Combined Effects of Cytokinin and UV-C Light on Phenolic Pattern in Ceratonia siliqua Shoot Cultures
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Explant Material, Culture Medium and Growth Conditions
2.2. Benzyl Adenine and UV Treatments
2.3. Extraction and Quantification of Photosynthetic Pigments
2.4. Extraction and Quantification of Sugars, DPPH Radical Scavenging Activity and Phenolic Compounds
2.5. Statistical Analysis
3. Results
3.1. Effect of BA Concentrations on Carob Shoot Multiplication
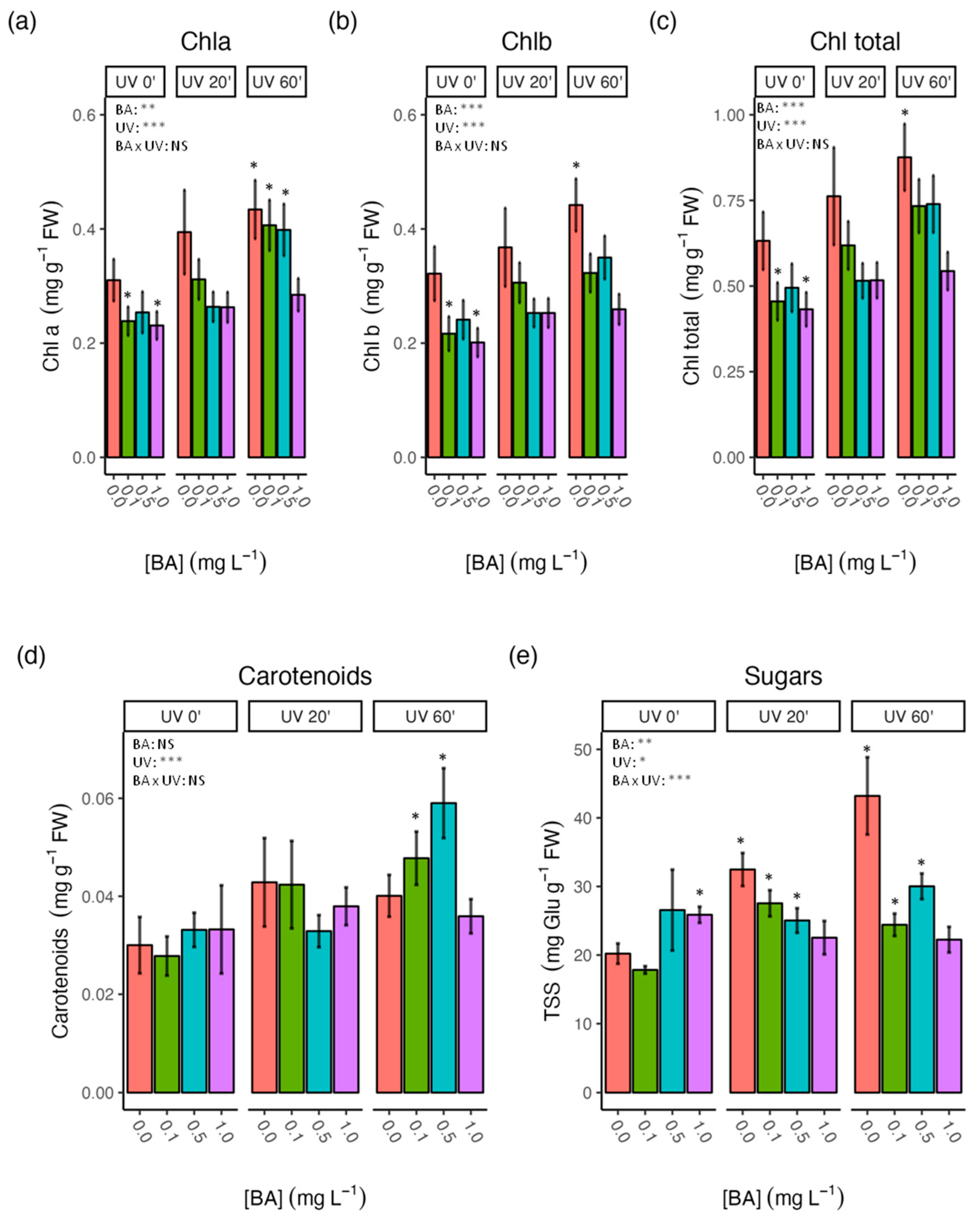
3.2. Effect of BA Concentrations and UV-C Treatments on the Physiological Performance of Carob Shoots
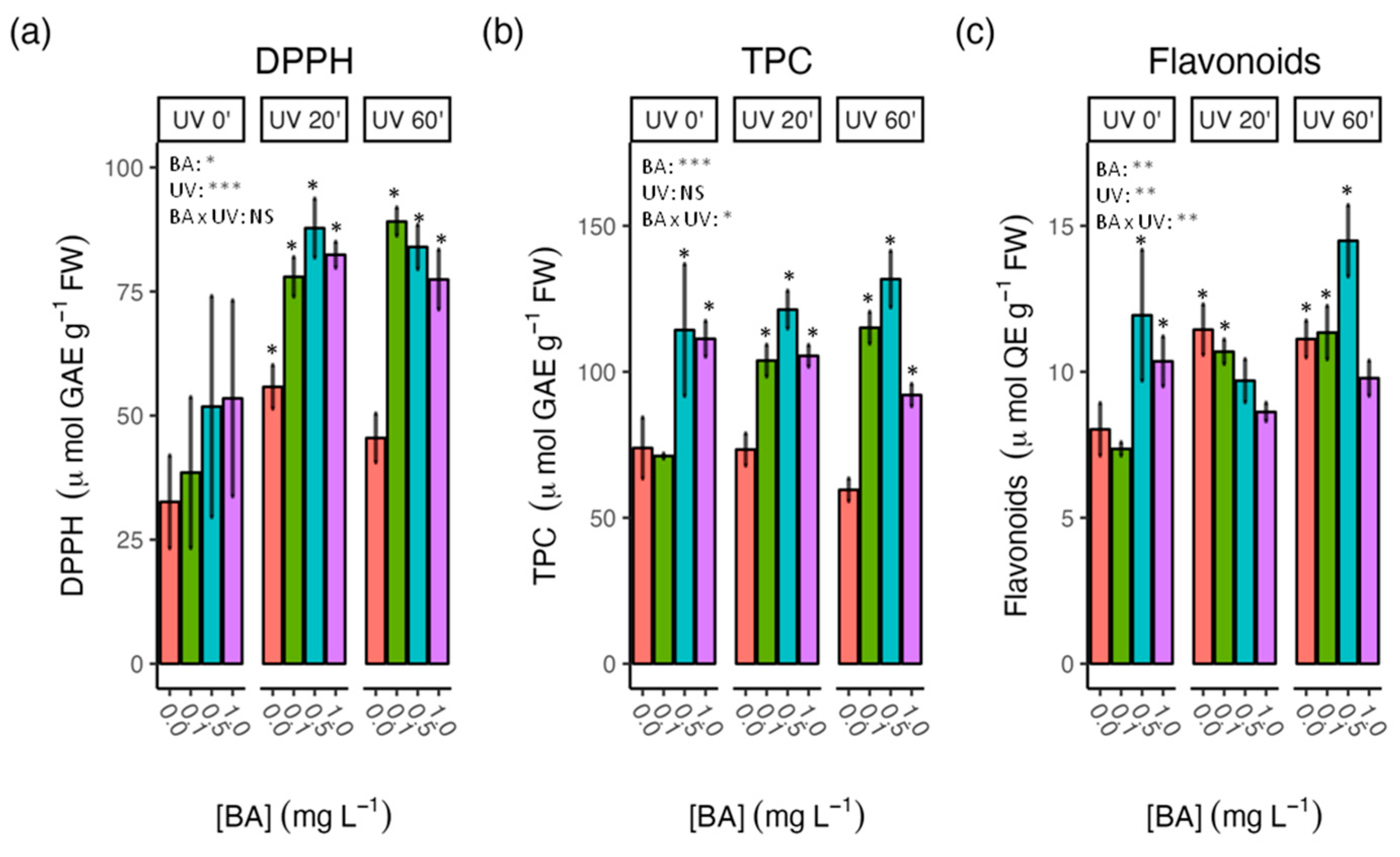
3.3. Effect of BA Concentrations and UV-C Treatments on Antioxidant Activity and Phenolic Content in Carob Shoots
3.4. Principal Component Analysis
4. Discussion
4.1. Increasing BA Doses Favored Carob Shoot Multiplication but also Increase the Development of Basal Callus
4.2. Sugar Contents Were Significantly Influenced by the Interaction between BA and UV-C Treatments
4.3. Combined BA and UV-C Treatments Further Enhanced the Antioxidant Capacity of Carob Shoots in Comparison with the Individual Treatments
4.4. Phenol Accumulation in Response to UV-C Treatments Was Influenced by BA Concentrations in the Culture Media
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alrteimei, H.A.; Ash’aari, Z.H.; Muharram, F.M. Last Decade Assessment of the Impacts of Regional Climate Change on Crop Yield Variations in the Mediterranean Region. Agriculture 2022, 12, 1787. [Google Scholar] [CrossRef]
- Harmanny, K.S.; Malek, Ž. Adaptations in Irrigated Agriculture in the Mediterranean Region: An Overview and Spatial Analysis of Implemented Strategies. Reg. Environ. Chang. 2019, 19, 1401–1416. [Google Scholar] [CrossRef] [Green Version]
- Vidal-Macua, J.J.; Ninyerola, M.; Zabala, A.; Domingo-Marimon, C.; Gonzalez-Guerrero, O.; Pons, X. Environmental and Socioeconomic Factors of Abandonment of Rainfed and Irrigated Crops in Northeast Spain. Appl. Geogr. 2018, 90, 155–174. [Google Scholar] [CrossRef]
- Batlle, I.; Tous, J. Carob Tree: Ceratonia siliqua L. Promoting the Conservation and Use of Underutilized and Neglected Crops; Institute of Plant Genetics and Crop Plant, Gatersleben/International Plant Genetic Resources Institute: Rome, Italy, 1997; Volume 17, ISBN 92-9043-328-X. [Google Scholar]
- Gioxari, A.; Amerikanou, C.; Nestoridi, I.; Gourgari, E.; Pratsinis, H.; Kalogeropoulos, N.; Andrikopoulos, N.K.; Kaliora, A.C. Carob: A Sustainable Opportunity for Metabolic Health. Foods 2022, 11, 2154. [Google Scholar] [CrossRef]
- Shahzad, A.; Akhtar, R.; Bukhari, N.A.; Perveen, K. High Incidence Regeneration System in Ceratonia siliqua L. Articulated with SEM and Biochemical Analysis during Developmental Stages. Trees 2017, 31, 1149–1163. [Google Scholar] [CrossRef]
- Pérez-García, F. Germination Characteristics and Intrapopulation Variation in Carob (Ceratonia siliqua L.) Seeds. Span. J. Agric. Res. 2009, 7, 398. [Google Scholar] [CrossRef] [Green Version]
- Altman, A.; Loberant, B. Micropropagation of Plants, Principles and Practices. In Encyclopedia of Cell Technology; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2003. [Google Scholar]
- Romano, A.; Barros, S.; Martins-Loução, M.A. Micropropagation of the Mediterranean Tree Ceratonia siliqua. Plant Cell Tissue Organ Cult. 2002, 68, 35–41. [Google Scholar] [CrossRef] [Green Version]
- Saïdi, R.; Rahmouni, S.; el Ansari, Z.N.; Maouni, A.; Badoc, A.; Lamarti, A. Effect of Cytokinins on the Micropropagation of Carob (Ceratonia siliqua L.) through Shoot Tip Culture. Am. J. Plant Sci. 2019, 10, 1469–1481. [Google Scholar] [CrossRef] [Green Version]
- Gonçalves, S.; Correia, P.J.; Martins-Loução, M.A.; Romano, A. A New Medium Formulation for in vitro Rooting of Carob Tree Based on Leaf Macronutrients Concentrations. Biol. Plant. 2005, 49, 277–280. [Google Scholar] [CrossRef]
- Hönig, M.; Plíhalová, L.; Husičková, A.; Nisler, J.; Doležal, K. Role of Cytokinins in Senescence, Antioxidant Defence and Photosynthesis. Int. J. Mol. Sci. 2018, 19, 4045. [Google Scholar] [CrossRef] [Green Version]
- Vasconcelos, J.N.C.; Brito, A.L.; Pinheiro, A.L.; Pinto, D.I.J.G.E.C.; Almeida, J.R.G.D.S.; Soares, T.L.; de Santana, J.R.F. Stimulation of 6-Benzylaminopurine and Meta-Topolin-Induced in vitro Shoot Organogenesis and Production of Flavonoids of Amburana cearensis (Allemão) A.C. Smith. Biocatal. Agric. Biotechnol. 2019, 22, 101408. [Google Scholar] [CrossRef]
- Hlophe, N.P.; Aremu, A.O.; Gruz, J.; van Staden, J.; Finnie, J.F. Influence of Different Cytokinins on the Phenolic Acids and Antioxidant Activity of Two Brachystelma Species. Plant Cell Tissue Organ Cult. 2021, 145, 689–699. [Google Scholar] [CrossRef]
- Grzegorczyk-Karolak, I.; Hnatuszko-Konka, K.; Zarzycka, M.; Kuźma, Ł. The Stimulatory Effect of Purine-Type Cytokinins on Proliferation and Polyphenolic Compound Accumulation in Shoot Culture of Salvia viridis. Biomolecules 2020, 10, 178. [Google Scholar] [CrossRef] [Green Version]
- Benson, E. Do Free Radicals Have a Role in Plant Tissue Culture Recalcitrance? Vitr. Plant Cell. Dev. Biol. 2000, 36, 163–170. [Google Scholar] [CrossRef]
- Vasconsuelo, A.; Boland, R. Molecular Aspects of the Early Stages of Elicitation of Secondary Metabolites in Plants. Plant Sci. 2007, 172, 861–875. [Google Scholar] [CrossRef]
- Yun, C.; Zhao, Z.; Ri, I.; Gao, Y.; Shi, Y.; Miao, N.; Gu, L.; Wang, W.; Wang, H. How Does UV-B Stress Affect Secondary Metabolites of Scutellaria baicalensis in vitro Shoots Grown at Different 6-benzyl Aminopurine Concentrations? Physiol. Plant. 2022, 174, e13778. [Google Scholar] [CrossRef]
- López-Orenes, A.; Ferrer, M.A.; Calderón, A.A. Microwave Radiation as an Inducer of Secondary Metabolite Production in Drosera rotundifolia in vitro Plantlets. J. Nat. Prod. 2022, 85, 2104–2109. [Google Scholar] [CrossRef] [PubMed]
- Alsoufi, A.S.M.; Pączkowski, C.; Długosz, M.; Szakiel, A. Influence of Selected Abiotic Factors on Triterpenoid Biosynthesis and Saponin Secretion in Marigold (Calendula officinalis L.) in vitro Hairy Root Cultures. Molecules 2019, 24, 2907. [Google Scholar] [CrossRef] [Green Version]
- Murashige, T.; Skoog, F. A Revised Medium for Rapid Growth and Bio Assays with Tobacco Tissue Cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- López-Orenes, A.; Bueso, M.C.; Conesa, H.; Calderón, A.A.; Ferrer, M.A. Seasonal Ionomic and Metabolic Changes in Aleppo Pines Growing on Mine Tailings under Mediterranean Semi-Arid Climate. Sci. Total Environ. 2018, 637–638, 625–635. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of Total Carotenoids and Chlorophylls a and b of Leaf Extracts in Different Solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Tortosa, V.; López-Orenes, A.; Martínez-Pérez, A.; Ferrer, M.A.; Calderón, A.A. Antioxidant Activity and Rosmarinic Acid Changes in Salicylic Acid-Treated Thymus membranaceus Shoots. Food Chem. 2012, 130, 362–369. [Google Scholar] [CrossRef]
- Kosalec, I.; Bakmaz, M.; Pepeljnjak, S.; Vladimir-Knezević, S. Quantitative Analysis of the Flavonoids in Raw Propolis from Northern Croatia. Acta Pharm. 2004, 1, 65–72. [Google Scholar]
- Zheng, W.; Wang, S.Y. Antioxidant Activity and Phenolic Compounds in Selected Herbs. J. Agric. Food Chem. 2001, 49, 5165–5170. [Google Scholar] [CrossRef]
- Stavrou, I.J.; Christou, A.; Kapnissi-Christodoulou, C.P. Polyphenols in Carobs: A Review on Their Composition, Antioxidant Capacity and Cytotoxic Effects, and Health Impact. Food Chem. 2018, 269, 355–374. [Google Scholar] [CrossRef]
- Baťková, P.; Pospíšilová, J.; Synková, H. Production of Reactive Oxygen Species and Development of Antioxidative Systems during in vitro Growth and ex vitro Transfer. Biol. Plant. 2008, 52, 413–422. [Google Scholar] [CrossRef]
- Lozzi, A.; Abdelwahd, R.; Mentag, R.; Abousalim, A. Development of a New Culture Medium and Efficient Protocol for in vitro Micropropagation of Ceratonia siliqua L. Vitr. Cell. Dev. Biol.-Plant 2019, 55, 615–624. [Google Scholar] [CrossRef]
- Ahmed, M.; El-Fadl, R.; Hegazi, G.; Elaziem, T. Improving Micropropagation Protocol for Carob (Ceratonia siliqua). Plant Cell Biotechnol. Mol. Biol. 2021, 22, 84–94. [Google Scholar]
- Ikeuchi, M.; Sugimoto, K.; Iwase, A. Plant Callus: Mechanisms of Induction and Repression. Plant Cell 2013, 25, 3159–3173. [Google Scholar] [CrossRef] [Green Version]
- Cassells, A.C.; Curry, R.F. Oxidative Stress and Physiological, Epigenetic and Genetic Variability in Plant Tissue Culture: Implications for Micropropagators and Genetic Engineers. Plant Cell Tissue Organ Cult. 2001, 64, 145–157. [Google Scholar] [CrossRef]
- Xu, K.; Qiu, B.S. Responses of Superhigh-Yield Hybrid Rice Liangyoupeijiu to Enhancement of Ultraviolet-B Radiation. Plant Sci. 2007, 172, 139–149. [Google Scholar] [CrossRef]
- Siddiqui, A.; Dawar, S.; Javed Zaki, M.; Hamid, N. Role of Ultraviolet (UV-C) Radiation in the Control of Root Infecting Fungi on Groundnut and Mung Bean. Pak. J. Bot. 2011, 43, 2221–2224. [Google Scholar]
- Thomas, D.T.T.; Puthur, J.T. UV Radiation Priming: A Means of Amplifying the Inherent Potential for Abiotic Stress Tolerance in Crop Plants. Environ. Exp. Bot. 2017, 138, 57–66. [Google Scholar] [CrossRef]
- Bornman, J.F.; Evert, R.F.; Mierzwa, R.J. The Effect of UV-B and UV-C Radiation on Sugar Beet Leaves. Protoplasma 1983, 117, 7–16. [Google Scholar] [CrossRef]
- Lin, Q.; Xie, Y.; Liu, W.; Zhang, J.; Cheng, S.; Xie, X.; Guan, W.; Wang, Z. UV-C Treatment on Physiological Response of Potato (Solanum tuberosum L.) during Low Temperature Storage. J. Food Sci. Technol. 2017, 54, 55–61. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.; Ge, Z.; Limwachiranon, J.; Li, L.; Li, W.; Luo, Z. UV-C Treatment Affects Browning and Starch Metabolism of Minimally Processed Lily Bulb. Postharvest Biol. Technol. 2017, 128, 105–111. [Google Scholar] [CrossRef]
- Aremu, A.O.; Bairu, M.W.; Szüčová, L.; Finnie, J.F.; van Staden, J. The Role of Meta-Topolins on the Photosynthetic Pigment Profiles and Foliar Structures of Micropropagated ‘Williams’ Bananas. J. Plant Physiol. 2012, 169, 1530–1541. [Google Scholar] [CrossRef]
- Urban, L.; Chabane Sari, D.; Orsal, B.; Lopes, M.; Miranda, R.; Aarrouf, J. UV-C Light and Pulsed Light as Alternatives to Chemical and Biological Elicitors for Stimulating Plant Natural Defenses against Fungal Diseases. Sci. Hortic. 2018, 235, 452–459. [Google Scholar] [CrossRef]
- Agati, G.; Brunetti, C.; di Ferdinando, M.; Ferrini, F.; Pollastri, S.; Tattini, M. Functional Roles of Flavonoids in Photoprotection: New Evidence, Lessons from the Past. Plant Physiol. Biochem. 2013, 72, 35–45. [Google Scholar] [CrossRef]
- Fabón, G.; Martínez-Abaigar, J.; Tomás, R.; Núñez-Olivera, E. Effects of Enhanced UV-B Radiation on Hydroxycinnamic Acid Derivatives Extracted from Different Cell Compartments in the Aquatic Liverwort Jungermannia exsertifolia subsp. cordifolia. Physiol. Plant. 2010, 140, 269–279. [Google Scholar] [CrossRef]
- Morales, L.O.; Tegelberg, R.; Brosche, M.; Keinanen, M.; Lindfors, A.; Aphalo, P.J. Effects of Solar UV-A and UV-B Radiation on Gene Expression and Phenolic Accumulation in Betula pendula Leaves. Tree Physiol. 2010, 30, 923–934. [Google Scholar] [CrossRef] [Green Version]
- Urban, L.; Charles, F.; de Miranda, M.R.A.; Aarrouf, J. Understanding the Physiological Effects of UV-C Light and Exploiting Its Agronomic Potential before and after Harvest. Plant Physiol. Biochem. 2016, 105, 1–11. [Google Scholar] [CrossRef]
- López-Orenes, A.; Ros-Marín, A.F.; Ferrer, M.A.; Calderón, A.A. Antioxidant Capacity as a Marker for Assessing the in vitro Performance of the Endangered Cistus heterophyllus. Sci. World J. 2013, 2013, 176295. [Google Scholar] [CrossRef] [Green Version]
- Dawood, M.F.A.; Abu-Elsaoud, A.M.; Sofy, M.R.; Mohamed, H.I.; Soliman, M.H. Appraisal of Kinetin Spraying Strategy to Alleviate the Harmful Effects of UVC Stress on Tomato Plants. Environ. Sci. Pollut. Res. 2022, 29, 52378–52398. [Google Scholar] [CrossRef]
- Singh, M.; Bashri, G.; Prasad, S.M.; Singh, V.P. Kinetin Alleviates UV-B-Induced Damage in Solanum lycopersicum: Implications of Phenolics and Antioxidants. J. Plant Growth Regul. 2019, 38, 831–841. [Google Scholar] [CrossRef]
- Zwack, P.J.; Rashotte, A.M. Interactions between Cytokinin Signalling and Abiotic Stress Responses. J. Exp. Bot. 2015, 66, 4863–4871. [Google Scholar] [CrossRef] [Green Version]
- Li, S.-M.; Zheng, H.-X.; Zhang, X.-S.; Sui, N. Cytokinins as Central Regulators during Plant Growth and Stress Response. Plant Cell Rep. 2021, 40, 271–282. [Google Scholar] [CrossRef]
- Dudonné, S.; Vitrac, X.; Coutière, P.; Woillez, M.; Mérillon, J.-M. Comparative Study of Antioxidant Properties and Total Phenolic Content of 30 Plant Extracts of Industrial Interest Using DPPH, ABTS, FRAP, SOD, and ORAC Assays. J. Agric. Food Chem. 2009, 57, 1768–1774. [Google Scholar] [CrossRef]
- López-Orenes, A.; Martínez-Moreno, J.M.; Calderón, A.A.; Ferrer, M.A. Changes in Phenolic Metabolism in Salicylic Acid-Treated Shoots of Cistus heterophyllus. Plant Cell Tissue Organ Cult. 2013, 113, 417–427. [Google Scholar] [CrossRef]
- Ferreyra, M.L.F.; Serra, P.; Casati, P. Recent Advances on the Roles of Flavonoids as Plant Protective Molecules after UV and High Light Exposure. Physiol. Plant. 2021, 173, 736–749. [Google Scholar] [CrossRef]
- Aremu, A.O.; Gruz, J.; Šubrtová, M.; Szüčová, L.; Doležal, K.; Bairu, M.W.; Finnie, J.F.; van Staden, J. Antioxidant and Phenolic Acid Profiles of Tissue Cultured and Acclimatized Merwilla plumbea Plantlets in Relation to the Applied Cytokinins. J. Plant Physiol. 2013, 170, 1303–1308. [Google Scholar] [CrossRef]
- Moyo, M.; Amoo, S.O.; Aremu, A.O.; Gruz, J.; Šubrtová, M.; Doležal, K.; van Staden, J. Plant Regeneration and Biochemical Accumulation of Hydroxybenzoic and Hydroxycinnamic Acid Derivatives in Hypoxis hemerocallidea Organ and Callus Cultures. Plant Sci. 2014, 227, 157–164. [Google Scholar] [CrossRef]
- de Falco, B.; Grauso, L.; Fiore, A.; Bonanomi, G.; Lanzotti, V. Metabolomics and Chemometrics of Seven Aromatic Plants: Carob, Eucalyptus, Laurel, Mint, Myrtle, Rosemary and Strawberry Tree. Phytochem. Anal. 2022, 33, 696–709. [Google Scholar] [CrossRef]
- Ramirez-Estrada, K.; Vidal-Limon, H.; Hidalgo, D.; Moyano, E.; Golenioswki, M.; Cusidó, R.; Palazon, J. Elicitation, an Effective Strategy for the Biotechnological Production of Bioactive High-Added Value Compounds in Plant Cell Factories. Molecules 2016, 21, 182. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| BA Concentration (mg L−1) | Number of Branches per Explant | Shoot Length (cm) | Presence of Basal Callus (%) | Size of Basal Callus (Diameter, mm) |
|---|---|---|---|---|
| 0.0 | No ramification | 2.1 ± 0.2 a | 33.3 d | 1.0 ± 0.1 d |
| 0.1 | 1.53 ± 0.13 c | 1.8 ± 0.2 a | 66.7 c | 3.0 ± 0.1 c |
| 0.5 | 2.52 ± 0.13 b | 1.3 ± 0.1 b | 88.9 b | 3.5 ± 0.2 b |
| 1.0 | 2.93 ± 0.12 a | 1.0 ± 0.0 c | 100 a | 4.6 ± 0.2 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costa-Pérez, A.; Ferrer, M.A.; Calderón, A.A. Combined Effects of Cytokinin and UV-C Light on Phenolic Pattern in Ceratonia siliqua Shoot Cultures. Agronomy 2023, 13, 621. https://doi.org/10.3390/agronomy13030621
Costa-Pérez A, Ferrer MA, Calderón AA. Combined Effects of Cytokinin and UV-C Light on Phenolic Pattern in Ceratonia siliqua Shoot Cultures. Agronomy. 2023; 13(3):621. https://doi.org/10.3390/agronomy13030621
Chicago/Turabian StyleCosta-Pérez, Antonio, María A. Ferrer, and Antonio A. Calderón. 2023. "Combined Effects of Cytokinin and UV-C Light on Phenolic Pattern in Ceratonia siliqua Shoot Cultures" Agronomy 13, no. 3: 621. https://doi.org/10.3390/agronomy13030621
APA StyleCosta-Pérez, A., Ferrer, M. A., & Calderón, A. A. (2023). Combined Effects of Cytokinin and UV-C Light on Phenolic Pattern in Ceratonia siliqua Shoot Cultures. Agronomy, 13(3), 621. https://doi.org/10.3390/agronomy13030621







