Responses of the Growth Characteristics of Spinach to Different Moisture Contents in Soil under Irrigation with Magnetoelectric Water
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site and Soil Description
2.2. Determination Content and Method
2.3. Models Used
2.3.1. Logistic Model
2.3.2. Light Response Model
2.4. Data Processing
3. Results and Analysis
3.1. Dynamic Characteristics of the Total Nitrogen Content in Spinach Leaves
3.2. Dynamic Characteristics of the Fresh Weight of Spinach Shoots
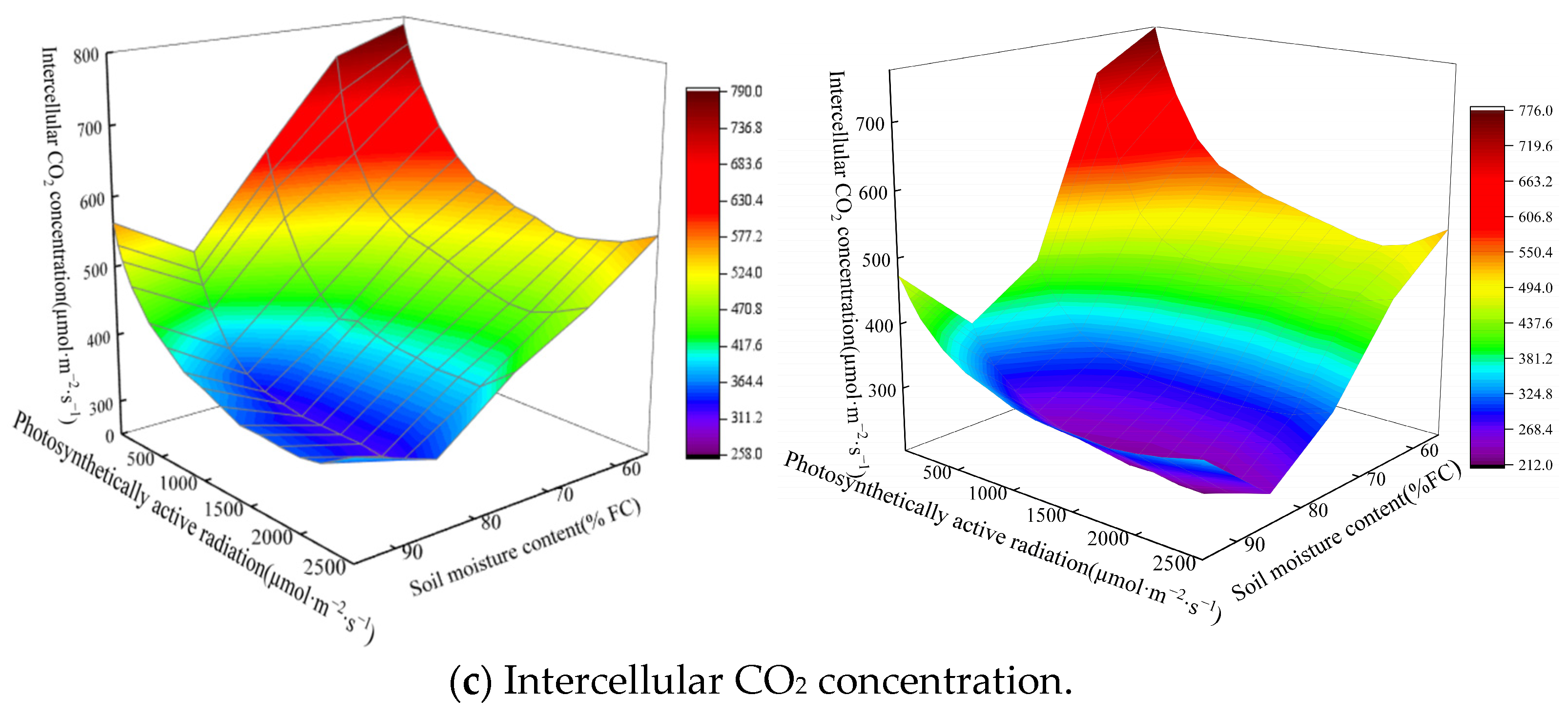
3.3. Analysis of Chlorophyll Content in Spinach and Its Photosynthetic Characteristics
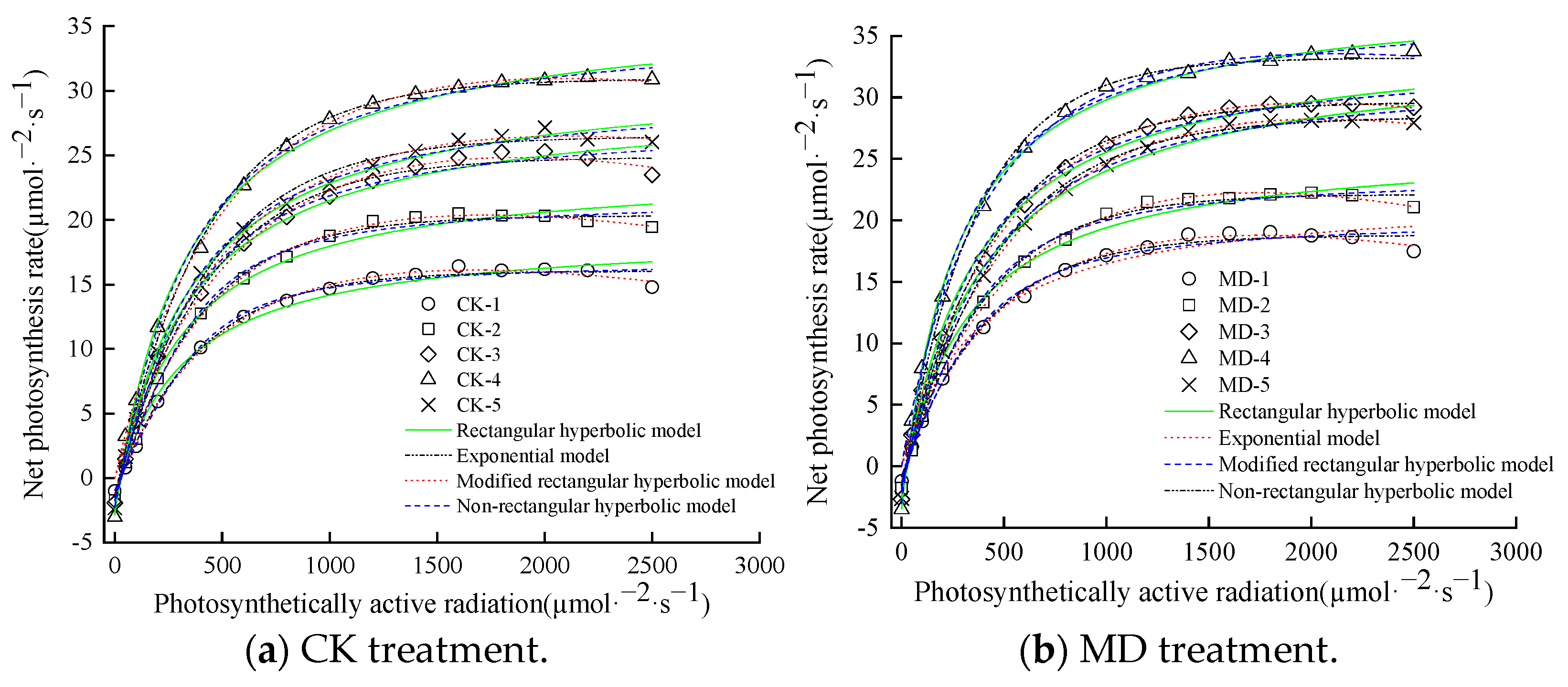
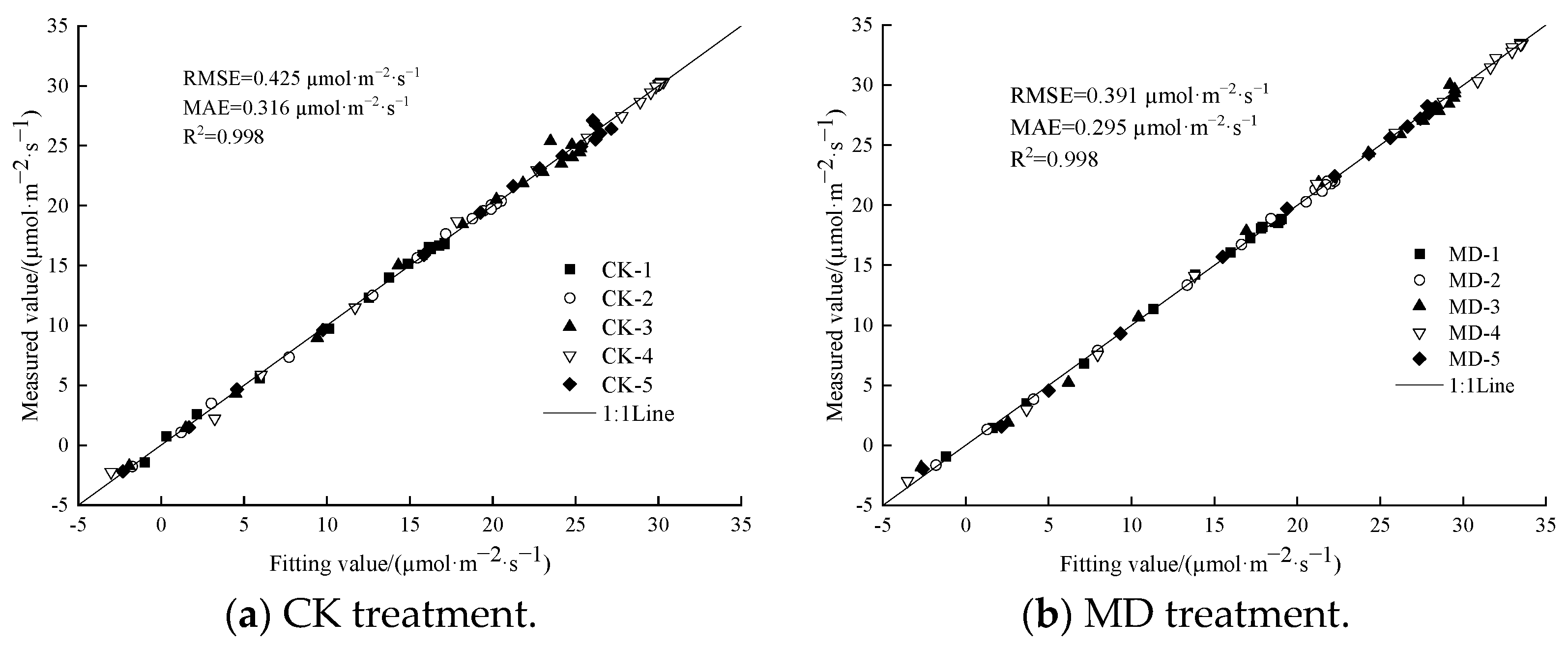
3.4. Determination of a Suitable Model for the Photoresponse Curve
3.5. Light Response Fitting and Parameter Variation Characteristics of Spinach as Shown by the Modified Rectangular Hyperbolic Model
4. Discussion
4.1. Effects of Soil Moisture Content and Magnetoelectric Water on the Total Nitrogen Content and Chlorophyll in Spinach Leaves
4.2. Effects of Soil Moisture Content and Magnetoelectric Water on Photosynthetic Characteristics and Aboveground Fresh Weight of Spinach
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, J.J.; Fu, Z.H.; Liu, W.F. Impacts of Precipitation Variations on Agricultural Water Scarcity under Historical and Future Climate Change. J. Hydrol. 2023, 617, 128999. [Google Scholar] [CrossRef]
- Mu, Y.; Zhao, G.Q.; Zhao, Q.Q.; Liu, H.; Wang, Q.J. Advances in the Application of Activated Water Irrigation. J. Agric. Resour. Environ. 2019, 36, 403–411. [Google Scholar] [CrossRef]
- Mian, S.H.; Ghulam, D.; Amir, M.A.; Shahbaz, H.; Rana, R.K. Eco-friendly Magnetic Field Treatment to Enhance Wheat Yield and Seed Germination Growth. Environ. Nanotechnol. Monit. Manag. 2020, 14, 100299. [Google Scholar] [CrossRef]
- Georgii, S.; Yurii, K.; Alla, B. Formation of the Quality of Wheat Grain by Extremely Low Frequency Electromagnetic Field Treatment. Tech. Audit Prod. Rsrv. 2022, 4, 38–44. [Google Scholar] [CrossRef]
- Abdulrahman, S.A.; Halimah, O.A.K. The Effect of Magnetically Treated Water on the Germination and Growth of a Local Variety of Millet Pennisetum Glaucum. Life Sci. J. 2018, 15, 16–28. [Google Scholar] [CrossRef]
- Zareei, E.; Zaare-Nahandi, F.; Oustan, S.; Hajilou, J. Effects of Magnetic Solutions on Some Biochemical Properties and Production of Some Phenolic Compounds in Grapevine (Vitis vinifera L.). Sci. Hortic. 2019, 253, 217–226. [Google Scholar] [CrossRef]
- Mohammadi, R.; Dastorani, M.T.; Akbari, M.; Ahani, H. The Impacts of Magnetized Water Treatment on Different Morphological and Physiological Factors of Plant Species in Arid Regions. Water Supply 2019, 19, 2016–2026. [Google Scholar] [CrossRef]
- Li, J.; Fan, J.; Zhu, Z.M. Effects of Activated Water Irrigation on Growth Characteristics of Soybean under Drought Stress. J. Appl. Ecol. 2020, 31, 3711–3718. [Google Scholar] [CrossRef]
- Wang, H.; Fan, J.; Fu, W. Effect of Activated Water Irrigation on the Yield and Water Use Efficiency of Winter Wheat under Irrigation Deficit. Agronomy 2022, 12, 1315. [Google Scholar] [CrossRef]
- Wang, Q.J.; Sun, Y.; Ning, S.R.; Zhang, J.H.; Zhou, B.B.; Su, L.J.; Shan, Y.Y. Effects of Activated Irrigation Water on Soil Physicochemical Properties. Adv. Earth Sci. 2019, 34, 660–670. [Google Scholar] [CrossRef]
- Scott, A.C.; Zubot, W.; Davis, C.W.; Brogly, J. Bioaccumulation Potential of Naphthenic Acids and Other Ionizable Dissolved Organics in Oil Sands Process Water (OSPW)-a Review. Sci. Total Environ. 2019, 712, 134558. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.J.; Xu, Z.Y.; Shan, Y.Y.; Zhang, J.H. Effect of Salinity of De-electronic Brackish Water on Characteristics of Water and Salt Movement in Soil. Trans. Chin. Soc. Agric. Eng. 2018, 34, 125–132. [Google Scholar] [CrossRef]
- Zhang, W.W.; Zhang, Z.F.; Bo, H.T.; Chen, D.F.; Sun, X. Study on the Timeliness of Performance of Magnetized Water. Mech. Electr. Eng. Technol. 2020, 49, 71–73. [Google Scholar] [CrossRef]
- Li, Z.Y.; Wang, Q.J.; Zhang, J.H.; Xie, J.B.; Wei, K. Effect of Magnetization-de-electronic Integrated Activation Water on Water-salt Transport Characteristics of Salinized Soil. J. Soil Water Conserv. 2021, 35, 290–295. [Google Scholar] [CrossRef]
- Magnee, K.J.H.; Scholten, O.E.; Postma, J.; Lammerts, V.B.; Edith, T.; Groot, S.P.C. Sensitivity of Spinach Seed Germination to Moisture is Driven by Oxygen Availability and Influenced by Seed Size and Pericarp. Seed Sci. Technol. 2020, 48, 117–131. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of Total Carotenoids and Chlorophylls a and b of Leaf Extracts in Different Solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef]
- Lewis, J.D.; Lucash, M.; Olszyk, D.; Tingey, D.T. Seasonal Patterns of Photosynthesis in Douglas Fir Seedlings During the Third and Fourth Year of Exposure to Elevated CO2 and Temperature. Plant Cell Environ. 2001, 24, 539–548. [Google Scholar] [CrossRef]
- Gates, D.M. Mathematical Models in Plant Physiology: A Quantitative Qpproach to Problems in Plant and Crop Physiology. Q. Rev. Biol. 1976, 1, 97. [Google Scholar] [CrossRef]
- Bassman, J.H.; Zwier, J.C. Gas Exchange Characteristics of Populus Trichocarpa, Populus Deltoides and Populus Trichocarpa × p. Deltoides Clones. Tree Physiol. 1991, 8, 145–159. [Google Scholar] [CrossRef]
- Zhu, T.T.; Li, J.; Liu, Y.Y.; Tong, X.J.; Yu, Q. Leaf Photosynthetic Light Response of Summer Maize: Comparison of Models and Analysis of Parameters. Photosynthetica 2020, 58, 19–28. [Google Scholar] [CrossRef]
- Song, X.; Yue, X.; Chen, W.; Jiang, H.; Li, X. Detection of Cadmium Risk to The Photosynthetic Performance of Hybrid Pennisetum. Front. Plant Sci. 2019, 10, 798–812. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Jie, W.; Liu, B.; Nara, K.; Lian, C.; Shen, Z.; Yan, X.; Chen, Y. Ectomycorrhizal Fungi Reduce the Light Compensation Point and Promote Carbon Fixation of Pinus Thunbergii Seedlings to Adapt to Shade Environments. Mycorrhiza 2017, 27, 823–830. [Google Scholar] [CrossRef]
- Liu, Q.; Li, F.R.; Xie, L.F. Optimal Model of Photosynthesis-Light Response Curve in Canopy of Planted Larix Olgensis Tree. Chin. J. Appl. Ecol. 2016, 27, 2420–2428. [Google Scholar] [CrossRef]
- Li, Y.H.; Liu, J.H.; Lv, P.; Mi, J.Z.; Zhao, B.P. Silicon Improves the Photosynthetic Performance of Oat Leaves Infected with Puccinia Graminis f. sp. Avenae. Front. Plant Sci. 2022, 13, 1037136. [Google Scholar] [CrossRef]
- Coast, O.; Shah, S.; Ivakov, A.; Gaju, O.; Wilson, P.B.; Posch, B.C.; Bryant, C.J.; Negrini, A.C.; Evans, J.R.; Condon, A.G.; et al. Predicting Dark Respiration Rates of Wheat Leaves from Hyperspectral Reflectance. Plant Cell Environ. 2019, 42, 2133–2150. [Google Scholar] [CrossRef] [PubMed]
- Gendy, A.; Gohary, A.; Omer, E.A.; Hendawya, S.F.; Husseina, M.S.; Stancheva, I. Effect of Nitrogen and Potassium Fertilizer on Herbage and Oil Yield of Chervil Plant (Anthriscus cerefolium L.). Ind. Crops Prod. 2015, 69, 167–174. [Google Scholar] [CrossRef]
- Fei, C.; Fan, H.; Fan, X.X.; Xu, G. Estimation of Total Nitrogen content in Sugar Beet Leaves Based on Chlorophyll Fluorescence Parameters. Photosynthetica 2020, 58, 869–872. [Google Scholar] [CrossRef]
- Budiyanto, G. The Influence of Organic Materials to Nitrogen Content of Leaf Tissue of Corn Planted in Coastal Sand soil. Adv. Sci. Lett. 2018, 24, 9138–9140. [Google Scholar] [CrossRef]
- Yao, X.; Xiao, B.; Kidron, G.J. Respiration Rate of Moss-Dominated Biocrusts and their Relationships with Temperature and Moisture in Asemiarid Ecosystem-sciencedirect. Catena 2019, 183, 104195. [Google Scholar] [CrossRef]
- Rania, M.A.N.; Mohamed, S.B.; Mercedes, B. Physiological and Nnatomical Studies of Two Wheat Cultivars Irrigated with Magnetic Water under Drought Stress Conditions-Sciencedirect. Plant Physiol. Biochem. 2019, 135, 480–488. [Google Scholar] [CrossRef]
- Selim, A.F.H.; El-Nady, M.F. Physio-Anatomical Responses of Drought Stressed Tomato Plants to Magnetic Field. Acta Astronaut 2011, 69, 387–396. [Google Scholar] [CrossRef]
- Radhakrishnan, R.; Kumari, B. Pulsed Magnetic Field: A Contemporary Approach Offers to Enhance Plant Growth and Yield of Soybean. Plant Physiol. Biochem. 2012, 51, 139–144. [Google Scholar] [CrossRef]
- Zhang, F.J.; Zhang, K.K.; Du, C.Z.; Li, J.; Xing, Y.X.; Yang, L.T.; Li, Y.R. Effect of Drought Stress on Anatomical Structure and Chloroplast Ultrastructure in Leaves of Sugarcane. Sugar Tech. 2015, 17, 41–48. [Google Scholar] [CrossRef]
- Al-Khazan, M.; Abdullatif, B.M.; Al-Assaf, N. Effects of Magnetically Treated Water on Water Status, Chlorophyll Pigments and Some Elements Content of jojoba (Simmondsia chinensis L.) at Different Growth Stages. Afr. J. Environ. Sci. Technol. 2011, 5, 722–731. [Google Scholar] [CrossRef]
- Wang, X.H.; Kong, X.J.; Li, L.J.; Jia, P.P.; Cheng, X.G.; Zhang, X.T.; Zhang, L.; Xue, H.Y.; Khan, A.; Zhang, Z.Y. Rubisco Small Subunits’ Genome-Wide Identification and their Function from Gene Expression to Rubisco Activity and Photosynthesis Among Peanut Genotypes under Different Nitrogen Levels. Agronomy 2022, 12, 2316. [Google Scholar] [CrossRef]
- Akita, R.; Kamiyama, C.; Hikosaka, K. Polygonum Sachalinense Alters the Balance Between Capacities of Regeneration and Carboxylation of Ribulose1, 5-Bisphosphate in Response to Growth CO2 Increment But Not the Nitrogen Allocation Within the Photosynthetic Apparatus. Physiol. Plant 2012, 146, 404–412. [Google Scholar] [CrossRef] [PubMed]
- Jiao, L.; Wang, L.; Zhou, Q.; Huang, X. Stomatal and Non-stomatal Factors Regulated the Photosynthesis of Soybean Seedlings in the Present of Exogenous Bisphenol a. Ecotoxicol. Environ. Saf. 2017, 145, 150–160. [Google Scholar] [CrossRef]
- Buckley, T.N. Modeling Stomatal Conductance. Plant Physiol. 2017, 174, 572–582. [Google Scholar] [CrossRef]
- Farquhar, G.D.; Sharkey, T.D. Stomatal Conductance and Photosynthesis. Annu. Rev. Plant Physiol. 1982, 33, 317–345. [Google Scholar] [CrossRef]
- Ogbe, A.A.; Finnie, J.F.; Van Staden, J. The Role of Endophytes in Secondary Metabolites Accumulation in Medicinal Plants under Abiotic Stress. S. Afr. J. Bot. 2020, 134, 126–134. [Google Scholar] [CrossRef]
- Vellosillo, T.; Vicente, J.; Kulasekaran, S.; Hamberg, M.; Castresana, C. Emerging Complexity in Reactive Oxygen Species Production and Signaling During the Response of Plants to Pathogens. Plant Physiol. 2010, 154, 444–448. [Google Scholar] [CrossRef]
- Yu, X.C.; Li, Y.; Chang, J.Z.; Ihsan, M.; Xun, B.Z.; Hong, D.Z. Effects of Soaking Seeds in Exogenous Vitamins on Active Oxygen Metabolism and Seedling Growth under Low-Temperature Stress. Saudi J. Biol. Sci. 2021, 28, 3254–3261. [Google Scholar] [CrossRef]
- Wang, Q.J.; Zhang, J.H.; Meng, Q.; Tan, S.; Zhou, L.W.; Liu, X.Y. Experiment on Physical and Chemical Characteristics of Activated Brackish Water. Trans. Chin. Soc. Agric. Eng. 2016, 32, 60–66. [Google Scholar] [CrossRef]
- Kilani, B.R.; Delphine, L.V.; Isabel, L.D.; Leprince, A.S.; Marianne, B.; Maldiney, R.; Asma, J.; Chedly, A.; Arnould, S. Hydrogen Peroxide Produced by NADPH Oxidases Increases Proline Accumulation During Salt or Mannitol Stress in Arabidopsis Thaliana. New Phytol. 2015, 208, 1138–1148. [Google Scholar] [CrossRef]
- Liao, W.; Huang, G.; Yu, J.; Zhang, M.; Shi, X. Nitric Oxide and Hydrogen Peroxide are Involved in Indole-3-Butyric Acid-Induced Adventitious root Development in Marigold. J. Pomol. Hortic. Sci. 2015, 86, 159–165. [Google Scholar] [CrossRef]
- Hu, J.Y.; Yan, C.; Li, S.; Tang, H.; Chen, Y.H. Comparative Physiological Responses and Transcriptome Analysis Revealing the Metabolic Regulatory Mechanism of Prunella vulgaris L. Induced by Exogenous Application of Hydrogen Peroxide. Ind. Crops Prod. 2023, 192, 116065. [Google Scholar] [CrossRef]
- Li, X.; Bao, Z.Y.; Chen, Y.N.; Lan, Q.Q.; Song, C.B.; Shi, L.Y.; Chen, W.; Cao, S.F.; Yang, Z.F.; Zheng, Q.B. Exogenous Glutathione Modulates Redox Homeostasis in Okra (Abelmoschus esculentus) During Storage. Postharvest Biol. Technol. 2023, 195, 112145. [Google Scholar] [CrossRef]
- Liu, T.; Ye, X.L.; Li, M.; Li, J.M.; Qi, H.Y.; Hu, X.H. H2O2 and NO are Involved in Trehalose-regulated Oxidative Stress Tolerance in Cold-stressed Tomato Plants. Environ. Exp. Bot. 2020, 171, 103961. [Google Scholar] [CrossRef]
- Yao, M.M.; Ge, W.Y.; Zhou, Q.; Zhou, X.; Luo, M.L.; Zhao, Y.B.; Wei, B.D.; Ji, S.J. Exogenous Glutathione Alleviates Chilling Injury in Postharvest Bell Pepper by Modulating the Ascorbate-Glutathione (AsA-GSH) Cycle. Food Chem. 2021, 352, 129458. [Google Scholar] [CrossRef] [PubMed]
- Xu, A.Q.; Cheng, F.; Zhou, S.; Hu, H.; Bie, Z.L. Chilling-Induced H2O2 Signaling Activates the Antioxidant Enzymes in Alleviating the Photooxidative Damage Caused by Loss of Function of 2-Cys Peroxiredoxin in Watermelon. Plant Stress 2022, 6, 100108. [Google Scholar] [CrossRef]
- Rahman, M.A.; Alam, I.; Sharmin, S.A.; Kabir, A.H.Y.; Kim, Y.G.; Liu, G.S.; Lee, B.H. Physiological and Proteomic Analyses Reveal the Protective Roles of Exogenous Hydrogen Peroxide in Alleviating Drought Stress in Soybean Plants. Plant Biotechnol. Rep. 2021, 15, 805–818. [Google Scholar] [CrossRef]
- Diana, S.L.O.; Morales, L.O.; Strid, A.; Torres, P.I.; Guevara, G.R.G. UV-B Exposure and Exogenous Hydrogen Peroxide Application Lead to Cross-tolerance Toward Drought in Nicotiana tabacum L. Physiol. Plant 2021, 173, 666–679. [Google Scholar] [CrossRef]
- Lee, B.R.; La, V.H.; Park, S.H.; Mamun, M.A.; Bae, D.W.; Kim, T.H. H2O2-Responsive Hormonal Status Involves Oxidative Burst Signaling and Proline Metabolism in Rapeseed Leaves. Antioxidants 2022, 11, 566. [Google Scholar] [CrossRef] [PubMed]
- Shaila, S.T.; Moklasur, R.; Farjana, R.; Mehera, A.S.; Muhammad, H.K.; Anamul, H.; Mohammad, S.R. Seed Priming with Salicylic Acid (SA) and Hydrogen Peroxide (H2O2) Improve Germination and Seedling Growth of Wheat (Triticum aestivum) under Salt Stress. Asian J. Res. Crop Sci. 2021, 6, 60–69. [Google Scholar] [CrossRef]
- Yang, J.Y.; Wang, P.J.; Li, S.Z.; Liu, T.; Hu, X.H. Polyamine Oxidase Triggers H2O2-Mediated Spermidine Improved Oxidative Stress Tolerance of Tomato Seedlings Subjected to Saline-Alkaline Stress. Int. J. Mol. Sci. 2022, 23, 1625. [Google Scholar] [CrossRef] [PubMed]
- Tania, S.S.; Rhaman, M.S.; Rauf, F.; Rahaman, M.M.; Kabir, M.H.; Hoque, M.A.; Murata, Y. Alleviation of Salt-Inhibited Germination and Seedling Growth of Kidney Bean by Seed Priming and Exogenous Application of Salicylic Acid (SA) and Hydrogen Peroxide (H2O2). Seeds 2022, 1, 87–98. [Google Scholar] [CrossRef]
- Yao, X.; Zhou, M.L.; Ruan, J.J.; Peng, Y.; Ma, C.; Wu, W.J.; Gao, A.J.; Weng, W.F.; Cheng, J.P. Physiological and Biochemical Regulation Mechanism of Exogenous Hydrogen Peroxide in Alleviating NaCl Stress Toxicity in Tartary Buckwheat (Fagopyrum tataricum (L.) Gaertn). Int. J. Mol. Sci. 2022, 23, 10698. [Google Scholar] [CrossRef]
- Darmanti, S.; Hastuti, E.D.; Suedy, S.W.A. Exogenous Hydrogen Peroxide Induce an Antioxidative Defense System Against Drought Stress in Soybeanglycine Max (L.) Merr Crops. J. Biol. Sci. 2020, 31, 213–220. [Google Scholar] [CrossRef]
- Moussa, H.R. The Impact of Magnetic Water Application for Improving Common Bean (Phaseolus vulgaris L.) Production. Nyc. Sci. J. 2015, 4, 15–20. [Google Scholar] [CrossRef]
- Gao, Y.; Sun, Y.F.; Zhang, R.X.; Chu, G. Effects of Magnetic Water Irrigation on the Growth, N Uptake and Antioxidant Enzyme Activities of Cotton Seedlings. J. Agric. Sci. Technol. 2017, 7, 9. [Google Scholar] [CrossRef]
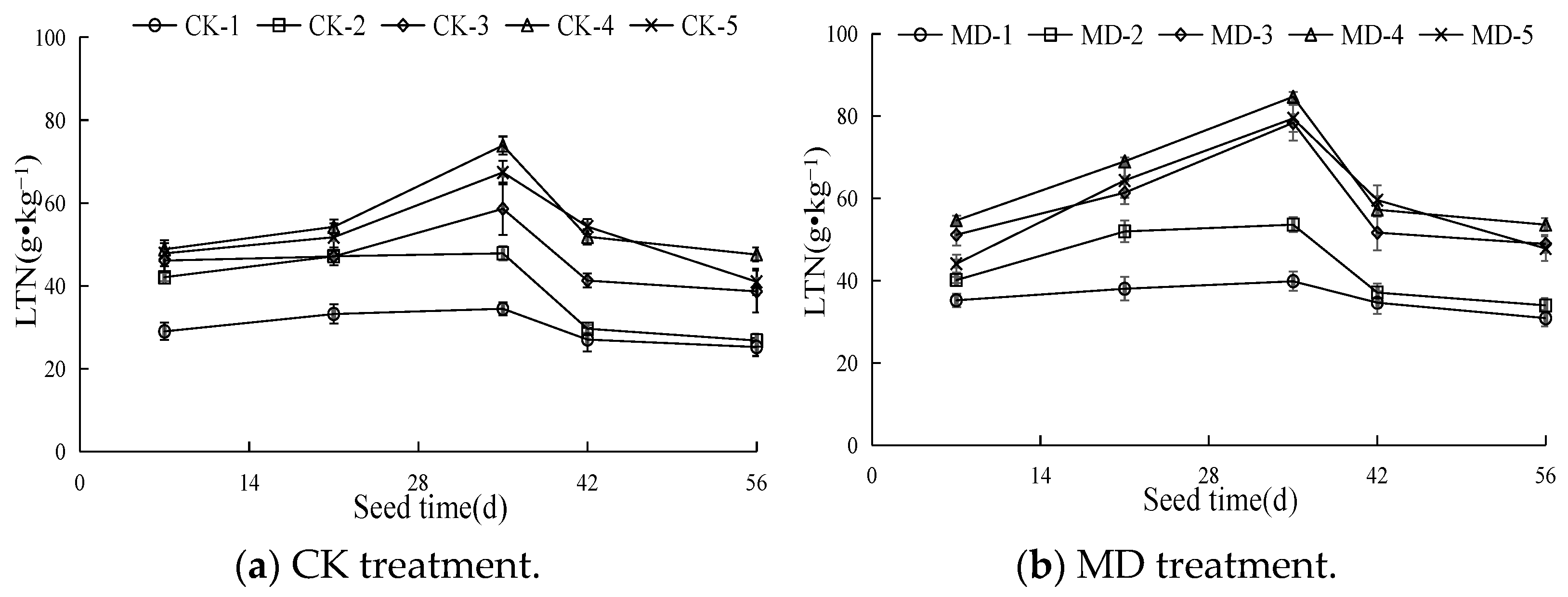

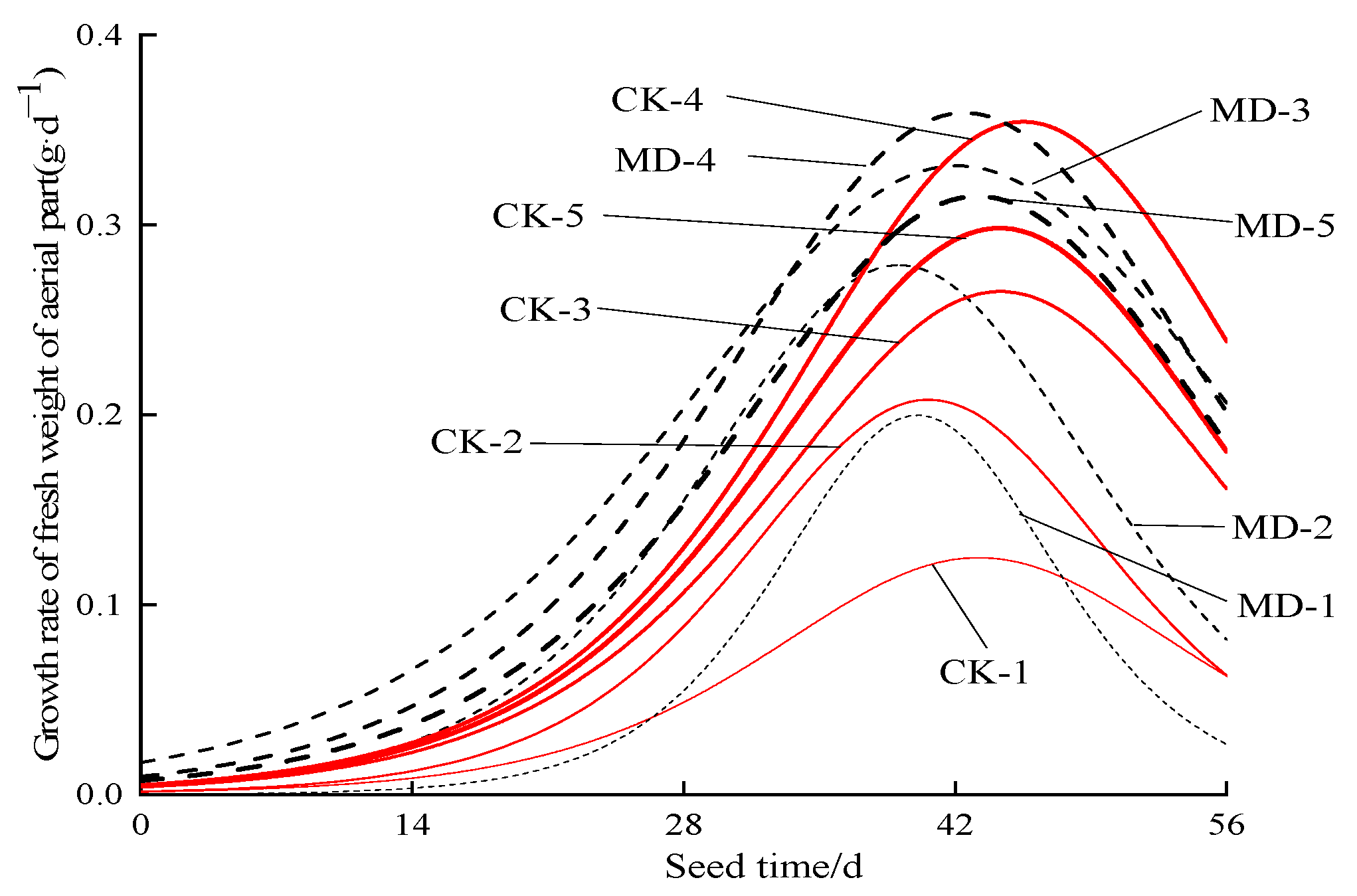
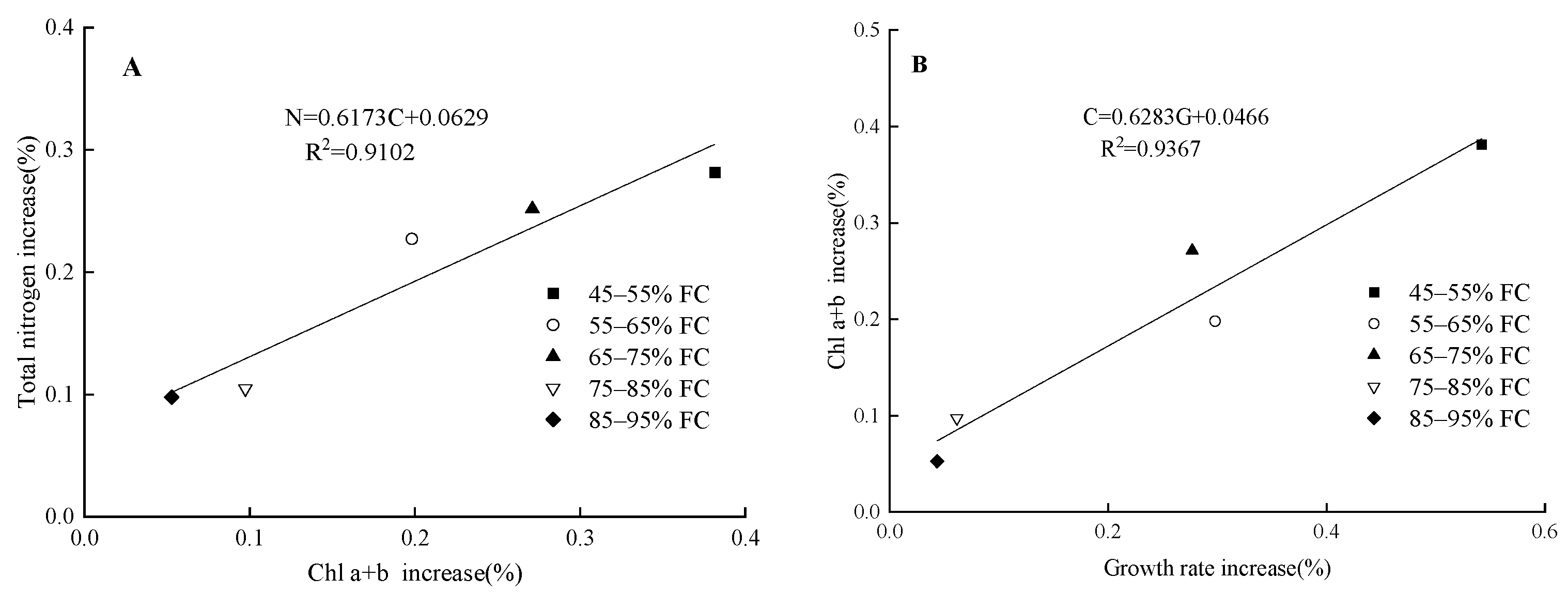
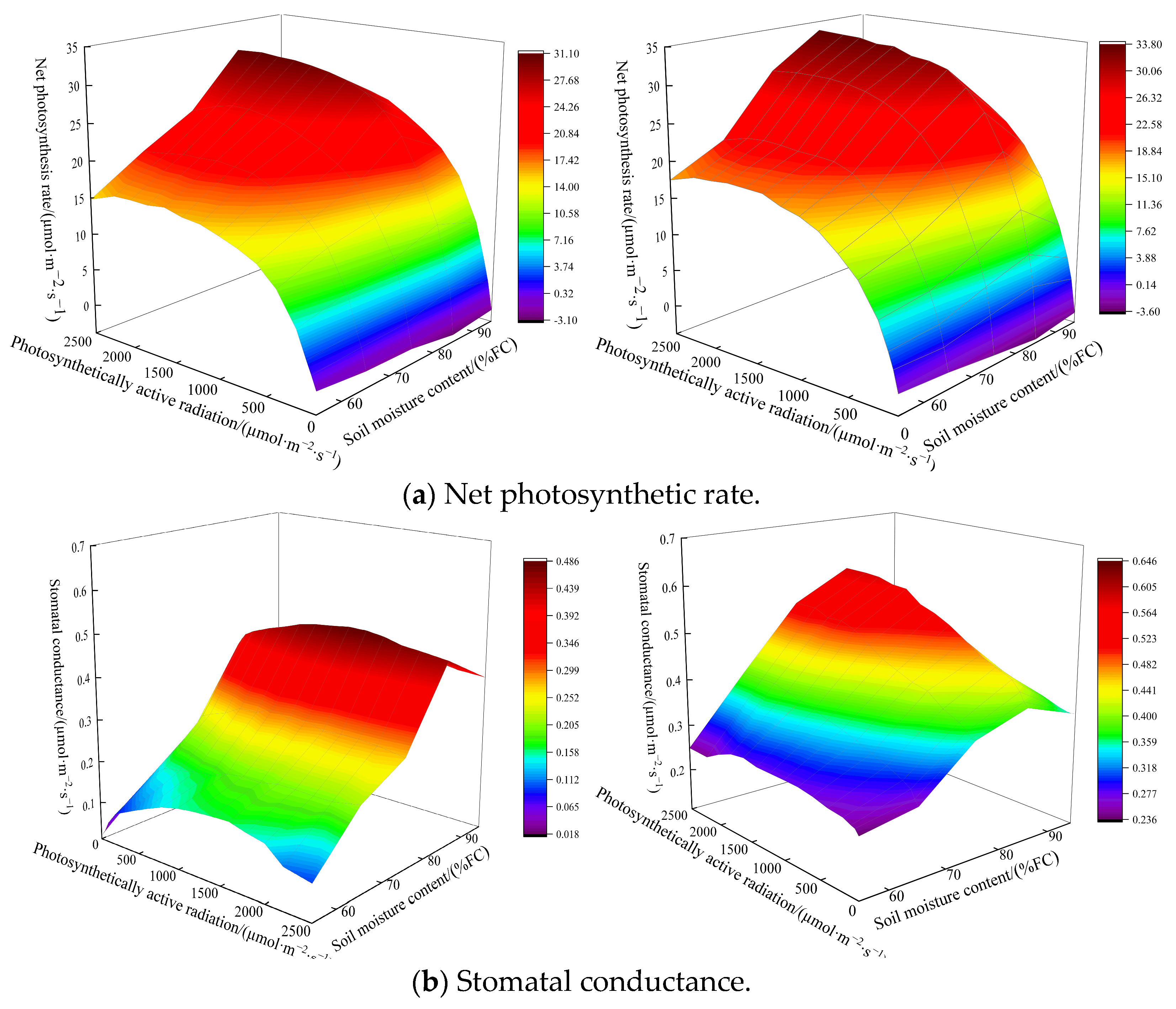
| Treatment | Irrigation Type | Soil Moisture Content |
|---|---|---|
| CK-1 | conventional water | 45–55%FC |
| CK-2 | conventional water | 55–65%FC |
| CK-3 | conventional water | 65–75%FC |
| CK-4 | conventional water | 75–85%FC |
| CK-5 | conventional water | 85–95%FC |
| MD-1 | magnetoelectric water | 45–55%FC |
| MD-2 | magnetoelectric water | 55–65%FC |
| MD-3 | magnetoelectric water | 65–75%FC |
| MD-4 | magnetoelectric water | 75–85%FC |
| MD-5 | magnetoelectric water | 85–95%FC |
| Treatment | Logistic Model Parameter Values | RMSE | R2 | ||
|---|---|---|---|---|---|
| FWAmax | A | B | |||
| CK-1 | 3.62 ± 0.55 i | 5.958 | 0.138 | 0.022 | 0.998 |
| MD-1 | 3.81 ± 0.28 i | 8.397 | 0.210 | 0.068 | 0.993 |
| CK-2 | 5.3 ± 0.43 h | 6.378 | 0.157 | 0.034 | 0.998 |
| MD-2 | 7.69 ± 0.12 g | 5.671 | 0.145 | 0.007 | 0.999 |
| CK-3 | 8.41 ± 0.35 f | 5.585 | 0.126 | 0.028 | 0.999 |
| MD-3 | 12.86 ± 0.19 a | 4.337 | 0.103 | 0.092 | 0.998 |
| CK-4 | 11.43 ± 0.26 c | 5.645 | 0.124 | 5.013 | 0.998 |
| MD-4 | 12.17 ± 0.23 b | 5.013 | 0.118 | 0.015 | 0.999 |
| CK-5 | 9.74 ± 0.24 e | 5.580 | 0.126 | 0.026 | 0.997 |
| MD−5 | 10.59 ± 0.32 d | 5.136 | 0.119 | 0.018 | 0.999 |
| Treatment | LTN | FWA | ||
|---|---|---|---|---|
| F | P | F | P | |
| Irrigation water type | 49.285 | 8.24 × 10−7 | 175.38 | 2.33 × 10−11 |
| Soil moisture content | 81.619 | 4.28 × 10−12 | 505.36 | 8.88 × 10−20 |
| Irrigation water type × Soil moisture content | 0.707 | 0.597 | 35.66 | 7.73 × 10−9 |
| R2 | 0.927 | 0.988 | ||
| Treatment | Chl a (mg·g−1) | Chl b (mg·g−1) | Chl a/b (mg·g−1) | Chl a + b (mg·g−1) |
|---|---|---|---|---|
| CK-1 | 0.87 ± 0.13 h | 0.38 ± 0.02 e | 2.29 ± 0.07 i | 1.25 ± 0.12 i |
| MD-1 | 1.24 ± 0.06 g | 0.51 ± 0.04 d | 2.43 ± 0.05 f | 1.75 ± 0.09 h |
| CK-2 | 1.49 ± 0.11 f | 0.56 ± 0.04 d | 2.66 ± 0.1 e | 2.04 ± 0.06 g |
| MD-2 | 1.84 ± 0.05 e | 0.62 ± 0.02 cd | 2.97 ±0.06 cd | 2.46 ± 0.07 f |
| CK-3 | 1.87 ± 0.08 de | 0.63 ± 0.05 bcd | 2.98 ±0.05 c | 2.48 ± 0.09 ef |
| MD-3 | 2.41 ± 0.11 b | 0.75 ± 0.0 8 b | 3.21 ±0.07 ab | 3.16 ± 0.15 c |
| CK-4 | 3.32 ± 0.31 a | 1.05 ± 0.08 a | 3.16 ±0.02 b | 4.37 ± 0.11 b |
| MD-4 | 3.47 ± 0.22 a | 1.11 ± 0.11 a | 3.13 ±0.06 a | 4.58 ± 0.12 a |
| CK-5 | 1.97 ± 0.09 cd | 0.69 ± 0.06 bc | 2.86 ±0.09 d | 2.66 ± 0.11 de |
| MD-5 | 2.11 ± 0.08 c | 0.7 ± 0.08 b | 3.01 ±0.07 bc | 2.81 ± 0.08 d |
| Models | Treatment | RMSE µmol·m−2·s−1 | MAE µmol·m−2·s−1 | R2 | Models | Treatments | RMSE µmol·m−2·s−1 | MAE µmol·m−2·s−1 | R2 |
|---|---|---|---|---|---|---|---|---|---|
| Rectangular hyperbolic model | CK-1 | 0.583 | 0.453 | 0.992 | Non- rectangular hyperbolic model | CK-1 | 0.373 | 0.302 | 0.997 |
| MD-1 | 0.656 | 0.533 | 0.991 | MD-1 | 0.510 | 0.409 | 0.994 | ||
| CK-2 | 0.795 | 0.635 | 0.989 | CK-2 | 0.512 | 0.434 | 0.995 | ||
| MD-2 | 0.777 | 0.599 | 0.991 | MD-2 | 0.534 | 0.44 | 0.996 | ||
| CK-3 | 0.758 | 0.555 | 0.993 | CK-3 | 0.664 | 0.49 | 0.995 | ||
| MD-3 | 0.863 | 0.695 | 0.993 | MD-3 | 0.771 | 0.662 | 0.995 | ||
| CK-4 | 0.768 | 0.641 | 0.995 | CK-4 | 0.674 | 0.555 | 0.996 | ||
| MD-4 | 0.588 | 0.478 | 0.998 | MD-4 | 0.534 | 0.449 | 0.998 | ||
| CK-5 | 0.529 | 0.392 | 0.997 | CK-5 | 0.458 | 0.354 | 0.998 | ||
| MD-5 | 0.511 | 0.407 | 0.998 | MD-5 | 0.469 | 0.395 | 0.998 | ||
| Modified rectangular hyperbolic model | CK-1 | 0.315 | 0.292 | 0.998 | Exponential model | CK-1 | 0.355 | 0.31 | 0.997 |
| MD-1 | 0.221 | 0.19 | 0.999 | MD-1 | 0.389 | 0.302 | 0.997 | ||
| CK-2 | 0.233 | 0.185 | 0.999 | CK-2 | 0.392 | 0.327 | 0.997 | ||
| MD-2 | 0.217 | 0.174 | 0.999 | MD-2 | 0.385 | 0.298 | 0.998 | ||
| CK-3 | 0.439 | 0.369 | 0.998 | CK-3 | 0.602 | 0.504 | 0.996 | ||
| MD-3 | 0.445 | 0.364 | 0.998 | MD-3 | 0.63 | 0.546 | 0.997 | ||
| CK-4 | 0.429 | 0.296 | 0.999 | CK-4 | 0.586 | 0.432 | 0.997 | ||
| MD-4 | 0.367 | 0.303 | 0.999 | MD-4 | 0.723 | 0.578 | 0.996 | ||
| CK-5 | 0.359 | 0.301 | 0.999 | CK-5 | 0.577 | 0.487 | 0.996 | ||
| MD-5 | 0.316 | 0.258 | 0.999 | MD-5 | 0.502 | 0.411 | 0.998 |
| Treatment | α | Pnmax µmol·m−2·s−1 | Ic µmol·m−2·s−1 | Is µmol·m−2·s−1 | Rd µmol·m−2·s−1 | R2 | ΔI = Is − Ic µmol·m−2·s−1 |
|---|---|---|---|---|---|---|---|
| CK-1 | 0.038 ± 0.003 i | 13.88 ± 0.59 g | 29.98 ± 0.42 a | 1680.73 ± 12.35 h | 1.15 ± 0.05 h | 0.998 | 1650.75 |
| MD-1 | 0.049 ± 0.004 h | 16.99 ± 0.97 f | 26.96 ± 0.36 c | 1748.88 ± 8.52 f | 1.32 ± 0.06 g | 0.999 | 1721.91 |
| CK-2 | 0.048 ± 0.005 h | 16.97 ± 0.68 f | 27.80 ± 0.51 b | 1726.72 ± 10.37 g | 1.36 ± 0.04 g | 0.999 | 1698.92 |
| MD-2 | 0.061 ± 0.002 g | 22.77 ± 0.51 e | 24.90 ± 1.14 e | 2001.42 ± 45.54 e | 1.51 ± 0.06 f | 0.999 | 1976.5 |
| CK-3 | 0.067 ± 0.003 f | 24.68 ± 0.33 d | 26.82 ± 0.63 e | 1991.70 ± 28.73 e | 1.80 ± 0.06 e | 0.998 | 1964.88 |
| MD-3 | 0.091 ± 0.003 c | 26.53 ± 0.82 c | 23.26 ± 0.31 f | 2278.81 ± 16.22 c | 2.12 ± 0.02 c | 0.998 | 2255.54 |
| CK-4 | 0.099 ± 0.003 b | 31.13 ± 1.25 b | 22.76 ± 0.24 g | 2325.34 ±14.84 b | 2.24 ± 0.05 b | 0.999 | 2302.58 |
| MD-4 | 0.129 ± 0.005 a | 33.73 ± 1.13 a | 20.29 ± 0.53 h | 2517.71 ± 65.41 a | 2.61 ± 0.14 a | 0.999 | 2497.43 |
| CK-5 | 0.072 ± 0.002 e | 24.46 ± 0.65 d | 25.09 ± 0.22 d | 2212.51 ± 23.68 d | 1.80 ± 0.04 e | 0.999 | 2187.42 |
| MD-5 | 0.079 ± 0.003 d | 31.03 ± 1.37 b | 24.64 ± 0.21 e | 2251.21 ± 9.34 d | 1.95 ± 0.08 d | 0.999 | 2226.61 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Y.; Wang, J.; Wang, Q.; Wang, C. Responses of the Growth Characteristics of Spinach to Different Moisture Contents in Soil under Irrigation with Magnetoelectric Water. Agronomy 2023, 13, 657. https://doi.org/10.3390/agronomy13030657
Sun Y, Wang J, Wang Q, Wang C. Responses of the Growth Characteristics of Spinach to Different Moisture Contents in Soil under Irrigation with Magnetoelectric Water. Agronomy. 2023; 13(3):657. https://doi.org/10.3390/agronomy13030657
Chicago/Turabian StyleSun, Yan, Jian Wang, Quanjiu Wang, and Chunhong Wang. 2023. "Responses of the Growth Characteristics of Spinach to Different Moisture Contents in Soil under Irrigation with Magnetoelectric Water" Agronomy 13, no. 3: 657. https://doi.org/10.3390/agronomy13030657
APA StyleSun, Y., Wang, J., Wang, Q., & Wang, C. (2023). Responses of the Growth Characteristics of Spinach to Different Moisture Contents in Soil under Irrigation with Magnetoelectric Water. Agronomy, 13(3), 657. https://doi.org/10.3390/agronomy13030657






