Effect of Foliar Treatments with Calcium and Nitrogen on Oregano Yield
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Site and Plant Material
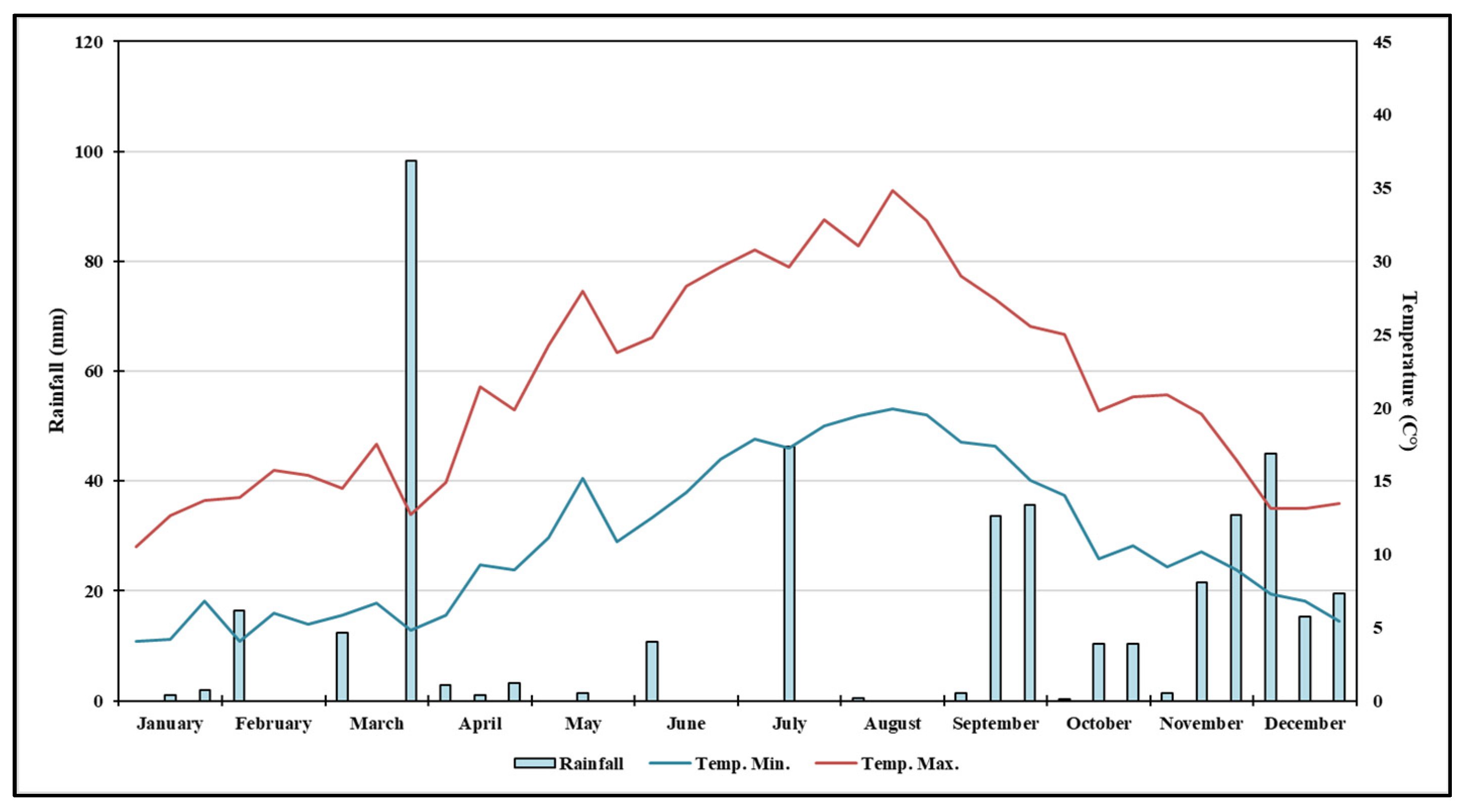
2.2. Weather Data
2.3. Foliar Treatments
2.4. Plant Measurement
2.5. Collection of Plant Material
2.6. Essential Oil Extraction
2.7. Experimental Design
2.8. Statistical Analysis
3. Results
3.1. Analysis of Rainfall and Air Temperature Trends at the Experimental Site
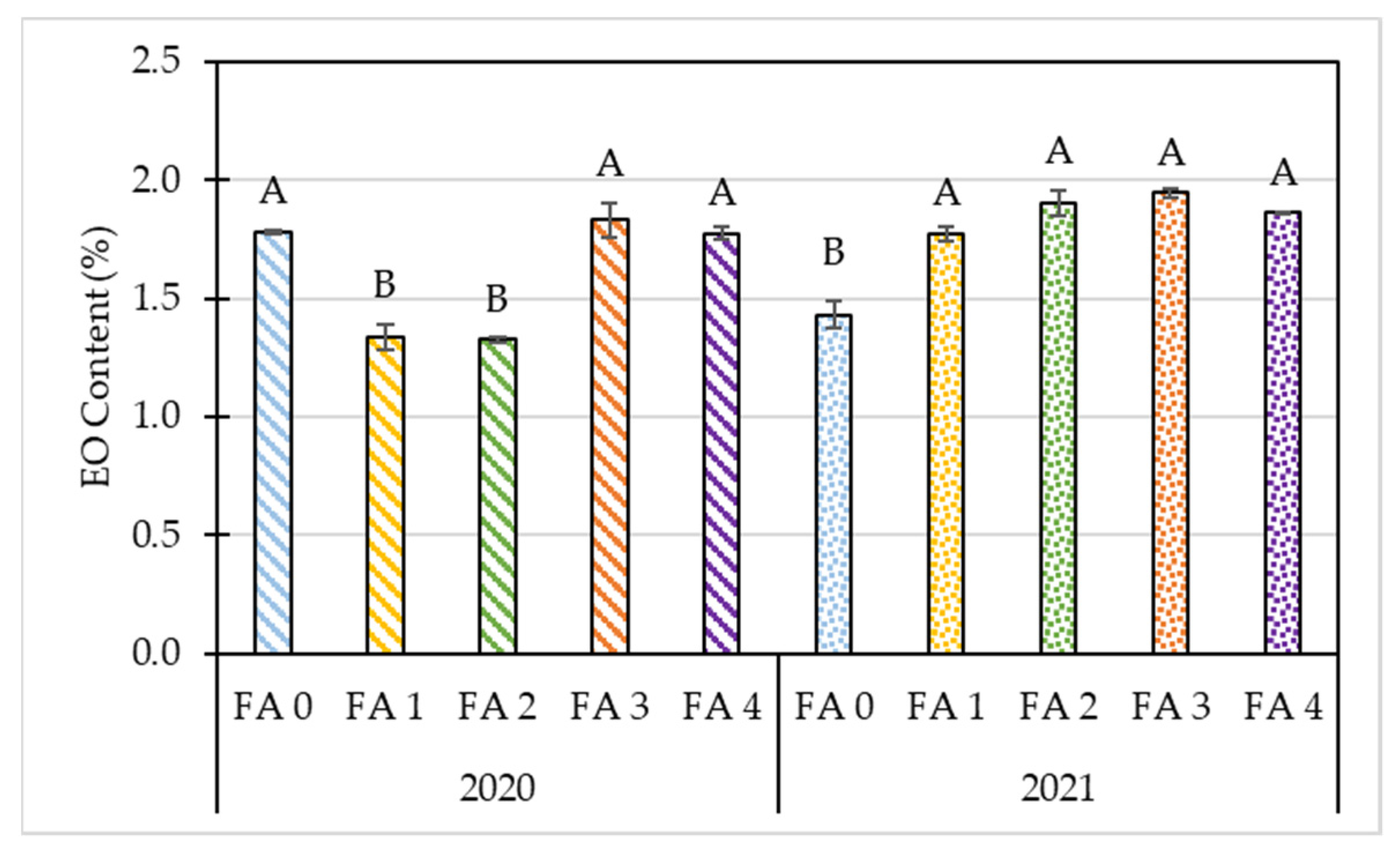
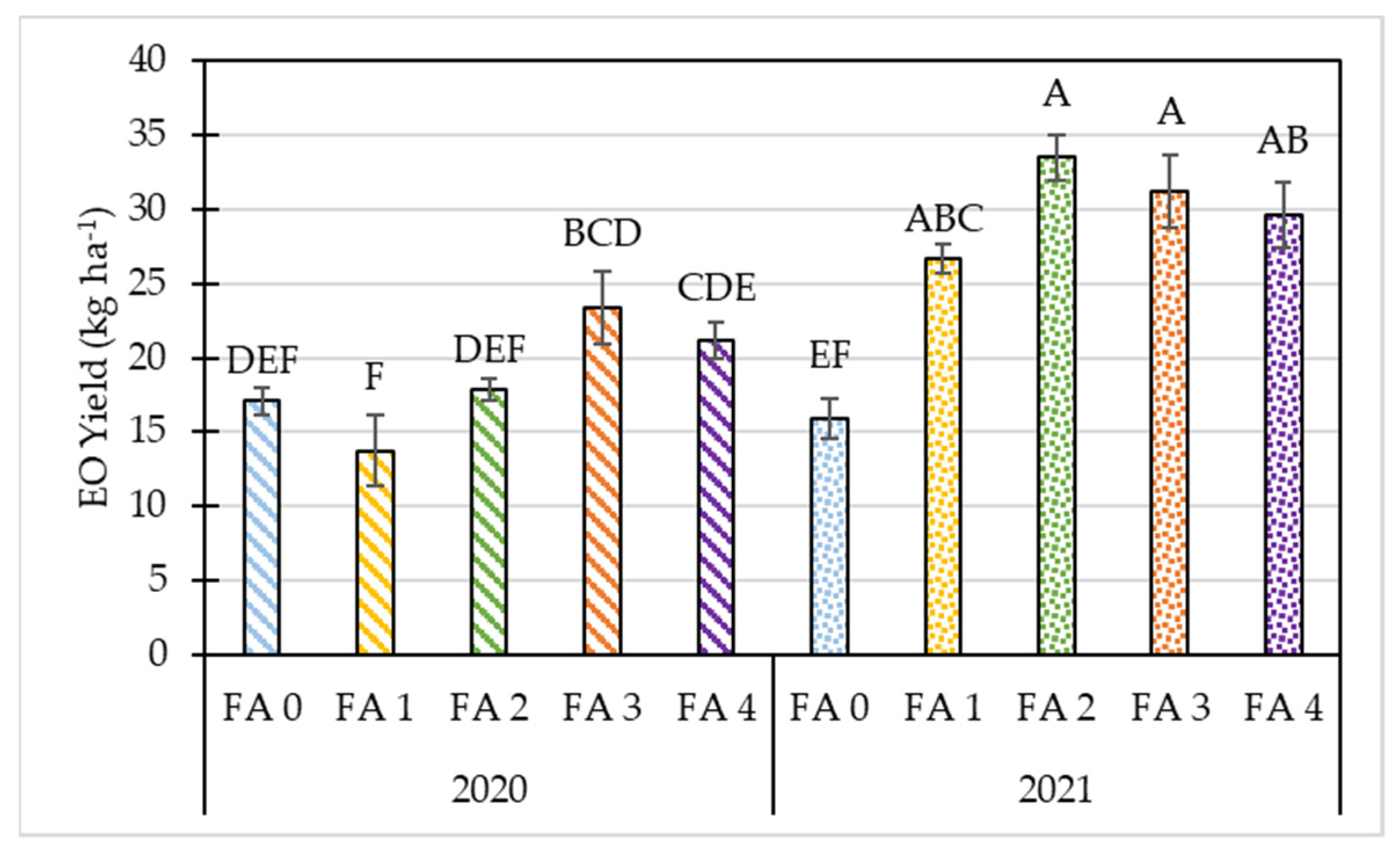
3.2. Effects of Year and Foliar Treatment on Morphological and Yield Parameters of Oregano
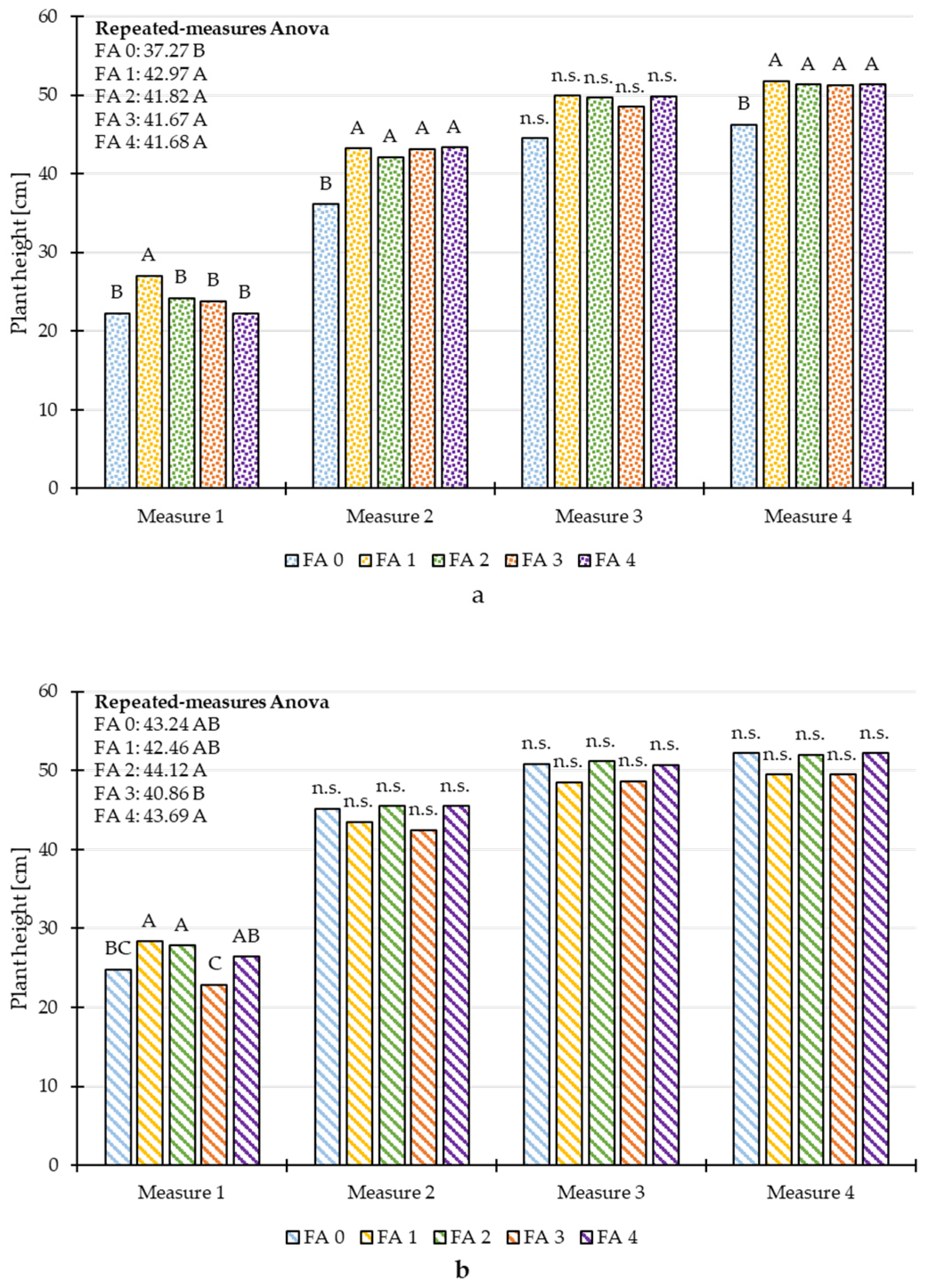
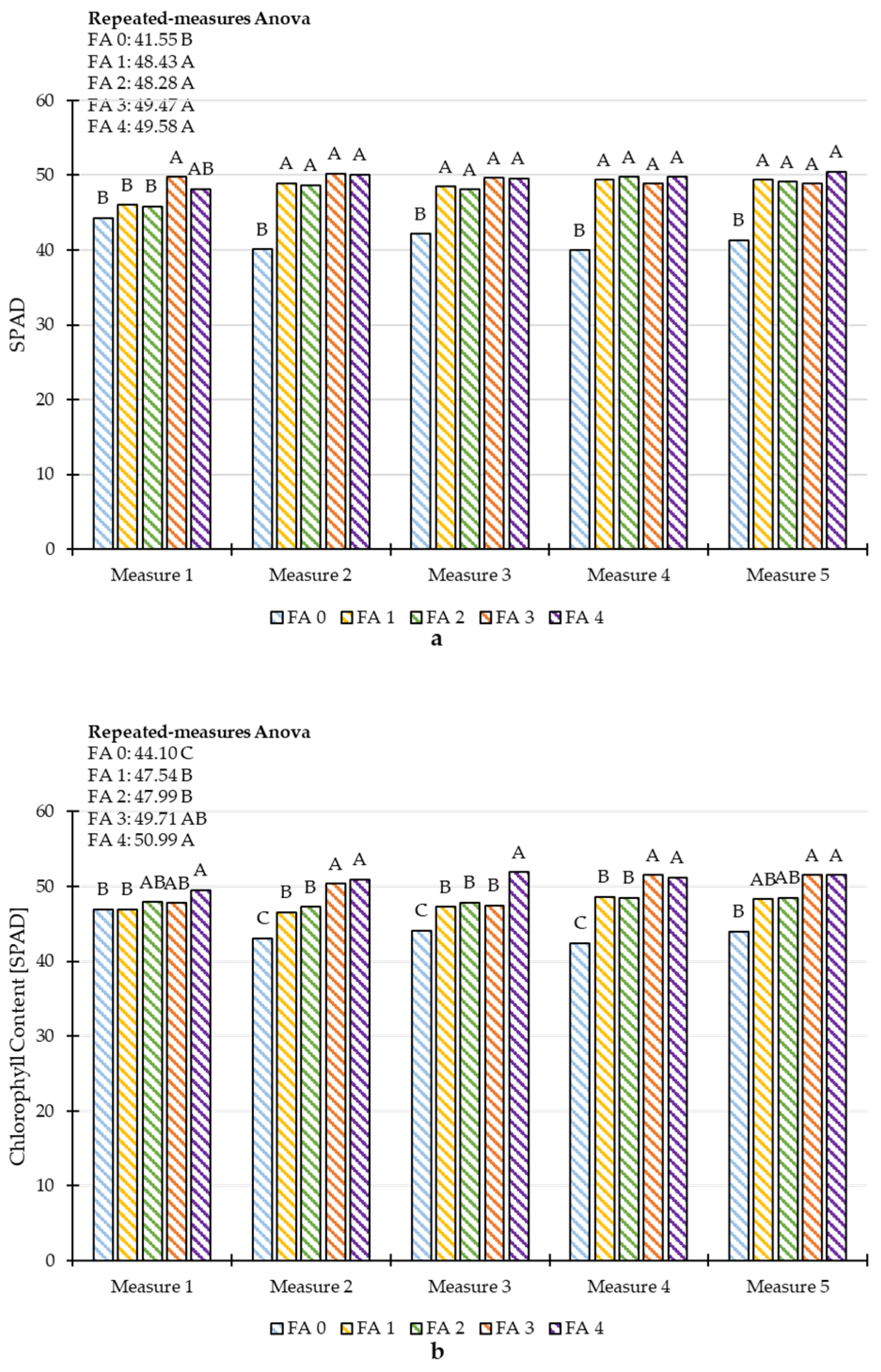
3.3. Repeated Measures ANOVA of Plant Height and Chlorophyll Content
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Global Status Report on Alcohol and Health 2018; WHO: Genova, Italy, 2018. [Google Scholar]
- De Falco, E.; Mancini, E.; Roscigno, G.; Mignola, E.; Taglialatela-Scafati, O.; Senatore, F. Chemical composition and biological activity of essential oils of Origanum vulgare L. subsp. vulgare L. under different growth conditions. Molecules 2013, 18, 14948–14960. [Google Scholar] [CrossRef]
- Tuttolomondo, T.; La Bella, S.; Licata, M.; Virga, G.; Leto, C.; Saija, A.; Trombetta, D.; Tomaino, A.; Speciale, A.; Napoli, E.M.; et al. Biomolecular characterization of wild sicilian oregano: Phytochemical screening of essential oils and extracts, and evaluation of their antioxidant activities. Chem. Biodivers 2013, 10, 411–433. [Google Scholar] [CrossRef]
- Tuttolomondo, T.; Martinelli, F.; Mariotti, L.; Leto, C.; Maggio, A.; La Bella, S. Agronomic, metabolomic and lipidomic characterisation of Sicilian Origanum vulgare (L.) ecotypes. Nat. Prod. Res. 2016, 30, 1103–1107. [Google Scholar] [CrossRef]
- Virga, G.; Sabatino, L.; Licata, M.; Tuttolomondo, T.; Leto, C.; La Bella, S. Effects of irrigation with different sources of water on growth, yield and essential oil compounds in oregano. Plants 2020, 9, 1618. [Google Scholar] [CrossRef]
- Bonfanti, C.; Iannì, R.; Mazzaglia, A.; Lanza, C.M.; Napoli, E.M.; Ruberto, G. Emerging cultivation of oregano in Sicily: Sensory evaluation of plants and chemical composition of essential oils. Ind. Crop. Prod. 2012, 35, 160–165. [Google Scholar] [CrossRef]
- Węglarz, Z.; Kosakowska, O.; Przybył, J.L.; Pióro-Jabrucka, E.; Bączek, K. The quality of Greek oregano (O. vulgare L. subsp. hirtum (Link) Ietswaart) and common oregano (O. vulgare L. subsp. vulgare) cultivated in the temperate climate of central Europe. Foods 2020, 9, 1671. [Google Scholar] [CrossRef]
- Gavaric, N.; Mozina, S.S.; Kladar, N.; Bozin, B. Chemical profile, antioxidant and antibacterial activity of thyme and oregano essential oils, thymol and carvacrol and their possible synergism. J. Essent. Oil Bear. Plants 2015, 18, 1013–1021. [Google Scholar] [CrossRef]
- Adame-Gallegos, J.R.; Andrade-Ochoa, S.; Nevarez-Moorillon, G.V. Potential use of Mexican oregano essential oil against parasite, fungal and bacterial pathogens. J. Essent. Oil Bear. Plants 2016, 19, 553–567. [Google Scholar] [CrossRef]
- Vokou, D.; Kokkini, S.; Bessiere, J.M. Geographic variation of Greek oregano (Origanum vulgare ssp. hirtum) essential oils. Biochem. Syst. Ecol. 1993, 21, 287–295. [Google Scholar] [CrossRef]
- Kokkini, S.; Karousou, R.; Dardioti, A.; Krigas, N.; Lanaras, T. Autumn essential oils of Greek oregano. Phytochemistry 1997, 44, 883–886. [Google Scholar] [CrossRef]
- Gavalas, N.P.; Kalburtji, K.L.; Kokkini, S.; Mamolos, A.P.; Veresoglou, D.S. Ecotypic variation in plant characteristics for Origanum vulgare subsp. hirtum populations. Biochem. Syst. Ecol. 2011, 39, 562–569. [Google Scholar] [CrossRef]
- Ninou, E.; Paschalidis, K.; Mylonas, I. Essential oil responses to water stress in greek oregano populations. J. Essent. Oil Bear. Plants 2017, 20, 12–23. [Google Scholar] [CrossRef]
- Tuttolomondo, T.; Virga, G.; Licata, M.; Iacuzzi, N.; Farruggia, D.; Bella, S.L. Assessment of Production and Qualitative Characteristics of Different Populations of Salvia sclarea L. Found in Sicily (Italy). Agronomy 2021, 11, 1508. [Google Scholar] [CrossRef]
- Licata, M.; Tuttolomondo, T.; Dugo, G.; Ruberto, G.; Leto, C.; Napoli, E.M.; Virga, G.; Leone, R.; La Bella, S. Study of quantitative and qualitative variations in essential oils of Sicilian oregano biotypes. J. Essent. Oil Res. 2015, 27, 293–306. [Google Scholar] [CrossRef]
- Ninou, E.; Cook, C.M.; Papathanasiou, F.; Aschonitis, V.; Avdikos, I.; Tsivelikas, A.L.; Stefanou, S.; Ralli, P.; Mylonas, I. Nitrogen Effects on the Essential Oil and Biomass Production of Field Grown Greek Oregano (Origanum vulgare subsp. hirtum) Populations. Agronomy 2021, 11, 1722. [Google Scholar] [CrossRef]
- Shaw, B.; Thomas, T.H.; Cooke, D.T. Responses of sugar beet (Beta vulgaris L.) to drought and nutrient deficiency stress. Plant Growth Regul. 2002, 37, 77–83. [Google Scholar] [CrossRef]
- Farooq, M.; Wahid, A.; Kobayashi, N.; Fujita, D.; Basra, S. Plant drought stress: Effects, mechanisms and management. In Sustainable Agriculture; Springer: Dordrecht, The Netherlands, 2009; pp. 153–188. [Google Scholar]
- Morshedloo, M.R.; Craker, L.E.; Salami, A.; Nazeri, V.; Sang, H.; Maggi, F. Effect of prolonged water stress on essential oil content, compositions and gene expression patterns of mono-and sesquiterpene synthesis in two oregano (Origanum vulgare L.) subspecies. Plant Physiol. Biochem. 2017, 111, 119–128. [Google Scholar] [CrossRef]
- Yadav, R.K.; Sangwan, R.S.; Sabir, F.; Srivastava, A.K.; Sangwan, N.S. Effect of prolonged water stress on specialized secondary metabolites, peltate glandular trichomes, and pathway gene expression in Artemisia annua L. Plant Physiol. Biochem. 2014, 74, 70–83. [Google Scholar] [CrossRef]
- Marschner, P.; Rengel, Z. Nutrient availability in soils. In Marschner’s Mineral Nutrition of Higher Plants; Academic Press: Cambridge, MA, USA, 2012; pp. 315–330. [Google Scholar]
- Giannoulis, K.D.; Kamvoukou, C.A.; Gougoulias, N.; Wogiatzi, E. Irrigation and nitrogen application affect Greek oregano (Origanum vulgare ssp. hirtum) dry biomass, essential oil yield and composition. Ind. Crop. Prod. 2020, 150, 112392. [Google Scholar] [CrossRef]
- Król, B.; Sęczyk, Ł.; Kołodziej, B.; Paszko, T. Biomass production, active substance content, and bioaccessibility of Greek oregano (Origanum vulgare ssp. hirtum (Link) Ietswaart) following the application of nitrogen. Ind. Crop. Prod. 2020, 148, 112271. [Google Scholar] [CrossRef]
- Omer, E.A. Response of wild Egyptian oregano to nitrogen fertilization in a sandy soil. J. Plant Nutr. 1999, 22, 103–114. [Google Scholar] [CrossRef]
- Sotiropoulou, D.E.; Karamanos, A.J. Field studies of nitrogen application on growth and yield of Greek oregano (Origanum vulgare ssp. hirtum (Link) Ietswaart). Ind. Crop. Prod. 2010, 32, 450–457. [Google Scholar] [CrossRef]
- Dordas, C.A. Chlorophyll meter readings, N leaf concentration and their relationship with N use efficiency in oregano. J. Plant Nutr. 2017, 40, 391–403. [Google Scholar] [CrossRef]
- Karamanos, A.J.; Sotiropoulou, D.E. Field studies of nitrogen application on Greek oregano (Origanum vulgare ssp. hirtum (Link) Ietswaart) essential oil during two cultivation seasons. Ind. Crop. Prod. 2013, 46, 246–252. [Google Scholar] [CrossRef]
- Nieves-Cordones, M.; Martinez, V.; Benito, B.; Rubio, F. Comparison between Arabidopsis and Rice for Main Pathways of K+ and Na+ Uptake by Roots. Front. Plant Sci. 2016, 7, 992. [Google Scholar] [CrossRef] [PubMed]
- Haro, R.; Benito, B. The Role of Soil Fungi In K+ Plant Nutrition. Int. J. Mol. Sci. 2019, 20, 3169. [Google Scholar] [CrossRef] [PubMed]
- Chrysargyris, A.; Xylia, P.; Botsaris, G.; Tzortzakis, N. Antioxidant and antibacterial activities, mineral and essential oil composition of spearmint (Mentha spicata L.) affected by the potassium levels. Ind. Crop. Prod. 2017, 103, 202–212. [Google Scholar] [CrossRef]
- Khalid, A.K. Effect of potassium uptake on the composition of essential oil content in Calendula officinalis L. flowers. Emirates J. Food Agricult. 2013, 25, 189–195. [Google Scholar] [CrossRef]
- Nell, M.; Vötsch, M.; Vierheilig, H.; Steinkellner, S.; Zitterl-Eglseer, K.; Franz, C.; Novak, J. Effect of phosphorus uptake on growth and secondary metabolites of garden (Salvia officinalis L.). J. Sci. Food Agric. 2009, 89, 1090–1096. [Google Scholar] [CrossRef]
- Ramezani, S.; Rezaei, M.R.; Sotoudehnia, P. Improved growth, yield, and essential oil content of basil grown under different levels of phosphorus sprays in the field. J. Appl. Biol. Sci. 2009, 3, 96–101. [Google Scholar]
- Kapoor, R.; Giri, B.; Mukerji, K.G. Improved growth and essential oil yield and quality in Foeniculum vulgare Mill on mycorrhizal inoculation supplemented with P-fertilizer. Bioresour. Technol. 2004, 93, 307–311. [Google Scholar] [CrossRef] [PubMed]
- Trivino, M.G.; Johnson, C.B. Season has a major effect on the essential oil yield response to nutrient supply in Origanum majorana. J. Hortic. Sci. Biotechnol. 2000, 75, 520–527. [Google Scholar] [CrossRef]
- Hochmal, A.K.; Schulze, S.; Trompelt, K.; Hippler, M. Calcium-dependent regulation of photosynthesis. Biochim. Biophys. Acta 2015, 1847, 993–1003. [Google Scholar] [CrossRef] [PubMed]
- Ettinger, W.F.; Clear, A.M.; Fanning, K.J.; Peck, M.L. Identification of a Ca2+/H+ antiport in the plant chloroplast thylakoid membrane. Plant Phys. 1999, 119, 1379–1386. [Google Scholar] [CrossRef] [PubMed]
- Vainonen, J.P.; Sakuragi, Y.; Stael, S.; Tikkanen, M.; Allahverdiyeva, Y.; Paakkarinen, V.; Aro, E.; Suorsa, M.; Scheller, H.V.; Vener, A.V.; et al. Light regulation of CAS, a novel phosphoprotein in the thylakoid membrane of Arabidopsis thaliana. FEBS J. 2008, 275, 1767–1777. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; Peng, L.; Liu, Y.; Yao, R.; Liu, Z.; Li, X.; Yang, Y.; Wang, J. Arabidopsis calcium-dependent protein kinase AtCPK1 plays a positive role in salt/drought-stress response. Biochem. Biophys. Res. Commun. 2018, 498, 92–98. [Google Scholar] [CrossRef]
- Naeem, M.; Muhammad, S.N.; Rashid, A.; Muhammad, Z.I.; Muhammad, Y.A.; Yasir, H.; Fahad, S. Foliar calcium spray confers drought stress tolerance in maize via modulation of plant growth, water relations, proline content and hydrogen peroxide activity. Arch. Agron. Soil Sci. 2018, 64, 116–131. [Google Scholar] [CrossRef]
- Cakmak, I.; Yazici, A.M. Magnesium: A forgotten element in crop production. Better Crops 2010, 94, 23–25. [Google Scholar]
- Igamberdiev, A.U.; Kleczkowski, L.A. Optimization of ATP synthase function in mitochondria and chloroplasts via the adenylate kinase equilibrium. Front. Plant Sci. 2015, 6, 10. [Google Scholar] [CrossRef]
- Ceylan, Y.; Kutman, U.B.; Mengutay, M.; Cakmak, I. Magnesium applications to growth medium and foliage affect the starch distribution, increase the grain size and improve the seed germination in wheat. Plant Soil 2016, 406, 145–156. [Google Scholar] [CrossRef]
- ur Rehman, H.; Alharby, H.F.; Alzahrani, Y.; Rady, M.M. Magnesium and Organic Biostimulant Integrative Application Induces Physiological and Biochemical Changes in Sun Flower Plants and Its Harvested Progeny on Sandy Soil. Plant Physiol. Biochem. 2018, 126, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Borowski, E.; Michalek, S. The effect of foliar nutrition of spinach (Spinacia oleracea L.) with magnesium salts and urea on gas exchange, leaf yield and quality. Acta Agrobot. 2010, 63, 77–85. [Google Scholar] [CrossRef]
- Servizio Informativo Agrometeorologico Siciliano. Available online: www.sias.regione.sicilia.it (accessed on 24 May 2022).
- Alyemeni, M.N.; Ahanger, M.A.; Wijaya, L.; Alam, P.; Bhardwaj, R.; Ahmad, P. Selenium mitigates cadmium-induced oxidative stress in tomato (Solanum lycopersicum L.) plants by modulating chlorophyll fluorescence, osmolyte accumulation, and antioxidant system. Protoplasma 2018, 255, 459–469. [Google Scholar] [CrossRef] [PubMed]
- Rouphael, Y.; and Colla, G. Toward a sustainable agriculture through plant biostimulants: From experimental data to practical applications. Agronomy 2020, 10, 1461. [Google Scholar] [CrossRef]
- Van Oosten, M.J.; Pepe, O.; De Pascale, S.; Silletti, S.; Maggio, A. The role of biostimulants and bioeffectors as alleviators of abiotic stress in crop plants. Chem. Biol. Technol. Agric. 2017, 4, 5. [Google Scholar] [CrossRef]
- Zardak, S.G.; Dehnavi, M.M.; Salehi, A.; Gholamhoseini, M. Effects of using arbuscular mycorrhizal fungi to alleviate drought stress on the physiological traits and essential oil yield of fennel. Rhizosphere 2018, 6, 31–38. [Google Scholar] [CrossRef]
- Di Miceli, G.; Farruggia, D.; Iacuzzi, N.; Bacarella, S.; La Bella, S.; Consentino, B.B. Planting Date and Different N-Fertilization Rates Differently Modulate Agronomic and Economic Traits of a Sicilian Onion Landrace and of a Commercial Variety. Horticulturae 2022, 8, 454. [Google Scholar] [CrossRef]
- Wick, K.; Heumesser, C.; Schmid, E. Groundwater nitrate contamination: Factors and indicators. J. Environ. Manag. 2012, 111, 178–186. [Google Scholar] [CrossRef]
- Shahrajabian, M.H.; Sun, W.; Cheng, Q. Foliar application of nutrients on medicinal and aromatic plants, the sustainable approaches for higher and better production. Beni-Suef Univ. J. Basic Appl. Sci. 2022, 11, 26. [Google Scholar] [CrossRef]
- Jones, R.G.; and Lunt, O.R. The function of calcium in plants. Bot. Rev. 1967, 33, 407–426. [Google Scholar] [CrossRef]
- Jaleel, C.A.; Manivannan, P.; Sankar, B.; Kishorekumar, A.; Panneerselvam, R. Calcium chloride effects on salinity-induced oxidative stress, proline metabolism and indole alkaloid accumulation in Catharanthus roseus. C. R. Biol. 2007, 330, 674–683. [Google Scholar] [CrossRef] [PubMed]
- Murillo-Amador, B.; Nieto-Garibay, A.; López-Aguilar, R.; Troyo-Diéguez, E.; Rueda-Puente, E.O.; Flores-Hernández, A.; Ruiz-Espinoza, F.H. Physiological, morphometric characteristics and yield of Origanum vulgare L. and Thymus vulgaris L. exposed to open-field and shade-enclosure. Ind. Crop. Prod. 2013, 49, 659–667. [Google Scholar] [CrossRef]
- Economakis, C.D.; Fournaraki, C.E. Growth and nutrient uptake of Origanum vulgare ssp. hirtum in solution culture. In Proceedings of the WOCMAP I-Medicinal and Aromatic Plants Conference: Part 3 of 4 331, Maastricht, The Netherlands, 19–25 July 1992; pp. 345–350. [Google Scholar]
- Carrubba, A.; La Torre, R.; Matranga, A. Effect of the choice of different row arrangements on the bioagronomical behaviour of Origanum heracleoticum. In Proceedings of the International Conference on Medicinal and Aromatic Plants. Possibilities and Limitations of Medicinal and Aromatic Plant 576, Budapest, Hungary, 8–11 July 2001; pp. 247–252. [Google Scholar]
- Tuttolomondo, T.; La Bella, S.; Virga, G.; D’anna, E.; Ruberto, G. Comparative study of different populations of oregano (Origanum vulgare ssp. hirtum) found in Sicily. In Proceedings of the I International Symposium on Medicinal, Aromatic and Nutraceutical Plants from Mountainous Areas (MAP-Mountain 2011) 955, Saas-Fee, Switzerland, 6 July 2011; pp. 119–123. [Google Scholar]
- De Falco, E.; Roscigno, G.; Landolfi, S.; Scandolera, E.; Senatore, F. Growth, essential oil characterization, and antimicrobial activity of three wild biotypes of oregano under cultivation condition in Southern Italy. Ind. Crop. Prod. 2014, 62, 242–249. [Google Scholar] [CrossRef]
- Gerami, F.; Moghaddam, P.R.; Ghorbani, R.; Hassani, A. Effects of irrigation intervals and organic manure on morphological traits, essential oil content and yield of oregano (Origanum vulgare L.). An. Acad. Bras. Cienc. 2016, 88, 2375–2385. [Google Scholar] [CrossRef] [PubMed]
- Kimera, F.; Sewilam, H.; Fouad, W.M.; Suloma, A. Efficient utilization of aquaculture effluents to maximize plant growth, yield, and essential oils composition of Origanum majorana cultivation. An. Agricul. Sci. 2021, 66, 1–7. [Google Scholar] [CrossRef]
- Hancioglu, N.E.; Kurunc, A.; Tontul, I.; Topuz, A. Irrigation water salinity effects on oregano (Origanum onites L.) water use, yield and quality parameters. Sci. Hort. 2019, 247, 327–334. [Google Scholar] [CrossRef]
- Dordas, C. Foliar application of calcium and magnesium improves growth, yield, and essential oil yield of oregano (Origanum vulgare ssp. hirtum). Ind. Crop. Prod. 2009, 29, 599–608. [Google Scholar] [CrossRef]
- Murillo-Amador, B.; Morales-Prado, L.E.; Troyo-Diéguez, E.; Córdoba-Matson, M.V.; Hernández-Montiel, L.G.; Rueda-Puente, E.O.; Nieto-Garibay, A. Changing environmental conditions and applying organic fertilizers in Origanum vulgare L. Front. Plant Sci. 2015, 6, 549. [Google Scholar] [CrossRef]
- Nasar, J.; Wang, G.Y.; Ahmad, S.; Muhammad, I.; Zeeshan, M.; Gitari, H.; Adnan, M.; Fahad, S.; Zhou, X.; Hasan, M.E. Nitrogen fertilization coupled with iron foliar application improves the photosynthetic characteristics, photosynthetic nitrogen use efficiency, and the related enzymes of maize crops under different planting patterns. Front. Plant Sci. 2022, 13, 988055. [Google Scholar] [CrossRef]
- Storey, R.; Jones, R.G.W.; Schachtman, D.P.; Treeby, M.T. Calcium-accumulating cells in the meristematic region of grapevine root apices. Funct. Plant Biol. 2003, 30, 719–727. [Google Scholar] [CrossRef]
- Clarkson, D.T. Calcium transport between tissues and its distribution in the plant. Plant Cell Environ. 1984, 7, 449–456. [Google Scholar] [CrossRef]
- Kara, Z.; Sabir, A. Effects of HerbaGreen application on vegetative developments of some grapevine rootstocks during nursery propagation in glasshouse. In Proceedings of the 2nd International Symposium on Sustainable Development, Sarajevo, Bosnia and Herzegovina, 8–9 June 2010; pp. 127–132. [Google Scholar]
- Sabir, A.; Yazar, K.; Sabir, F.; Kara, Z.; Yazici, M.A.; Goksu, N. Vine growth, yield, berry quality attributes and leaf nutrient content of grapevines as influenced by seaweed extract (Ascophyllum nodosum) and nanosize fertilizer pulverizations. Sci. Hort. 2014, 175, 1–8. [Google Scholar] [CrossRef]
- Emrahi, R.; Morshedloo, M.R.; Ahmadi, H.; Javanmard, A.; Maggi, F. Intraspecific divergence in phytochemical characteristics and drought tolerance of two carvacrol-rich Origanum vulgare subspecies: Subsp. hirtum and subsp. gracile. Ind. Crops Prod. 2021, 168, 113557. [Google Scholar] [CrossRef]
- Tester, M.; Langridge, P. Breeding technologies to increase crop production in a changing world. Science 2010, 327, 818–822. [Google Scholar] [CrossRef]
- Sun, J.; Gu, J.; Zeng, J.; Han, S.; Song, A.P.; Chen, F.; Fang, W.; Jiang, J.; Chen, S. Changes in leaf morphology, antioxidant activity and photosynthesis capacity in two different drought-tolerant cultivars of chrysanthemum during and after water stress. Sci. Hortic. 2013, 161, 249–258. [Google Scholar] [CrossRef]
- Razi, K.; Muneer, S. Drought Stress-Induced Physiological Mechanisms, Signaling Pathways and Molecular Response of Chloroplasts in Common Vegetable Crops. Crit. Rev. Biotechnol. 2021, 41, 669–691. [Google Scholar] [CrossRef]
- Anami, S.; De Block, M.; Machuka, J.; Van Lijsebettens, M. Molecular Improvement of Tropical Maize for Drought Stress Tolerance in Sub-Saharan Africa. Crit. Rev. Plant Sci. 2009, 28, 16–35. [Google Scholar] [CrossRef]
- Harb, A.; Krishnan, A.; Ambavaram, M.M.R.; Pereira, A. Molecular and physiological analysis of drought stress in Arabidopsis reveals early responses leading to acclimation in plant growth. Plant Physiol. 2010, 154, 1254–1271. [Google Scholar] [CrossRef]
- Mohammadi-Cheraghabadi, M.; Modarres-Sanavy, S.A.M.; Sefidkon, F.; Rashidi-Monfared, S.; Mokhtassi-Bidgoli, A. Improving water deficit tolerance of Salvia officinalis L. using putrescine. Sci. Rep. 2021, 11, 21997. [Google Scholar] [CrossRef]
- Khorasaninejad, S.; Ahmadabadi, A.; Hemmati, K. The Effect of Humic Acid on Leaf Morphophysiological and Phytochemical Properties of Echinacea purpurea L. under Water Deficit Stress. Sci. Hortic. 2018, 239, 314–323. [Google Scholar] [CrossRef]
- Ghasemi, N.; Omidi, H.; Bostani, A. Morphological Properties of Catharanthus roseus L. Seedlings Affected by Priming Techniques under Natural Salinity Stress. J. Plant Growth Regul. 2021, 40, 550–557. [Google Scholar] [CrossRef]
- Morshedloo, M.R.; Salami, S.A.; Nazeri, V.; Craker, L.E. Prolonged Water Stress on Growth and Constituency of Iranian of Oregano (Origanum vulgare L.). J. Med. Act. Plants 2017, 5, 7–19. [Google Scholar]
- Upadhyaya, H.; Panda, S.K.; Dutta, B.K. CaCl2 improves post-drought recovery potential in Camellia sinensis (L) O. Kuntze. Plant Cell Rep. 2011, 30, 495–503. [Google Scholar] [CrossRef]
- Matłok, N.; Stępień, A.E.; Gorzelany, J.; Wojnarowska-Nowak, R.; Balawejder, M. Effects of organic and mineral fertilization on yield and selected quality parameters for dried herbs of two varieties of oregano (Origanum vulgare L.). Appl. Sci. 2020, 10, 5503. [Google Scholar] [CrossRef]
- Tuttolomondo, T.; Iapichino, G.; Licata, M.; Virga, G.; Leto, C.; La Bella, S. Agronomic evaluation and chemical characterization of Sicilian Salvia sclarea L. accessions. Agronomy 2020, 10, 1114. [Google Scholar] [CrossRef]
- La Bella, S.; Virga, G.; Iacuzzi, N.; Licata, M.; Sabatino, L.; Consentino, B.B.; Leto, C.; Tuttolomondo, T. Effects of Irrigation, Peat-Alternative Substrate and Plant Habitus on the Morphological and Production Characteristics of Sicilian Rosemary (Rosmarinus officinalis L.) Biotypes Grown in Pot. Agriculture 2021, 11, 13. [Google Scholar] [CrossRef]
- Militello, M.; Carrubba, A.; Blázquez, M.A. Artemisia arborescens L.: Essential oil composition and effects of plant growth stage in some genotypes from Sicily. J. Essent. Oil Res. 2012, 24, 229–235. [Google Scholar] [CrossRef]
- Napoli, E.M.; Siracusa, L.; Saija, A.; Speciale, A.; Trombetta, D.; Tuttolomondo, T.; La Bella, S.; Licata, M.; Virga, G.; Leone, R.; et al. Wild Sicilian rosemary: Phytochemical and morphological screening and antioxidant activity evaluation of extracts and essential oils. Chem. Biodivers. 2015, 12, 1075–1094. [Google Scholar] [CrossRef]
- White, P.J.; and Broadley, M.R. Calcium in plants. Ann. Bot. 2003, 92, 487–511. [Google Scholar] [CrossRef]
- Khalid, N.R.; Ahmed, E.; Hong, Z.; Zhang, Y.; Ahmad, M. Nitrogen doped TiO2 nanoparticles decorated on graphene sheets for photocatalysis applications. Curr. Appl. Phys. 2012, 12, 1485–1492. [Google Scholar] [CrossRef]
- Kutlu, M.; Cakmakci, R.A.M.A.Z.A.N.; Hosseinpour, A.; Karagöz, H. The use of plant growth promoting rhizobacteria (PGPR)’s effect on essential oil rate, essential oil content, some morphological parameters and nutrient uptake of Turkish oregano. Appl. Ecol. Environ. Res. 2019, 17, 1641–1653. [Google Scholar] [CrossRef]
- Vrataric, M.; Sudaric, A.; Kovacevic, V.; Duvnjak, T.; Krizmanic, M.; Mijic, A. Response of soybean to foliar fertilization with magnesium sulfate (Epsom salt). Cereal Res. Commun. 2006, 34, 709–712. [Google Scholar] [CrossRef]
- Prasad, A.; Chattopadhyay, A.; Yadav, A.; Kumari, R. Variation in the chemical composition and yield of essential oil of rose-scented geranium (Pelargonium sp.) by the foliar application of metallic salts. Flavour Frag. J. 2008, 23, 133–136. [Google Scholar] [CrossRef]




| Plant Height | Inflorescence Length | Chlorophyll Content | RWC | Total Fresh Yield | Total Dry Yield | Inflorescence Dry Yield | Leaf Dry Yield | Stem Dry Yield | EO Content | EO Yield | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| (cm) | (cm) | (SPAD) | (%) | (t ha−1) | (t ha−1) | (t ha−1) | (t ha−1) | (t ha−1) | (%w/w) | (kg ha−1) | |
| Year (Y) | |||||||||||
| 2020 | n.s. | n.s. | n.s. | n.s. | 4.13 B | 2.19 B | 0.58 B | 0.58 B | 1.03 B | 1.60 B | 18.63 B |
| 2021 | n.s. | n.s. | n.s. | n.s. | 4.87 A | 2.68 A | 0.79 A | 0.73 A | 1.17 A | 1.78 A | 27.38 A |
| F | 0.41 | 0.05 | 1.74 | 0.81 | 17.75 | 51.09 | 41.90 | 49.88 | 17.34 | 20.70 | 27.65 |
| p-value | 0.53 | 0.82 | 0.20 | 0.38 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Foliar Application (FA) | |||||||||||
| FA0 | n.s. | n.s. | 42.6 B | 80.0 C | 3.48 C | 1.82 C | 0.49 B | 0.53 B | 0.79 C | 1.60 B | 16.48 B |
| FA1 | n.s. | n.s. | 48.8 A | 88.8 A | 4.14 BC | 2.31 B | 0.58 B | 0.68 A | 1.05 B | 1.55 B | 20.17 B |
| FA2 | n.s. | n.s. | 48.8 A | 85.9 B | 5.11 A | 2.70 A | 0.76 A | 0.73 A | 1.21 A | 1.60 B | 24.55 A |
| FA3 | n.s. | n.s. | 50.2 A | 86.6 AB | 5.02 A | 2.73 A | 0.82 A | 0.68 A | 1.25 A | 1.89 A | 28.42 A |
| FA4 | n.s. | n.s. | 51.0 A | 88.1 AB | 4.73 AB | 2.60 AB | 0.75 A | 0.64 A | 1.21 A | 1.82 A | 25.40 A |
| F | 0.25 | 1.91 | 16.54 | 31.31 | 11.94 | 24.51 | 14.46 | 10.35 | 25.07 | 12.14 | 9.31 |
| p-value | 0.91 | 0.15 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Y × FA | |||||||||||
| F | 0.49 | 1.37 | 1.19 | 23.42 | 1.09 | 2.74 | 0.50 | 4.16 | 3.35 | 18.62 | 44.23 |
| p-value | 0.74 | 0.28 | 0.34 | 0.00 | 0.39 | 0.36 | 0.74 | 0.013 | 0.03 | 0.00 | 0.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farruggia, D.; Iacuzzi, N.; La Bella, S.; Sabatino, L.; Consentino, B.B.; Tuttolomondo, T. Effect of Foliar Treatments with Calcium and Nitrogen on Oregano Yield. Agronomy 2023, 13, 719. https://doi.org/10.3390/agronomy13030719
Farruggia D, Iacuzzi N, La Bella S, Sabatino L, Consentino BB, Tuttolomondo T. Effect of Foliar Treatments with Calcium and Nitrogen on Oregano Yield. Agronomy. 2023; 13(3):719. https://doi.org/10.3390/agronomy13030719
Chicago/Turabian StyleFarruggia, Davide, Nicolò Iacuzzi, Salvatore La Bella, Leo Sabatino, Beppe Benedetto Consentino, and Teresa Tuttolomondo. 2023. "Effect of Foliar Treatments with Calcium and Nitrogen on Oregano Yield" Agronomy 13, no. 3: 719. https://doi.org/10.3390/agronomy13030719
APA StyleFarruggia, D., Iacuzzi, N., La Bella, S., Sabatino, L., Consentino, B. B., & Tuttolomondo, T. (2023). Effect of Foliar Treatments with Calcium and Nitrogen on Oregano Yield. Agronomy, 13(3), 719. https://doi.org/10.3390/agronomy13030719











