Impact of Fusarium Head Blight on Wheat Flour Quality: Examination of Protease Activity, Technological Quality and Rheological Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Wheat Samples, Field Experiments, FHB Inoculations, and Disease Assessment
2.2. Specific Properties of Grain and Flour
2.2.1. Technological Properties
2.2.2. Amylolytic Activity
2.2.3. Proteolytic Activity
2.3. Rheological Properties of Dough
2.4. Statistical Analysis
3. Results
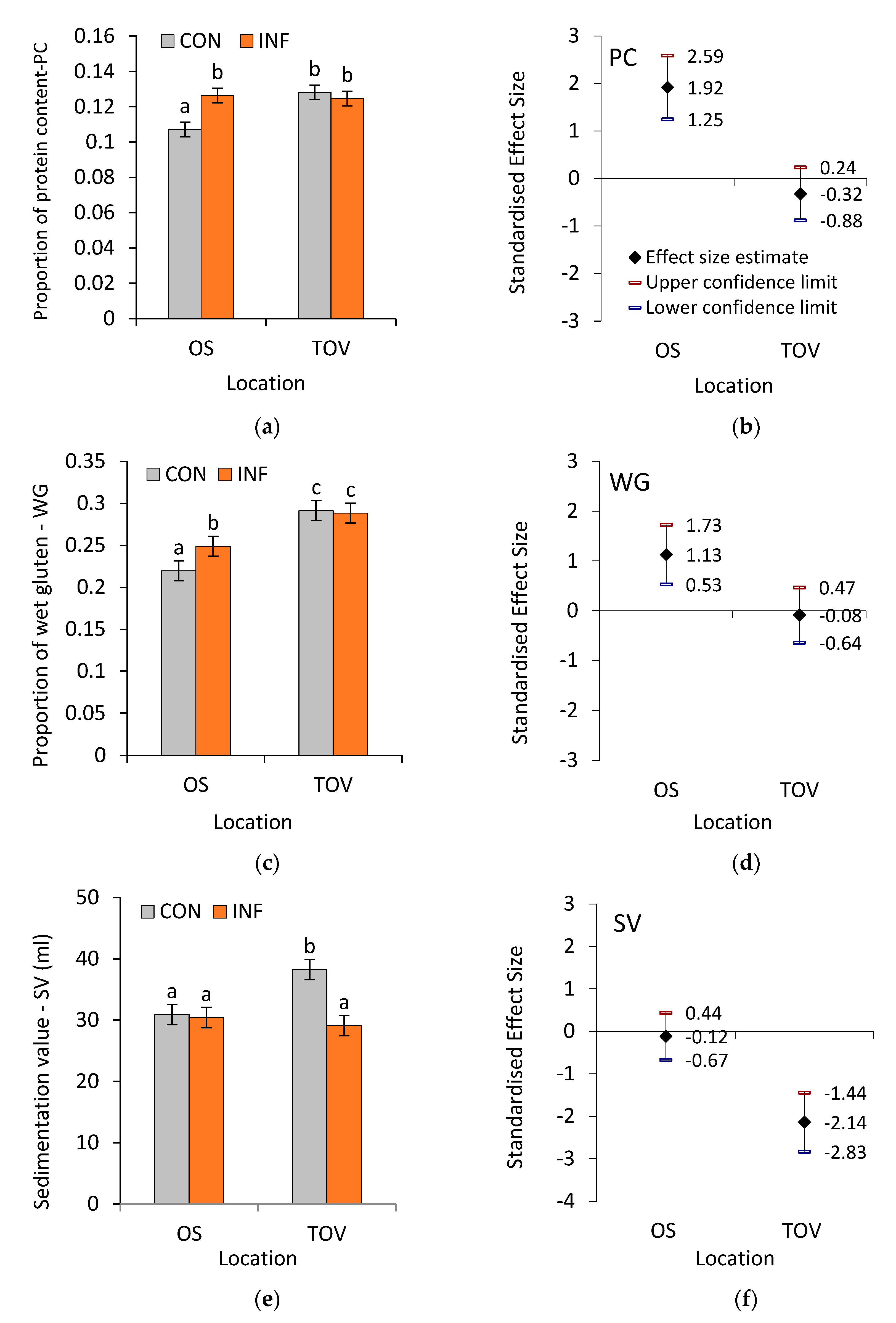
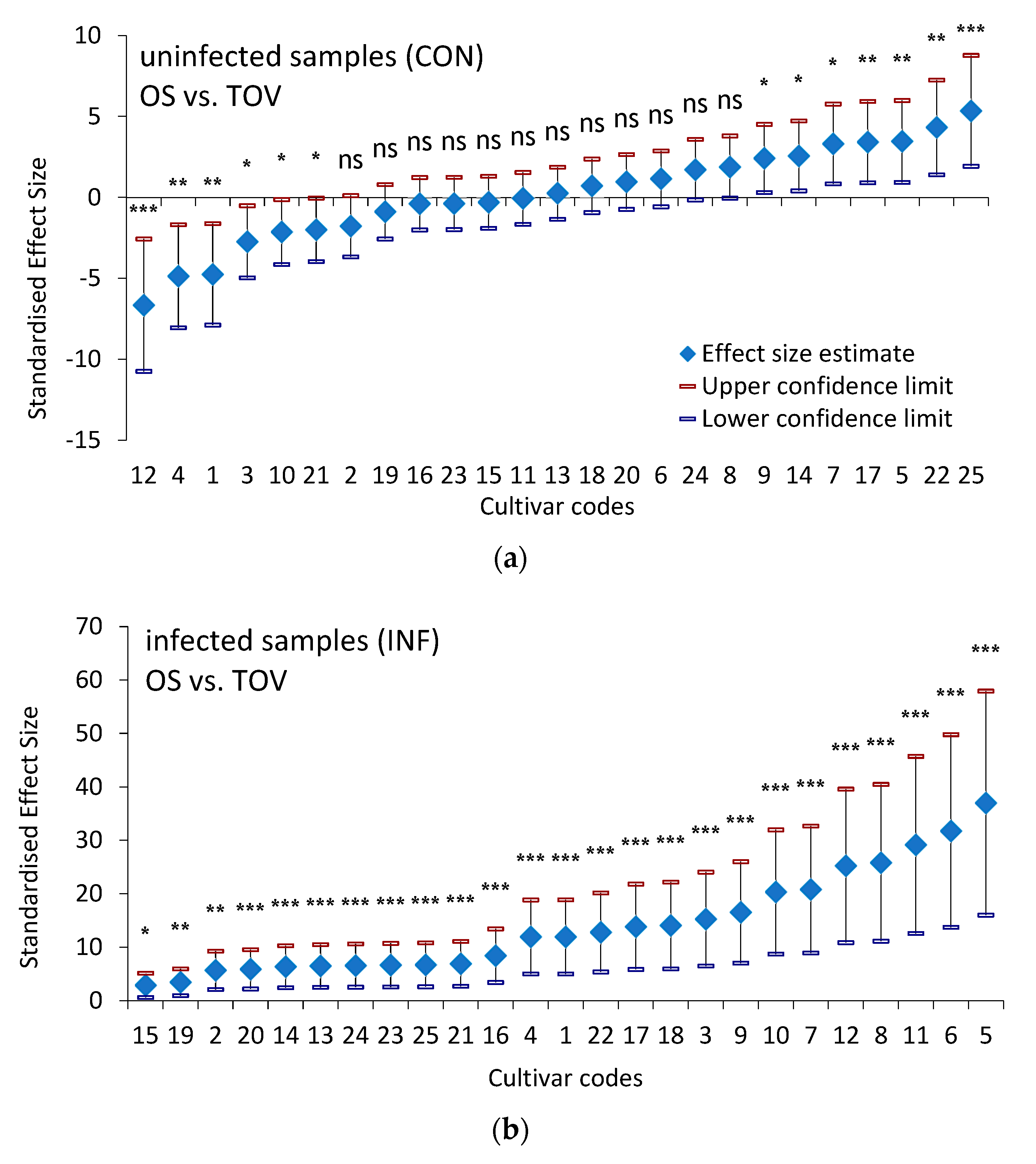
3.1. Technological Quality of Wheat Samples
3.2. Proteolytic Activity of Wheat Samples
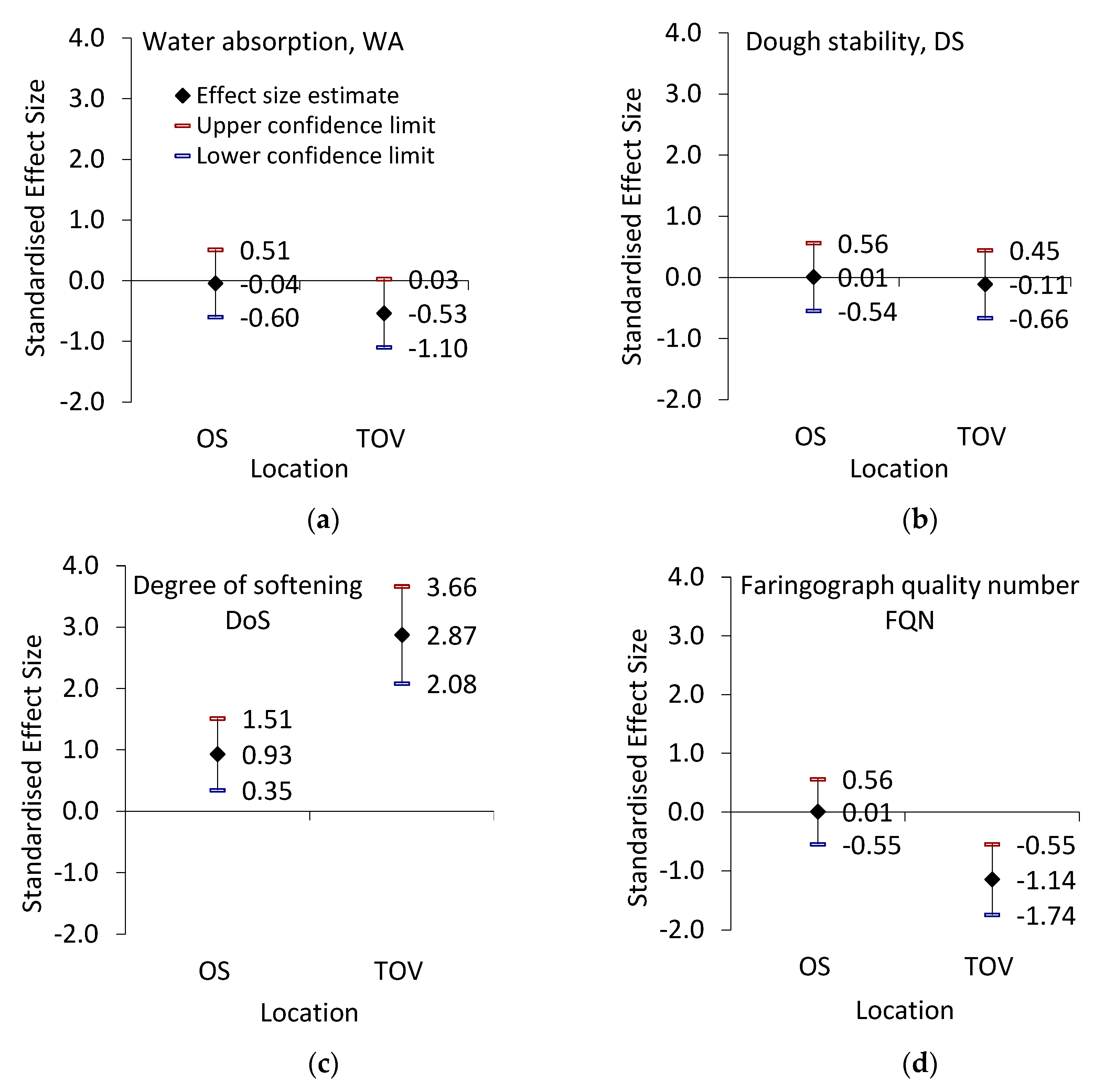
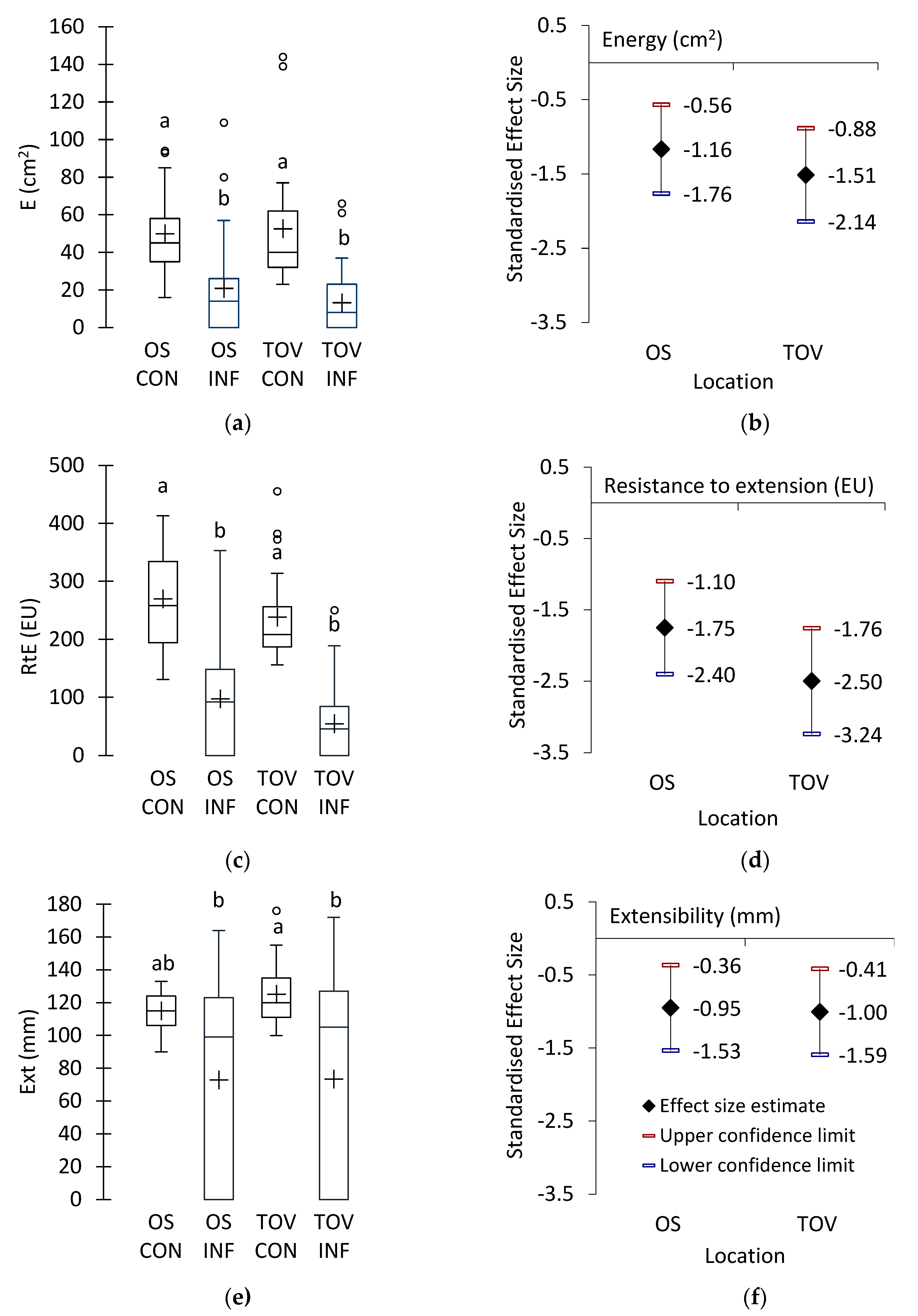
3.3. Rheological Quality of Wheat Samples
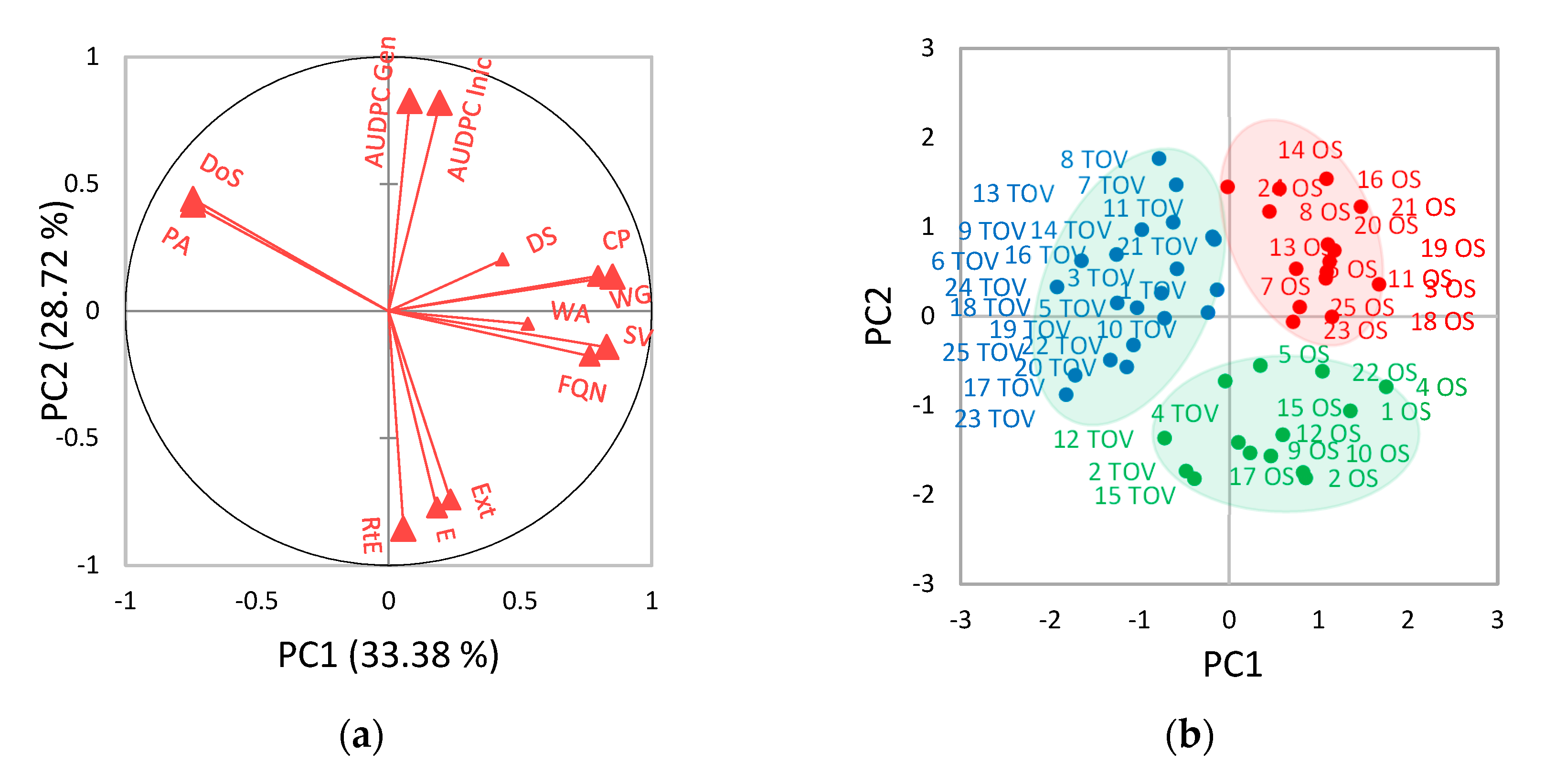
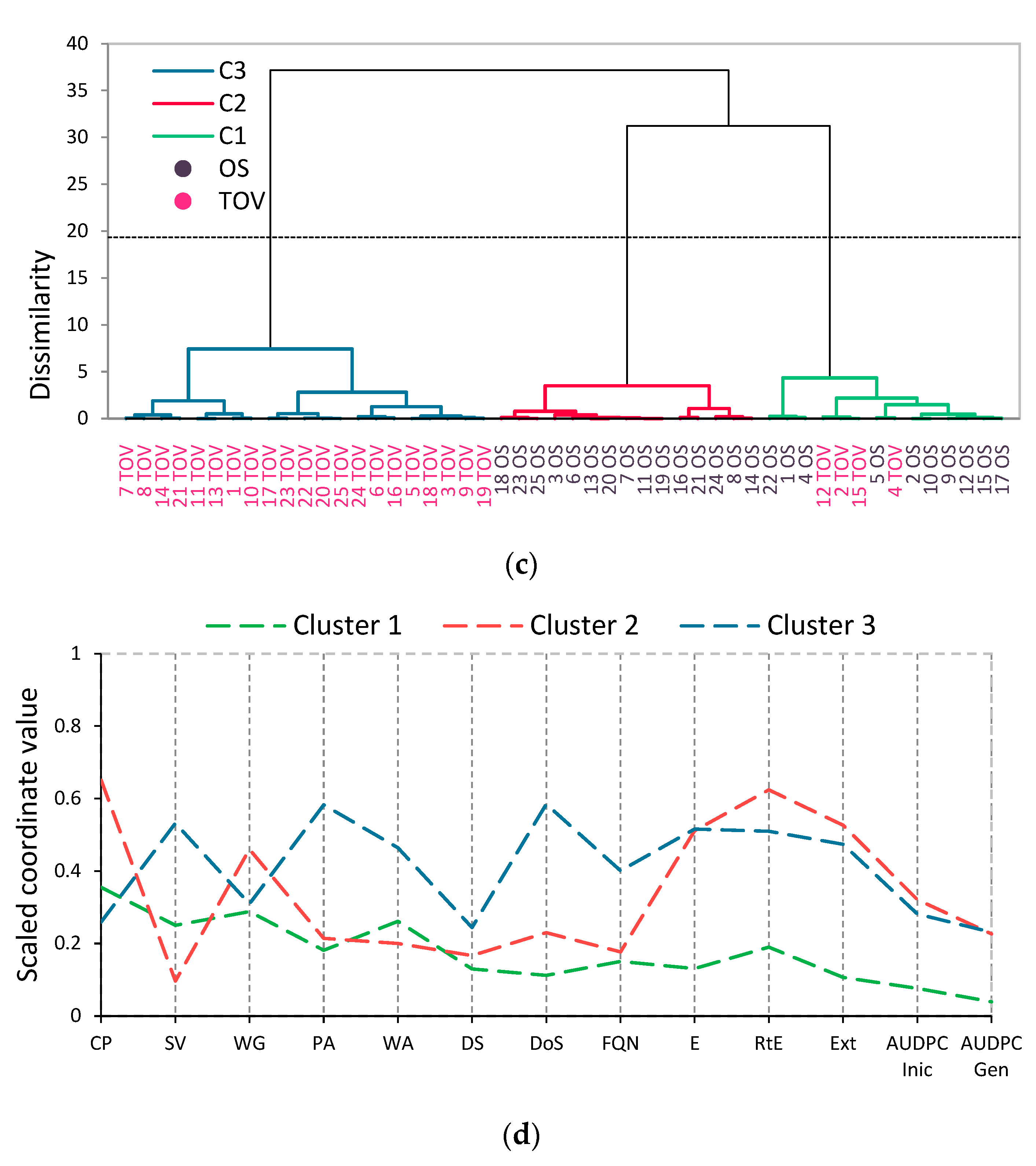
3.4. Multivariate Analysis
4. Discussion
4.1. Influence of Fusarium Infection and Nonspecific Proteolytic Activity on Technological Quality of Wheat Cultivars Grown at Two Locations
4.2. Influence of Fusarium Infection and Nonspecific Proteolytic Activity on Rheological Quality of Wheat Cultivars Grown at Two Locations
4.3. Multivariate Analysis of Quality Parameters among 25 Winter Wheat Varieties at Two Locations under Two Treatments
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Leslie, J.F.; Moretti, A.; Mesterházy, Á.; Ameye, M.; Audenaert, K.; Singh, P.K.; Richard-Forget, F.; Chulze, S.N.; Ponte, E.M.; del Chala, A.; et al. Key Global Actions for Mycotoxin Management in Wheat and Other Small Grains. Toxins 2021, 13, 725. [Google Scholar] [CrossRef]
- Alconada, T.M.; Moure, M.C.; Ortega, L.M. Fusarium Infection in Wheat, Aggressiveness and Changes in Grain Quality: A Review. Vegetos 2019, 32, 441–449. [Google Scholar] [CrossRef]
- Bellesi, F.J.; Arata, A.F.; Martínez, M.; Arrigoni, A.C.; Stenglein, S.A.; Dinolfo, M.I. Degradation of Gluten Proteins by Fusarium Species and Their Impact on the Grain Quality of Bread Wheat. J. Stored Prod. Res. 2019, 83, 1–8. [Google Scholar] [CrossRef]
- O’Donnell, K.; Kistler, H.C.; Tacke, B.K.; Casper, H.H. Gene Genealogies Reveal Global Phylogeographic Structure and Reproductive Isolation among Lineages of Fusarium graminearum, the Fungus Causing Wheat Scab. Proc. Natl. Acad. Sci. USA 2000, 97, 7905–7910. [Google Scholar] [CrossRef] [Green Version]
- Goswami, R.S.; Kistler, H.C. Heading for Disaster: Fusarium Graminearum on Cereal Crops. Mol. Plant Pathol. 2004, 5, 515–525. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.-M.; Nicholson, P.; Thomsett, M.A.; Simpson, D.; Cooke, B.M.; Doohan, F.M.; Brennan, J.; Monaghan, S.; Moretti, A.; Mule, G.; et al. Relationship between the Fungal Complex Causing Fusarium Head Blight of Wheat and Environmental Conditions. Phytopathology 2008, 98, 69–78. [Google Scholar] [CrossRef] [Green Version]
- Marček, T.; Vuletić, M.V.; Bakula, I.; Alivojvodić, S.; Španić, V. Time-Course Experiment of Fusarium Infestation of Wheat Genotypes with the Emphasis on the Physiological Response. Croat. J. Food Sci. Technol. 2018, 10, 58–63. [Google Scholar] [CrossRef]
- Španić, V.; Zdunić, Z.; Drezner, G.; Vuletić, M. Differences in Physiological Traits at the Initial Stage of Fusarium Head Blight Infection in Wheat. Biol. Plant 2020, 64, 174–181. [Google Scholar] [CrossRef]
- Martínez, M.; Biganzoli, F.; Arata, A.; Dinolfo, M.I.; Rojas, D.; Cristos, D.; Stenglein, S. Warm Nights Increase Fusarium Head Blight Negative Impact on Barley and Wheat Grains. Agric. For. Meteorol. 2022, 318, 108909. [Google Scholar] [CrossRef]
- Siou, D.; Gélisse, S.; Laval, V.; Repinçay, C.; Canalès, R.; Suffert, F.; Lannou, C. Effect of Wheat Spike Infection Timing on Fusarium Head Blight Development and Mycotoxin Accumulation. Plant Pathol. 2014, 63, 390–399. [Google Scholar] [CrossRef]
- del Ponte, E.M.; Fernandes, J.M.C.; Bergstrom, G.C. Influence of Growth Stage on Fusarium Head Blight and Deoxynivalenol Production in Wheat. J. Phytopathol. 2007, 155, 577–581. [Google Scholar] [CrossRef] [Green Version]
- Schaafsma, A.W.; Tamburic-Ilincic, L.; Hooker, D.C. Effect of Previous Crop, Tillage, Field Size, Adjacent Crop, and Sampling Direction on Airborne Propagules of Gibberella Zeae/Fusarium Graminearum, Fusarium Head Blight Severity, and Deoxynivalenol Accumulation in Winter Wheat. Can. J. Plant Pathol. 2005, 27, 217–224. [Google Scholar] [CrossRef]
- Wegulo, S.N.; Baenziger, P.S.; Hernandez Nopsa, J.; Bockus, W.W.; Hallen-Adams, H. Management of Fusarium Head Blight of Wheat and Barley. Crop Protection 2015, 73, 100–107. [Google Scholar] [CrossRef]
- Champeil, A.; Fourbet, J.F.; Doré, T.; Rossignol, L. Influence of Cropping System on Fusarium Head Blight and Mycotoxin Levels in Winter Wheat. Crop Protection 2004, 23, 531–537. [Google Scholar] [CrossRef]
- Kollers, S.; Rodemann, B.; Ling, J.; Korzun, V.; Ebmeyer, E.; Argillier, O.; Hinze, M.; Plieske, J.; Kulosa, D.; Ganal, M.W.; et al. Whole Genome Association Mapping of Fusarium Head Blight Resistance in European Winter Wheat (Triticum Aestivum L.). PLoS ONE 2013, 8, e0057500. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Z.; Hao, Y.; Mergoum, M.; Bai, G.; Humphreys, G.; Cloutier, S.; Xia, X.; He, Z. Breeding Wheat for Resistance to Fusarium Head Blight in the Global North: China, USA, and Canada. Crop J. 2019, 7, 730–738. [Google Scholar] [CrossRef]
- Wu, F.; Zhou, Y.; Shen, Y.; Sun, Z.; Li, L.; Li, T. Linking Multi-Omics to Wheat Resistance Types to Fusarium Head Blight to Reveal the Underlying Mechanisms. Int. J. Mol. Sci. 2022, 23, 2280. [Google Scholar] [CrossRef]
- Schroeder, H.W.; Christensen, J.J.; Christensen, J.D.; Platz-Christensen, J.; Schroeder, H.W. Factors Affecting Resistance of Wheat to Scab Caused by Gibberella zeae. Phytopathology 1963, 53, 831–838. [Google Scholar]
- Steiner, B.; Lemmens, M.; Griesser, M.; Scholz, U.; Schondelmaier, J.; Buerstmayr, H. Molecular Mapping of Resistance to Fusarium Head Blight in the Spring Wheat Cultivar Frontana. Theor. Appl. Genet. 2004, 109, 215–224. [Google Scholar] [CrossRef]
- Buerstmayr, M.; Wagner, C.; Nosenko, T.; Omony, J.; Steiner, B.; Nussbaumer, T.; Mayer, K.F.X.; Buerstmayr, H. Fusarium Head Blight Resistance in European Winter Wheat: Insights from Genome-Wide Transcriptome Analysis. BMC Genom. 2021, 22, 470. [Google Scholar] [CrossRef]
- Drakopoulos, D.; Kägi, A.; Six, J.; Zorn, A.; Wettstein, F.E.; Bucheli, T.D.; Forrer, H.-R.; Vogelgsang, S. The Agronomic and Economic Viability of Innovative Cropping Systems to Reduce Fusarium Head Blight and Related Mycotoxins in Wheat. Agric. Syst. 2021, 192, 103198. [Google Scholar] [CrossRef]
- Magan, N.; Aldred, D. Post-Harvest Control Strategies: Minimizing Mycotoxins in the Food Chain. Int. J. Food Microbiol. 2007, 119, 131–139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gärtner, B.H.; Munich, M.; Kleijer, G.; Mascher, F. Characterisation of Kernel Resistance against Fusarium Infection in Spring Wheat by Baking Quality and Mycotoxin Assessments. Eur. J. Plant Pathol. 2007, 120, 61–68. [Google Scholar] [CrossRef]
- Kreuzberger, M.; Limsuwan, S.; Eggert, K.; Karlovsky, P.; Pawelzik, E.; Eggert, M. Impact of Fusarium Spp. Infection of Bread Wheat (Triticum Aestivum L.) on Composition and Quality of Flour in Association with EU Maximum Level for Deoxynivalenol. J. Appl. Bot. Food Qual. 2015, 88, 177–185. [Google Scholar] [CrossRef]
- Matthäus, K.; Dänicke, S.; Vahjen, W.; Simon, O.; Wang, J.; Valenta, H.; Meyer, K.; Strumpf, A.; Ziesenib, H.; Flachowsky, G. Progression of Mycotoxin and Nutrient Concentrations in Wheat after Inoculation with Fusarium Culmorum. Arch. Anim. Nutr. 2004, 58, 19–35. [Google Scholar] [CrossRef] [PubMed]
- Bacala, R.; Fu, B.X.; Cordova, K.; Hatcher, D.W. Wheat Fusarium Protease Specificity and Effect on Dough Properties. Foods 2021, 10, 1585. [Google Scholar] [CrossRef]
- Horvat, D.; Španić, V.; Dvojković, K.; Šimić, G.; Magdić, D.; Nevistić, A. The Influence of Fusarium Infection on Wheat (Triticum Aestivum L.) Proteins Distribution and Baking Quality. Cereal Res. Commun. 2015, 43, 61–71. [Google Scholar] [CrossRef] [Green Version]
- Berghofer, L.K.; Hocking, A.D.; Miskelly, D.; Jansson, E. Microbiology of Wheat and Flour Milling in Australia. Int. J. Food Microbiol. 2003, 85, 137–149. [Google Scholar] [CrossRef]
- Španić, V.; Dvojković, K.; Babić, J.; Drezner, G.; Zdunić, Z. Fusarium Head Blight Infestation in Relation to Winter Wheat End-Use Quality—A Three-Year Study. Agronomy 2021, 11, 1648. [Google Scholar] [CrossRef]
- Diaz-Mendoza, M.; Diaz, I.; Martinez, M. Insights on the Proteases Involved in Barley and Wheat Grain Germination. Int. J. Mol. Sci. 2019, 20, 2087. [Google Scholar] [CrossRef] [Green Version]
- Nightingale, M.J.; Marchylo, B.A.; Clear, R.M.; Dexter, J.E.; Preston, K.R. Fusarium Head Blight: Effect of Fungal Proteases on Wheat Storage Proteins. Cereal Chem. J. 1999, 76, 150–158. [Google Scholar] [CrossRef]
- Eggert, K.; Rawel, H.M.; Pawelzik, E. In Vitro Degradation of Wheat Gluten Fractions by Fusarium Graminearum Proteases. Eur. Food Res. Technol. 2011, 233, 697–705. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Wieser, H.; Pawelzik, E.; Weinert, J.; Keutgen, A.J.; Wolf, G.A. Impact of the Fungal Protease Produced by Fusarium Culmorum on the Protein Quality and Breadmaking Properties of Winter Wheat. Eur. Food Res. Technol. 2005, 220, 552–559. [Google Scholar] [CrossRef]
- van Oort, M. Enzymes in Bread Making. In Enzymes in Food Technology; Wiley-Blackwell: Oxford, UK, 2009; pp. 103–143. [Google Scholar]
- Koga, S.; Rieder, A.; Ballance, S.; Uhlen, A.K.; Veiseth-Kent, E. Gluten-Degrading Proteases in Wheat Infected by Fusarium Graminearum—Protease Identification and Effects on Gluten and Dough Properties. J. Agric. Food Chem. 2019, 67, 11025–11034. [Google Scholar] [CrossRef] [PubMed]
- Pekkarinen, A.I.; Jones, B.L. Purification and Identification of Barley (Hordeum Vulgare L.) Proteins That Inhibit the Alkaline Serine Proteinases of Fusarium Culmorum. J. Agric. Food Chem. 2003, 51, 1710–1717. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Wang, H.; Zhang, C.; Wang, J.; Chen, A.; Chen, Y.; Ma, Z. System-Wide Characterization of Subtilases Reveals That Subtilisin-like Protease FgPrb1 of Fusarium Graminearum Regulates Fungal Development and Virulence. Fungal Genet. Biol. 2020, 144, 103449. [Google Scholar] [CrossRef]
- Elagamey, E.; Abdellatef, M.A.E.; Arafat, M.Y. Proteomic Insights of Chitosan Mediated Inhibition of Fusarium Oxysporum f. Sp. Cucumerinum. J. Proteomics 2022, 260, 104560. [Google Scholar] [CrossRef]
- Pekkarinen, A.I.; Longstaff, C.; Jones, B.L. Kinetics of the Inhibition of Fusarium Serine Proteinases by Barley (Hordeum Vulgare L.) Inhibitors. J. Agric. Food Chem. 2007, 55, 2736–2742. [Google Scholar] [CrossRef]
- Snijders, C.H.A.; van Eeuwijk, F.A. Genotype x Strain Interactions for Resistance to Fusarium Head Blight Caused by Fusarium Culmorum in Winter Wheat. Theor. Appl. Genet. 1991, 81, 239–244. [Google Scholar] [CrossRef]
- Zadoks, J.C.; Chang, T.T.; Konzak, C.F. A Decimal Code for the Growth Stages of Cereals. Weed Res. 1974, 14, 415–421. [Google Scholar] [CrossRef]
- ICC Standard No. 155; Determination of Wet Gluten Quantity and Quality (Gluten Index Ac. to Perten) of Whole Wheat Meal and Wheat Flour (Triticum Aestivum). International Association for Cereal Science and Technology: Vienna, Austria, 1994.
- HRN EN ISO 5529; Wheat—Determination of the Sedimentation Index—Zeleny Test (ISO 5529:2007). ISO: Geneva, Switzerland, 2010.
- HR EN ISO 3093; Wheat, Rye and Their Flours, Durum Wheat and Durum Wheat Semolina—Determination of the Falling Number According to Hagberg-Perten. ISO: Geneva, Switzerland, 2009.
- Cupp-Enyard, C. Sigma’s Nonspecific Protease Activity Assay—Casein as a Substrate. J. Vis. Exp. 2008, 17, e899. [Google Scholar] [CrossRef]
- HRN EN ISO 5530-1; Wheat Flour—Physical Characteristics of Doughs—Part 1: Determination of Water Absorption and Rheological Properties Using a Farinograph. ISO: Geneva, Switzerland, 2015.
- HRN EN ISO 5530-2; Wheat Flour—Physical Characteristics of Doughs—Part 2: Determination of Rheological Properties Using an Extensograph. ISO: Geneva, Switzerland, 2015.
- Microsoft Corporation Microsoft Excel 2019. Available online: https://office.microsoft.com/excel (accessed on 17 January 2023).
- Addinsoft XLSTAT Statistical and Data Analysis Solution 2022. Available online: https://www.xlstat.com (accessed on 17 January 2023).
- Hedges, L.V.; Olkin, I. Estimation of a Single Effect Size: Parametric and Nonparametric Methods. In Statistical Methods for Meta-Analysis; Elsevier: Amsterdam, The Netherlands, 1985; pp. 75–106. [Google Scholar]
- Ellis, P.D. The Essential Guide to Effect Sizes: Statistical Power, Meta-Analysis, and the Interpretation of Research Results; Cambridge University Press: Cambridge, UK, 2010. [Google Scholar]
- Eltaher, S.; Baenziger, P.S.; Belamkar, V.; Emara, H.A.; Nower, A.A.; Salem, K.F.M.; Alqudah, A.M.; Sallam, A. GWAS Revealed Effect of Genotype × Environment Interactions for Grain Yield of Nebraska Winter Wheat. BMC Genom. 2021, 22, 2. [Google Scholar] [CrossRef]
- Surma, M.; Adamski, T.; Banaszak, Z.; Kaczmarek, Z.; Kuczyńska, A.; Majcher, M.; Ługowska, B.; Obuchowski, W.; Salmanowicz, B.; Krystkowiak, K. Effect of Genotype, Environment and Their Interaction on Quality Parameters of Wheat Breeding Lines of Diverse Grain Hardness. Plant Prod. Sci. 2012, 15, 192–203. [Google Scholar] [CrossRef]
- Nehe, A.; Akin, B.; Sanal, T.; Evlice, A.K.; Ünsal, R.; Dinçer, N.; Demir, L.; Geren, H.; Sevim, I.; Orhan, Ş.; et al. Genotype x Environment Interaction and Genetic Gain for Grain Yield and Grain Quality Traits in Turkish Spring Wheat Released between 1964 and 2010. PLoS ONE 2019, 14, e0219432. [Google Scholar] [CrossRef] [Green Version]
- Williams, R.M.; O’Brien, L.; Eagles, H.A.; Solah, V.A.; Jayasena, V. The Influences of Genotype, Environment, and Genotypeenvironment Interaction on Wheat Quality. Aust. J. Agric. Res. 2008, 59, 95–111. [Google Scholar] [CrossRef]
- Abedi, T.; Alemzadeh, A.; Kazemeini, S.A. Wheat Yield and Grain Protein Response to Nitrogen Amount and Timing. Aust. J. Crop Sci. 2011, 5, 330. [Google Scholar]
- Wang, R.; Wang, H.; Jiang, G.; Yin, H.; Che, Z. Effects of Nitrogen Application Strategy on Nitrogen Enzyme Activities and Protein Content in Spring Wheat Grain. Agriculture 2022, 12, 1891. [Google Scholar] [CrossRef]
- Ghimire, D.; Das, S.; Mueller, N.D.; Creech, C.F.; Santra, D.; Baenziger, P.S.; Easterly, A.C.; Maust, B.; Maharjan, B. Effects of Cultivars and Nitrogen Management on Wheat Grain Yield and Protein. Agron. J. 2021, 113, 4348–4368. [Google Scholar] [CrossRef]
- Stupar, V.; Paunović, A.; Madić, M.; Knežević, D.; Đurović, D. Influence of Genotype, Nitrogen Fertilisation, and Weather Conditions on Yield Variability and Grain Quality in Spring Malting Barley. J. Cent. Eur. Agric. 2021, 22, 86–95. [Google Scholar] [CrossRef]
- Xue, C.; Matros, A.; Mock, H.-P.; Mühling, K.-H. Protein Composition and Baking Quality of Wheat Flour as Affected by Split Nitrogen Application. Front. Plant Sci. 2019, 10, 642. [Google Scholar] [CrossRef] [PubMed]
- Xue, C.; Schulte auf’m Erley, G.; Rücker, S.; Koehler, P.; Obenauf, U.; Mühling, K.H. Late Nitrogen Application Increased Protein Concentration but Not Baking Quality of Wheat. J. Plant Nutr. Soil Sci. 2016, 179, 591–601. [Google Scholar] [CrossRef]
- Xue, C.; Erley, G.S.A.; Rossmann, A.; Schuster, R.; Koehler, P.; Mühling, K.H. Split Nitrogen Application Improves Wheat Baking Quality by Influencing Protein Composition Rather than Concentration. Front. Plant Sci. 2016, 7, 738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malik, A.H.; Kuktaite, R.; Johansson, E. Combined Effect of Genetic and Environmental Factors on the Accumulation of Proteins in the Wheat Grain and Their Relationship to Breadmaking Quality. J. Cereal Sci. 2013, 57, 170–174. [Google Scholar] [CrossRef]
- Zecevic, V.; Knezevic, D.; Boskovic, J.; Madic, M. Effect of Genotype and Environment on Wheat Quality. Genetika 2009, 41, 247–253. [Google Scholar] [CrossRef]
- Boyacioǧlu, D.; Hettiarachchy, N.S. Changes in Some Biochemical Components of Wheat Grain That Was Infected with Fusarium Graminearum. J. Cereal Sci. 1995, 21, 57–62. [Google Scholar] [CrossRef]
- Barneix, A.J. Physiology and Biochemistry of Source-Regulated Protein Accumulation in the Wheat Grain. J. Plant Physiol. 2007, 164, 581–590. [Google Scholar] [CrossRef]
- Leitgeb, M.; Knez, Ž.; Hojnik Podrepšek, G. Effect of Green Food Processing Technology on the Enzyme Activity in Spelt Flour. Foods 2022, 11, 3832. [Google Scholar] [CrossRef]
- Tóth, B.; Mesterházy, Á.; Horváth, Z.; Bartók, T.; Varga, M.; Varga, J. Genetic Variability of Central European Isolates of the Fusarium Graminearum Species Complex. Eur. J. Plant Pathol. 2005, 113, 35–45. [Google Scholar] [CrossRef]
- Akinsanmi, O.A.; Backhouse, D.; Simpfendorfer, S.; Chakraborty, S. Mycelial Compatibility Reactions of Australian Fusarium Graminearum and F. Pseudograminearum Isolates Compared with AFLP Groupings. Plant Pathol. 2008, 57, 251–261. [Google Scholar] [CrossRef]
- Malbrán, I.; Mourelos, C.A.; Girotti, J.R.; Aulicino, M.B.; Balatti, P.A.; Lori, G.A. Aggressiveness Variation of Fusarium Graminearum Isolates from Argentina Following Point Inoculation of Field Grown Wheat Spikes. Crop Prot. 2012, 42, 234–243. [Google Scholar] [CrossRef]
- Ortega, L.M.; Moure, M.C.; González, E.M.; Alconada, T.M. Wheat Storage Proteins: Changes on the Glutenins after Wheat Infection with Different Isolates of Fusarium Graminearum. Int. Microbiol. 2019, 22, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Eggert, K.; Wieser, H.; Pawelzik, E. The Influence of Fusarium Infection and Growing Location on the Quantitative Protein Composition of (Part I) Emmer (Triticum Dicoccum). Eur. Food Res. Technol. 2010, 230, 837–847. [Google Scholar] [CrossRef] [Green Version]
- Pawelzik, E.; Permady, H.H.; Weinert, J.; Wolf, G.A. Effect of Fusarium Contamination on Selected Quality Criteria of Wheat. Getreide Mehl. Brot. 1998, 52, 264–266. [Google Scholar]
- Juodeikiene, G.; Basinskiene, L.; Vidmantiene, D.; Bartkiene, E.; Bakutis, B.; Baliukoniene, V. Acoustic Sensing of Deoxynivalenol in Co-Occurrence with Zearalenone and T-2/HT-2 Toxin in Winter Wheat Cultivar Sirvinta from Lithuania. World Mycotoxin J. 2011, 4, 395–404. [Google Scholar] [CrossRef]
- Khatkar, B.S.; Bell, A.E.; Schofield, J.D. A Comparative Study of the Inter-Relationships Between Mixograph Parameters and Bread-Making Qualities of Wheat Flours and Glutens. J. Sci. Food Agric. 1996, 72, 71–85. [Google Scholar] [CrossRef]
- Hadnađev, T.D.; Torbica, A.; Hadnađev, M. Rheological Properties of Wheat Flour Substitutes/Alternative Crops Assessed by Mixolab. Procedia Food Sci. 2011, 1, 328–334. [Google Scholar] [CrossRef] [Green Version]
- Martin, C.; Schöneberg, T.; Vogelsgang, S.; Vincenti, J.; Bertossa, M.; Mauch-Mani, B.; Mascher, F. Factors of Wheat Grain Resistance to Fusarium Head Blight. Phytopathol. Mediterr. 2017, 56, 154–166. [Google Scholar] [CrossRef]
- Okuda, R.; Tabara, A.; Okusu, H.; Seguchi, M. Measurement of Water Absorption in Wheat Flour by Mixograph Test. Food Sci. Technol. Res. 2016, 22, 841–846. [Google Scholar] [CrossRef] [Green Version]
- Zaidul, I.; Karim, A.A.; Manan, D.; Ariffin, A.; Norulaini, N.N.; Omar, A.M. A Farinograph Study on the Viscoelastic Properties of Sago/Wheat Flour Dough Systems. J. Sci. Food Agric. 2004, 84, 616–622. [Google Scholar] [CrossRef]
- Martínez, M.; Ramírez Albuquerque, L.; Arata, A.F.; Biganzoli, F.; Pinto, V.F.; Stenglein, S.A. Effects of Fusarium Graminearum and Fusarium Poae on Disease Parameters, Grain Quality and Mycotoxins Contamination in Bread Wheat (Part I). J. Sci. Food Agric. 2020, 100, 863–873. [Google Scholar] [CrossRef]
- Aydoğan, S.; Şahin, M.; Akçacık, A.; Sümerya, A.; Seyfi, H.; Bahri, T.; Uluslararası, D.; Araştırma, T.; Müdürlüğü, E. Relationships between Farinograph Parameters and Bread Volume, Physicochemical Traits in Bread Wheat Flours. J. Bahri Dagdas. Crop Res. 2015, 3(1), 14–18. [Google Scholar]
- Scala, V.; Aureli, G.; Cesarano, G.; Incerti, G.; Fanelli, C.; Scala, F.; Reverberi, M.; Bonanomi, G. Climate, Soil Management, and Cultivar Affect Fusarium Head Blight Incidence and Deoxynivalenol Accumulation in Durum Wheat of Southern Italy. Front. Microbiol. 2016, 7, 1014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Havrlentová, M.; Šliková, S.; Gregusová, V.; Kovácsová, B.; Lančaričová, A.; Nemeček, P.; Hendrichová, J.; Hozlár, P. The Influence of Artificial Fusarium Infection on Oat Grain Quality. Microorganisms 2021, 9, 2108. [Google Scholar] [CrossRef] [PubMed]
- Surma, M.; Adamski, T.; Wiśniewska, H.; Kaczmarek, Z.; Mejza, I.; Mejza, S.; Kuczyńska, A.; Krystkowiak, K.; Mikołajczak, K.; Ogrodowicz, P. Uni- and Multivariate Approaches to Evaluating the Susceptibility of Wheat Hybrids to Fusarium Head Blight. Czech J. Genet. Plant Breed. 2016, 52, 132–138. [Google Scholar] [CrossRef] [Green Version]
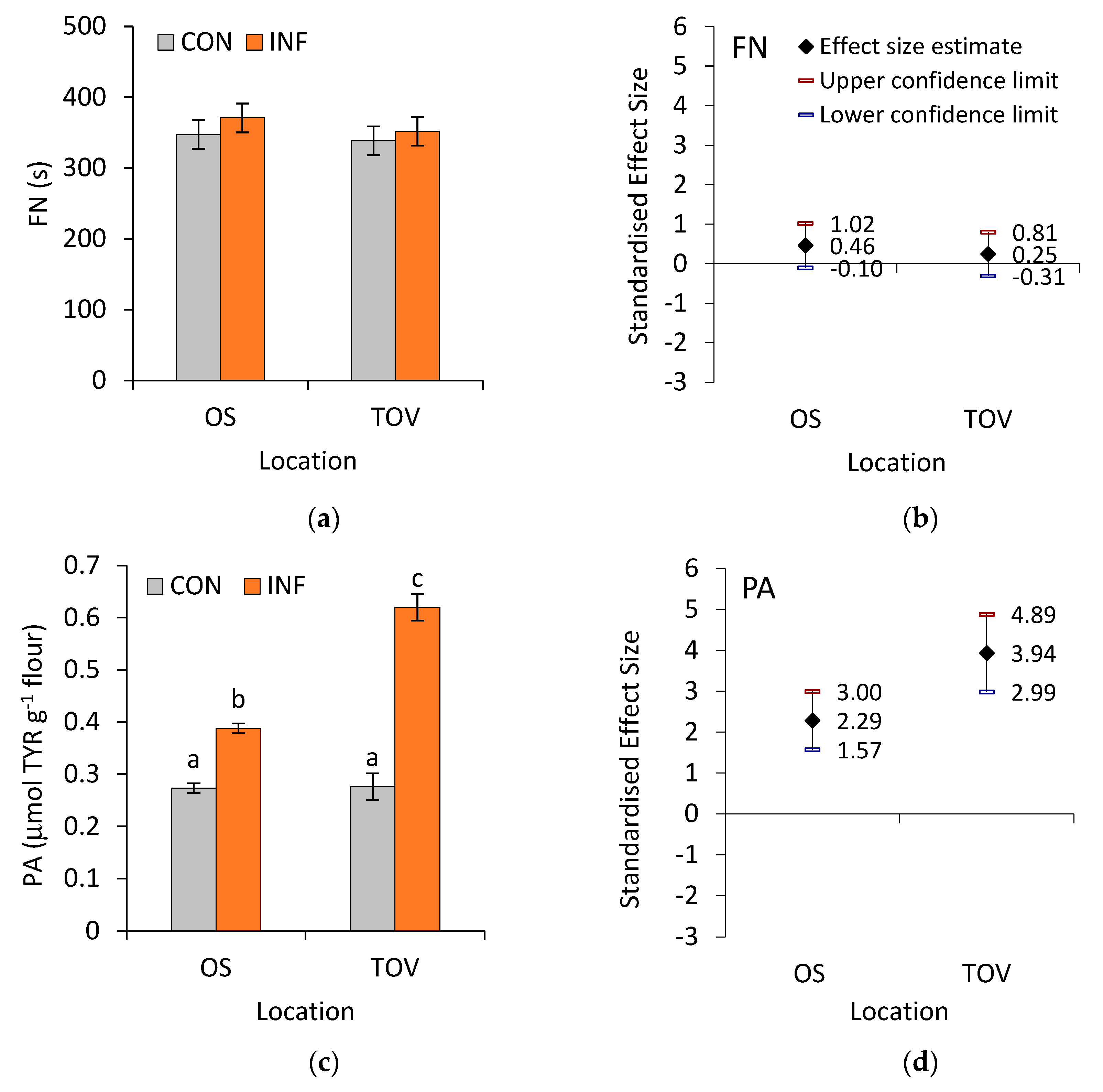
| Code | Winter Wheat Cultivars | Code | Winter Wheat Cultivars |
|---|---|---|---|
| 1 | Osk.107/15 MS/S | 14 | Imported * W2 S |
| 2 | Osk. 51/15 R | 15 | Imported * W3 R |
| 3 | Osk. 54/15 MS/MR | 16 | Osk. 108/04 S |
| 4 | Osk. 84/15 MR/R | 17 | Imported * W4 R |
| 5 | Osk. 251/02 MR/R | 18 | Osk. 106/03 S |
| 6 | Osk.111/08 MR/R | 19 | Osk. 44/11 MS/MR |
| 7 | Osk. 70/14 S | 20 | Osk.116/09 MR |
| 8 | Osk. 78/14 S | 21 | Osk. 4.40/7–82 S |
| 9 | Osk.138/14 MR/R | 22 | Osk. 381/06 MR |
| 10 | Osk. 52/13 R | 23 | Osk. 114/08 MR |
| 11 | Osk. 89/05 S/MS | 24 | Osk. 10287 S |
| 12 | Imported * W1 R | 25 | Osk 120/06 MS/MR |
| 13 | Osk.7733 S/MS |
| Location OS | Location TOV | |||
|---|---|---|---|---|
| CON (n = 25) | INF (n = 25) | CON (n = 25) | INF (n = 25) | |
| WA (%) | ANOVA (F3, 96 = 3.02, p = 0.03, R2 = 0.09) | |||
| 56.28 ± 0.49 ab | 56.18 ± 0.47 a | 57.91 ± 0.48 b | 56.74 ± 0.37 ab | |
| DS (min) | ANOVA (F3, 96 = 0.69, p = 0.56, R2 = 0.02) | |||
| 0.46 ± 0.07 a | 0.46 ± 0.09 a | 0.63 ± 0.09 a | 0.57 ± 0.13 a | |
| DoS (FU) | ANOVA (F3, 96 = 46.99, p < 0.0001, R2 = 0.59) | |||
| 83.88 ± 4.54 b | 107.96 ± 5.42 c | 56.08 ± 4.81 a | 150.04 ± 7.51 d | |
| FQN | ANOVA (F3, 96 = 8.71, p < 0.0001, R2 = 0.214) | |||
| 42.68 ± 6.8 a | 42.92 ± 4.9 a | 78.92 ± 8.4 b | 41.40 ± 3.6 a | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peršić, V.; Božinović, I.; Varnica, I.; Babić, J.; Španić, V. Impact of Fusarium Head Blight on Wheat Flour Quality: Examination of Protease Activity, Technological Quality and Rheological Properties. Agronomy 2023, 13, 662. https://doi.org/10.3390/agronomy13030662
Peršić V, Božinović I, Varnica I, Babić J, Španić V. Impact of Fusarium Head Blight on Wheat Flour Quality: Examination of Protease Activity, Technological Quality and Rheological Properties. Agronomy. 2023; 13(3):662. https://doi.org/10.3390/agronomy13030662
Chicago/Turabian StylePeršić, Vesna, Iva Božinović, Ivan Varnica, Jurislav Babić, and Valentina Španić. 2023. "Impact of Fusarium Head Blight on Wheat Flour Quality: Examination of Protease Activity, Technological Quality and Rheological Properties" Agronomy 13, no. 3: 662. https://doi.org/10.3390/agronomy13030662
APA StylePeršić, V., Božinović, I., Varnica, I., Babić, J., & Španić, V. (2023). Impact of Fusarium Head Blight on Wheat Flour Quality: Examination of Protease Activity, Technological Quality and Rheological Properties. Agronomy, 13(3), 662. https://doi.org/10.3390/agronomy13030662








