Evaluation of Silicon Supplementation for Drought Stress under Water-Deficit Conditions: An Application of Sustainable Agriculture
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Collection, Experimental Treatments, and Layout
2.2. Measurement of Morphological Parameters
2.3. Determination of Amino Acids and Antioxidants Enzyme Activity
2.4. Detection of Pigments
2.5. Determination of Glycinebetaine (GB) and Total Soluble Proteins
2.6. Free Proline and MDA Contents Estimation
2.7. Determination of Photosynthetic Attribute
2.8. Statistical Analysis
3. Results
3.1. Morphological Attributes
3.2. Total Free Amino Acids and Antioxidants Enzyme
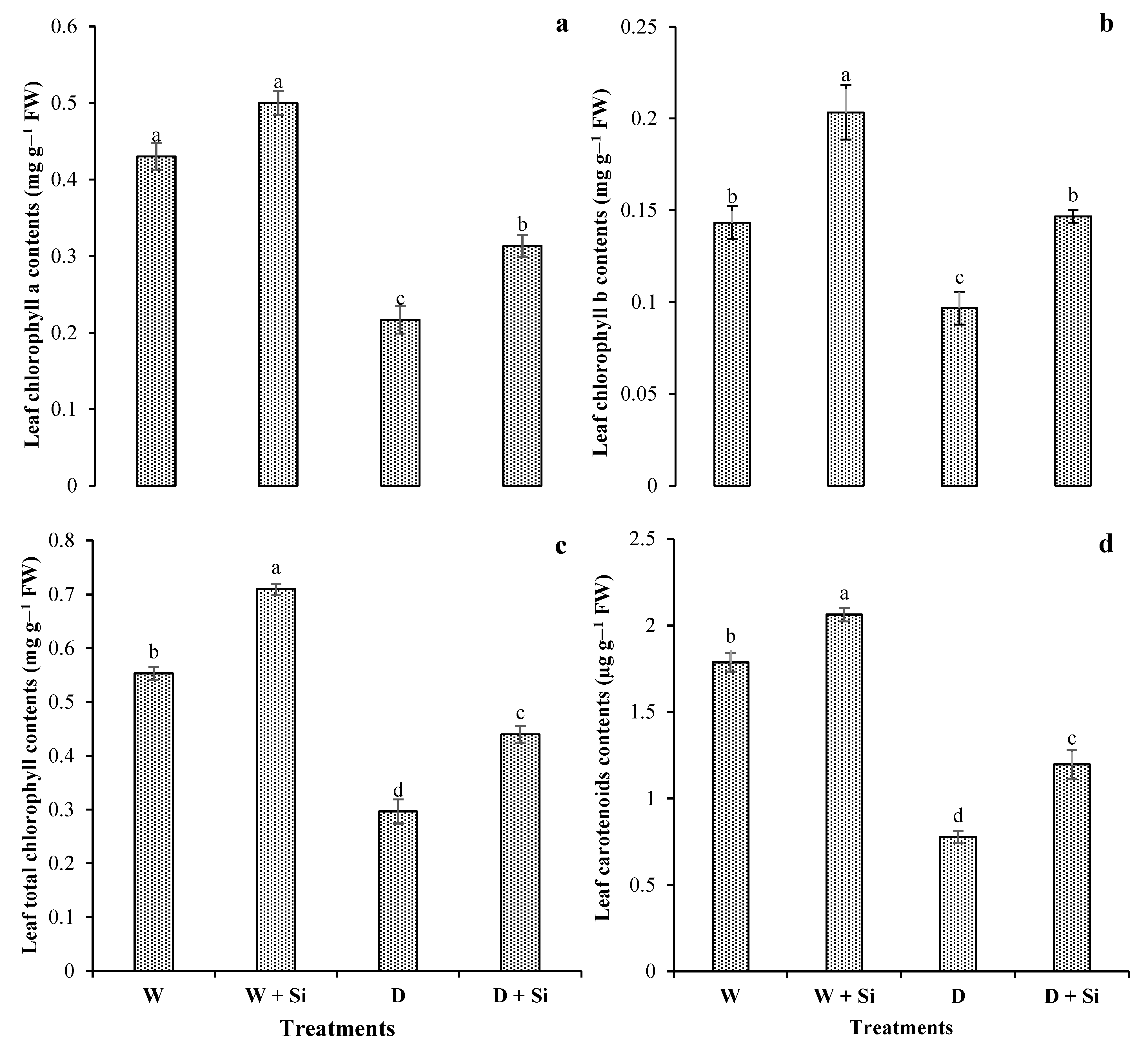
3.3. Pigments
3.4. GB and Soluble Proteins
3.5. Free Proline and MDA
3.6. Photosynthetic Attributes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, M.; Wang, R.; Mur, L.A.J.; Ruan, J.; Shen, Q.; Guo, S. Functions of silicon in plant drought stress responses. Hortic. Res. 2021, 8, 254. [Google Scholar] [CrossRef]
- Coskun, D.; Deshmukh, R.; Sonah, H.; Menzies, J.G.; Reynolds, O.; Ma, J.F.; Kronzucker, H.J.; Bélanger, R.R. The controversies of silicon’s role in plant biology. New Phytol. 2019, 221, 67–85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, J.F.; Yamaji, N. A cooperative system of silicon transport in plants. Trends Plant Sci. 2015, 20, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Luyckx, M.; Hausman, J.-F.; Lutts, S.; Guerriero, G. Silicon and Plants: Current Knowledge and Technological Perspectives. Front. Plant Sci. 2017, 8, 411. [Google Scholar] [CrossRef] [Green Version]
- Yin, J.L.; Jia, J.H.; Lian, Z.Y.; Hu, Y.H.; Guo, J.; Huo, H.Q.; Zhu, Y.X.; Gong, H.J. Silicon enhances the salt tolerance of cucumber through increasing polyamine accumulation and decreasing oxidative damage. Ecotoxicol. Environ. Saf. 2019, 169, 8–17. [Google Scholar] [CrossRef]
- Gao, H.; Wenying, Y.; Xiaoqing, Y.; Jiahui, L.; Xiwu, S.; Maoxiang, S.; Yuansong, X.; Futian, P. Silicon enhances the drought resistance of peach seedlings by regulating hormone, amino acid, and sugar metabolism. BMC Plant Biol. 2022, 22, 422. [Google Scholar] [CrossRef] [PubMed]
- Seifikalhor, M.; Niknam, V.; Aliniaeifard, S.; Didaran, F.; Tsaniklidis, G.; Fanourakis, D.; Teymoorzadeh, M.; Mousavi, S.H.; Bosacchi, M.; Li, T. The regulatory role of γ-aminobutyric acid in chickpea plants depends on drought tolerance and water scarcity level. Sci. Rep. 2022, 12, 7034. [Google Scholar] [CrossRef]
- Ali, N.; Schwarzenberg, A.; Yvin, J.-C.; Hosseini, S.A. Regulatory Role of Silicon in Mediating Differential Stress Tolerance Responses in Two Contrasting Tomato Genotypes under Osmotic Stress. Front. Plant Sci. 2018, 9, 1475. [Google Scholar] [CrossRef] [Green Version]
- Ahsan, M.; Nafees, M.; Amin, M.; Nawaz, F.; Tufail, A.; Sardar, H.; Shokralla, S.; Mahmoud, E.A.; El-Sabrout, A.M.; Elansary, H.O. Nutrients Uptake and Accumulation in Plant Parts of Fragrant Rosa Species Irrigated with Treated and Untreated Wastewater. Plants 2022, 11, 1260. [Google Scholar] [CrossRef]
- Schwalm, C.R.; Anderegg, W.R.L.; Michalak, A.M.; Fisher, J.B.; Biondi, F.; Koch, G.; Litvak, M.; Ogle, K.; Shaw, J.D.; Wolf, A.; et al. Global patterns of drought recovery. Nature 2017, 548, 202–205. [Google Scholar] [CrossRef]
- Hessini, K.; Wasli, H.; Al-Yasi, H.M.; Ali, E.F.; Issa, A.A.; Hassan, F.A.S.; Siddique, K.H.M. Graded Moisture Deficit Effect on Secondary Metabolites, Antioxidant, and Inhibitory Enzyme Activities in Leaf Extracts of Rosa damascena Mill. var. trigentipetala. Horticulturae 2022, 8, 177. [Google Scholar] [CrossRef]
- Jafari, S.; Garmdareh, S.E.H.; Azadegan, B. Effects of drought stress on morphological, physiological, and biochemical characteristics of stock plant (Matthiola incana L.). Sci. Hortic. 2019, 253, 128–133. [Google Scholar] [CrossRef]
- IPCC. Intergovernmental panel on climate change. In 5th Assessment Report, WGII, Climate Change 2014: Impacts, Adaptation, and Vulnerability; Cambridge University Press: Cambridge, UK, 2014; Available online: http://www.ipcc.ch/report/ar5/wg2/ (accessed on 16 July 2018).
- Toscano, S.; Romano, D. Morphological, Physiological, and Biochemical Responses of Zinnia to Drought Stress. Horticulturae 2021, 7, 362. [Google Scholar] [CrossRef]
- Heidari, Z.; Nazarideljou, M.J.; Danesh, Y.R.; Khezrinejad, N. Morphophysiological and Biochemical Responses of Zinnia elegans to Different Irrigation Regimes in Symbiosis with Glomus mosseae. Int. J. Hortic. Sci. 2016, 3, 19–32. [Google Scholar] [CrossRef]
- Talbi, S.; Rojas, J.A.; Sahrawy, M.; Rodríguez-Serrano, M.; Cárdenas, K.E.; Debouba, M.; Sandalio, L.M. Effect of drought on growth, photosynthesis and total antioxidant capacity of the saharan plant Oudeneya africana. Environ. Exp. Bot. 2020, 176, 104099. [Google Scholar] [CrossRef]
- Cal, A.J.; Sanciangco, M.; Rebolledo, M.C.; Luquet, D.; Torres, R.O.; McNally, K.L.; Henry, A. Leaf morphology, rather than plant water status, underlies genetic variation of rice leaf rolling under drought. Plant Cell Environ. 2019, 42, 1532–1544. [Google Scholar] [CrossRef] [Green Version]
- Nemali, K.S.; Bonin, C.; Dohleman, F.G.; Stephens, M.; Reeves, W.R.; Nelson, D.E.; Castiglioni, P.; Whitsel, J.E.; Sammons, B.; Silady, R.A.; et al. Physiological responses related to increased grain yield under drought in the first biotechnology-derived drought-tolerant maize. Plant Cell Environ. 2015, 38, 1866–1880. [Google Scholar] [CrossRef] [Green Version]
- Seleiman, M.F.; Al-Suhaibani, N.; Ali, N.; Akmal, M.; Alotaibi, M.; Refay, Y.; Dindaroglu, T.; Abdul-Wajid, H.H.; Battaglia, M.L. Drought Stress Impacts on Plants and Different Approaches to Alleviate Its Adverse Effects. Plants 2021, 10, 259. [Google Scholar] [CrossRef]
- Yousefzadeh, K.; Houshmand, S.; Shiran, B.; Mousavi-Fard, S.; Zeinali, H.; Nikoloudakis, N.; Gheisari, M.M.; Fanourakis, D. Joint Effects of Developmental Stage and Water Deficit on Essential Oil Traits (Content, Yield, Composition) and Related Gene Expression: A Case Study in Two Thymus Species. Agronomy 2022, 12, 1008. [Google Scholar] [CrossRef]
- Ahmadi-Majd, M.; Mousavi-Fard, S.; Nejad, A.R.; Fanourakis, D. Carbon nanotubes in the holding solution stimulate flower opening and prolong vase life in carnation. Chem. Biol. Technol. Agric. 2022, 9, 15. [Google Scholar] [CrossRef]
- Ahmadi-Majd, M.; Nejad, A.R.; Mousavi-Fard, S.; Fanourakis, D. Postharvest application of single, multi-walled carbon nanotubes and nanographene oxide improves rose keeping quality. J. Hortic. Sci. Biotechnol. 2021, 97, 346–360. [Google Scholar] [CrossRef]
- Al-Yasi, H.; Attia, H.; Alamer, K.; Hassan, F.; Ali, E.; Elshazly, S.; Siddique, K.H.; Hessini, K. Impact of drought on growth, photosynthesis, osmotic adjustment, and cell wall elasticity in Damask rose. Plant Physiol. Biochem. 2020, 150, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Uzma, J.; Talla, S.K.; Madam, E.; Mamidala, P. Assessment of Salinity Tolerance Deploying Antioxidant Defense Systems in Gerbera Jamesonii. Biosci. Biotechnol. Res. Asia 2022, 19, 243–254. [Google Scholar] [CrossRef]
- Hajizadeh, H.S.; Asadi, M.; Zahedi, S.M.; Hamzehpour, N.; Rasouli, F.; Helvacı, M.; Alas, T. Silicon dioxide-nanoparticle nutrition mitigates salinity in gerbera by modulating ion accumulation and antioxidants. Folia Hortic. 2021, 33, 91–105. [Google Scholar] [CrossRef]
- da Silva, D.P.C.; Paiva, P.D.D.O.; Herrera, R.C.; Porto, J.M.P.; dos Reis, M.V.; Paiva, R. Effectiveness of silicon sources for in vitro development of gerbera. Plant Cell Tissue Organ Cult. 2020, 141, 77–85. [Google Scholar] [CrossRef]
- Amin, M.; Ahmad, R.; Ali, A.; Hussain, I.; Mahmood, R.; Aslam, M.; Lee, D.J. Influence of Silicon Fertilization on Maize Performance under Limited Water Supply. Silicon 2016, 10, 177–183. [Google Scholar] [CrossRef]
- Álvarez, S.; Sánchez-Blanco, M.J. Comparison of individual and combined effects of salinity and deficit irrigation on physiological, nutritional and ornamental aspects of tolerance in Callistemon laevis plants. J. Plant Physiol. 2015, 185, 65–74. [Google Scholar] [CrossRef] [Green Version]
- Cirillo, C.; De Micco, V.; Rouphael, Y.; Balzano, A.; Caputo, R.; De Pascale, S. Morpho-anatomical and physiological traits of two Bougainvillea genotypes trained to two shapes under deficit irrigation. Trees 2017, 31, 173–187. [Google Scholar] [CrossRef] [Green Version]
- Elansary, H.O.; Salem, M.Z.M. Morphological and physiological responses and drought resistance enhancement of ornamental shrubs by trinexapac ethyl application. Sci. Hortic. 2015, 189, 1–11. [Google Scholar] [CrossRef]
- Hajipour, H.; Zohreh, J. Effect of foliar application of silicon on physiological responses of chrysanthemum (Dendranthema × grandiflorum) at two different growth stages. J. Ornam. Plants 2015, 6, 39–47. [Google Scholar]
- Hosseini, S.A.; Maillard, A.; Hajirezaei, M.R.; Ali, N.; Schwarzenberg, A.; Jamois, F.; Yvin, J.-C. Induction of Barley Silicon Transporter HvLsi1 and HvLsi2, increased silicon concentration in the shoot and regulated Starch and ABA Homeostasis under Osmotic stress and Concomitant Potassium Deficiency. Front. Plant Sci. 2017, 8, 1359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamenidou, S.; Cavins, T.J.; Marek, S. Silicon supplements affect floricultural quality traits and elemental nutrient concentrations of greenhouse produced gerbera. Sci. Hortic. 2010, 123, 390–394. [Google Scholar] [CrossRef]
- Al-Maitah, S.S. Silicon mitigate salinity stress on gerbera cut flower. Nat. App. Sci. 2022, 37, 2. [Google Scholar]
- Hamilton, P.B.; Van Slyke, D.D. Amino acids determination with ninhydrin. J. Biol. Chem. 1943, 150, 231–233. [Google Scholar] [CrossRef]
- Dixit, V.; Pandey, V.; Shyam, R. Differential antioxidative responses to cadmium in roots and leaves of pea (Pisum sativum L. cv. Azad). J. Exp. Bot. 2001, 52, 1101–1109. [Google Scholar] [CrossRef] [Green Version]
- Giannopolitis, C.N.; Ries, S.K. Superoxide dismutase I. Occurrence in higher plants. Plant Physiol. 1997, 59, 309–314. [Google Scholar] [CrossRef]
- Cakmak, I. Activity of ascorbate-dependent H2O2-scavenging enzymes and leaf chlorosis are enhanced in magnesium- and potassium-deficient leaves, but not in phosphorus-deficient leaves. J. Exp. Bot. 1994, 45, 1259–1266. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Q.; Yang, X.; Xia, Z. Effects of Sodium Selenite and Germination on the Sprouting of Chickpeas (Cicer arietinum L.) and Its Content of Selenium, Formononetin and Biochanin A in the Sprouts. Biol. Trace Elem. Res. 2012, 146, 376–380. [Google Scholar] [CrossRef]
- Davies, B. “Carotenoids”, in Chemistry and Biochemistry of Plant Pigments; Goodwin, T.W., Ed.; Academic Press: London, UK, 1976; pp. 38–165. [Google Scholar]
- Arnon, D.I. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grieve, C.M.; Grattan, S.R. Rapid assay for determination of water soluble quaternary ammonium compounds. Plant Soil 1983, 70, 303–307. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Annu. Rev. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Ahmad, P.; John, R.; Sarwat, M.; Umar, S. Responses of proline, lipid peroxidation and antioxidative enzymes in two varieties of Pisum sativum L. under salt stress. Int. J. Plant. Prod. 2008, 2, 353–366. [Google Scholar] [CrossRef]
- Li, G.; Wan, S.; Zhou, J.; Yang, Z.; Qin, P. Leaf chlorophyll fluorescence, hyperspectral reflectance, pigments content, malondialdehyde and proline accumulation responses of castor bean (Ricinus communis L.) seedlings to salt stress levels. Ind. Crops Prod. 2010, 31, 13–19. [Google Scholar] [CrossRef]
- Toscano, S.; Ferrante, A.; Tribulato, A.; Romano, D. Leaf physiological and anatomical responses of Lantana and Ligustrum species under different water availability. Plant Physiol. Biochem. 2018, 127, 380–392. [Google Scholar] [CrossRef] [PubMed]
- Chyliński, W.K.; Łukaszewska, A.J.; Kutnik, K. Drought response of two bedding plants. Acta Physiol. Plant. 2007, 29, 399–406. [Google Scholar] [CrossRef]
- Zomorrodi, N.; Nejad, A.R.; Mousavi-Fard, S.; Feizi, H.; Tsaniklidis, G.; Fanourakis, D. Potency of Titanium Dioxide Nanoparticles, Sodium Hydrogen Sulfide and Salicylic Acid in Ameliorating the Depressive Effects of Water Deficit on Periwinkle Ornamental Quality. Horticulturae 2022, 8, 675. [Google Scholar] [CrossRef]
- Gao, S.; Wang, Y.; Yu, S.; Huang, Y.; Liu, H.; Chen, W.; He, X. Effects of drought stress on growth, physiology and secondary metabolites of Two Adonis species in Northeast China. Sci. Hortic. 2020, 259, 108795. [Google Scholar] [CrossRef]
- Damalas, C.A. Improving drought tolerance in sweet basil (Ocimum basilicum) with salicylic acid. Sci. Hortic. 2019, 246, 360–365. [Google Scholar] [CrossRef]
- Hessini, K.; Issaoui, K.; Ferchichi, S.; Saif, T.; Abdelly, C.; Siddique, K.H.; Cruz, C. Interactive effects of salinity and nitrogen forms on plant growth, photosynthesis and osmotic adjustment in maize. Plant Physiol. Biochem. 2019, 139, 171–178. [Google Scholar] [CrossRef]
- Bukhari, M.A.; Ahmad, Z.; Ashraf, M.Y.; Afzal, M.; Nawaz, F.; Nafees, M.; Jatoi, W.N.; Malghani, N.A.; Shah, A.N.; Manan, A. Silicon Mitigates Drought Stress in Wheat (Triticum aestivum L.) through Improving Photosynthetic Pigments, Biochemical and Yield Characters. Silicon 2020, 13, 4757–4772. [Google Scholar] [CrossRef]
- Hameed, A.; Farooq, T.; Hameed, A.; Sheikh, M.A. Silicon mediated priming induces acclimation to mild water-deficit stress by altering physio-biochemical attributes in wheat plants. Front. Plant Sci. 2021, 12, 117. [Google Scholar] [CrossRef] [PubMed]
- Sarvandi, S.; Ehtesham, A.N.; Rezaei, A.N.; Azimi, M.H. Morpho-physiological responses of some iris cultivars under drought and salinity stresses. J. Agric. Sci. Tech. 2020, 22, 535–546. [Google Scholar]
- Nawaz, F.; Naeem, M.; Ashraf, M.Y.; Tahir, M.N.; Zulfiqar, B.; Salahuddin, M.; Shabbir, R.N.; Aslam, M. Selenium Supplementation Affects Physiological and Biochemical Processes to Improve Fodder Yield and Quality of Maize (Zea mays L.) under Water Deficit Conditions. Front. Plant Sci. 2016, 7, 1438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahsan, M.; Zulfiqar, H.; Farooq, M.A.; Ali, S.; Tufail, A.; Kanwal, S.; Shaheen, M.R.; Sajid, M.; Gul, H.; Jamal, A.; et al. Strigolactone (GR24) Application Positively Regulates Photosynthetic Attributes, Stress-Related Metabolites and Antioxidant Enzymatic Activities of Ornamental Sunflower (Helianthus annuus cv. Vincent’s Choice) under Salinity Stress. Agriculture 2022, 13, 50. [Google Scholar] [CrossRef]
- Farman, M.; Nawaz, F.; Majeed, S.; Javeed, H.M.R.; Ahsan, M.; Ahmad, K.S.; Aurangzaib, M.; Bukhari, M.A.; Shehzad, M.A.; Hussain, M.B. Silicon Seed Priming Combined with Foliar Spray of Sulfur Regulates Photosynthetic and Antioxidant Systems to Confer Drought Tolerance in Maize (Zea mays L.). Silicon 2022, 14, 7901–7917. [Google Scholar] [CrossRef]
- Khoshbakht, D.; Asghari, M.R.; Haghighi, M. Effects of foliar applications of nitric oxide and spermidine on chlorophyll fluorescence, photosynthesis and antioxidant enzyme activities of citrus seedlings under salinity stress. Photosynthetica 2018, 56, 1313–1325. [Google Scholar] [CrossRef]
- Amiri, R.; Nikbakht, A.; Etemadi, N. Alleviation of drought stress on rose geranium [Pelargonium graveolens (L.) Herit.] in terms of antioxidant activity and secondary metabolites by mycorrhizal inoculation. Sci. Hortic. 2015, 197, 373–380. [Google Scholar] [CrossRef]
- Dehghanipoodeh, S.; Ghobadi, C.; Baninasab, B.; Gheysari, M.; Shiranibidabadi, S. Effect of Silicon on Growth and Development of Strawberry under Water Deficit Conditions. Hortic. Plant J. 2018, 4, 226–232. [Google Scholar] [CrossRef]
- Naeem, M.; Naeem, M.S.; Ahmad, R.; Ahmad, R. Foliar-applied calcium induces drought stress tolerance in maize by manipulating osmolyte accumulation and antioxidative responses. Pak. J. Bot. 2017, 49, 427–434. [Google Scholar]
- Parveen, A.; Liu, W.; Hussain, S.; Asghar, J.; Perveen, X.Y. Silicon priming regulates morpho-physiological growth and oxidative metabolism in maize under drought stress. Plants 2019, 8, 431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anjum, S.A.; Ashraf, U.; Tanveer, M.; Khan, I.; Hussain, S.; Shahzad, B.; Zohaib, A.; Abbas, F.; Saleem, M.F.; Ali, I.; et al. Drought Induced Changes in Growth, Osmolyte Accumulation and Antioxidant Metabolism of Three Maize Hybrids. Front. Plant Sci. 2017, 8, 69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dhami, N.; Tissue, D.T.; Cazzonelli, C.I. Leaf-age dependent response of carotenoid accumulation to elevated CO2 in Arabidopsis. Arch. Biochem. Biophys. 2018, 647, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Seif, M.; Aliniaeifard, S.; Arab, M.; Mehrjerdi, M.Z.; Shomali, A.; Fanourakis, D.; Li, T.; Woltering, E. Monochromatic red light during plant growth decreases the size and improves the functionality of stomata in chrysanthemum. Funct. Plant Biol. 2021, 48, 515. [Google Scholar] [CrossRef] [PubMed]
- Asrar, A.-W.A.; Elhindi, K.M. Alleviation of drought stress of marigold (Tagetes erecta) plants by using arbuscular mycorrhizal fungi. Saudi J. Biol. Sci. 2011, 18, 93–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kiani, S.P.; Maury, P.; Sarrafi, A.; Grieu, P. QTL analysis of chlorophyll fluorescence parameters in sunflower (Helianthus annuus L.) under well-watered and water-stressed conditions. Plant Sci. 2008, 175, 565–573. [Google Scholar] [CrossRef]
- Parveen, A.; Saleem, M.H.; Kamran, M.; Haider, M.Z.; Chen, J.-T.; Malik, Z.; Rana, M.S.; Hassan, A.; Hur, G.; Javed, M.T.; et al. Effect of Citric Acid on Growth, Ecophysiology, Chloroplast Ultrastructure, and Phytoremediation Potential of Jute (Corchorus capsularis L.) Seedlings Exposed to Copper Stress. Biomolecules 2020, 10, 592. [Google Scholar] [CrossRef]
- Blum, A. Osmotic adjustment is a prime drought stress adaptive engine in support of plant production. Plant Cell Environ. 2017, 40, 4–10. [Google Scholar] [CrossRef]
- Souri, Z.; Khanna, K.; Karimi, N.; Ahmad, P. Silicon and Plants: Current Knowledge and Future Prospects. J. Plant Growth Regul. 2020, 14, 1–20. [Google Scholar] [CrossRef]
- Rastogi, A.; Tripathi, D.K.; Yadav, S.; Chauhan, D.K.; Živčák, M.; Ghorbanpour, M.; El-Sheery, N.I.; Brestic, M. Application of silicon nanoparticles in agriculture. 3 Biotech 2019, 9, 90. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Wang, Y.; Pan, K.; Li, W.; Zhang, L.; Shen, X.; Liu, L.; Deng, M. Responses of the antioxidant defense system to drought stress in the leaves of Fargesia denudata seedlings, the staple food of the giant panda. Russ. J. Plant Physiol. 2019, 3, 374–383. [Google Scholar] [CrossRef]
- Ben Ahmed, C.; Ben Rouina, B.; Sensoy, S.; Boukhris, M.; Ben Abdallah, F. Changes in gas exchange, proline accumulation and antioxidative enzyme activities in three olive cultivars under contrasting water availability regimes. Environ. Exp. Bot. 2009, 67, 345–352. [Google Scholar] [CrossRef]
- Anjum, S.A.; Xie, X.; Wang, L.; Saleem, M.F.; Man, C.; Lei, W. Morphological, physiological and biochemical responses of plants to drought stress. Afr. J. Agric. Res. 2011, 6, 2026–2032. [Google Scholar]
- Babaei, K.; Moghaddam, M.; Farhadi, N.; Pirbalouti, A.G. Morphological, physiological and phytochemical responses of Mexican marigold (Tagetes minuta L.) to drought stress. Sci. Hortic. 2021, 284, 110116. [Google Scholar] [CrossRef]
- Coskun, D.; Britto, D.T.; Huynh, W.Q.; Kronzucker, H.J. The Role of Silicon in Higher Plants under Salinity and Drought Stress. Front. Plant Sci. 2016, 7, 1072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zahedi, S.M.; Hosseini, M.S.; Hoveizeh, N.F.; Kadkhodaei, S.; Vaculík, M. Comparative morphological, physiological and molecular analyses of drought-stressed strawberry plants affected by SiO2 and SiO2-NPs foliar spray. Sci. Hortic. 2023, 309, 111686. [Google Scholar] [CrossRef]
- Oraee, A.; Tehranifar, A.; Nezami, A.; Shoor, M. Effects of drought stress on cold hardiness of non-acclimated viola (Viola × wittrockiana ‘Iona Gold with Blotch’) in controlled conditions. Sci. Hortic. 2018, 238, 98–106. [Google Scholar] [CrossRef]
- Drake, P.L.; Froend, R.H.; Franks, P.J. Smaller, faster stomata: Scaling of stomatal size, rate of response, and stomatal conductance. J. Exp. Bot. 2013, 64, 495–505. [Google Scholar] [CrossRef] [Green Version]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahsan, M.; Valipour, M.; Nawaz, F.; Raheel, M.; Abbas, H.T.; Sajid, M.; Manan, A.; Kanwal, S.; Mahmoud, E.A.; Casini, R.; et al. Evaluation of Silicon Supplementation for Drought Stress under Water-Deficit Conditions: An Application of Sustainable Agriculture. Agronomy 2023, 13, 599. https://doi.org/10.3390/agronomy13020599
Ahsan M, Valipour M, Nawaz F, Raheel M, Abbas HT, Sajid M, Manan A, Kanwal S, Mahmoud EA, Casini R, et al. Evaluation of Silicon Supplementation for Drought Stress under Water-Deficit Conditions: An Application of Sustainable Agriculture. Agronomy. 2023; 13(2):599. https://doi.org/10.3390/agronomy13020599
Chicago/Turabian StyleAhsan, Muhammad, Mohammad Valipour, Fahim Nawaz, Muhammad Raheel, Hafiz Tassawar Abbas, Mateen Sajid, Abdul Manan, Shamsa Kanwal, Eman A. Mahmoud, Ryan Casini, and et al. 2023. "Evaluation of Silicon Supplementation for Drought Stress under Water-Deficit Conditions: An Application of Sustainable Agriculture" Agronomy 13, no. 2: 599. https://doi.org/10.3390/agronomy13020599
APA StyleAhsan, M., Valipour, M., Nawaz, F., Raheel, M., Abbas, H. T., Sajid, M., Manan, A., Kanwal, S., Mahmoud, E. A., Casini, R., Elansary, H. O., Radicetti, E., & Zulfiqar, H. (2023). Evaluation of Silicon Supplementation for Drought Stress under Water-Deficit Conditions: An Application of Sustainable Agriculture. Agronomy, 13(2), 599. https://doi.org/10.3390/agronomy13020599









