Action of Different Exposures of Chilled Atmospheric Treatments on the Mortality of Granary Weevil and Embryo Viability of the Treated Wheat
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Sample Preparation
2.2. Insect Eradication Test
2.3. Determination of Viability of Wheat Grain by TTC Test
2.4. Statistical Analysis
3. Results
3.1. Insect Mortalities Triggered by Freezing
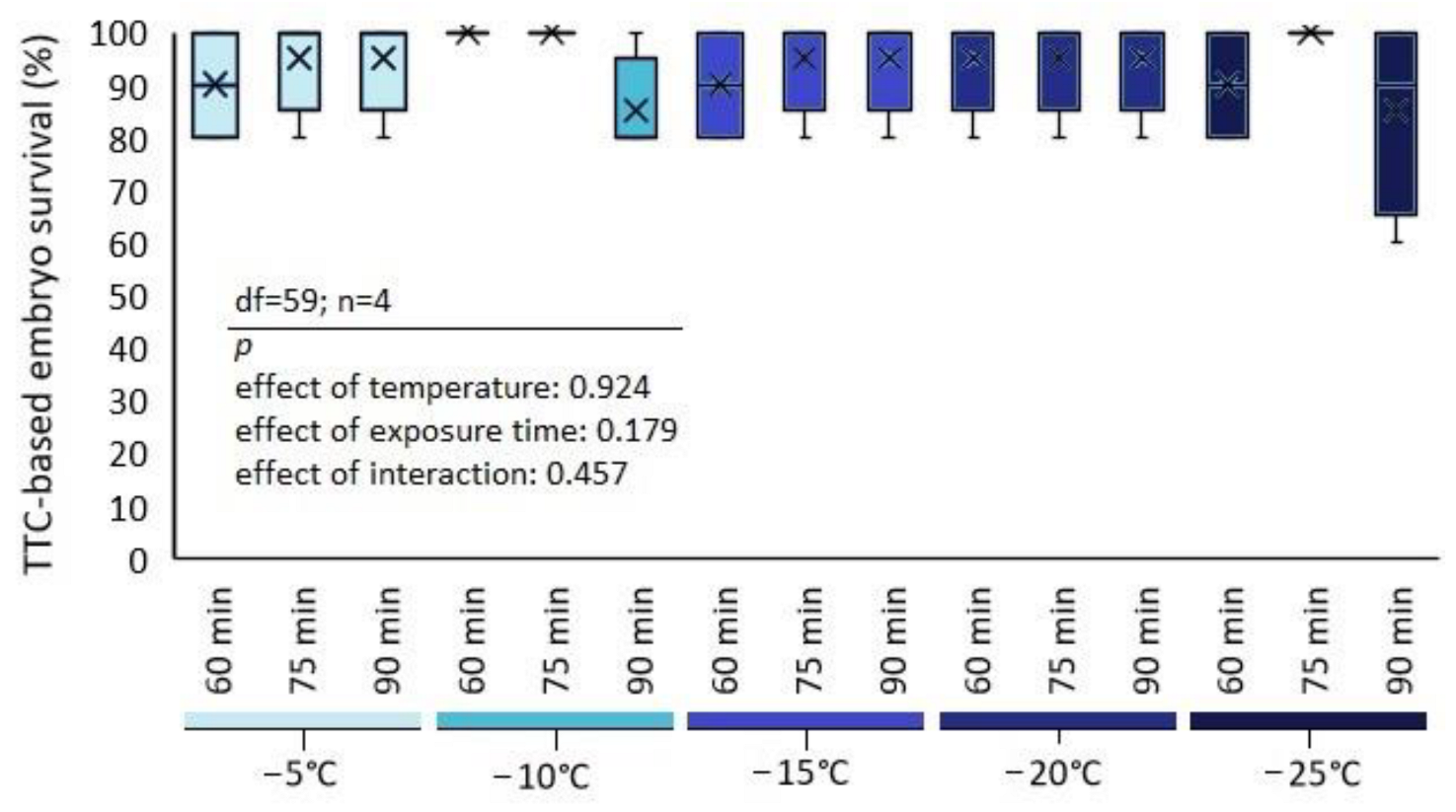
3.2. Viability of the Treated Wheat
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bell, C.H. Insect and mite penetration and contamination of packaged food. In Food and Beverage Stability and Shelf Life. Woodhead Publishing Series in Food Science, Technology and Nutrition; Kilcast, D., Subramaniam, P., Eds.; Woodhead Publishing: Sawston, UK, 2011; pp. 106–131. [Google Scholar]
- Abass, A.B.; Ndunguru, G.; Mamiro, P.; Alenkhe, B.; Mlingi, N.; Bekunda, M. Post-harvest food losses in a maize-based farming system of semi-arid savannah area of Tanzania. J. Stored Prod. Res. 2014, 57, 49–57. [Google Scholar] [CrossRef]
- Lazzari, S.M.; Lazzari, F.A. Insect pests in stored grain. In. Insect Bioecology and Nutrition for Integrated Pest Management; Panizzi, A.R., Parra, J.R.P., Eds.; Taylor & Francis Group: Boca Raton, FL, USA, 2012; pp. 417–450. [Google Scholar]
- Nietupski, M.; Ludwiczak, E.; Cabaj, R.; Purwin, C.; Kordan, B. Fatty acids present in wheat kernels influence the development of the grain weevil (Sitophilus granarius L.). Insects 2021, 12, 806. [Google Scholar] [CrossRef]
- Baker, J.E. Nitrogenous excretory products of adults of Sitophilus oryzae and Sitophilus granarius. Comp. Biochem. Physiol. B Comp. Biochem. 1976, 53, 107–109. [Google Scholar] [CrossRef]
- Zettler, J.L.; Arthur, F.H. Chemical control of stored product insects with fumigants and residual treatments. Crop Prot. 2000, 19, 577–582. [Google Scholar] [CrossRef]
- Hallman, G.J. Control of stored product pests by ionizing radiation. J. Stored Prod. Res. 2013, 52, 36–41. [Google Scholar] [CrossRef]
- Indiarto, R.; Qonit, M.A.H. A review of irradiation technologies on food and agricultural products. Int. J. Sci. Technol. Res. 2020, 9, 4411–4414. [Google Scholar]
- Cao, Y.; Xu, K.; Zhu, X.; Bai, Y.; Yang, W.; Li, C. Role of modified atmosphere in pest control and mechanism of its effect on insects. Front. Physiol. 2019, 10, 206. [Google Scholar] [CrossRef]
- Mason, L.J.; Strait, C.A. Stored product integrated pest management with extreme temperatures. In. Temperature Sensitivity in Insects and Application in Integrated Pest Management; Hallman, G.J., Denlinger, D.L., Eds.; CRC Pres, Taylor & Francis Group: Boca Raton, FL, USA, 2019; pp. 141–177. [Google Scholar]
- Korunić, Z.; Liška, A.; Lucić, P.; Hamel, D.; Rozman, V. Evaluation of diatomaceous earth formulations enhanced with natural products against stored product insects. J. Stored Prod. Res. 2020, 86, 101565. [Google Scholar] [CrossRef]
- Ziaee, M.; Ebadollahi, A.; Wakil, W. Integrating inert dusts with other technologies in stored products protection. Toxin Rev. 2021, 40, 404–419. [Google Scholar] [CrossRef]
- Longstaff, B.C. Biology of the grain pest species of the genus Sitophilus (Coleoptera: Curculionidae: A critical review. Prot. Ecol. 1981, 3, 83–130. [Google Scholar]
- Nakakita, H.; Ikenata, H. Action of low temperature on physiology of Sitophilus zeamais Motschulsky and Sitophilus oryzae (L.) (Coleoptera: Curculionidae) in rice storage. J. Stored Prod. Res. 1997, 33, 31–38. [Google Scholar] [CrossRef]
- Ileleji, K.E.; Maier, D.E.; Woloshuk, C.P. Evaluation of different temperature management strategies for suppression of Sitophilus zeamais (Motschulsky) in stored maize. J. Stored Prod. Res. 2007, 43, 480–488. [Google Scholar] [CrossRef]
- Youssef, M.O.; Youssef, A.D.; Hassan, R.A.; Mahmoud, A.M. Threshold temperature and heat unit requirements for the development of the granary weevil, Sitophilus granarius (L.). Arch. Phytopathol. Plant Prot. 2014, 47, 555–563. [Google Scholar]
- Remmert, H. Ecology; Springer: Berlin/Heidelberg, Germany, 1980. [Google Scholar]
- Fields, P.G. The control of stored-product insects and mites with extreme temperatures. J. Stored Prod. Res. 1992, 28, 89–118. [Google Scholar] [CrossRef]
- Thaung, M.; Collins, P.J. Joint effects of temperature and insecticides on mortality and fecundity of Sitophilus oryzae (Coleoptera: Curculionidae) in wheat and maize. J. Econ. Entomol. 1986, 79, 909–914. [Google Scholar] [CrossRef]
- Lü, J.; Zhang, H. The effect of acclimation to sublethal temperature on subsequent susceptibility of Sitophilus zeamais Mostchulsky (Coleoptera: Curculionidae) to high temperatures. PLoS ONE 2016, 11, e0159400. [Google Scholar] [CrossRef]
- Kljajić, P.; Andrić, G.; Pražić-Golić, M.; Inđić, D.; Vuković, S. The effects of cold pre-treatment on the toxicity of several contact insecticides on adults of three Sitophilus granarius (L.) populations. J. Pest Sci. 2014, 87, 301–308. [Google Scholar] [CrossRef]
- Holubová, Ľ.; Švubová, R.; Slováková, Ľ.; Bokor, B.; Chobotová Kročková, V.; Renčko, J.; Uhrin, F.; Medvecka, V.; Zahoranová, A.; Gálová, E. Cold atmospheric pressure plasma treatment of maize grains—Induction of growth, enzyme activities and heat shock proteins. Int. J. Mol. Sci. 2021, 22, 8509. [Google Scholar] [CrossRef]
- Eliopoulos, P.A.; Prasodimou, G.Z.; Pouliou, A.V. Time–mortality relationships of larvae and adults of grain beetles exposed to extreme cold. Crop Prot. 2011, 30, 1097–1102. [Google Scholar] [CrossRef]
- Lukács, H.; Pál-Fám, F.; Varga-Visi, É.; Rolbiecki, R.; Percze, A.; Keszthelyi, S. Impact of short-term atmospheric heat transfer on the survival of granary weevil in stored winter wheat. Agronomy 2022, 12, 1313. [Google Scholar] [CrossRef]
- Howe, R.W. A summary of estimates of optimal and minimal conditions for population increase of some stored products insects. J. Stored Prod. Res. 1965, 1, 177–184. [Google Scholar] [CrossRef]
- Carvalho, T.C.; Krzyzanowski, F.C.; Ohlson, O.C. Tetrazolium test adjustment for wheat seeds. J. Seed Sci. 2013, 35, 361–367. [Google Scholar] [CrossRef]
- Abbott, W.S. A method of computing the effectiveness of an insecticide. J. Econ Entomol. 1925, 8, 265–267. [Google Scholar] [CrossRef]
- Scotti, G. Les Insectes et les Acariens des ce ’re ’Alesstocke ’es; ITCF/AFNOR: Paris, France, 1978. [Google Scholar]
- Wellington, W.G. Returning the insect to insect ecology: Some consequences for pest management. Environ. Entomol. 1977, 6, 1–8. [Google Scholar] [CrossRef]
- Marpaung, D.S.S. Mortality rate of Sitophilus zeamais in low temperature storage. Agrointek J. Tek. Ind. Pert. 2021, 15, 1046–1053. [Google Scholar] [CrossRef]
- Grgac, R.; Rozsypal, J.; Des Marteaux, L.; Štětina, T.; Koštál, V. Stabilization of insect cell membranes and soluble enzymes by accumulated cryoprotectants during freezing stress. Proc. Nat. Acad. Sci. USA 2022, 119, e2211744119. [Google Scholar] [CrossRef]
- Steinbrecht, R.A.; Müller, M. Freeze-substitution and freeze-drying. In Cryotechniques in Biological Electron Microscopy; Steinbrecht, R.A., Zierold, K., Eds.; Springer: Berlin/Heidelberg, Germany, 1987; pp. 149–179. [Google Scholar]
- Gurruchaga, H.; Del Burgo, L.S.; Hernandez, R.M.; Orive, G.; Selden, C.; Fuller, B.; Ciriza, J.; Pedraz, J.L. Advances in the slow freezing cryopreservation of microencapsulated cells. J. Contr. Rel. 2018, 281, 119–138. [Google Scholar] [CrossRef]
- Thorpe, G.R.; Cuff, W.R.; Longstaff, B. Control of Sitophilus oryzae infestation of stored wheat: An ecosystem model of the use of aeration. Ecol. Mod. 1982, 15, 331–351. [Google Scholar] [CrossRef]
- Ke, D.; Kader, A.A. Potential of controlled atmospheres for postharvest insect disinfestation of fruits and vegetables. Postharv. News Inform. 1992, 3, 31N–37N. [Google Scholar]
- Meyer, G.L. How insects react to the cold. PSA J. 1994, 60, 20–22. [Google Scholar]
- Rust, M.K.; Paine, E.O.; Reierson, D.A. Evaluation of freezing to control wood-destroying insects (Isoptera, Coleoptera). J. Econ Entomol. 1997, 90, 1215–1221. [Google Scholar] [CrossRef]
- Kenkel, P.; Criswell, J.T.; Cuperus, G.W.; Noyes, R.T.; Anderson, K.; Fargo, W.S. Stored product integrated pest management. Food Rev. Int. 1994, 10, 177–193. [Google Scholar] [CrossRef]
- Fields, P.G.; White, N.D.G. Alternatives to methyl bromide treatments for stored-product and quarantine insects. Ann. Rev. Entomol. 2002, 47, 331–359. [Google Scholar] [CrossRef]
- Vincent, C.; Hallman, G.; Panneton, B.; Fleurat-Lessard, F. Management of agricultural insects with physical control methods. Ann. Rev. Entomol. 2003, 48, 261–281. [Google Scholar] [CrossRef]
- Phillips, T.W.; Throne, J.E. Biorational approaches to managing stored-product insects. Ann. Rev. Entomol. 2010, 55, 375–397. [Google Scholar] [CrossRef]
- Du, X.; He, W.; Gao, S.; Liu, D.; Wu, W.; Tu, D.; Kong, L.; Xi, M. Raised bed planting increases economic efficiency and energy use efficiency while reducing the environmental footprint for wheat after rice production. Energy 2022, 245, 123256. [Google Scholar] [CrossRef]



| Adjusted Atmospheric Temperature | −5 ± 0.50 | −10 ± 0.50 | −15 ± 0.50 | −20 ± 0.50 | −25 ± 0.50 | |
|---|---|---|---|---|---|---|
| stored grains temperature | at 60 min of exposure | 7.8 ± 0.23 | 2.8 ± 1.25 | 0.2 ± 0.54 | −3.9 ± 0.65 | −6.4 ± 0.21 |
| at 75 min of exposure | 5.3 ± 0.65 | 0.7 ± 0.44 | −2.7 ± 0.76 | −7 ± 0.26 | −10.5 ± 0.97 | |
| at 90 min of exposure | 3.8 ± 1.11 | −1.4 ± 0.85 | −4.2 ± 1.28 | −9.2 ± 1.06 | −15.3 ± 1.52 | |
| Treatments | No. Progeny | |||||||
|---|---|---|---|---|---|---|---|---|
| control | 6.75 ± 0.75 | statistical relationships (df = 19) | ||||||
| set up the atmospheric temperature | −5 °C | −10 °C | −15 °C | −20 °C | −25 °C | F | p | |
| exposure times | 60 min | 3.25 ± 0.62 | 1.75 ± 0.75 | 0.75 ± 0.47 | 0.75 ± 0.75 | 0.75 ± 0.25 | 11.277 | 0.001 |
| 75 min | 3.00 ± 2.04 | 2.00 ± 0.40 | 3.50 ± 2.06 | 1.00 ± 0.41 | 1.00 ± 0.41 | 1.834 | 0.003 | |
| 90 min | 0.75 ± 0.25 | 0.75 ± 0.47 | 2.00 ± 1.68 | 0.50 ± 0.28 | 0 ± 0 | 21.091 | 4.7 × 10−5 | |
| statistical relationships (df = 11) | F | 1.434 | 1.434 | 0.008 | 11.301 | 20.509 | ||
| p | 0.243 | 0.243 | 0.928 | 0.002 | 0.001 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Keszthelyi, S.; Lukács, H.; Gibicsár, S.; Rolbiecki, R.; Pál-Fám, F. Action of Different Exposures of Chilled Atmospheric Treatments on the Mortality of Granary Weevil and Embryo Viability of the Treated Wheat. Agronomy 2023, 13, 597. https://doi.org/10.3390/agronomy13020597
Keszthelyi S, Lukács H, Gibicsár S, Rolbiecki R, Pál-Fám F. Action of Different Exposures of Chilled Atmospheric Treatments on the Mortality of Granary Weevil and Embryo Viability of the Treated Wheat. Agronomy. 2023; 13(2):597. https://doi.org/10.3390/agronomy13020597
Chicago/Turabian StyleKeszthelyi, Sándor, Helga Lukács, Szilvia Gibicsár, Roman Rolbiecki, and Ferenc Pál-Fám. 2023. "Action of Different Exposures of Chilled Atmospheric Treatments on the Mortality of Granary Weevil and Embryo Viability of the Treated Wheat" Agronomy 13, no. 2: 597. https://doi.org/10.3390/agronomy13020597
APA StyleKeszthelyi, S., Lukács, H., Gibicsár, S., Rolbiecki, R., & Pál-Fám, F. (2023). Action of Different Exposures of Chilled Atmospheric Treatments on the Mortality of Granary Weevil and Embryo Viability of the Treated Wheat. Agronomy, 13(2), 597. https://doi.org/10.3390/agronomy13020597












