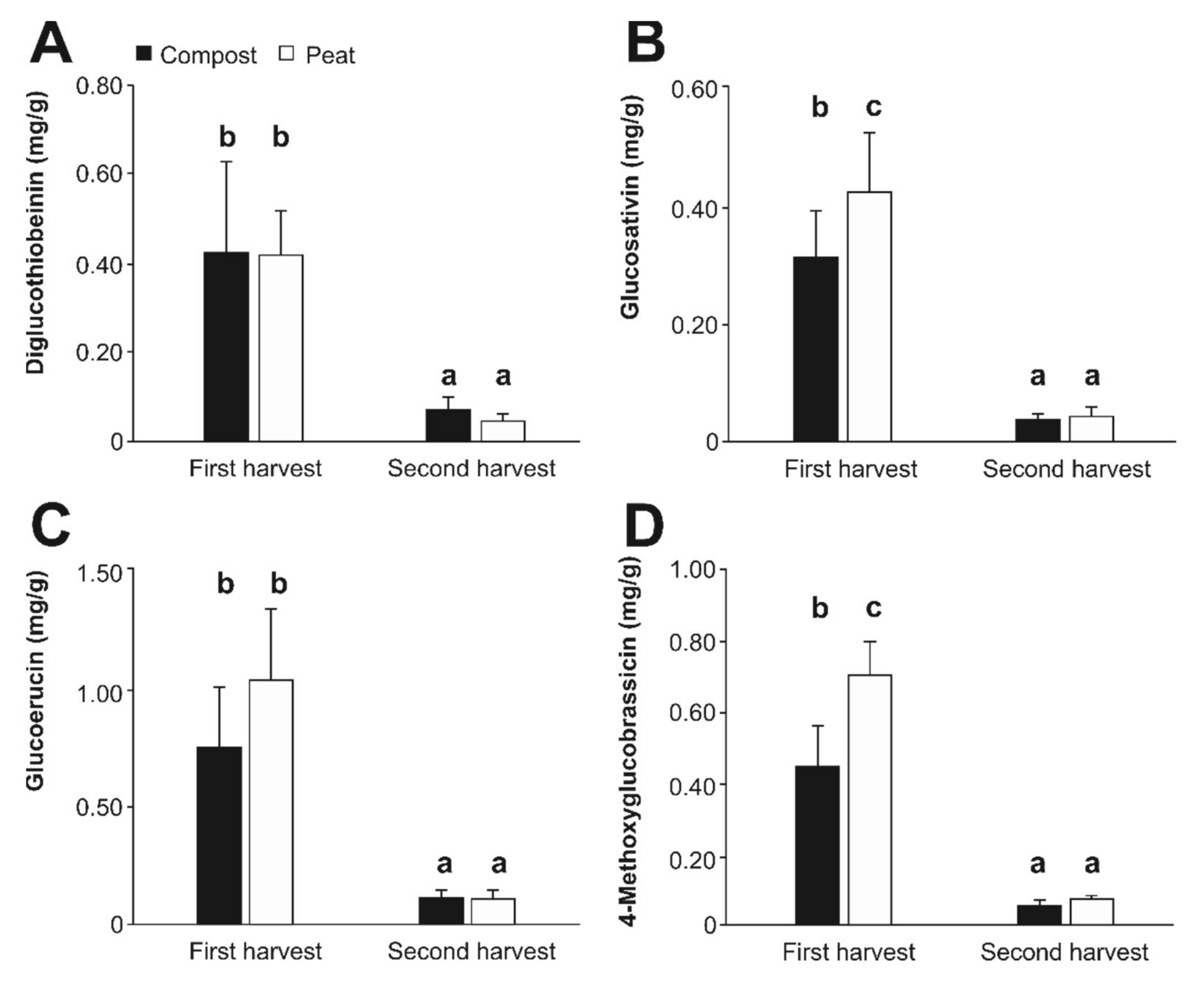
3.2.2. Glucosinolate Content
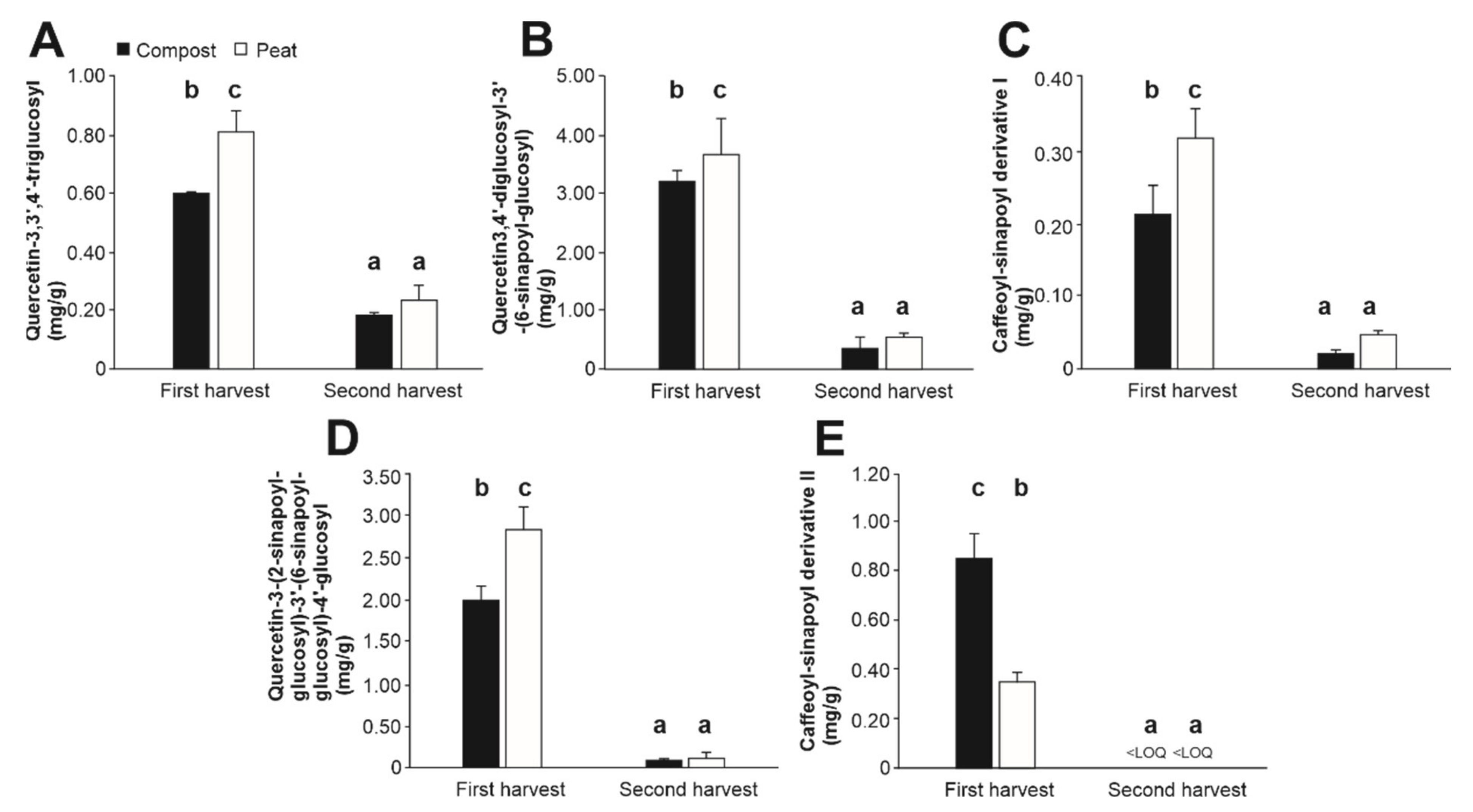
3.2.3. (Poly)phenolic Content
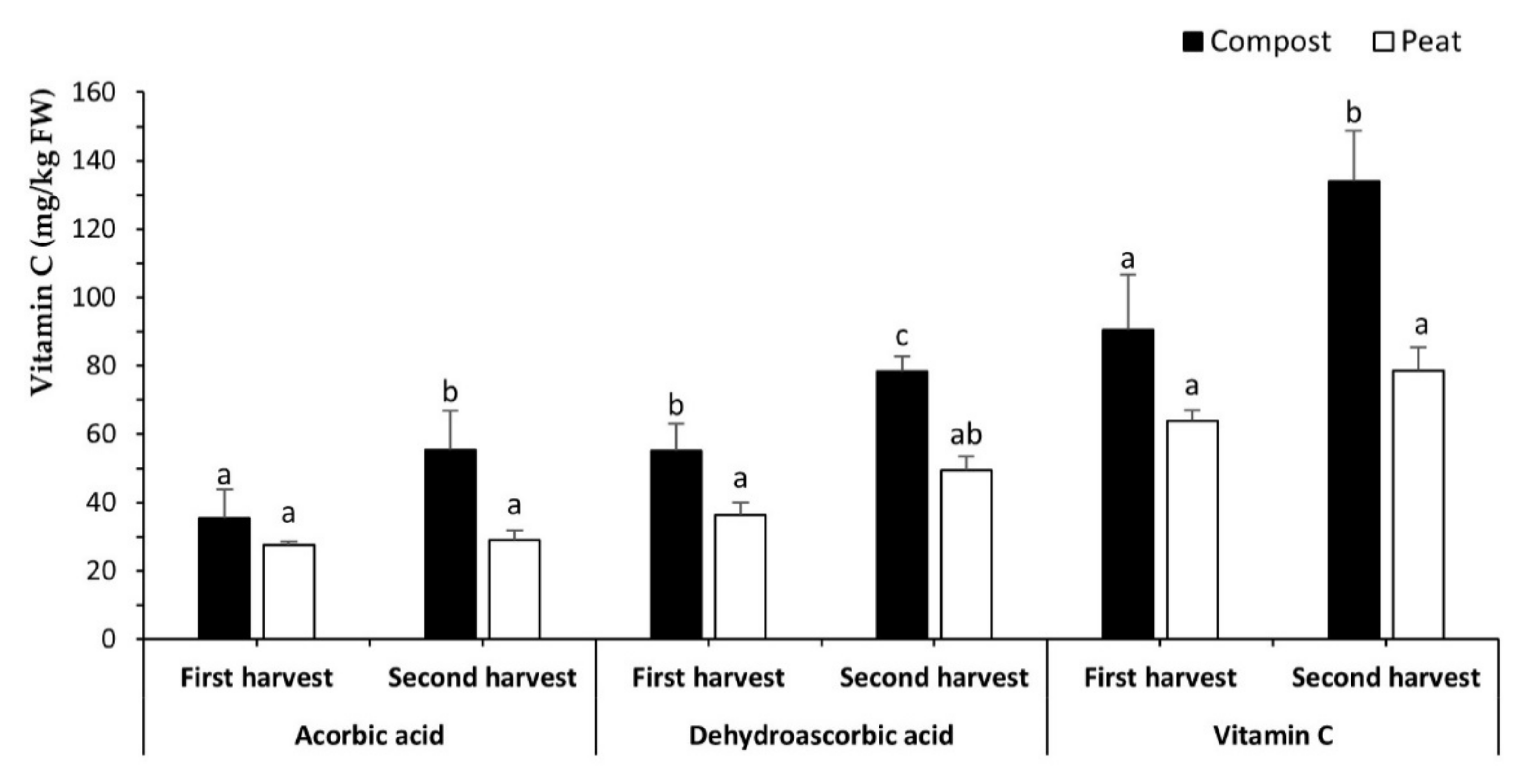
3.3. Vitamin C Content
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kris-Etherton, P.M.; Hecker, K.D.; Bonanome, A.; Coval, S.M.; Binkoski, A.E.; Hilpert, K.F.; Griel, A.E.; Etherton, T.D. Bioactive compounds in foods: Their role in the prevention of cardiovascular disease and cancer. Am. J. Med. 2002, 113, 71–88. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Ispizua, E.; Calatayud, Á.; Marsal, J.I.; Cannata, C.; Basile, F.; Abdelkhalik, A.; Soler, S.; Valcárcel, J.V.; Martínez-Cuenca, M.R. The Nutritional Quality Potential of Microgreens, Baby Leaves, and Adult Lettuce: An Underexploited Nutraceutical Source. Foods 2022, 11, 423. [Google Scholar] [CrossRef] [PubMed]
- Signore, A.; Renna, M.; Santamaria, P. Agrobiodiversity of Vegetable Crops: Aspect, Needs, and Future Perspectives. Annu. Plant Rev. Online 2019, 2, 41–64. [Google Scholar] [CrossRef]
- European Commission. Commission Regulation (EU) No 752/2014. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/HTML/?uri=CELEX:32014R0752&from=EN (accessed on 15 July 2022).
- Verbeek, M.; Hardeweg, B. From consumer to prosumer: Are small-scale home indoor farms economically viable? Eur. J. Hortic. Sci. 2022, 87, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Department of Economic and Social Affairs. World Urbanization Prospects: The 2018 Revision; Department of Economic and Social Affairs: New York, NY, USA, 2018.
- Tietjen, S.; Graubner, I.; Sradnick, A. Reducing peat in substrate mixture formulations for press pots using the Taguchi method. Sci. Hortic. 2022, 295, 110838. [Google Scholar] [CrossRef]
- Blok, C.; Eveleens, B.; van Winkel, A. Growing media for food and quality of life in the period 2020–2050. Acta Hortic. 2021, 1305, 341–355. [Google Scholar] [CrossRef]
- Atzori, G.; Pane, C.; Zaccardelli, M.; Cacini, S.; Massa, D. The role of peat-free organic substrates in the sustainable management of soilless cultivations. Agronomy 2021, 11, 1236. [Google Scholar] [CrossRef]
- Kern, J.; Tammeorg, P.; Shanskiy, M.; Sakrabani, R.; Knicker, H.; Kammann, C.; Tuhkanen, E.-M.; Smidt, G.; Prasad, M.; Tiilikkala, K.; et al. Synergistic use of peat and charred material in growing media—An option to reduce the pressure on peatlands? J. Environ. Eng. Landsc. Manag. 2017, 25, 160–174. [Google Scholar] [CrossRef]
- Ceglie, F.G.; Bustamante, M.A.; Ben Amara, M.; Tittarelli, F. The Challenge of Peat Substitution in Organic Seedling Production: Optimization of Growing Media Formulation through Mixture Design and Response Surface Analysis. PLoS ONE 2015, 10, e0128600. [Google Scholar] [CrossRef]
- Parada, F.; Ercilla-Montserrat, M.; Arcas-Pilz, V.; Lopez-Capel, E.; Carazo, N.; Montero, J.I.; Gabarrell, X.; Villalba, G.; Rieradevall, J.; Muñoz, P. Comparison of organic substrates in urban rooftop agriculture, towards improving crop production resilience to temporary drought in Mediterranean cities. J. Sci. Food Agric. 2021, 101, 5888–5897. [Google Scholar] [CrossRef]
- Zawadzińska, A.; Salachna, P.; Nowak, J.S.; Kowalczyk, W.; Piechocki, R.; Łopusiewicz, Ł.; Pietrak, A. Compost based on pulp and paper mill sludge, fruit-vegetable waste, mushroom spent substrate and rye straw improves yield and nutritional value of tomato. Agronomy 2022, 12, 13. [Google Scholar] [CrossRef]
- Ober Allen, J.; Alaimo, K.; Elam, D.; Perry, E. Growing Vegetables and Values: Benefits of Neighborhood-Based Community Gardens for Youth Development and Nutrition. J. Hunger Environ. Nutr. 2008, 3, 418–439. [Google Scholar] [CrossRef]
- Daneshvar, H.; Babalar, M.; Díaz-Pérez, J.C.; Nambeesan, S.; Delshad, M.; Tabrizi, L. Evaluation of organic and mineral fertilizers on plant growth, minerals, and postharvest quality of celery (Apium graveolens L.). J. Plant Nutr. 2022, 1–18. [Google Scholar] [CrossRef]
- Pant, A.; Radovich, T.J.K.; Hue, N.V.; Arancon, N.Q. Effects of Vermicompost Tea (Aqueous Extract) on Pak Choi Yield, Quality, and on Soil Biological Properties. Compost. Sci. Util. 2011, 19, 279–292. [Google Scholar] [CrossRef]
- Neugart, S.; Wiesner-Reinhold, M.; Frede, K.; Jander, E.; Homann, T.; Rawel, H.M.; Schreiner, M.; Baldermann, S. Effect of Solid Biological Waste Compost on the Metabolite Profile of Brassica rapa ssp. chinensis. Front. Plant Sci. 2018, 9, 305. [Google Scholar] [CrossRef]
- Pascual, J.A.; Ceglie, F.; Tuzel, Y.; Koller, M.; Koren, A.; Hitchings, R.; Tittarelli, F. Organic substrate for transplant production in organic nurseries. A review. Agron. Sustain. Dev. 2018, 38, 35. [Google Scholar] [CrossRef]
- Giménez, A.; Fernández, J.A.; Pascual, J.A.; Ros, M.; Saez-Tovar, J.; Martinez-Sabater, E.; Gruda, N.S.; Egea-Gilabert, C. Promising composts as growing media for the production of baby leaf lettuce in a floating system. Agronomy 2020, 10, 1540. [Google Scholar] [CrossRef]
- Giménez, A.; Fernández, J.A.; Pascual, J.A.; Ros, M.; López-Serrano, M.; Egea-Gilabert, C. An agroindustrial compost as alternative to peat for production of baby leaf red lettuce in a floating system. Sci. Hortic. 2019, 246, 907–915. [Google Scholar] [CrossRef]
- Dominguez-Perles, R.; Martinez-Ballesta, M.C.; Riquelme, F.; Carvajal, M.; Garcia-Viguera, C.; Moreno, D.A. Novel varieties of broccoli for optimal bioactive components under saline stress. J. Sci. Food Agric. 2011, 91, 1638–1647. [Google Scholar] [CrossRef]
- Miean, K.H.; Mohamed, S. Flavonoid (Myricetin, Quercetin, Kaempferol, Luteolin, and Apigenin) Content of Edible Tropical Plants. J. Agric. Food Chem. 2001, 49, 3106–3112. [Google Scholar] [CrossRef]
- Valdés, R.; Miralles, J.; Ochoa, J.; Sánchez-Blanco, M.J.; Bañón Arias, S. Saline reclaimed wastewater can be used to produce potted weeping fig (Ficus benjamina L.) with minimal effects on plant quality. Spanish J. Agric. Res. 2012, 10, 1167. [Google Scholar] [CrossRef]
- Baenas, N.; Villaño, D.; García-Viguera, C.; Moreno, D.A. Optimizing elicitation and seed priming to enrich broccoli and radish sprouts in glucosinolates. Food Chem. 2016, 204, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Abellán, Á.; Domínguez-Perles, R.; García-Viguera, C.; Moreno, D.A. Evidence on the Bioaccessibility of Glucosinolates and Breakdown Products of Cruciferous Sprouts by Simulated In Vitro Gastrointestinal Digestion. Int. J. Mol. Sci. 2021, 22, 11046. [Google Scholar] [CrossRef] [PubMed]
- Zapata, S.; Dufour, J.-P. Ascorbic, Dehydroascorbic and Isoascorbic Acid Simultaneous Determinations by Reverse Phase Ion Interaction HPLC. J. Food Sci. 1992, 57, 506–511. [Google Scholar] [CrossRef]
- Thapa, U.; Nandi, S.; Rai, R.; Upadhyay, A. Effect of nitrogen levels and harvest timing on growth, yield and quality of lettuce under floating hydroponic system. J. Plant Nutr. 2022, 45, 2563–2577. [Google Scholar] [CrossRef]
- Adamczewska-Sowińska, K.; Sowiński, J.; Jama-Rodzeńska, A. The Effect of Sowing Date and Harvest Time on Leafy Greens of Quinoa (Chenopodium quinoa Willd.) Yield and Selected Nutritional Parameters. Agriculture 2021, 11, 405. [Google Scholar] [CrossRef]
- Nerlich, A.; Dannehl, D. Soilless Cultivation: Dynamically Changing Chemical Properties and Physical Conditions of Organic Substrates Influence the Plant Phenotype of Lettuce. Front. Plant Sci. 2021, 11, 601455. [Google Scholar] [CrossRef]
- Oberpaur, C.; Fernández, C.; Délano, G.; Arévalo, M.E. Inclusion of various controlled release fertilizers in moss substrates(Sphagnum magellanicum). Cienc. E Investig. Agrar. 2012, 39, 435–443. [Google Scholar] [CrossRef]
- Isaka, T.; Clark, S.; Meyer, J. Compost Functions as Effective Replacement for Peat-Based Potting Media in Organic Greenhouse Transplant Production. J 2021, 4, 394–403. [Google Scholar] [CrossRef]
- Zhang, L.; Sun, X.; Tian, Y.; Gong, X. Composted green waste as a substitute for peat in growth media: Effects on growth and nutrition of Calathea insignis. PLoS ONE 2013, 8, e78121. [Google Scholar] [CrossRef] [Green Version]
- Vos, J.; Van Der Putten, P.E.L.; Birch, C.J. Effect of nitrogen supply on leaf appearance, leaf growth, leaf nitrogen economy and photosynthetic capacity in maize (Zea mays L.). F. Crop. Res. 2005, 93, 64–73. [Google Scholar] [CrossRef]
- Bhattacharya, A. Water-Use Efficiency Under Changing Climatic Conditions. In Changing Climate and Resource Use Efficiency in Plants; Elsevier: Amsterdam, The Netherlands, 2019; pp. 111–180. [Google Scholar]
- Hatfield, J.L.; Dold, C. Water-Use Efficiency: Advances and Challenges in a Changing Climate. Front. Plant Sci. 2019, 10, 103. [Google Scholar] [CrossRef] [PubMed]
- Kimball, B.A.; Kobayashi, K.; Bindi, M. Responses of Agricultural Crops to Free-Air CO2 Enrichment. Adv. Agron. 2002, 77, 293–368. [Google Scholar]
- Kumar, U.; Singh, P.; Boote, K.J. Effect of Climate Change Factors on Processes of Crop Growth and Development and Yield of Groundnut (Arachis hypogaea L.). Adv. Agron. 2012, 116, 41–69. [Google Scholar]
- Muhammad Arif, S.I. Effect of Nitrogen Levels and Plant Population on Yield and Yield Components of Maize. Adv. Crop Sci. Technol. 2015, 3, 2. [Google Scholar] [CrossRef]
- Vile, D.; Garnier, É.; Shipley, B.; Laurent, G.; Navas, M.-L.; Roumet, C.; Lavorel, S.; Díaz, S.; Hodgson, J.G.; Lloret, F.; et al. Specific Leaf Area and Dry Matter Content Estimate Thickness in Laminar Leaves. Ann. Bot. 2005, 96, 1129–1136. [Google Scholar] [CrossRef]
- Gong, H.; Gao, J. Soil and climatic drivers of plant SLA (specific leaf area). Glob. Ecol. Conserv. 2019, 20, e00696. [Google Scholar] [CrossRef]
- Bell, L.; Oruna-Concha, M.J.; Wagstaff, C. Identification and quantification of glucosinolate and flavonol compounds in rocket salad (Eruca sativa, Eruca vesicaria and Diplotaxis tenuifolia) by LC–MS: Highlighting the potential for improving nutritional value of rocket crops. Food Chem. 2015, 172, 852–861. [Google Scholar] [CrossRef]
- Cataldi, T.R.I.; Rubino, A.; Lelario, F.; Bufo, S.A. Naturally occurring glucosinolates in plant extracts of rocket salad (Eruca sativa L.) identified by liquid chromatography coupled with negative ion electrospray ionization and quadrupole ion-trap mass spectrometry. Rapid Commun. Mass Spectrom. 2007, 21, 2374–2388. [Google Scholar] [CrossRef]
- D’Antuono, L.F.; Elementi, S.; Neri, R. Glucosinolates in Diplotaxis and Eruca leaves: Diversity, taxonomic relations and applied aspects. Phytochemistry 2008, 69, 187–199. [Google Scholar] [CrossRef]
- Ferreres, F.; Llorach, R.; Gil-Izquierdo, A. Characterization of the interglycosidic linkage in di-, tri-, tetra- and pentaglycosylated flavonoids and differentiation of positional isomers by liquid chromatography/electrospray ionization tandem mass spectrometry. J. Mass Spectrom. 2004, 39, 312–321. [Google Scholar] [CrossRef]
- Martínez-Sánchez, A.; Llorach, R.; Gil, M.I.; Ferreres, F. Identification of New Flavonoid Glycosides and Flavonoid Profiles To Characterize Rocket Leafy Salads (Eruca vesicaria and Diplotaxis tenuifolia). J. Agric. Food Chem. 2007, 55, 1356–1363. [Google Scholar] [CrossRef] [PubMed]
- dos Santos PR, D.; de Lima Moreira, D.; Guimarães, E.F.; Kaplan, M.A.C. Essential oil analysis of 10 Piperaceae species from the Brazilian Atlantic forest. Phytochemistry 2001, 58, 547–551. [Google Scholar] [CrossRef]
- Ma, C.; Whitaker, B.D.; Kennelly, E.J. New 5-O-Caffeoylquinic Acid Derivatives in Fruit of the Wild Eggplant Relative Solanum viarum. J. Agric. Food Chem. 2010, 58, 11036–11042. [Google Scholar] [CrossRef]
- Durazzo, A.; Azzini, E.; Lazzè, M.; Raguzzini, A.; Pizzala, R.; Maiani, G. Italian Wild Rocket [Diplotaxis Tenuifolia (L.) DC.]: Influence of Agricultural Practices on Antioxidant Molecules and on Cytotoxicity and Antiproliferative Effects. Agriculture 2013, 3, 285–298. [Google Scholar] [CrossRef]
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Rémésy, C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am. J. Clin. Nutr. 2005, 81, 230S–242S. [Google Scholar] [CrossRef] [PubMed]
- Bell, L.; Wagstaff, C. Rocket science: A review of phytochemical & health-related research in Eruca & Diplotaxis species. Food Chem. X 2019, 1, 100002. [Google Scholar] [CrossRef] [PubMed]
- Bennett, R.N.; Carvalho, R.; Mellon, F.A.; Eagles, J.; Rosa, E.A.S. Identification and Quantification of Glucosinolates in Sprouts Derived from Seeds of Wild Eruca sativa L. (Salad Rocket) and Diplotaxis tenuifolia L. (Wild Rocket) from Diverse Geographical Locations. J. Agric. Food Chem. 2007, 55, 67–74. [Google Scholar] [CrossRef]
- Kim, H.W.; Ko, H.C.; Baek, H.J.; Cho, S.M.; Jang, H.H.; Lee, Y.M.; Kim, J.B. Identification and quantification of glucosinolates in Korean leaf mustard germplasm (Brassica juncea var. integrifolia) by liquid chromatography–electrospray ionization/tandem mass spectrometry. Eur. Food Res. Technol. 2016, 242, 1479–1484. [Google Scholar] [CrossRef]
- Lee, M.-K.; Chun, J.-H.; Byeon, D.H.; Chung, S.-O.; Park, S.U.; Park, S.; Arasu, M.V.; Al-Dhabi, N.A.; Lim, Y.-P.; Kim, S.-J. Variation of glucosinolates in 62 varieties of Chinese cabbage (Brassica rapa L. ssp. pekinensis) and their antioxidant activity. LWT—Food Sci. Technol. 2014, 58, 93–101. [Google Scholar] [CrossRef]
- Park, S.; Arasu, M.V.; Lee, M.-K.; Chun, J.-H.; Seo, J.M.; Al-Dhabi, N.A.; Kim, S.-J. Analysis and metabolite profiling of glucosinolates, anthocyanins and free amino acids in inbred lines of green and red cabbage (Brassica oleracea L.). LWT—Food Sci. Technol. 2014, 58, 203–213. [Google Scholar] [CrossRef]
- Avato, P.; Argentieri, M.P. Brassicaceae: A rich source of health improving phytochemicals. Phytochem. Rev. 2015, 14, 1019–1033. [Google Scholar] [CrossRef]
- Variyar, P.S.; Banerjee, A.; Akkarakaran, J.J.; Suprasanna, P. Role of Glucosinolates in Plant Stress Tolerance. In Emerging Technologies and Management of Crop Stress Tolerance; Elsevier: Amsterdam, The Netherlands, 2014; pp. 271–291. [Google Scholar]
- Taranto, F.; Francese, G.; Di Dato, F.; D’Alessandro, A.; Greco, B.; Onofaro Sanajà, V.; Pentangelo, A.; Mennella, G.; Tripodi, P. Leaf Metabolic, Genetic, and Morphophysiological Profiles of Cultivated and Wild Rocket Salad (Eruca and Diplotaxis Spp.). J. Agric. Food Chem. 2016, 64, 5824–5836. [Google Scholar] [CrossRef] [PubMed]
- Locato, V.; Cimini, S.; Gara, L. De Strategies to increase vitamin C in plants: From plant defense perspective to food biofortification. Front. Plant Sci. 2013, 4, 152. [Google Scholar] [CrossRef]
- Ruxton, C.H.S.; Gardner, E.J.; Walker, D. Can pure fruit and vegetable juices protect against cancer and cardiovascular disease too? A review of the evidence. Int. J. Food Sci. Nutra. 2006, 57, 249–272. [Google Scholar] [CrossRef]
- Cisternas, P.; Silva-Alvarez, C.; Martínez, F.; Fernandez, E.; Ferrada, L.; Oyarce, K.; Salazar, K.; Bolaños, J.P.; Nualart, F. The oxidized form of vitamin C, dehydroascorbic acid, regulates neuronal energy metabolism. J. Neurochem. 2014, 129, 663–671. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.Y.; Lin, H.-S. Compost as a Soil Supplement Increases the Level of Antioxidant Compounds and Oxygen Radical Absorbance Capacity in Strawberries. J. Agric. Food Chem. 2003, 51, 6844–6850. [Google Scholar] [CrossRef] [PubMed]
- Tortosa, G.; González-Gordo, S.; Ruiz, C.; Bedmar, E.; Palma, J. “Alperujo” Compost Improves the Ascorbate (Vitamin C) Content in Pepper (Capsicum annuum L.) Fruits and Influences Their Oxidative Metabolism. Agronomy 2018, 8, 82. [Google Scholar] [CrossRef]
- Paciolla, C.; Fortunato, S.; Dipierro, N.; Paradiso, A.; De Leonardis, S.; Mastropasqua, L.; de Pinto, M.C. Vitamin C in Plants: From Functions to Biofortification. Antioxidants 2019, 8, 519. [Google Scholar] [CrossRef] [Green Version]
| Parameter 1 | Peat | Compost |
|---|---|---|
| Physical characteristics | ||
| Bulk density (g/cm3) | 0.5 ± 0.1 | 0.2 ± 0.0 |
| Total pore space (%) | 75.1 ± 0.1 | 87.6 ± 0.1 |
| Air capacity (AC—%) | 20.6 ± 1.2 | 32.7 ± 0.3 |
| Water holding capacity (WHC—%) | 57.0 ± 6.0 | 44.3 ± 5.0 |
| Physico-chemical and chemical characteristics | ||
| pH | 5.3 ± 0.1 | 8.4 ± 0.1 |
| EC (dS/m) | 1.2 ± 0.1 | 1.6 ± 0.1 |
| C/N | 49.6 ± 0.1 | 9.7 ± 0.1 |
| TOC (g/L) | 233 ± 2.0 | 70 ± 1.0 |
| Total N (g/L) | 4.7 ± 2.6 | 7.2 ± 0.6 |
| Organic N (g/L) | 4.6 ± 0.6 | 7.0 ± 0.2 |
| Nitric (mg/L) | 4.5 ± 1.5 | 1.0 ± 0.4 |
| Ammonium N (mg/L) | 1.5 ± 0.1 | 0.4 ± 0.2 |
| Total P (P2O5 g/L) | 2.2 ± 0.1 | 15.3 ± 0.1 |
| Available P (P2O5 g/L) | 2.1 ± 0.1 | 6.2 ± 0.1 |
| Total K (K2O g/L) | 1.6 ± 0.2 | 2.0 ± 0.1 |
| Available K (K2O g/L) | 1.4 ± 0.1 | 0.8 ± 0.1 |
| Ca (g/L) | 9.0 ± 1.5 | 10.7 ± 0.2 |
| Mg (g/L) | 0.9 ± 0.3 | 0.16 ± 0.1 |
| Fe (g/L) | 0.6 ± 0.1 | 55.6 ± 0.1 |
| B (mg/L) | 0.2 ± 0.1 | 9.4 ± 0.1 |
| Cu (mg/L) | 2.8 ± 0.5 | 7.4 ± 0.2 |
| Mn (mg/L) | 35.5 ± 0.3 | 37.2 ± 1.4 |
| Mo (mg/L) | 0.8 ± 0.1 | 0.4 ± 0.1 |
| Zn (mg/L) | 7.2 ± 0.5 | 20.8 ± 0.5 |
| Parameter | Yield (kg/m2) | Leaves (Number/Plant) | Leaf Area (cm2/Plant) | WUE (kg/m3) | DW (%) | SLA (cm2/g DW) |
|---|---|---|---|---|---|---|
| Substrate (S) | ||||||
| Compost | 1.34 ± 0.60 | 33 ± 5 | 427 ± 10 | 7.00 ± 2.46 | 6.60 ± 0.12 | 261.1 ± 80.88 |
| Peat | 1.17 ± 0.45 | 38 ± 2 | 367 ± 25 | 6.47 ± 1.84 | 6.40 ± 0.19 | 199.1 ± 9.80 |
| Harvest (H) | ||||||
| 1st | 0.79 ± 0.11 | 18 ± 2 | 295 ± 19 | 4.88 ± 0.66 | 6.50 ± 0.23 | 266.70 ± 75.67 |
| 2nd | 1.72 ± 0.23 | 53 ± 5 | 498 ± 16 | 8.58 ± 0.98 | 6.50 ± 0.15 | 193.50 ± 2.78 |
| Significance | ||||||
| S | n.s. | n.s. | *** | n.s. | n.s. | ** |
| H | *** | *** | *** | *** | n.s. | ** |
| S x H | n.s. | ** | *** | n.s. | n.s. | ** |
| Glucosinolate | R-name | Rt (min) | m/z [M-H] | m/z MS2[M-H] | m/z MS3[M-H] |
|---|---|---|---|---|---|
| Diglucothiobeinin | 4-(β-D-Glucopyranosuldisulfanyl)-butyl | 17.4 | 600 | 420, 259, 241, 195 | 420: 259, 97 |
| Glucosativin | 4-Mercaptobutyl | 17.7 | 406 | 259, 209, 195 | 259: 97 |
| Glucoerucin | 4-methylthiobutyl | 19.3 | 420 | 339, 259, 241 | 340: 259, 97 |
| 4-Methoxyglucobrassicin | 4-methoxy-3-indolylmethyl | 23.5 | 477 | 285, 259, 227 | N.d. |
| Phenolic Compound | Rt (min) | m/z [M-H] | m/z MS2[M-H] | m/z MS3[M-H] |
|---|---|---|---|---|
| Quercetin-3,3′,4′-triglucosyl | 24.2 | 787 | 625, 463 | 625: 463, 301 |
| Quercetin-3,4′-diglucosyl-3′-(6-sinapoyl-glucosyl) | 29.5 | 993 | 831 | 831: 669, 463, 301 |
| Caffeoyl sinapoyl derivative I | 30.6 | 993 | 445, 341, 220 | 445: 223, 179 |
| Quercetin-3-(2-sinapoyl-glucosyl)-3′-(6-sinapoyl-glucosyl)-4′glucosyl | 32.3 | 1199 | 1037, 831 | 831: 669, 463, 301 |
| Caffeoyl sinapoyl derivative II | 35.3 | 891 | 401, 357, 341 | 401: 223, 179 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Signore, A.; Amoruso, F.; Gallegos-Cedillo, V.M.; Gómez, P.A.; Ochoa, J.; Egea-Gilabert, C.; Costa-Pérez, A.; Domínguez-Perles, R.; Moreno, D.A.; Pascual, J.A.; et al. Agro-Industrial Compost in Soilless Cultivation Modulates the Vitamin C Content and Phytochemical Markers of Plant Stress in Rocket Salad (Diplotaxis tenuifolia (L.) DC.). Agronomy 2023, 13, 544. https://doi.org/10.3390/agronomy13020544
Signore A, Amoruso F, Gallegos-Cedillo VM, Gómez PA, Ochoa J, Egea-Gilabert C, Costa-Pérez A, Domínguez-Perles R, Moreno DA, Pascual JA, et al. Agro-Industrial Compost in Soilless Cultivation Modulates the Vitamin C Content and Phytochemical Markers of Plant Stress in Rocket Salad (Diplotaxis tenuifolia (L.) DC.). Agronomy. 2023; 13(2):544. https://doi.org/10.3390/agronomy13020544
Chicago/Turabian StyleSignore, Angelo, Fabio Amoruso, Victor M. Gallegos-Cedillo, Perla A. Gómez, Jesús Ochoa, Catalina Egea-Gilabert, Antonio Costa-Pérez, Raúl Domínguez-Perles, Diego A. Moreno, José Antonio Pascual, and et al. 2023. "Agro-Industrial Compost in Soilless Cultivation Modulates the Vitamin C Content and Phytochemical Markers of Plant Stress in Rocket Salad (Diplotaxis tenuifolia (L.) DC.)" Agronomy 13, no. 2: 544. https://doi.org/10.3390/agronomy13020544
APA StyleSignore, A., Amoruso, F., Gallegos-Cedillo, V. M., Gómez, P. A., Ochoa, J., Egea-Gilabert, C., Costa-Pérez, A., Domínguez-Perles, R., Moreno, D. A., Pascual, J. A., & Fernández, J. A. (2023). Agro-Industrial Compost in Soilless Cultivation Modulates the Vitamin C Content and Phytochemical Markers of Plant Stress in Rocket Salad (Diplotaxis tenuifolia (L.) DC.). Agronomy, 13(2), 544. https://doi.org/10.3390/agronomy13020544











