Integrative Transcriptome and Chlorophyll Fluorescence Test Analysis Shed New Light on the Leaf Senescence Mechanism of Zoysia japonica
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
2.2. Chlorophyll, Soluble Sugar, and Plant Hormones Measurements
2.3. Chlorophyll a Fluorescence Measurement and JIP-Test Parameters Analysis
2.4. Illumina cDNA Library Construction and Sequencing
2.5. Transcriptomic Analysis
2.6. qRT-PCR Verification of DEGs
2.7. GO and Pathway Enrichment Analysis
3. Results
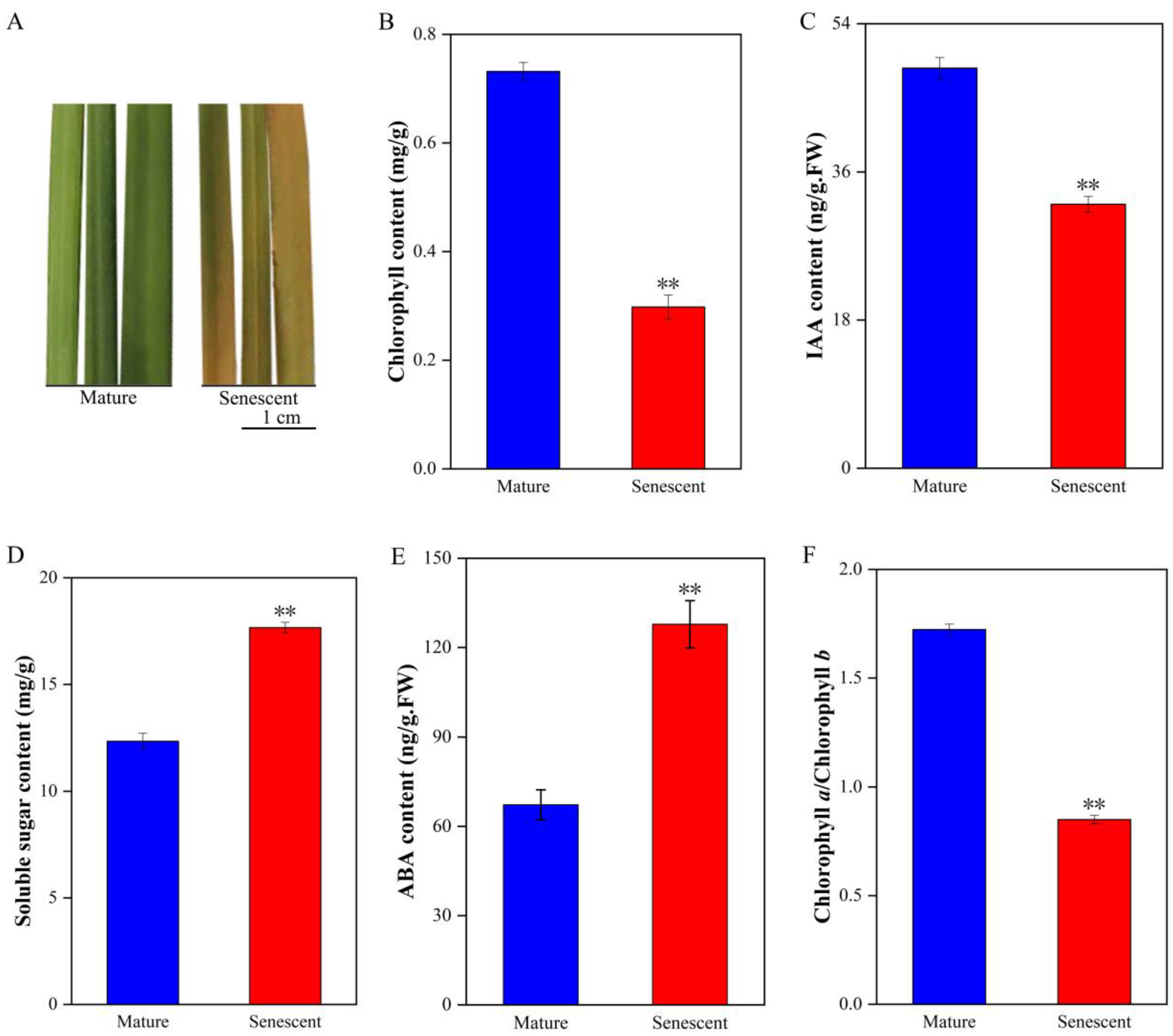
3.1. Physiological Changes and Hormone Levels Differences in Mature and Senescent Leaves
3.2. RNA-Seq Analysis of Z. japonica and the Identification of DEGs
3.3. SAG Conservation Analyses
3.4. GO and KEGG Analysis
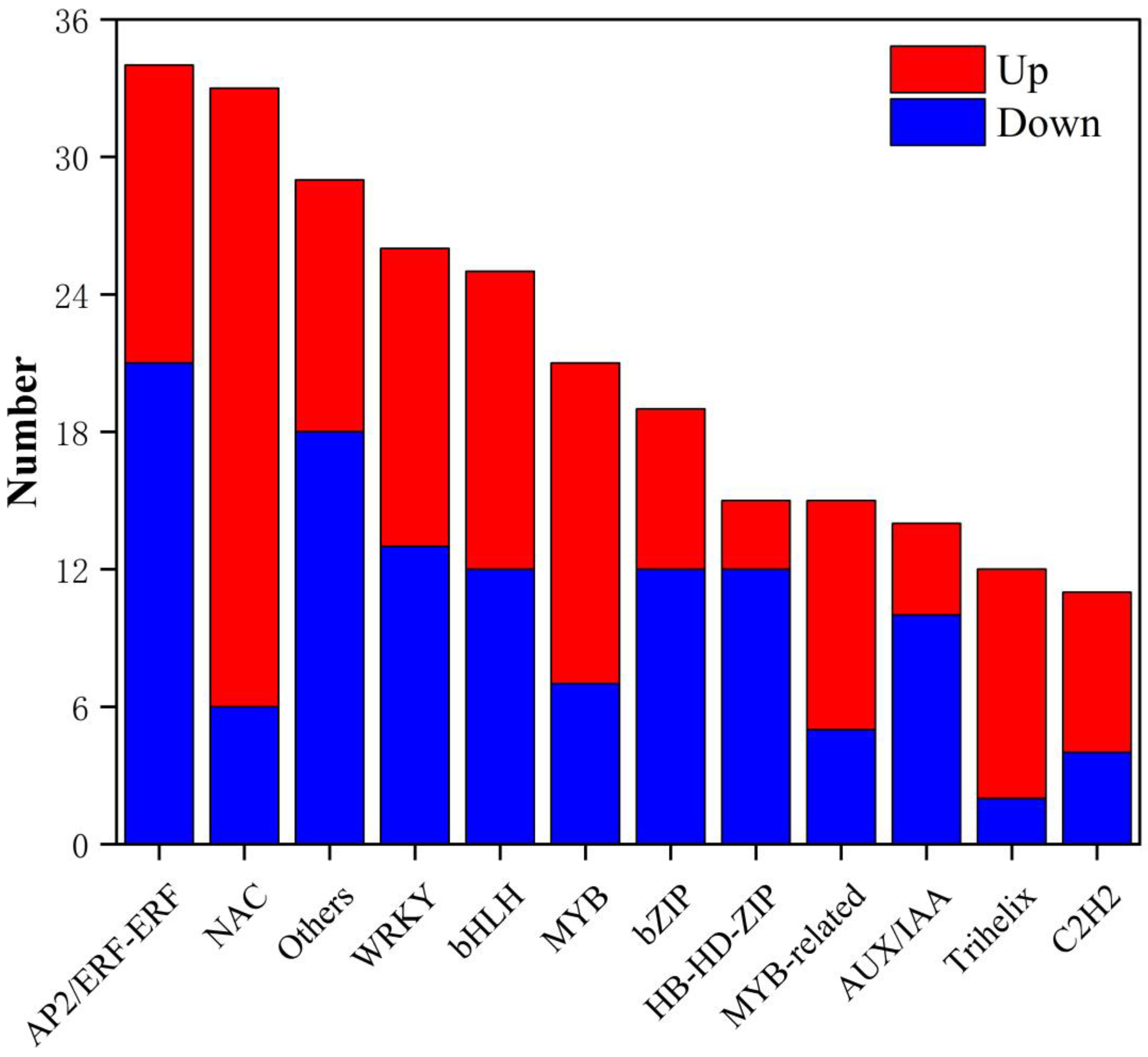
3.5. Transcription Factor Analysis
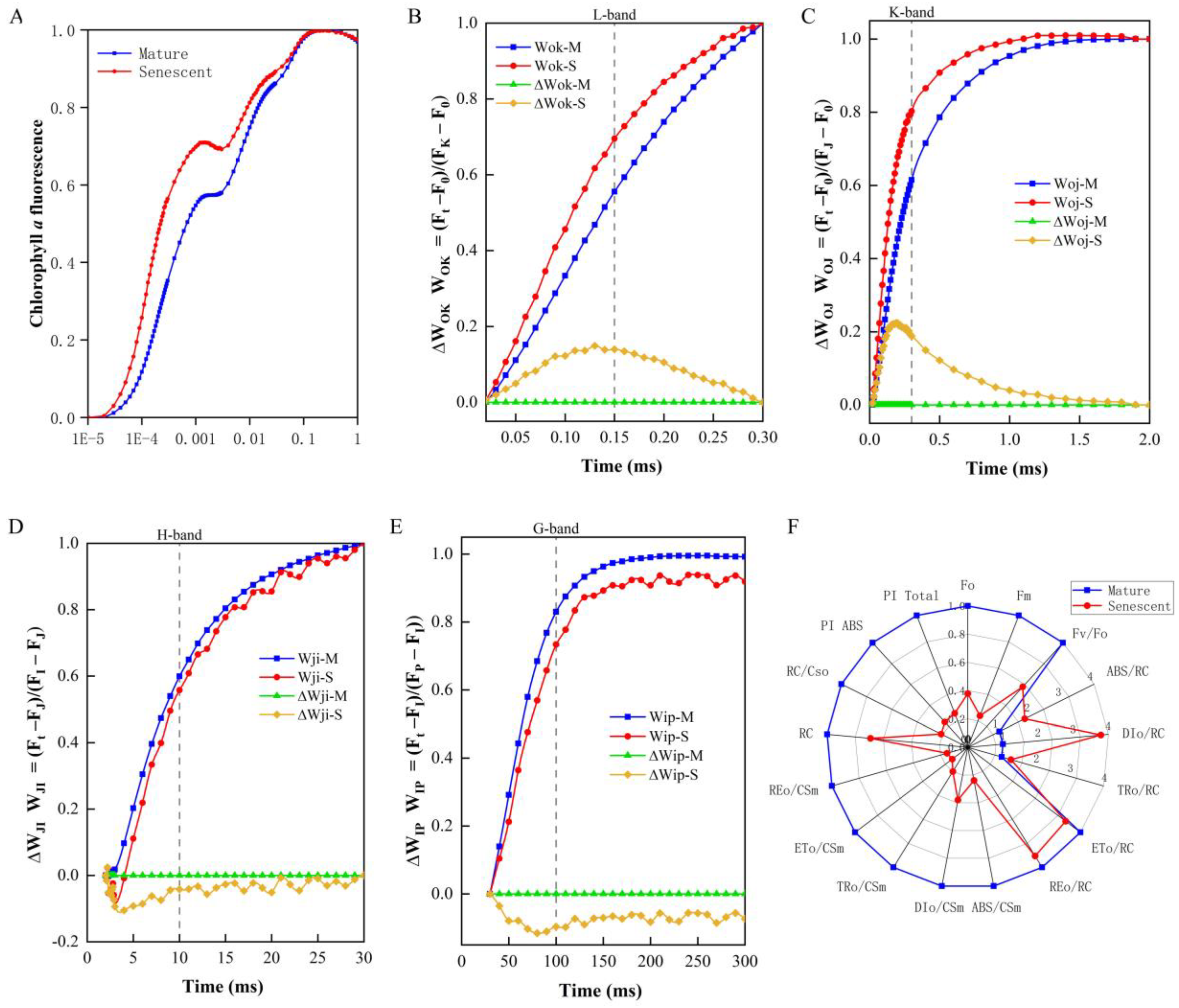
3.6. JIP-Test Demonstrated Senescence Inhibited Leaf Photosynthetic Capacity
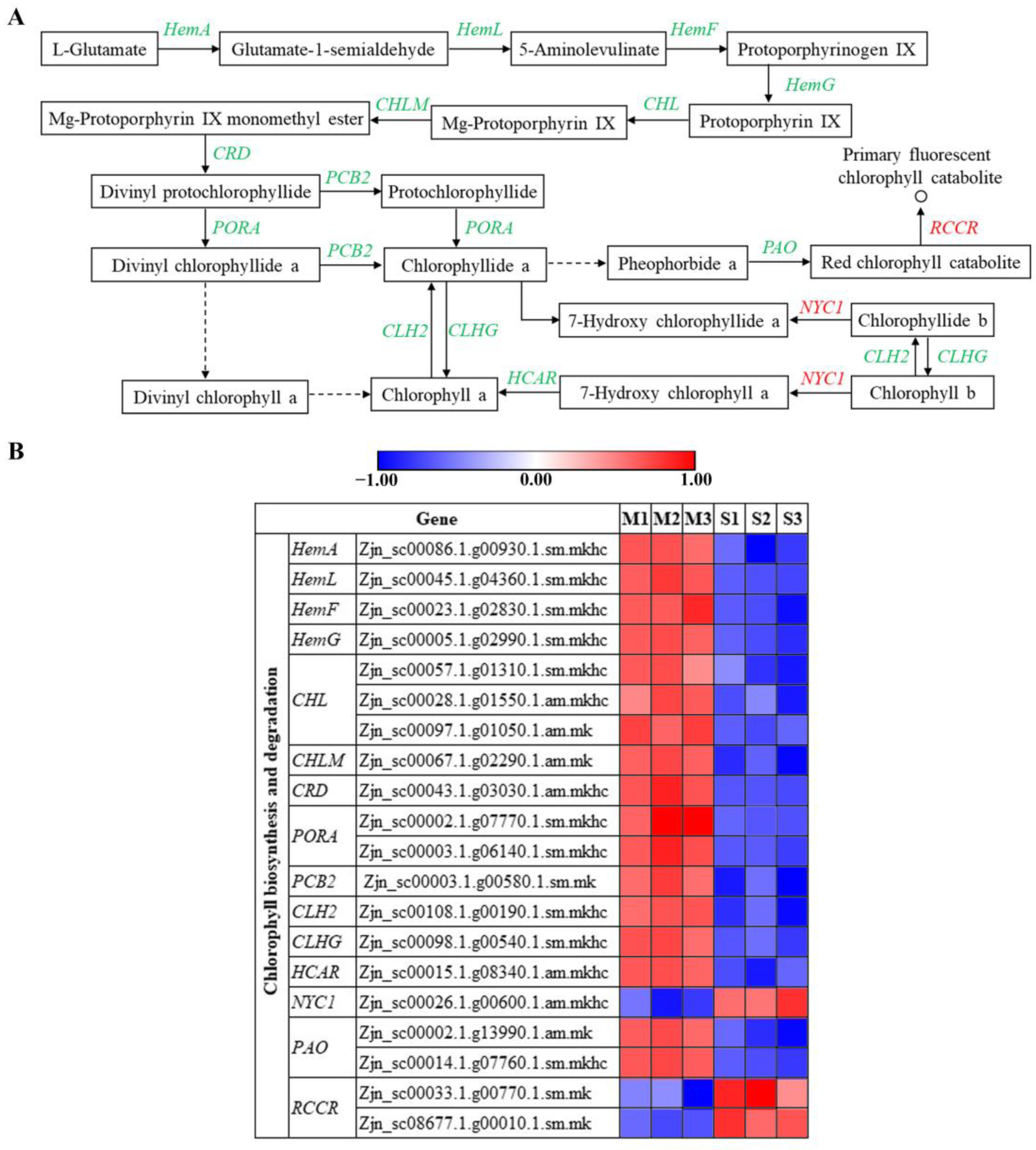
3.7. Genes Regulating Chlorophyll Biosynthesis and Metabolism Are Involved in Leaf Senescence
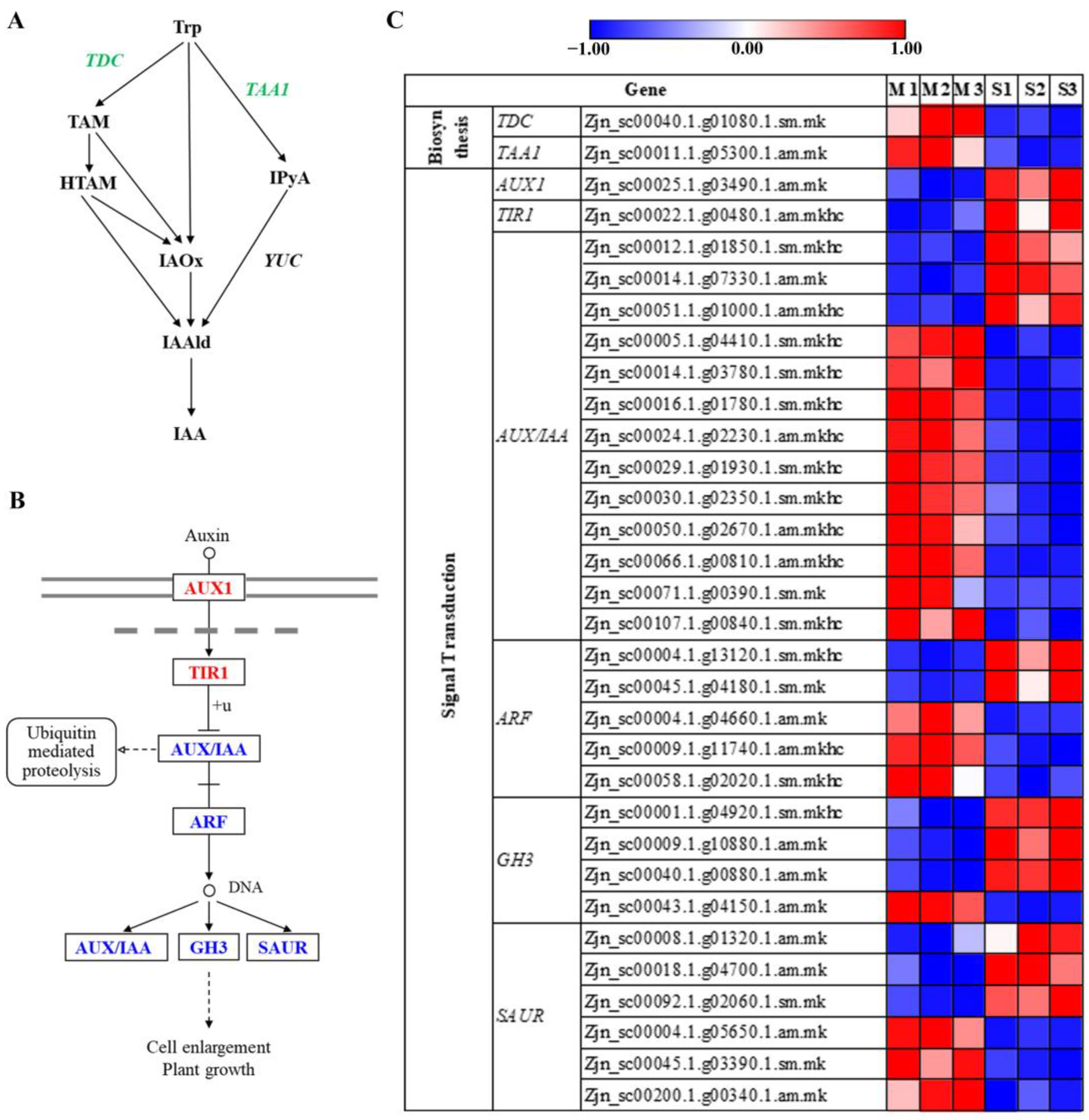
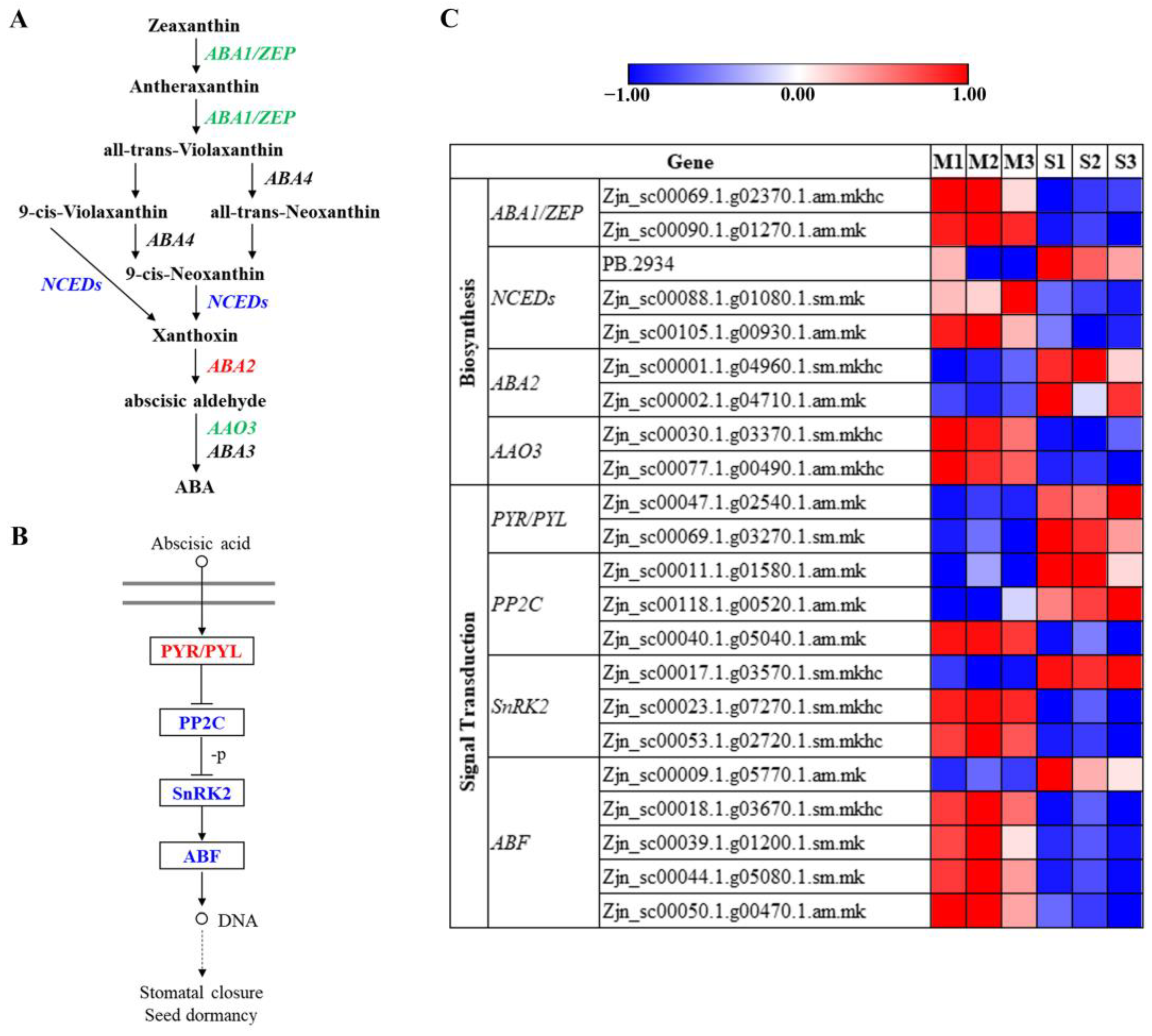
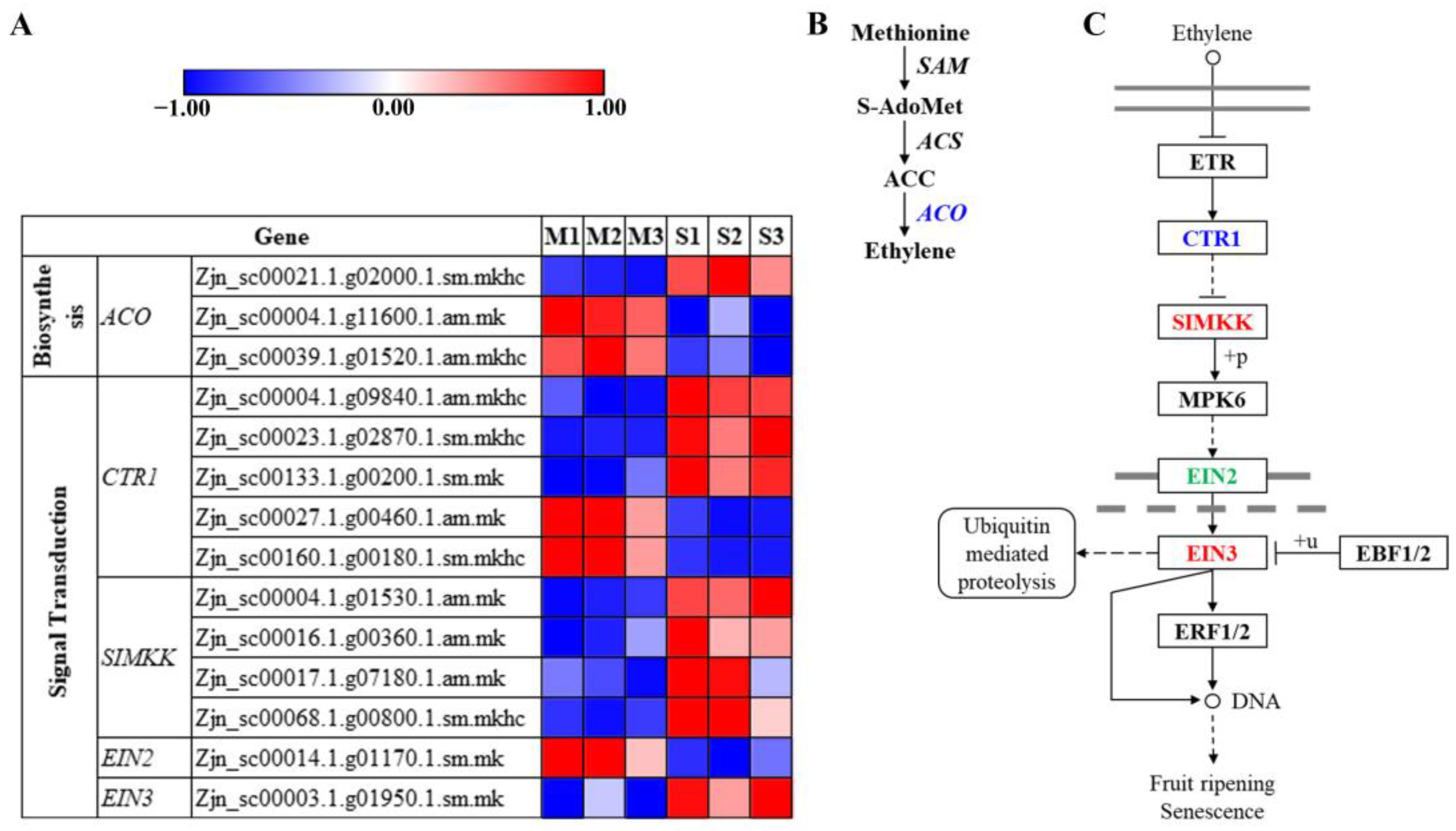
3.8. Plant Hormone Responses during Leaf Senescence in Z. japonica
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lim, P.O.; Kim, H.J.; Nam, H.G. Leaf Senescence. Annu. Rev. Plant Biol. 2007, 58, 115–136. [Google Scholar] [CrossRef]
- Gao, S.; Gao, J.; Zhu, X.; Song, Y.; Li, Z.; Ren, G.; Zhou, X.; Kuai, B. ABF2, ABF3, and ABF4 promote ABA-mediated chlorophyll degradation and leaf senescence by transcriptional activation of chlorophyll catabolic genes and senescence-associated genes in Arabidopsis. Mol. Plant 2016, 9, 1272–1285. [Google Scholar] [CrossRef]
- Schippers, J.H.M.; Schmidt, R.; Wagstaff, C.; Jing, H.-C. Living to die and dying to live: The survival strategy behind leaf senescence. Plant Physiol. 2015, 169, 914–930. [Google Scholar] [CrossRef]
- Chao, Y.; Xie, L.; Yuan, J.; Guo, T.; Li, Y.; Liu, F.; Han, L. Transcriptome analysis of leaf senescence in red clover (Trifolium pratense L.). Physiol. Mol. Biol. Plants 2018, 24, 753–765. [Google Scholar] [CrossRef]
- Xu, B.; Li, H.; Li, Y.; Yu, G.; Zhang, J.; Huang, B. Characterization and transcriptional regulation of chlorophyll b reductase gene NON-YELLOW COLORING 1 associated with leaf senescence in perennial ryegrass (Lolium perenne L.). Environ. Exp. Bot. 2018, 149, 43–50. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Y.; Zou, D.; Zhao, Y.; Wang, H.-L.; Zhang, Y.; Xia, X.; Luo, J.; Guo, H.; Zhang, Z. LSD 3.0: A comprehensive resource for the leaf senescence research community. Nucleic Acids Res. 2020, 48, D1069–D1075. [Google Scholar] [CrossRef]
- Kim, J.; Woo, H.R.; Nam, H.G. Toward systems understanding of leaf senescence: An integrated multi-omics perspective on leaf senescence research. Mol. Plant 2016, 9, 813–825. [Google Scholar] [CrossRef]
- Lin, M.; Pang, C.; Fan, S.; Song, M.; Wei, H.; Yu, S. Global analysis of the Gossypium hirsutum L. Transcriptome during leaf senescence by RNA-Seq. BMC Plant Biol. 2015, 15, 43. [Google Scholar] [CrossRef]
- Zhang, J.; Yu, G.; Wen, W.; Ma, X.; Xu, B.; Huang, B. Functional characterization and hormonal regulation of the PHEOPHYTINASE gene LpPPH controlling leaf senescence in perennial ryegrass. J. Exp. Bot. 2016, 67, 935–945. [Google Scholar] [CrossRef]
- Teng, K.; Tan, P.; Guan, J.; Dong, D.; Liu, L.; Guo, Y.; Guo, W.; Yuesen, Y.; Fan, X.; Wu, J. Functional characterization of the chlorophyll b reductase gene NYC1 associated with chlorophyll degradation and photosynthesis in Zoysia japonica. Environ. Exp. Bot. 2021, 191, 104607. [Google Scholar] [CrossRef]
- Teng, K.; Han, C.; Yue, Y.; Xu, L.; Li, H.; Wu, J.; Fan, X. Functional characterization of the pheophytinase gene, ZjPPH, from Zoysia japonica in regulating chlorophyll degradation and photosynthesis. Front. Plant Sci. 2021, 12, 786570. [Google Scholar] [CrossRef]
- Yu, G.; Xie, Z.; Lei, S.; Li, H.; Xu, B.; Huang, B. The NAC factor LpNAL delays leaf senescence by repressing two chlorophyll catabolic genes in perennial ryegrass. Plant Physiol. 2022, 189, 595–610. [Google Scholar] [CrossRef]
- Balazadeh, S.; Riaño-Pachón, D.; Mueller-Roeber, B. Transcription factors regulating leaf senescence in Arabidopsis thaliana. Plant Biol. 2008, 10, 63–75. [Google Scholar] [CrossRef]
- Xu, H.; Wang, X.; Chen, J. Overexpression of the Rap2. 4f transcriptional factor in Arabidopsis promotes leaf senescence. Sci. China Life Sci. 2010, 53, 1221–1226. [Google Scholar] [CrossRef]
- Yang, S.-D.; Seo, P.J.; Yoon, H.-K.; Park, C.-M. The Arabidopsis NAC Transcription Factor VNI2 Integrates Abscisic Acid Signals into Leaf Senescence via the COR/RD Genes. Plant Cell 2011, 23, 2155–2168. [Google Scholar] [CrossRef]
- Farage-Barhom, S.; Burd, S.; Sonego, L.; Mett, A.; Belausov, E.; Gidoni, D.; Lers, A. Localization of the Arabidopsis senescence- and cell death-associated BFN1 nuclease: From the ER to fragmented nuclei. Mol. Plant 2011, 4, 1062–1073. [Google Scholar] [CrossRef]
- Matallana-Ramirez, L.P.; Rauf, M.; Farage-Barhom, S.; Dortay, H.; Xue, G.-P.; Dröge-Laser, W.; Lers, A.; Balazadeh, S.; Mueller-Roeber, B. NAC transcription factor ORE1 and senescence-induced BIFUNCTIONAL NUCLEASE1 (BFN1) constitute a regulatory cascade in Arabidopsis. Mol. Plant 2013, 6, 1438–1452. [Google Scholar] [CrossRef]
- Qiu, K.; Li, Z.; Yang, Z.; Chen, J.; Wu, S.; Zhu, X.; Gao, S.; Gao, J.; Ren, G.; Kuai, B.; et al. EIN3 and ORE1 Accelerate Degreening during Ethylene-Mediated Leaf Senescence by Directly Activating Chlorophyll Catabolic Genes in Arabidopsis. PLoS Genet. 2015, 11, e1005399. [Google Scholar] [CrossRef]
- Guo, Y.; Gan, S. AtNAP, a NAC family transcription factor, has an important role in leaf senescence. Plant J. 2006, 46, 601–612. [Google Scholar] [CrossRef]
- Zhang, K.; Gan, S.-S. An abscisic acid-AtNAP transcription factor-SAG113 protein phosphatase 2C regulatory chain for controlling dehydration in senescing Arabidopsis leaves. Plant Physiol. 2012, 158, 961–969. [Google Scholar] [CrossRef]
- Rinerson, C.I.; Scully, E.D.; Palmer, N.A.; Donze-Reiner, T.; Rabara, R.C.; Tripathi, P.; Shen, Q.J.; Sattler, S.E.; Rohila, J.S.; Sarath, G.; et al. The WRKY transcription factor family and senescence in switchgrass. BMC Genom. 2015, 16, 912. [Google Scholar] [CrossRef]
- Jaradat, M.R.; Feurtado, J.A.; Huang, D.; Lu, Y.; Cutler, A.J. Multiple roles of the transcription factor AtMYBR1/AtMYB44 in ABA signaling, stress responses, and leaf senescence. BMC Plant Biol. 2013, 13, 192. [Google Scholar] [CrossRef]
- Miao, Y.; Zentgraf, U. A HECT E3 ubiquitin ligase negatively regulates Arabidopsis leaf senescence through degradation of the transcription factor WRKY53. Plant J. 2010, 63, 179–188. [Google Scholar] [CrossRef]
- Besseau, S.; Li, J.; Palva, E.T. WRKY54 and WRKY70 co-operate as negative regulators of leaf senescence in Arabidopsis thaliana. J. Exp. Bot. 2012, 63, 2667–2679. [Google Scholar] [CrossRef]
- Li, Z.; Peng, J.; Wen, X.; Guo, H. Gene Network Analysis and Functional Studies of Senescence-associated Genes Reveal Novel Regulators of Arabidopsis Leaf Senescence. J. Integr. Plant Biol. 2012, 54, 526–539. [Google Scholar] [CrossRef]
- Bajguz, A.; Piotrowska, A. Conjugates of auxin and cytokinin. Phytochemistry 2009, 70, 957–969. [Google Scholar] [CrossRef]
- Chen, Q.; Westfall, C.S.; Hicks, L.M.; Wang, S.; Jez, J.M. Kinetic basis for the conjugation of auxin by a GH3 family indole-acetic acid-amido synthetase. J. Biol. Chem. 2010, 285, 29780–29786. [Google Scholar] [CrossRef]
- Kim, J.I.; Murphy, A.S.; Baek, D.; Lee, S.-W.; Yun, D.-J.; Bressan, R.A.; Narasimhan, M.L. YUCCA6 over-expression demonstrates auxin function in delaying leaf senescence in Arabidopsis thaliana. J. Exp. Bot. 2011, 62, 3981–3992. [Google Scholar] [CrossRef]
- Urano, K.; Maruyama, K.; Jikumaru, Y.; Kamiya, Y.; Yamaguchi-Shinozaki, K.; Shinozaki, K. Analysis of plant hormone profiles in response to moderate dehydration stress. Plant J. 2017, 90, 17–36. [Google Scholar] [CrossRef]
- Kieber, J.J.; Schaller, G.E. Cytokinins. Arab. Book/Am. Soc. Plant Biol. 2014, 12, e0168. [Google Scholar] [CrossRef]
- Wasternack, C.; Hause, B. Jasmonates: Biosynthesis, perception, signal transduction and action in plant stress response, growth and development. An update to the 2007 review in Annals of Botany. Ann. Bot. 2013, 111, 1021–1058. [Google Scholar] [CrossRef]
- Yu, X.; Xu, Y.; Yan, S. Salicylic acid and ethylene coordinately promote leaf senescence. J. Integr. Plant Biol. 2021, 63, 823–827. [Google Scholar] [CrossRef]
- Wang, C.; Dai, S.; Zhang, Z.L.; Lao, W.; Wang, R.; Meng, X.; Zhou, X. Ethylene and salicylic acid synergistically accelerate leaf senescence in Arabidopsis. J. Integr. Plant Biol. 2021, 63, 828–833. [Google Scholar] [CrossRef]
- Kim, H.J.; Hong, S.H.; Kim, Y.W.; Lee, I.H.; Jun, J.H.; Phee, B.-K.; Rupak, T.; Jeong, H.; Lee, Y.; Hong, B.S. Gene regulatory cascade of senescence-associated NAC transcription factors activated by ETHYLENE-INSENSITIVE2-mediated leaf senescence signalling in Arabidopsis. J. Exp. Bot. 2014, 65, 4023–4036. [Google Scholar] [CrossRef]
- Kim, J.H.; Woo, H.R.; Kim, J.; Lim, P.O.; Lee, I.C.; Choi, S.H.; Hwang, D.; Nam, H.G. Trifurcate feed-forward regulation of age-dependent cell death involving miR164 in Arabidopsis. Science 2009, 323, 1053–1057. [Google Scholar] [CrossRef]
- Li, Z.; Peng, J.; Wen, X.; Guo, H. Ethylene-insensitive3 is a senescence-associated gene that accelerates age-dependent leaf senescence by directly repressing miR164 transcription in Arabidopsis. Plant Cell 2013, 25, 3311–3328. [Google Scholar] [CrossRef]
- Zhao, Y.; Chan, Z.; Gao, J.; Xing, L.; Cao, M.; Yu, C.; Hu, Y.; You, J.; Shi, H.; Zhu, Y. ABA receptor PYL9 promotes drought resistance and leaf senescence. Proc. Natl. Acad. Sci. USA 2016, 113, 1949–1954. [Google Scholar] [CrossRef]
- Patton, A.J.; Reicher, Z.J. Zoysiagrass species and genotypes differ in their winter injury and freeze tolerance. Crop Sci. 2007, 47, 1619–1627. [Google Scholar] [CrossRef]
- Kimball, J.A.; Zuleta, M.C.; Kenworthy, K.E.; Lehman, V.G.; Harris-Shultz, K.R.; Milla-Lewis, S. Genetic relationships in Zoysia species and the identification of putative interspecific hybrids using simple sequence repeat markers and inflorescence traits. Crop Sci. 2013, 53, 285–295. [Google Scholar] [CrossRef]
- Tanaka, H.; Tokunaga, R.; Muguerza, M.; Kitazaki, Y.; Hashiguchi, M.; Sato, S.; Tabata, S.; Akashi, R. Genetic structure and speciation of zoysiagrass ecotypes collected in Japan. Crop Sci. 2016, 56, 818–826. [Google Scholar] [CrossRef]
- Teng, K.; Tan, P.; Xiao, G.; Han, L.; Chang, Z.; Chao, Y. Heterologous expression of a novel Zoysia japonica salt-induced glycine-rich RNA-binding protein gene, ZjGRP, caused salt sensitivity in Arabidopsis. Plant Cell Rep. 2017, 36, 179–191. [Google Scholar] [CrossRef]
- Teng, K.; Tan, P.; Guo, W.; Yue, Y.; Fan, X.; Wu, J. Heterologous expression of a novel Zoysia japonica C2H2 zinc finger gene, ZjZFN1, improved salt tolerance in Arabidopsis. Front. Plant Sci. 2018, 9, 1159. [Google Scholar] [CrossRef]
- Teng, K.; Chang, Z.; Li, X.; Sun, X.; Liang, X.; Xu, L.; Chao, Y.; Han, L. Functional and RNA-sequencing analysis revealed expression of a novel stay-green gene from Zoysia japonica (ZjSGR) caused chlorophyll degradation and accelerated senescence in Arabidopsis. Front. Plant Sci. 2016, 7, 1894. [Google Scholar] [CrossRef]
- Tanaka, H.; Hirakawa, H.; Kosugi, S.; Nakayama, S.; Ono, A.; Watanabe, A.; Hashiguchi, M.; Gondo, T.; Ishigaki, G.; Muguerza, M.; et al. Sequencing and comparative analyses of the genomes of zoysiagrasses. DNA Res. 2016, 23, 171–180. [Google Scholar] [CrossRef]
- Guan, J.; Yin, S.; Yue, Y.; Liu, L.; Guo, Y.; Zhang, H.; Fan, X.; Teng, K. Single-molecule long-read sequencing analysis improves genome annotation and sheds new light on the transcripts and splice isoforms of Zoysia japonica. BMC Plant Biol. 2022, 22, 263. [Google Scholar] [CrossRef]
- Hoagland, D.R.; Arnon, D.I. The water-culture method for growing plants without soil. Circ. Calif. Agric. Exp. Stn. 1950, 347, 32. [Google Scholar]
- Guan, J.; Teng, K.; Yue, Y.; Guo, Y.; Liu, L.; Yin, S.; Han, L. Zoysia japonica chlorophyll b reductase gene NOL participates in chlorophyll degradation and photosynthesis. Front. Plant Sci. 2022, 13, 906018. [Google Scholar] [CrossRef]
- Kim, D.; Paggi, J.M.; Park, C.; Bennett, C.; Salzberg, S.L. Graph-based genome alignment and genotyping with HISAT2 and HISAT-genotype. Nat. Biotechnol. 2019, 37, 907–915. [Google Scholar] [CrossRef]
- Trapnell, C.; Williams, B.A.; Pertea, G.; Mortazavi, A.; Kwan, G.; Van Baren, M.J.; Salzberg, S.L.; Wold, B.J.; Pachter, L. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat. Biotechnol. 2010, 28, 511–515. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Young, M.D.; Wakefield, M.J.; Smyth, G.K.; Oshlack, A. Gene ontology analysis for RNA-seq: Accounting for selection bias. Genome Biol. 2010, 11, R14. [Google Scholar] [CrossRef]
- Xie, C.; Mao, X.; Huang, J.; Ding, Y.; Wu, J.; Dong, S.; Kong, L.; Gao, G.; Li, C.-Y.; Wei, L. KOBAS 2.0: A web server for annotation and identification of enriched pathways and diseases. Nucleic Acids Res. 2011, 39, W316–W322. [Google Scholar] [CrossRef]
- Ahn, J.H.; Kim, J.-S.; Kim, S.; Soh, H.Y.; Shin, H.; Jang, H.; Ryu, J.H.; Kim, A.; Yun, K.-Y.; Kim, S.; et al. De Novo Transcriptome Analysis to Identify Anthocyanin Biosynthesis Genes Responsible for Tissue-Specific Pigmentation in Zoysiagrass (Zoysia japonica Steud.). PLoS ONE 2015, 10, e0124497. [Google Scholar] [CrossRef]
- Xie, Q.; Niu, J.; Xu, X.; Xu, L.; Zhang, Y.; Fan, B.; Liang, X.; Zhang, L.; Yin, S.; Han, L. De novo assembly of the Japanese lawngrass (Zoysia japonica Steud.) root transcriptome and identification of candidate unigenes related to early responses under salt stress. Front. Plant Sci. 2015, 6, 610. [Google Scholar]
- Wang, J.; An, C.; Guo, H.; Yang, X.; Chen, J.; Zong, J.; Li, J.; Liu, J. Physiological and transcriptomic analyses reveal the mechanisms underlying the salt tolerance of Zoysia japonica Steud. BMC Plant Biol. 2020, 20, 114. [Google Scholar] [CrossRef]
- Wu, X.-Y.; Hu, W.-J.; Luo, H.; Xia, Y.; Zhao, Y.; Wang, L.-D.; Zhang, L.-M.; Luo, J.-C.; Jing, H.-C. Transcriptome profiling of developmental leaf senescence in sorghum (Sorghum bicolor). Plant Mol. Biol. 2016, 92, 555–580. [Google Scholar] [CrossRef]
- Liu, J.; Li, J.; Wang, H.; Fu, Z.; Liu, J.; Yu, Y. Identification and expression analysis of ERF transcription factor genes in petunia during flower senescence and in response to hormone treatments. J. Exp. Bot. 2011, 62, 825–840. [Google Scholar] [CrossRef]
- Sharabi-Schwager, M.; Lers, A.; Samach, A.; Guy, C.L.; Porat, R. Overexpression of the CBF2 transcriptional activator in Arabidopsis delays leaf senescence and extends plant longevity. J. Exp. Bot. 2010, 61, 261–273. [Google Scholar] [CrossRef]
- Chen, Y.; Qiu, K.; Kuai, B.; Ding, Y. Identification of an NAP-like transcription factor BeNAC1 regulating leaf senescence in bamboo (Bambusa emeiensis ‘Viridiflavus’). Physiol. Plant. 2011, 142, 361–371. [Google Scholar] [CrossRef]
- Gan, S. The hormonal regulation of senescence. In Plant Hormones: Biosynthesis, Signal Transduction, Action! Davies, P.J., Ed.; Springer: Dordrecht, The Netherlands, 2010; pp. 597–617. [Google Scholar]
- Hebbar, K.B.; Rane, J.; Ramana, S.; Panwar, N.R.; Ajay, S.; Rao, A.S.; Prasad, P.V.V. Natural variation in the regulation of leaf senescence and relation to N and root traits in wheat. Plant Soil 2014, 378, 99–112. [Google Scholar] [CrossRef]
- Pruzinská, A.; Tanner, G.; Aubry, S.; Anders, I.; Moser, S.; Müller, T.; Ongania, K.-H.; Kräutler, B.; Youn, J.-Y.; Liljegren, S.J.; et al. Chlorophyll breakdown in senescent Arabidopsis leaves. characterization of chlorophyll catabolites and of chlorophyll catabolic enzymes involved in the degreening reaction. Plant Physiol. 2005, 139, 52–63. [Google Scholar] [CrossRef]
- Schelbert, S.; Aubry, S.; Burla, B.; Agne, B.; Kessler, F.; Krupinska, K.; Hörtensteiner, S. Pheophytin pheophorbide hydrolase (pheophytinase) is involved in chlorophyll breakdown during leaf senescence in Arabidopsis. Plant Cell 2009, 21, 767–785. [Google Scholar] [CrossRef]
- Baker, N.R. Chlorophyll fluorescence: A probe of photosynthesis in vivo. Annu. Rev. Plant Biol. 2008, 59, 89–113. [Google Scholar] [CrossRef]
- Bussotti, F.; Desotgiu, R.; Pollastrini, M.; Cascio, C. The JIP test: A tool to screen the capacity of plant adaptation to climate change. Scand. J. For. Res. 2010, 25, 43–50. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Oukarroum, A.; Alexandrov, V.; Kouzmanova, M.; Brestic, M.; Zivcak, M.; Samborska, I.A.; Cetner, M.D.; Allakhverdiev, S.I.; Goltsev, V. Identification of nutrient deficiency in maize and tomato plants by in vivo chlorophyll a fluorescence measurements. Plant Physiol. Biochem. 2014, 81, 16–25. [Google Scholar] [CrossRef]
- Sitko, K.; Rusinowski, S.; Kalaji, H.M.; Szopiński, M.; Małkowski, E. Photosynthetic efficiency as bioindicator of environmental pressure in A. halleri. Plant Physiol. 2017, 175, 290–302. [Google Scholar] [CrossRef]
- Zagorchev, L.; Atanasova, A.; Albanova, I.; Traianova, A.; Mladenov, P.; Kouzmanova, M.; Goltsev, V.; Kalaji, H.M.; Teofanova, D. Functional characterization of the photosynthetic machinery in Smicronix galls on the parasitic plant Cuscuta campestris by JIP-Test. Cells 2021, 10, 1399. [Google Scholar] [CrossRef]
- Eichelmann, H.; Price, D.; Badger, M.; Laisk, A. Photosynthetic parameters of leaves of wild type and Cyt b6/f deficient transgenic Tobacco studied by CO2 uptake and transmittance at 800 nm. Plant Cell Physiol. 2000, 41, 432–439. [Google Scholar] [CrossRef]
- Zhang, H.; Zhou, C. Signal transduction in leaf senescence. Plant Mol. Biol. 2013, 82, 539–545. [Google Scholar] [CrossRef]
| Sample Name | Read Sum | Base Sum | N (%) | GC (%) | Q20 (%) | Q30 (%) |
|---|---|---|---|---|---|---|
| Mature sample 1 | 24,158,349 | 7,199,286,172 | 0.00 | 55.06 | 98.45 | 95.69 |
| Mature sample 2 | 22,712,448 | 6,766,266,766 | 0.00 | 55.34 | 98.30 | 95.30 |
| Mature sample 3 | 24,681,745 | 7,352,597,992 | 0.00 | 55.03 | 98.42 | 95.57 |
| Senescent sample 1 | 24,651,354 | 7,368,053,620 | 0.00 | 54.66 | 98.34 | 95.36 |
| Senescent sample 2 | 26,404,815 | 7,870,804,198 | 0.00 | 55.08 | 98.34 | 95.36 |
| Senescent sample 3 | 23,812,657 | 7,122,890,816 | 0.00 | 53.96 | 98.24 | 95.03 |
| Gene ID | Annotation | Regulated | Senescent/Mature (log2FC) | |
|---|---|---|---|---|
| Digital Expression | qRT-PCR | |||
| Zjn_sc03174.1.g00010.1.am.mk | Protein phosphatase 2C 64 | up | 1.74 | 1.16 |
| Zjn_sc00001.1.g03760.1.sm.mkhc | Hypothetical protein TIFY 10B-like | up | 2.19 | 2.12 |
| Zjn_sc00003.1.g10610.1.sm.mkhc | Jasmonate ZIM domain protein, partial | up | 1.97 | 2.03 |
| Zjn_sc00059.1.g01300.1.sm.mk | Transcription factor MYC2 | up | 1.4 | 1.53 |
| Zjn_sc00003.1.g01950.1.sm.mk | Ethylene insensitive 3-like 1 protein | up | 1.86 | 1.12 |
| Zjn_sc00138.1.g00080.1.am.mk | Cytochrome b6-f complex iron-sulfur subunit | down | −8.76 | −2.42 |
| Zjn_sc00184.1.g00360.1.am.mk | plastocyanin, chloroplastic-like | down | −5.18 | −7.77 |
| Zjn_sc00152.1.g00120.1.sm.mkhc | Ferredoxin--NADP reductase, leaf isozyme 2 | down | −1.74 | −3.39 |
| PB.16272 | photosystem II protein D2 | down | −1.97 | −2.07 |
| PB.4217 | ATP synthase CF1 alpha subunit | down | −3.20 | −0.87 |
| Kegg_Pathway | ko_id | Input Number | Corrected_q-Value | Up- or Downregulated |
|---|---|---|---|---|
| Autophagy—other | ko04136 | 14 | 0.000200076 | Upregulated |
| Ether lipid metabolism | ko00565 | 11 | 0.004112946 | Upregulated |
| Phenylpropanoid biosynthesis | ko00940 | 40 | 0.015142676 | Upregulated |
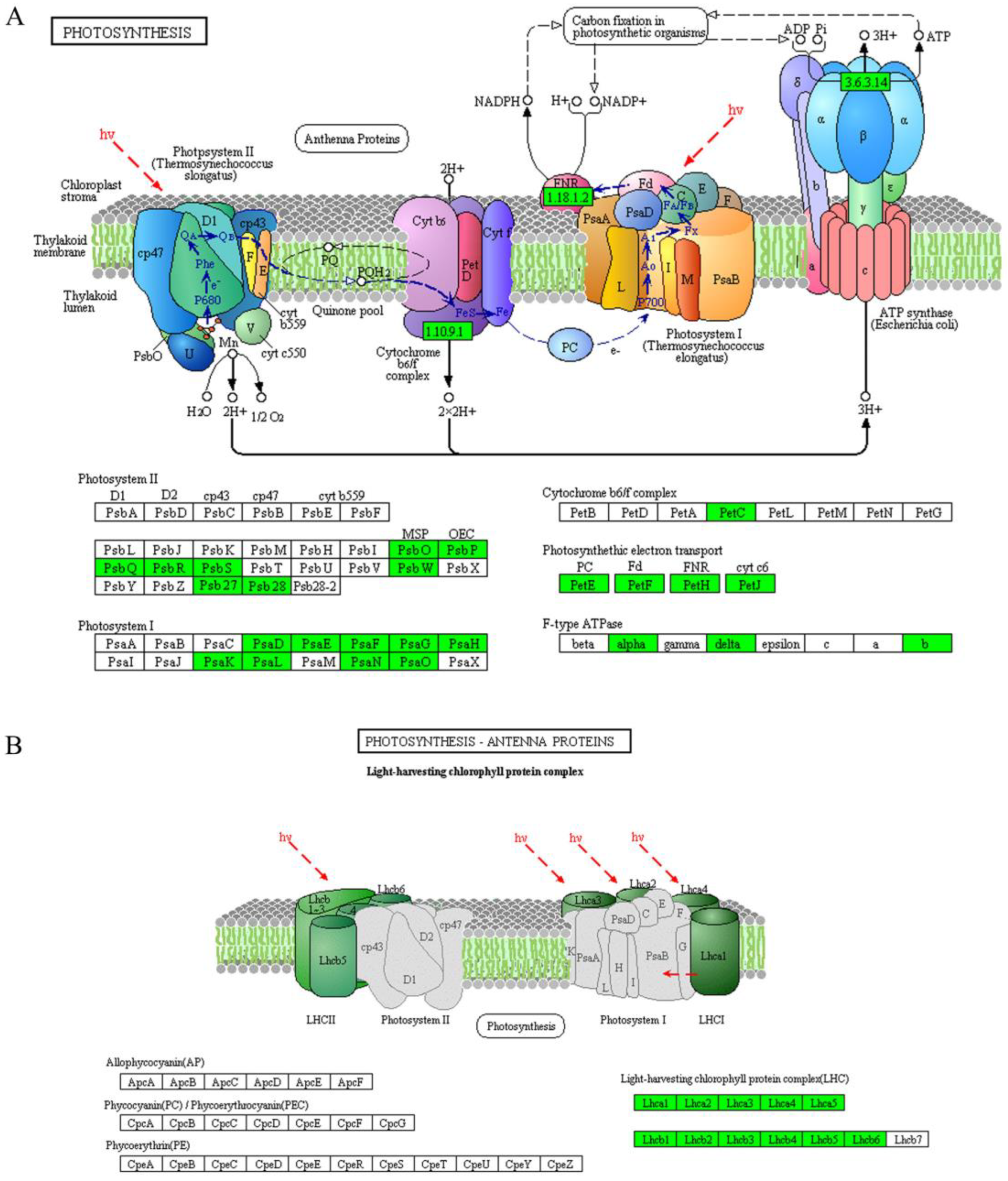
| Photosynthesis-antenna proteins | ko00196 | 22 | 8.09 × 10−19 | Downregulated |
| Carbon fixation in photosynthetic organisms | ko00710 | 46 | 1.27 × 10−11 | Downregulated |
| Photosynthesis | ko00195 | 49 | 8.94 × 10−11 | Downregulated |
| Ribosome | ko03010 | 61 | 8.21 × 10−6 | Downregulated |
| Porphyrin and chlorophyll metabolism | ko00860 | 21 | 1.89 × 10−5 | Downregulated |
| Carbon metabolism | ko01200 | 61 | 3.81 × 10−5 | Downregulated |
| Carotenoid biosynthesis | ko00906 | 16 | 0.007050826 | Downregulated |
| Pentose phosphate pathway | ko00030 | 18 | 0.017326576 | Downregulated |
| Glycolysis/gluconeogenesis | ko00010 | 34 | 0.019536307 | Downregulated |
| Ubiquinone and other terpenoid-quinone biosynthesis | ko00130 | 13 | 0.16294735 | Downregulated |
| Fructose and mannose metabolism | ko00051 | 18 | 0.259466752 | Downregulated |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guan, J.; Fan, X.; Yue, Y.; Xu, L.; Teng, K.; Yin, S. Integrative Transcriptome and Chlorophyll Fluorescence Test Analysis Shed New Light on the Leaf Senescence Mechanism of Zoysia japonica. Agronomy 2023, 13, 623. https://doi.org/10.3390/agronomy13030623
Guan J, Fan X, Yue Y, Xu L, Teng K, Yin S. Integrative Transcriptome and Chlorophyll Fluorescence Test Analysis Shed New Light on the Leaf Senescence Mechanism of Zoysia japonica. Agronomy. 2023; 13(3):623. https://doi.org/10.3390/agronomy13030623
Chicago/Turabian StyleGuan, Jin, Xifeng Fan, Yuesen Yue, Lixin Xu, Ke Teng, and Shuxia Yin. 2023. "Integrative Transcriptome and Chlorophyll Fluorescence Test Analysis Shed New Light on the Leaf Senescence Mechanism of Zoysia japonica" Agronomy 13, no. 3: 623. https://doi.org/10.3390/agronomy13030623
APA StyleGuan, J., Fan, X., Yue, Y., Xu, L., Teng, K., & Yin, S. (2023). Integrative Transcriptome and Chlorophyll Fluorescence Test Analysis Shed New Light on the Leaf Senescence Mechanism of Zoysia japonica. Agronomy, 13(3), 623. https://doi.org/10.3390/agronomy13030623







