Abstract
Brazil is the second-largest producer and the first exporter of beef, with herds mainly raised in extensive pastures, where Megathyrsus maximus occupies over 30 Mha. About 70% of the pastures are under degradation, and using plant growth-promoting bacteria (PGPB) may contribute to reversing this scenario. We investigated the effects of PGPB on the growth of six cultivars of M. maximus—Tanzania-1, Massai, BRS Zuri, Mombaça, BRS Tamani, and BRS Quênia—under greenhouse conditions. Plants were inoculated, or not, with the elite strains of Azospirillum brasilense CNPSo 2083 + CNPSo 2084, Bacillus subtilis CNPSo 2657, Pseudomonas fluorescens CNPSo 2719, or Rhizobium tropici CNPSo 103. At 35 days after emergence, plants were evaluated for ten root growth traits, shoot dry weight, and the levels of macro and micronutrients accumulated in shoots. Several root traits were increased due to inoculation in all genotypes, impacting plant growth and nutrient uptake. Despite the differences in effectiveness, all genotypes benefited from PGPB to some degree, but Mombaça and BRS Zuri were more responsive. Scanning electron microscopy indicated that bacterial species differed in their capacity to colonize seeds and rootlets. The results show that inoculation with elite PGPB strains may represent an important strategy for the sustainability of M. maximus pastures.
1. Introduction
Livestock farming is one of the most important economic activities in Brazil, which currently owns the world’s second-largest herd, totaling an estimated 218.15 million bovine heads, as well as being the first-ranked beef-exporting country. With a revenue of USD 70.72 billion in 2022, livestock is highly relevant to the Brazilian economy [1]. According to the Association for the Promotion of Research on the Improvement of Tropical Forages [2], the Brazilian livestock sector occupies 173 million hectares (Mha), equivalent to about 2.8 times the area occupied by grain crops, with 126 Mha consisting of cultivated pastures, and the remaining area represented by native pastures.
Based on the area used for forage seed production, 25.7% of the planted pastures in Brazil are occupied by the species Megathyrsus maximus (Jacq.) B.K. Simon and S.W.L. Jacobs (syn. Panicum maximum Jacq.), while 72.2% are occupied by by Urochloa spp. (syn. Brachiaria spp.) (Associação para Fomento à Pesquisa de Melhoramento de Forrageiras-UNIPASTO, unpublished data). Megathyrsus maximus has been increasingly used in Brazil, showing good adaptation to all edaphoclimatic conditions, as well as a high biomass production with good nutritional value and palatability [3].
Due to lower production costs, 93% of the cattle herd in Brazil has been raised in extensive pastures. In this system, animals are raised in pastures as a primary source of food, with lower inputs and labor costs, but across larger areas [4]. However, extensive growth is majorly associated with poor fertilizers and soil conservation investments, leading to pasture degradation and a decreased capacity of cattle support, altogether increasing the pressure to move to new areas of native vegetation [5]. As a result, about 70% of the pasture areas in Brazil are at some stage of degradation, mainly in the Central region, which is responsible for more than 55% of beef production [6]. The decrease in soil fertility due to inadequate management is among the main factors responsible for pasture degradation, leading to the progressive reduction in vigor and low productive and recovery capacities [7,8].
Nitrogen (N) is the most limiting nutrient required for pasture growth, followed by phosphorus (P). The low availability of N and P in tropical soils impairs forage production and quality, decreasing animal weight gain and reproductive performance. Besides its important role as a component of amino acids and proteins, N participates in photosynthesis, affecting light capture and favoring a greater production of proteins [9]. Phosphorus plays an important role in main metabolic processes such as photosynthesis, energy transference, signal transduction, macromolecule biosynthesis, and respiration [10].
Challenges to provide the needs of an ever-growing population, which is, globally, estimated to total 9.7 billion people by 2050, and concerns about the environmental impacts caused by livestock require new strategies and technologies in order to mitigate the impacts resulting from animal protein production [11]. In this context, several genera of plant growth-promoting bacteria (PGPB), mainly Azospirillum, Pseudomonas, Bacillus, and Rhizobium, have been studied and used as inoculants on grasses [10,11,12,13,14,15,16,17,18]. The main reported bacterial mechanisms of growth promotion in plants include the synthesis of phytohormones, such as indole-3-acetic acid (IAA), cytokinins, gibberellins, and ethylene, biological nitrogen fixation, the synthesis of enzymes such as ACC (1-aminocyclopropane-1-carboxylic acid) deaminase, nutrient mineralization, phosphate solubilization, and an increased tolerance to abiotic and biotic stresses and other benefits associated with a variety of other molecules [12,13,17,18,19,20,21,22]. However, the adoption of inoculants carrying PGPB requires increased knowledge of the interaction between the microorganisms and the host plant, as well as the development of good inoculation practices, including the adjustment of doses and methods of application [23,24]; currently, there are very few studies focusing on grass pastures, with modest information on M. maximus.
We aimed to evaluate the effects of the sole inoculation of four different species of PGPB, in six cultivars of M. maximus, on root parameters, biomass production, and total nutrient contents, in the shoots of plants grown under controlled conditions. Following this, the seed and root bacterial colonization in contrasting pairs of host x bacterium was evaluated with scanning electron microscopy, aiming to investigate the possible relationships between colonization and plant-growth-promotion performance.
2. Material and Methods
2.1. Biological Material
The bacterial strains used in the study are deposited at the “Diazotrophic and Plant-Growth-Promoting Bacteria Culture Collection of Embrapa Soja” (Collections WFCC #1213, WDCM #1054). Azospirillum brasilense strains CNPSo 2083 (=Ab-V5) and CNPSo 2084 (=Ab-V6) have been used in commercial inoculants in Brazil for maize (Zea mays L.), wheat (Triticum aestivum L.), rice (Oryza sativa L.), Brachiaria (Urochloa spp.), and in co-inoculation with the rhizobia of the common-bean (Phaseolus vulgaris L.) and soybean (Glycine max (L.) Merr.) [17]. Each strain was grown separately in dextrose yeast glucose sucrose (DYGS) medium [25] at 28 °C for 96 h, and then mixed, which resulted in a final concentration of 5 × 108 colony-forming units (CFU) mL−1. Strains of A. brasilense were mixed, because in Brazil, the commercial inoculants carry both strains [17]. Bacillus subtilis strain CNPSo 2657 (=PRBS-1, =A3-5), previously selected as a growth promoter for soybean [26], was grown in trypticase soy broth (TSB) medium at 28 °C for 48 h. Rhizobium tropici strain CNPSo 103 (=CIAT 899, =SEMIA 4077), a main symbiont of the common bean and other legumes [27], was grown in tryptone yeast extract (TY) medium at 28 °C for 48 h. Pseudomonas fluorescens strain CNPSo 2719 (=CCTB 03), first recommended for maize [28], and then for Urochloa spp. [29], was grown in King’s B broth medium at 28 °C for 48 h. As for A. brasilense, the final concentrations of B. subtilis, R. tropici and P. fluorescens were adjusted to 5 × 108 CFU mL−1.
The seeds of M. maximus cultivars BRS Tamani, Mombaça, Tanzânia-1, BRS Quênia, Massai, and BRS Zuri, broadly used as foraging plants in Brazilian pastures, were provided by Embrapa Gado de Corte, Campo Grande, Mato Grosso do Sul State, Brazil. From here, BRS Tamani, BRS Quênia and BRS Zuri will be nominated only as Tamani, Quênia and Zuri.
2.2. Plant-Growth-Promotion Evaluation under Greenhouse Conditions
2.2.1. Treatments and Growth Conditions
One experiment was carried out in the greenhouse at Embrapa Soja, in Londrina, Paraná State, southern Brazil (23°11′30.7″ S, 51°11′00.8″ W), in modified Leonard jars [30] of 500 cm³ capacity, containing a sterile substrate composed of a mix of sand and milled coal (3:1, v/v).
The treatments consisted of (i) a non-inoculated group (control); (ii) inoculation with A. brasilense CNPSo 2083 + CNPSo 2084; (iii) inoculation with B. subtilis CNPSo 2657; (iv) inoculation with P. fluorescens CNPSo 2719; (v) inoculation with R. tropici CNPSo 103. The experimental design was completely randomized, with six replicates.
Seeds were surface-sterilized as described before [29], and six seeds were sown per jar, receiving 1 mL of each inoculant adjusted to 5 × 105 CFU seed−1 at the sowing hole, according to the respective treatment. A week after the emergence, seedlings were thinned to one plantlet per jar. Plants received sterile nutrient solution of Hoagland and Arnon [31] with the N supply corresponding to 60 kg ha−1 of N.
2.2.2. Evaluation of Root Growth Traits
Thirty-five days after emergence (DAE), the plants were removed from the jars and roots and shoots were separated. The root system was washed with tap water, weighed, and the root volume (RV) was estimated by the displacement of water in a graduated cylinder. Total root length (TRL) was determined by the modified line-intersection method [32]. Basically, about 1 g of fresh roots was randomly arranged on plates with 1 × 1 cm grid squares, and the intersections with the vertical and horizontal grid lines were counted; TRL was calculated with the formula TRL = N × 0.7857, where N is the number of intersections and 0.7857 is the conversion factor [32].
Root mean diameter (RMD) was calculated by the formula 2[(RV/TRL)π]0.5 [33]. Root area (RA) was estimated by the formula π × RMD × TRL. Subsamples of approximately 0.15 g of thin roots of each plant were stored in formaldehyde–acetic acid–ethanol-70% solution (FAA) (5%:5%:90%), for the determination of root-hair length (RHL), root-hair incidence (RHI), and the total number of root branches (TNB). RHL was determined by the average of 100 root hairs in at least 20 thin root fragments per sample, using a microscope at ×100 magnification equipped with an ocular micrometer. RHI was determined by the presence or absence of root hairs on at least 100 fine-root intersections using the gridline method [34]. TNB was determined, in a stereomicroscope at ×30 magnification, by counting 120–150 ramifications in a root fraction, and using the formula [(RW × NB)/FW], where RW is the root system fresh weight, NB the number of branches in the root fraction, and FW the fresh weight of the root fraction [33].
After these measurements, roots were oven-dried at 60 °C for 72 h until a constant weight, in order to obtain the root dry weight (RDW). The specific root length (SRL) was calculated by the ratio between the total length and RDW. Root-tissue density (RTD) was determined by the ratio between RDW and RV [33].
2.2.3. Biomass Production and Accumulation of Nutrients in Shoots
After being collected, shoots of each replicate were put in paper bags and oven-dried at 60 °C for 72 h until reaching a constant weight. After weighing, tissues were ground in a Wiley mill and submitted to digestion (sulfuric acid for N; nitro-perchloric acid for P, K, Ca, Mg, S, Cu, Fe, Mn, and Zn; incineration for B), in order to determine the concentrations of macro- (N, P, K, Ca, Mg, and S) and micronutrients (B, Cu, Fe, Mn, and Zn) in the leaves, as described before [35].
2.2.4. Statistical Analysis
The data were first submitted to the analyses of normality, by the Shapiro–Wilk’s test, and homoscedasticity, by Levene’s test. When necessary, transformation to the square root, or the use of formulae provided by the software AgroEstat, were applied [36]. Root-hair incidence data were transformed to arcsine (x/100)0.5 before analysis. Means were submitted to ANOVA and the Duncan’s test at 5% significance. All analyses were performed with STATISTICA v.12.0 (Statsoft Inc., Tulsa, OK, USA).
2.3. Evaluation of Seed and Root Colonization with Scanning Electron Microscopy
2.3.1. Treatments, Plant Growth Conditions
A second experiment was performed, also under controlled conditions, with two contrasting cultivars identified in the first experiment, Zuri and Massai, which had the highest and the lowest responses to inoculation, respectively.
The same five inoculation and control treatments described in Section 2.2.1. were applied to both cultivars. Seeds of each cultivar were treated with the respective bacterial inoculant, in order to provide 5 × 105 CFU seed−1 per seed. Seeds were placed on a Petri dish containing germination paper and moistened with sterilized distilled water, and were incubated in a germination chamber at 25 °C and 70% relative humidity for 7 days.
2.3.2. Evaluation of Seed and Root Colonization
After seven days of growth, four germinated seeds from each treatment were prepared for scanning electron microscopic (SEM) analysis. Samples were fixed with glutaraldehyde 2.5% and sodium cacodylate buffer at 0.1 M for 24 h, and then were dehydrated with increasing concentrations of ethanol for 15 min at each concentration (30, 50, 70, 90, and 100%). The samples were then dried to a critical point with CO2 (BALTEC CPD 030 Critical Point Dryer), and were then attached to a metal stub and covered with gold (BALTEC SDC 050 Sputter Coater) for SEM visualization (FEI Quanta 200, FEI Company, Hillsboro, Oregon, EUA).
3. Results
3.1. Plant Growth Promotion
3.1.1. Root Traits
The results for ten root growth traits, including all combinations of plant genotypes and bacterial inoculation treatments, are shown in Table 1, and differences were observed among plant genotypes and bacterial species.

Table 1.
Root growth traits of six Megathyrsus maximus cultivars (Tamani, Mombaça, Tanzânia-1, Quênia, Massai, and Zuri) either inoculated with Azospirillum brasilense (CNPSo 2083 + CNPSo 2084), Bacillus subtillis (CNPSo 2657), Pseudomonas fluorescens (CNPSo 2719), or Rhizobium tropici (CNPSo 103), or remaining non-inoculated (control). The experiment was carried out under greenhouse conditions in a sterile substrate, and plants were sampled 35 days after emergence.
For the cultivar Tamani, A. brasilense increased the root dry weight (RDW), root volume (RV), and root area (RA); B. subtilis increased the total root length (TRL) and the total number of branches (TNB), whereas R. tropici increased TNB (Table 1). For Mombaça, A. brasilense increased the root tissue density (RTD), P. fluorescens and R. tropici increased RDW, and all strains increased TNB. Inoculation of A. brasilense in Tanzania-1 increased RDW, RV, TRL, RA, and TNB, in addition to root hair incidence (RHI) by 67%, when compared with the non-inoculated control. Still, in Tanzania-1, B. subtilis increased RV, TRL, RA, SRL and TNB values, and P. fluorescens showed similar effects to A. brasilense, in addition to an increase in RDW and a lack of significant response in RHI, while R. tropici only increased TNB. For cultivar Quênia, inoculation with A. brasilense increased RDW, RV, TRL, and RA values; B. subtilis increased TNB, but no effects were observed for inoculation with R. tropici or P. fluorescens. In Massai, A. brasilense increased the RHI and root mean diameter (RMD). The best results in Massai were achieved with P. fluorescens, which enhanced RV, TRL, RA, and RHI values; there were no effects observed when inoculating B. subtilis or R. tropici. Finally, all strains showed increased RV, RTL, and RA values in Zuri; in addition, inoculation with B. subtilis, P. fluorescens or R. tropici increased RTD, inoculation with A. brasilense increased RHI, whereas inoculation with B. subtilis, A. brasilense, and R. tropici increased TNB (Table 1).
Despite differences among plant genotypes and bacterial strains, all inoculation treatments enhanced root growth traits in all cultivars of M. maximus (Table 1). However, some cultivars, such as Mombaça, Tanzânia-1, and Zuri, were more responsive to inoculation, irrespective of the bacterial species. In contrast, others, such as Quênia and Massai, were more specific, each one responding to only two bacterial species. Differences were also observed among bacteria, with A. brasilense affecting more root morphological traits across all genotypes.
3.1.2. Shoot Biomass Production and Nutrient Accumulation
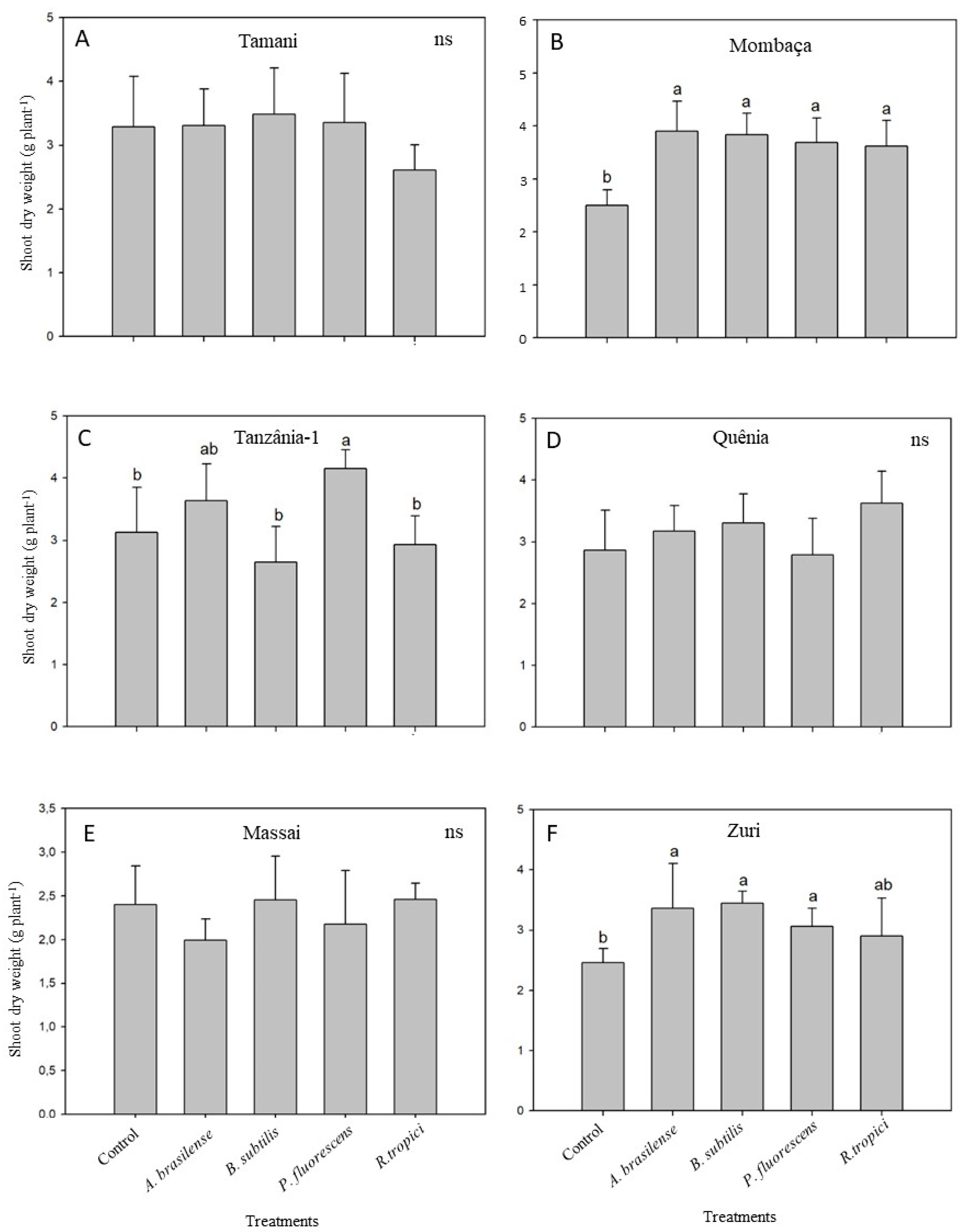
At 35 DAE, there were no improvements in shoot dry weight (SDW) due to inoculation with any of the bacterial strains in the cultivar Tamani (Figure 1A). Conversely, all inoculated strains increased the SDW of Mombaça when compared with the non-inoculated control (Figure 1B), while Tanzânia-1 responded only to inoculation with P. fluorescens (Figure 1C). No statistical differences were observed for Quênia (Figure 1D) and Massai (Figure 1E) with any of the bacterial strains. Zuri presented higher shoot dry biomass (SDW) when inoculated with A. brasilense, B. subtilis, or P. fluorescens (Figure 1F).
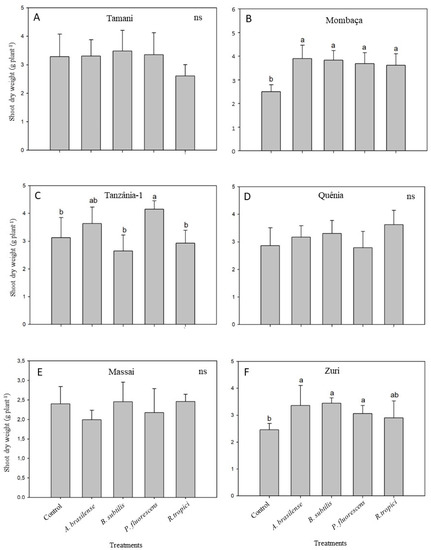
Figure 1.
Shoot dry weight of six Megathyrsus maximus cultivars [Tamani (A), Mombaça (B), Tanzânia-1 (C), Quênia (D), Massai (E), and Zuri (F)] either inoculated with Azospirillum brasilense (CNPSo 2083 + CNPSo 2084), Bacillus subtillis (CNPSo 2657), Pseudomonas fluorescens (CNPSo 2719), or Rhizobium tropici (CNPSo 103), or remaining non-inoculated (Control). The experiment was carried out under greenhouse conditions in a sterile substrate and plants were sampled 35 days after emergence. Means (n = 6) followed by different letters differ from each other by the Duncan’s test at p ≤ 0.05.
Considering the bacterial species, an improvement in shoot growth due to inoculation was observed in two genotypes for A. brasilense, two for B. subtilis, three for P. fluorescens, and one for R. tropici (Figure 1).
Although no statistical differences were observed in the shoot growth of Tamani, Quênia and Massai (Figure 1), all bacterial strains showed increased Cu contents in the shoots of Tamani, especially R. tropici (196%) and B. subtilis (262%) (Table 2).

Table 2.
Total nutrient contents in the shoots of six Megathyrsus maximus cultivars (Tamani, Mombaça, Tanzânia-1, Quênia, Massai, and Zuri) either inoculated with Azospirillum brasilense (CNPSo 2083 + CNPSo 2084), Bacillus subtillis (CNPSo 2657), Pseudomonas fluorescens (CNPSo 2719), or Rhizobium tropici (CNPSo 103), or remaining non-inoculated (control). The experiment was carried out under greenhouse conditions in a sterile substrate and plants were sampled 35 days after emergence.
Inoculation affected the total content of 9 out of 11 nutrients in Mombaça, with at least one bacterium increasing the accumulation of P, Mg, S, B, Fe, Mn, and Zn in shoots (Table 2). All bacteria, except P. fluorescens, increased N contents, and all except for A. brasilense increased Ca. Inoculation with A. brasilense or P. fluorescens increased P, K, and Fe contents compared with the other treatments, whereas Ca, was also increased by B. subtilis, and Cu by P. fluorescens.
For Quênia, eight nutrients increased in tissues with at least one of the bacteria evaluated. All strains increased Mg and Zn, the latter by an average of 2.5 times. Boron was increased by 90% with A. brasilense, P. fluorescens, or R. tropici inoculation, whereas inoculation with R. tropici or B. subtilis increased N and P contents. Moreover, A. brasilense also increased Ca and Mn contents, P. fluorescens increased Mn, R. tropici increased Ca and S, and B. subtilis increased Mn contents (Table 2). For Massai, inoculation with P. fluorescens or R. tropici increased Mg; B. subtilis or R. tropici increased Mn, whereas all bacteria increased Zn accumulation in shoots (Table 2). Finally, in Zuri, significant increases were observed for N, P, K, S, and B.
3.2. Seed and Root Colonization
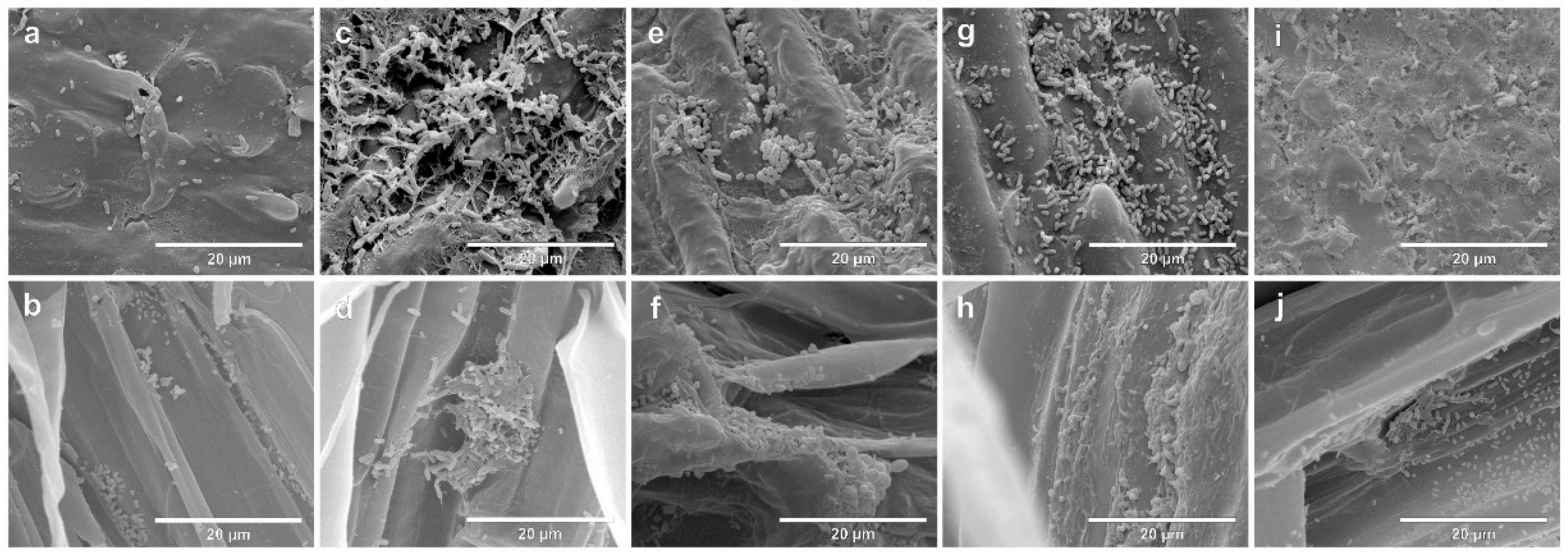
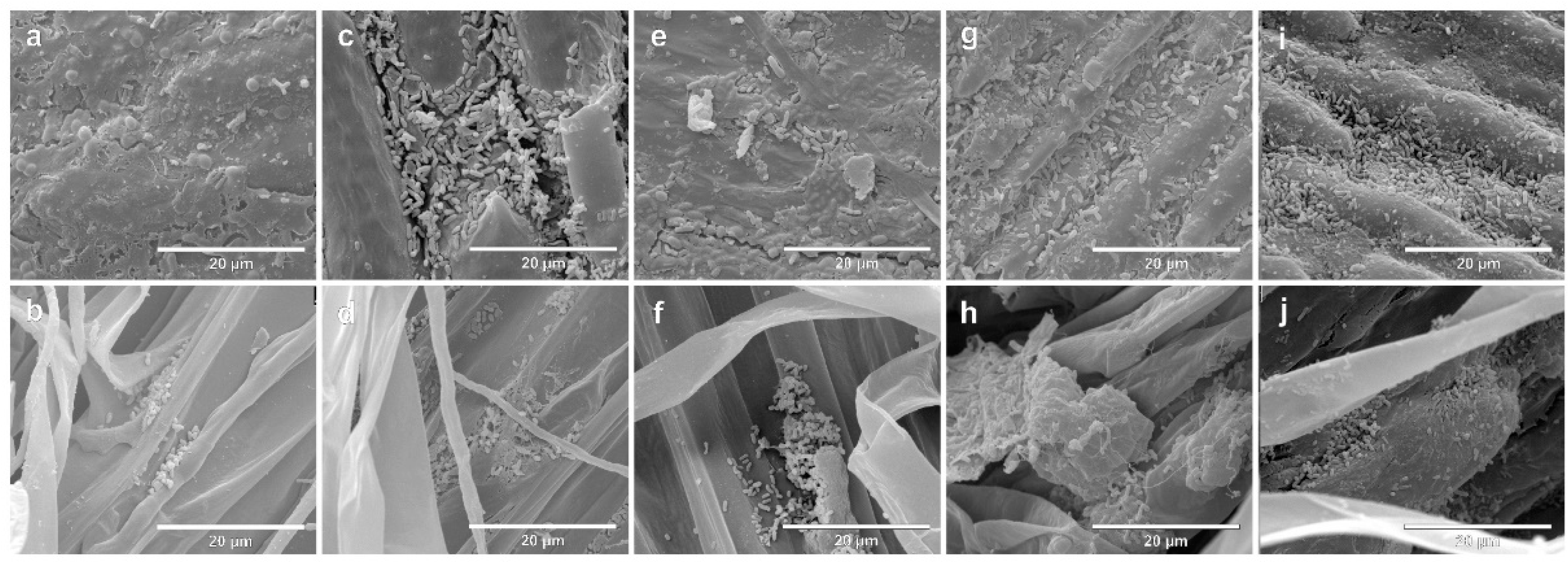
The colonization of seeds and rootlets of two contrasting genotypes, Massai (Figure 2) and Zuri (Figure 3), inoculated singularly with the four species A. brasilense, B. subtilis, P. fluorescens and R. tropici, or remaining non-inoculated, was evaluated using SEM. On both photomicrographs, the first line indicates seeds after seven days of growth, and the second line shows the rootlets. On both photomicrographs, a and b are negative controls (non-inoculated), c and d indicate Inoculation with A. brasilense, e and f indicate inoculation with B. subtilis, g and h indicate inoculation with P. fluorescens, and i and j indicate inoculation with R. tropici.
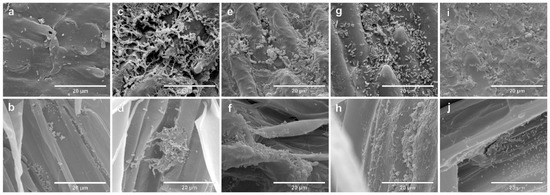
Figure 2.
Scanning electron micrographs of the seeds and rootlets of Megathyrsus maximus cultivar Massai, colonized by Azospirillum brasilense (CNPSo 2083 + CNPSo 2084), Bacillus subtillis (CNPSo 2657), Pseudomonas fluorescens (CNPSo 2719), or Rhizobium tropici (CNPSo 103), after seven days of growth. Non-inoculated seeds (a) and rootlets (b); seeds (c) and rootlets (d) of plants inoculated with A. brasilense; seeds (e) and rootlets (f) of plants inoculated with B. subtilis; seeds (g) and rootlets (h) of plants inoculated with P. fluorescens; seeds (i) and rootlets (j) of plants inoculated with R. tropici.
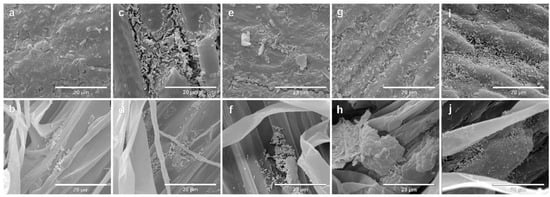
Figure 3.
Scanning electron micrographs of seeds and rootlets of Megathyrsus maximus cultivar Zuri, colonized by Azospirillum brasilense (CNPSo 2083 + CNPSo 2084), Bacillus subtillis (CNPSo 2657), Pseudomonas fluorescens (CNPSo 2719), or Rhizobium tropici (CNPSo 103), after 7 days of growth. Non-inoculated seeds (a) and rootlets (b); seeds (c) and rootlets (d) of plants inoculated with A. brasilense; seeds (e) and rootlets (f) of plants inoculated with B. subtilis; seeds (g) and rootlets (h) of plants inoculated with P. fluorescens; seeds (i) and rootlets (j) of plants inoculated with R. tropici.
After seven days, considering that the seeds in this experiment were not surface-disinfected, it was possible to observe a community of native microorganisms in the tegument that were colonizing the rootlets in the non-inoculated control for Massai seeds (Figure 2a) and rootlets (Figure 2b), as well as Zuri seeds (Figure 3a) and rootlets (Figure 3b). Seeds and rootlet sections that were bacterized were examined, and we confirmed that cells of all strains were consistently distributed on the surface of seed coats and rootlets in Massai (Figure 2c–j) and Zuri (Figure 3c–j).
A biofilm consisting of bacterial cells, and a net-like material, suggesting extracellular matrix formation, were observed in both Massai (Figure 2) and Zuri (Figure 3), being more abundant, in both genotypes, with A. brasilense (c,d) and R. tropici (i,j), and less abundant with P. fluorescens (g,h) inoculation. A lower adherence of bacterial cells was observed for B. subtilis (e,f) in the sections studied.
Observations in the most responsive cultivar Zuri have shown strong biofilm formation and roots colonized by dense biofilm development when inoculated with A. brasilense, R. tropici, and P. fluorescens when compared to B. subtilis (Figure 3).
4. Discussion
The importance of plant roots has been increasingly highlighted, especially in view of global climate changes, with increasing periods of drought, and the fertilizer crisis, with a shortage in the supply of nutrients. Enhancement in root growth implies in higher efficiency in the uptake of water and nutrients, and PGPB can help in achieving this goal. All four species were able to colonize seed coats and rootlets, but differences were observed, e.g., with higher colonization by A. brasilense. Despite differences among plant genotypes and bacterial strains, inoculation with PGPB increased root growth traits, including RDW, RV, TRL, RMD, RTD, RHI, RHL, and TNB. Improvements in root systems result in a higher efficiency in water and nutrient uptake, and more exudation that favors interactions with other beneficial microorganisms [37]. Therefore, our results indicate that PGPB may represent an important strategy for the management of grass pastures. It is worth mentioning that the search for elite strains within the native population of each site is highly recommended, as the probability of success in the adaptation is higher. In our study, all bacteria used are elite strains selected from native populations [17,26,28], except for R. tropici CNPSo 103, which is native to Colombia, but in this case, the strain has shown excellent adaptations to a variety of edaphoclimatic conditions in the tropics [16], including after more than 20 years of experimentation with the common bean crop in Brazil. Benefits in root growth traits can mostly be attributed to the synthesis of phytohormones, mainly auxins, as reported for A. brasilense strains CNPSo 2083 and CNPSo 2084 [25]. The phytohormones synthesized by these bacteria [38,39,40,41], with an emphasis on IAA and cytokinins, play important roles in root development. The auxin/cytokinin balance regulates the cell division and formation of new tissues, affecting shoot and root growth [42]
Auxins are involved in the activation of meristem, cell elongation, cell differentiation, and lateral root development, while cytokinins act towards the regulation of cell division and the induction of new tissues [43]. Barbieri and Galli [44], comparing a wild strain of A. brasilense with a mutant having a lower auxin production, found a decrease in the number of lateral roots colonized by Triticum durum var. Appula for the mutant strain, while Ortíz-Castro and collaborators [45] reported that the cytokinins produced by a strain of Bacillus megaterium (syn. Priesta megaterium) stimulated the production of lateral roots by Arabidopsis. Azospirillum brasilense also synthesizes nitric oxide, which acts as a signaling molecule in the pathway mediated by auxins, inducing the formation of branches and indirectly stimulating the formation of lateral roots [46]. Pseudomonas fluorescens, on the other hand, synthesizes cyclodipeptides, which regulate genes that are responsive to auxins in roots, making them key players in the modulation of root growth traits [47]. Indeed, A. brasilense favors the increase of root hair incidences in grasses [48,49], which was also evidenced in Arabidopsis inoculated with P. fluorescens [47], and in Urochloa brizantha and U. decumbens inoculated with A. brasilense [29].
Altogether, these mechanisms related to PGPB may explain the increase of favorable root traits in all six genotypes of M. maximus. However, despite improvements in root traits, these effects occurred to different degrees, depending on the interaction between the cultivar and strain. Three grass genotypes, Mombaça, Tanzânia-1, and Zuri, had at least one root trait that was significantly improved in response to all four bacterial species, A. brasilense, B. subtilis, P. fluorescens, and R. tropici. Tanzânia-1 and Zuri were also outstanding, showing improvements in six out of 10 traits. On the other hand, Quênia and Massai significantly increased the expression of five root traits, but only when inoculated with A. brasilense and either B subtilis for Quênia, or P. fluorescens for Massai. In relation to the bacteria, all grass genotypes responded to the inoculation with A. brasilense, five responded to B. subtillis, three responded to P. fluorescens, and three responded to R. tropici.
This increase in the root system of inoculated plants may also have been responsible for the higher accumulation of one or more nutrients in shoots. A higher uptake of water and nutrients is essential for the production of forage biomass, especially in tropical soils, where such factors are limited [50,51]. These results are in agreement with previous field trials in three geographic regions, showing that the inoculation of U. brizanta cv. Marandu with the strains Ab-V5 and Ab-V6 of A. brasilense increased the shoot dry mass production, ranging from 13 to 29.5%, as well as increased the accumulation of N from 2.9 to 11.2% in comparison with the non-inoculated plants [13].
Improvements in root traits are not always correlated with shoot biomass. We hypothesize that the lack of response could be due to the short time of plant growth and the optimized growth conditions in the greenhouse. For example, Pennisetum clandestinum inoculated with Pseudomonas sp. and Strenotrophomonas sp. had a lower shoot dry mass at 70 days when compared with the non-inoculated control, but, after 130 days, the control plants were outperformed by inoculated plants by 30% [52]. In our study, Tamani, Quênia, and Zuri did not show any improvement in shoot growth, and the latter two showed very specific interactions, responding only to A. brasilense and B. subtilis in terms of root growth traits.
Significant effects of inoculation on shoot growth were observed mainly in Mombaça, with the four bacterial species, and in Zuri, except with R. tropici, whereas Tanzâni-1 responded only to P. fluorescens. Higher biomass productivity is important to improve pasture recovery after grazing, allowing cattle to return earlier and more often, leading to a greater gain of animal protein without need to open new areas for pastures [29], which can be considered to be a land-saving technology [53]. Our results indicate that increased biomass due to the inoculation with PGPB can help in the process of the improvement of cattle raising.
Pasture reclamation is very important to maintain the viability of livestock activity, as well as to improve the efficiency of land use, soil and water conservation, and carbon sequestration [13]. Low soil fertility is one of the main causes of pasture degradation [8]. Outstanding results were observed in the accumulation of nutrients in shoots due to inoculation, even when no effects were observed for shoot growth. Increased nutrient accumulation in shoots, including macro and micronutrients (N, P, K, Ca. Mg, S, B, Cu, Fe, Mn, Zn) occurred in at least one bacterium–genotype inoculation. Differences among genotypes were once again observed, with the lowest performance for Tamani and Massai, with an increased uptake of only one and three micronutrients, respectively. Conversely, Mombaça, Quênia, Zuri, and Tanzânia-1 had an increased accumulation of nine, eight, six, and five macro- and micronutrients, respectively. Considering the bacterial species, although effects were observed according to the plant genotype, responsive genotypes, such as Mombaça, had enhanced nutrient contents in the shoots, irrespective of the inoculated bacteria. Therefore, the improved performance of plants inoculated with these four elite strains may represent an important strategy to reduce or avoid pasture degradation, increase forage longevity and nutritional quality, and minimize water and nutritional stresses [54,55].
Even though the experiment was carried under axenic conditions in the greenhouse and plants were supplied with ample nutrient solution, the improvement in root traits contributed to a higher accumulation of nutrients in plant biomass. We hypothesize that, under field conditions, plant responses to inoculation will be of higher magnitude, e.g., as reported for pastures inoculated with Azospirillum spp. under water restriction and low-fertility soil conditions [56], as well as for U. brizantha cv. Marandu grown in low-fertility soil [57].
While for the genus Brachiaria (Urochloa spp.), which occupies about 70% of the cultivated pastures in Brazil, no differences were observed among genotypes considering the response to inoculation [13,18,29], apparently, for M. maximus there are prominent differences. However, one must consider that our study aimed to perform a detailed analysis of parameters and performances that required axenic and controlled growth conditions. Previously, Mombaça and Zuri were described as having higher growth rates, which demands more nutrients compared with the slower growth rates of Tamani and Massai [58,59]. This difference among genotypes was also observed in a study on P uptake (M.C.M. Macedo, data unpublished). Therefore, responses in root traits, shoot growth and nutrient accumulation in Mombaça and Zuri could be related to their higher growth rates. Interestingly, Tamani-1 and Massai are known to accumulate sodium (Na) (M.C.M. Macedo, data unpublished), which might affect interactions with the bacteria. Plant–microbe interactions depend on recognition at a molecular level between partners [60], and the identification of differences in these molecules among genotypes represents an interesting subject for further studies. Furthermore, there may have differences in the bacterial ability to colonize roots and/or internal tissues, which would interfere with the plant-growth-promotion capacity [61]. Studies on the colonization of plant genotypes by plant-growth-promoting bacteria can help to clarify these points and maximize the benefits of inoculation, contributing for the sustainability of planted pastures.
5. Concluding Remarks
Globally, pastures occupy far more land than crops, and the same occurs in Brazil. Grasslands comprise the great majority of pastures worldwide, but, unfortunately, a significant percentage of them are in some stage of degradation. We investigated the performance of six genotypes of M. maximus when inoculated with elite strains of four PGPB species. All species were able to colonize seeds and rootlets, but differences were observed, with a higher capacity of A. brasilense. Improvements were observed in terms of the root traits, shoot biomass, and accumulation of nutrients in shoots. However, differences among genotypes were observed, with the best performance of Zuri and Mombaça, and a lower responsiveness of Tamani and Massai. Our results have shown the feasibility of improving biomass, as well as the quality of pastures containing M. maximus, by inoculation with PGPB. However, our results also indicate the need to search for the best plant genotype host x bacterium combinations. It will also be interesting to evaluate the consortia of bacterial species, as they can contribute with different biological processes. In all cases, preference should be given to the search for elite strains identified within the native population.
Author Contributions
M.H, M.A.N. and L.J. conceived and designed the experiments. G.S.G., A.B.L.R. and A.G.d.O.J. performed the experiments and analyses. All authors prepared the manuscript and agreed with the final version. M.H. and M.A.N. were responsible for funding. All authors have read and agreed to the published version of the manuscript.
Funding
Partially financed by the project INCT-Plant-Growth Promoting Microorganisms for Agricultural Sustainability and Environmental Responsibility (CNPq 465133/2014-4, Fundação Araucária-STI 043/2019), Project CNPq (405666/2022-5) and Projeto Rede FertBrasil (FINEP 01.22.0080.00).
Data Availability Statement
Data are included in the manuscript or will be available upon request.
Acknowledgments
F.R.B. acknowledges a fellowship from CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, Finance Code 001). M.A. Nogueira and M. Hungria acknowledge research fellows from CNPq.
Conflicts of Interest
The authors declare no conflict of interest.
References
- MAPA (Ministério da Agricultura, Pecuária e Abastecimento). Agropecuária Brasileira em Números. Secretaria de Política Agrícola. Available online: https://www.gov.br/agricultura/ptbr/assuntos/politica-agricola/todas-publicacoesde-politicaagricola/agropecuaria-brasileira-em-numeros (accessed on 15 November 2022).
- UNIPASTO (Associação para o Fomento à Pesquisa de Melhoramento de Forrageiras). 2022. Available online: https://www.unipasto.com.br (accessed on 14 April 2022).
- Jank, L. A história do Panicum maximum no Brasil. Ver. JC Maschietto. 2003, 1, 14. [Google Scholar]
- Bahlo, C.; Dahlhaus, P.; Thompson, H.; Trotter, M. The role of interoperable data standards in precision livestock farming in extensive livestock systems: A review. Comput. Electron. Agric. 2019, 156, 459–466. [Google Scholar] [CrossRef]
- Terra, A.B.; Florentino, L.A.; Rezende, A.D.; Silva, N.C. Leguminosas forrageiras na recuperação de pastagens no Brasil. Rev. Cienc. Agrícola. 2019, 42, 305–313. [Google Scholar] [CrossRef]
- Macedo, M.C.M.; Araújo, A.R. Sistemas de produção em integração: Alternativa para recuperação de pastagens degradadas. In ILPF: Inovação com Integração de Lavoura, Pecuária e Floresta, 1st ed.; Bungenstab, D.J., de Almeida, R.G., Laura, V.A., Balbino, L.C., Ferreira, A.D., Eds.; Embrapa: Brasília, Brazil, 2019; Volume 1, pp. 296–317. [Google Scholar]
- Peron, A.J.; Evangelista, A.R. Degradação de pastagens em regiões de cerrado. Ciênc. Agrotec. 2004, 28, 655–661. [Google Scholar] [CrossRef]
- Macedo, M.C.M.; Zimmer, A.H.; Kichel, N.A.; Almeida, R.G.; de Araujo, A.R. Degradação de pastagens, alternativas de recuperação e renovação, e formas de mitigação. In Encontro de Adubação de Pastagens da Scot Consultoria-Tec-Fértil; Scot Consultoria: Piracicaba, Brazil, 2013; pp. 158–181. [Google Scholar]
- Hungria, M.; Nogueira, M.A. Nitrogen fixation. In Marschner’s Mineral Nutrition of Plants, 4th ed.; Rengel, Z., Cakmak, I., White, P.J., Eds.; Elsevier: Amsterdam, The Netherlands; Academic Press: London, UK, 2023; pp. 615–650. ISBN 978-0-12-819773-8. [Google Scholar]
- Sharma, S.B.; Sayyed, R.Z.; Trived, M.H.; Gobi, T.A. Phosphate solubilizing microbes: Sustainable approach for managing phosphorus deficiency in agricultural soils. SpringerPlus 2013, 2, 1–14. [Google Scholar] [CrossRef]
- Abramovay, R. Alimentos versus população: Está ressurgindo o fantasma malthusiano? Ciênc. Cult. 2010, 62, 38–42. [Google Scholar]
- Hungria, M.; Campo, R.J.; Souza, E.M.; Pedrosa, F.O. Inoculation with selected strains of Azospirillum brasilense and A. lipoferum improves yields of maize and wheat in Brazil. Plant Soil. 2010, 331, 413–425. [Google Scholar] [CrossRef]
- Hungria, M.; Nogueira, M.A.; Araujo, R.S. Inoculation of Brachiaria spp. with the plant growth-promoting bacterium Azospirillum brasilense: An environment-friendly component in the reclamation of degraded pastures in the tropics. Agric. Ecosyst. Environ. 2016, 221, 125–131. [Google Scholar] [CrossRef]
- Hungria, M.; Barbosa, J.Z.; Rondina, A.B.L.; Nogueira, M.A. Improving maize sustainability with partial replacement of N-fertilizers by inoculation with Azospirillum brasilense. Agron. J. 2022, 114, 2969–2980. [Google Scholar] [CrossRef]
- Videira, S.S.; Oliveira, D.M.; Morais, R.F.; Borges, W.L.; Baldani, V.L.D.; Baldani, J.I. Genetic diversity and plant growth promoting traits of diazotrophic bacteria isolated from two Pennisetum purpureum Schum genotypes grown in the field. Plant Soil. 2012, 356, 51–66. [Google Scholar] [CrossRef]
- Santos, M.S.; Nogueira, M.A.; Hungria, M. Microbial inoculants: Reviewing the past, discussing the present and previewing an outstanding future for the use of beneficial bacteria in agriculture. AMB Express 2019, 9, 205. [Google Scholar] [CrossRef]
- Santos, M.S.; Nogueira, M.A.; Hungria, M. Outstanding impact of Azospirillum brasilense strains Ab-V5 and Ab-V6 on the Brazilian agriculture: Lessons that farmers are receptive to adopt new microbial inoculants. Rev. Bras. Cienc. Solo. 2021, 45, 1–31. [Google Scholar] [CrossRef]
- Guimarães, G.S.; Rondina, A.B.L.; Santos, M.S.; Nogueira, M.A.; Hungria, M. Pointing out opportunities to increase grassland pastures productivity via microbial inoculants: Attending the society’s demands for meat production with sustainability. Agronomy 2022, 12, 1748. [Google Scholar] [CrossRef]
- Ahmed, B.; Shahid, M.; Syed, A.; Rajput, V.D.; Elgorban, A.M.; Minkina, T.; Bahkali, A.H.; Lee, J. Drought tolerant Enterobacter sp. Leclercia adecarboxylata secretes indole-3-acetic scid and other biomolecules and enhances the biological sttributes of Vigna radiata (L.) R. Wilczek in water deficit conditions. Biology 2021, 10, 1149. [Google Scholar] [CrossRef] [PubMed]
- AL-Shwaiman, H.A.; Shahid, M.; Elgorban, A.M.; Siddique, K.H.M.; Syed, A. Beijerinckia fluminensis BFC-33, a novel multi-stress-tolerant soil bacterium: Deciphering the stress amelioration, phytopathogenic inhibition and growth promotion in Triticum aestivum (L.). Chemosphere 2022, 295, 133843. [Google Scholar] [CrossRef] [PubMed]
- Mariano, R.L.R.; Silveira, E.B.; Assis, S.M.P.; Gomes, A.M.A.; Nascimento, A.R.P.; Donato, V.M.T.S. Importância de bactérias promotoras de crescimento e de biocontrole de doenças de plantas para uma agricultura sustentável. An. Acad. Pernamb. Cienc. Agron. 2013, 1, 89–111. [Google Scholar]
- Fukami, J.; Abrantes, J.L.F.; Del Cerro, P.; Nogueira, M.A.; Ollero, F.J.; Megías, M.; Hungria, M. Revealing different strategies of quorum sensing in Azospirillum brasilense strains Ab-V5 and Ab-V6. Arch. Microbiol. 2018, 200, 47–56. [Google Scholar] [CrossRef]
- Msimbira, L.A.; Smith, D.L. The roles of plant growth promoting microbes in enhancing plant tolerance to acidity and alkalinity stresses. Front. Sustain. Food Syst. 2020, 4, 106. [Google Scholar] [CrossRef]
- Lopes, M.J.S.; Dias-Filho, M.B.; Castro, T.H.R.; Gurgel, E.S.C.; da Silva, G.B. Efficiency of biostimulants for alleviating shade effects on forage grass. J. Agric. Stud. 2021, 9, 14–30. [Google Scholar] [CrossRef]
- Fukami, J.; Cerezini, P.; Hungria, M. Azospirillum: Benefits that go far beyond biological nitrogen fixation. AMB Express 2018, 8, 73. [Google Scholar] [CrossRef]
- Araujo, F.F.; Hungria, M. Nodulação e rendimento de soja co-infectada com Bacillus subtilis e Bradyrhizobium japonicum/B. elkanii. Pesqui. Agropecuária Bras. 1999, 34, 1633–1643. [Google Scholar] [CrossRef]
- Ormeño-Orrillo, E.; Hungria, M.; Martínez-Romero, E. Dinitrogen-fixing prokaryotes. In The Prokaryotes—Prokaryotic Physiology and Biochemistry, 4th ed.; Rosemberg, E., De Long, E.F., Lory, S., Stackebrandt, E., Thompson, F., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 427–451. [Google Scholar] [CrossRef]
- Sandini, I.E.; Pacentchuk, F.; Hungria, M.; Nogueira, M.A.; Cruz, S.P.; Nakatani, A.S.; Araujo, R.S. Seed inoculation with Pseudomonas fluorescens promotes growth, yield and reduces nitrogen application in maize. Int. J. Agric. Biol. 2019, 22, 1369–1375. [Google Scholar] [CrossRef]
- Hungria, M.; Rondina, A.B.L.; Nunes, A.L.P.; Araujo, R.S.; Nogueira, M.A. Seed and leaf-spray inoculation of PGPR in Brachiaria (Urochloa spp.) as an economic and environmental opportunity to improve plant growth, forage yield and nutrient status. Plant Soil. 2021, 463, 171–186. [Google Scholar] [CrossRef]
- Yates, R.J.; Howieson, J.G.; Hungria, M.; Bala, A.; O’Hara, G.W.; Terpolilli, J. Authentication of rhizobia and assessment of the legume symbiosis in controlled plant growth systems. In Working with Rhizobia, 1st ed.; Howieson, J.G., Dilworth, M.J., Eds.; Australian Center for Interantional Agricultural Research (ACIAR): Canberra, Australia, 2016; pp. 73–108. [Google Scholar]
- Hoagland, D.R.; Arnon, D.I. The water-culture method for growing plants without soil. Calif. Agr. Exp. Sta. Cir. 1950, 347, 32. [Google Scholar]
- Tennant, D. A test of modified line intersect method of estimating root length. J. Ecol. 1975, 63, 995–1001. [Google Scholar] [CrossRef]
- Rondina, A.B.L.; Sanzovo, A.W.S.; Guimarães, G.S.; Wendling, J.R.; Nogueira, M.A.; Hungria, M. Changes in root morphological traits in soybean co-inoculated with Bradyrhizobium spp. and Azospirillum brasilense or treated with A. brasilense exudates. Biol. Fertil. Soils 2020, 56, 537–549. [Google Scholar] [CrossRef]
- Zangaro, W.; Nishidate, F.R.; Camargo, F.R.S.; Romagnoli, G.G.; Vandresen, J. Relationships among arbuscular mycorrhizas, root morphology and seedling growth of tropical native woody species in southern Brazil. J. Trop. Ecol. 2005, 21, 529–540. [Google Scholar] [CrossRef]
- Malavolta, E.; Vitti, G.C.; Oliveira, S.A. Princípios, métodos e técnicas de avaliação do estado nutricional. In Avaliação do Estado nutricional das Plantas: Princípios e Aplicações, 2nd ed.; Malavolta, E., Vitti., G.C., Oliveira., S.A., Eds.; Associação Brasileira para a Pesquisa da Potassa e do Fosfato: Piracicaba, Brazil, 1997; pp. 115–230. [Google Scholar]
- Barbosa, J.C.; Maldonado, J.W. AgroEstat: Sistema para análises estatísticas de ensaios agronômicos; FCAV/UNESP: Jaboticabal, Brazil, 2015; 396p. [Google Scholar]
- Freschet, G.T.; Roumet, C.; Comas, L.H.; Weemstra, M.; Bengough, A.G.; Rewald, B.; Bardgett, R.D.; de Deyn, G.B.; Johnson, D.; Klimešová, J.; et al. Root traits as drivers of plant and ecosystem functioning: Current understanding, pitfalls and future research needs. New Phytologist. 2021, 232, 1123–1158. [Google Scholar] [CrossRef]
- Přikryl, Z.; Vančura, V.; Wurst, M. Auxin formation by rhizosphere bacteria as a factor of root growth. Biol. Plant. 1985, 27, 159–163. [Google Scholar] [CrossRef]
- Lambrecht, M.; Okon, Y.; Broek, A.V.; Vanderleyden, J. Indole-3-acetic acid: A reciprocal signalling molecule in bacteria–plant interactions. Trends Microbiol. 2000, 8, 298–300. [Google Scholar] [CrossRef]
- Tahir, H.A.S.; Gun, Q.; Wu, H.; Raza, W.; Hanif, A.; Wu, L.; Colman, M.V.C.; Gao, X. Plant growth promotion by volatile organic compounds produced by Bacillus subtilis SYST2. Front. Microbiol. 2017, 8, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Tullio, L.D.; Nakatani, A.S.; Gomes, D.F.; Ollero, F.J.; Megías, M.; Hungria, M. Revealing the roles of y4wF and tidC genes in Rhizobium tropici CIAT 899: Biosynthesis of indolic compounds and impact on symbiotic properties. Arch. Microbiol. 2019, 201, 171–183. [Google Scholar] [CrossRef] [PubMed]
- Costacurta, A.; Vanderleyden, J. Synthesis of phytohormones by plant-associated bacteria. Crit. Rev. Microbiol. 1995, 21, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Duarte, C.F.D.; Cecato, U.; Biserra, T.T.; Mamédio, D.; Galbeiro, S. Azospirillum spp. in grasses and forages. Review. Rev. Mex. Cienc. Pecu. 2020, 11, 223–240. [Google Scholar] [CrossRef]
- Barbieri, P.; Galli, E. Effect on wheat root development of inoculation with an Azospirillum brasilense mutant with altered indole-3-acetic acid production. Res. Microbiol. 1993, 144, 69–75. [Google Scholar] [CrossRef]
- Ortíz-Castro, R.; Valencia-Cantero, E.; López-Bucio, J. Plant growth promotion by Bacillus megaterium involves cytokinin signaling. Plant Signal. Behav. 2008, 3, 263–265. [Google Scholar] [CrossRef] [PubMed]
- Creus, C.M.; Graziano, M.; Casanovas, E.M.; Pereyra, M.A.; Simontacchi, M.; Puntarulo, S.; Barrasi, C.A.; Lamattina, L. Nitric oxide is involved in the Azospirillum brasilense-induced lateral root formation in tomato. Planta 2005, 221, 297–303. [Google Scholar] [CrossRef]
- Ortiz-Castro, R.; Campos-García, J.; López-Bucio, J. Pseudomonas putida and Pseudomonas fluorescens influence Arabidopsis root system architecture through an auxin response mediated by bioactive cyclodipeptides. J. Plant Growth Regul. 2020, 39, 254–265. [Google Scholar] [CrossRef]
- El-Khawas, H.; Adachi, K. Identification and quantification of auxins in culture media of Azospirillum and Klebsiella and their effect on rice roots. Biol. Fertil. Soils. 1999, 28, 377–381. [Google Scholar] [CrossRef]
- Dobbelaere, S.; Croonenborghs, A.; Thys, A.; Broek, A.V.; Vanderleyden, J. Phytostimulatory effect of Azospirillum brasilense wild type and mutant strains altered in IAA production on wheat. Plant Soil 1999, 212, 153–162. [Google Scholar] [CrossRef]
- Garcia-Montiel, D.C.; Neill, C.; Melillo, J.; Thomas, S.; Steudler, P.A.; Cerri, C.C. Soil phosphorus transformations following forest clearing for pasture in the brazilian Amazon. Soil Sci. Soc. Am. J. 2000, 64, 1792–1804. [Google Scholar] [CrossRef]
- Dias-Filho, M.B. Diagnóstico das Pastagens no Brasil; Embrapa Amazônia Oriental: Belém, Brazil, 2014; 38p. [Google Scholar]
- Criollo, P.J.; Obando, M.; Sánchez, M.L.; Bonilla, R. Efecto de bacterias promotoras de crecimiento vegetal (PGPR) asociadas a Pennisetum clandestinum en el altiplano cundiboyacense. Corpoica Cienc. Tecnol. Agropec. 2012, 13, 189–195. [Google Scholar] [CrossRef]
- Telhado, S.F.P.; Capdeville, G. Land-Saving Technologies; Embrapa: Brasília, Brazil, 2021; 161p. [Google Scholar]
- Heinrichs, R.; Meirelles, G.C.; Santos, L.P.M.; Lira, M.C.S.; Lapaz, A.M.; Nogueira, M.A.; Bonini, C.S.B.; Soares Filho, C.V.; Moreira, A. Azospirillum inoculation of ‘Marandu’ palisade grass seeds: Effects on forage production and nutritional status. Semina. Cienc. Agrar. 2020, 41, 465–478. [Google Scholar] [CrossRef]
- Chu, T.N.; Bui, L.V.; Hoang, M.T.T. Pseudomonas PS01 isolated from maize rhizosphere alters root system architecture and promotes plant growth. Microorganisms 2020, 8, 471. [Google Scholar] [CrossRef] [PubMed]
- Itzigsohn, R.; Burdman, S.; Okon, Y.; Zaady, E.; Yonatan, R.; Perevolotsky, A. Plant-growth promotion in natural pastures by inoculation with Azospirillum brasilense under suboptimal growth conditions. Arid Soil Res. Rehabil. 2000, 13, 151–158. [Google Scholar] [CrossRef]
- Leite, R.D.C.; dos Santos, J.G.; Silva, E.L.; Alves, C.R.; Hungria, M.; Leite, R.D.C.; dos Santos, A.C. Productivity increase, reduction of nitrogen fertiliser use and drought-stress mitigation by inoculation of Marandu grass (Urochloa brizantha) with Azospirillum brasilense. Crop Past. Scienc. 2018, 70, 61–67. [Google Scholar] [CrossRef]
- Braga, G.J.; Ramos, A.K.B.; Carvalho, M.A.; Fonseca, C.E.L.; Fernandes, F.D.; Malaquias, J.V.; Santos, M.F.; Jank, L. Produção de Forragem e Valor Nutritivo de Híbridos de Panicum Maximum Jacq. em Resposta à Adubação; Brasilia, D.F., Ed.; Embrapa Cerrados: Brasília, Brasil, 2019; 19p. [Google Scholar]
- PASTOCERTO. Comparar Cultivares. 2019. Available online: https://www.pastocerto.com (accessed on 15 November 2022).
- Rothballer, M.; Schmid, M.; Hartmann, A. In situ localization and PGPR-effect of Azospirillum brasilense strains colonizing roots of different wheat varieties. Symbiosis 2003, 34, 261–279. [Google Scholar]
- Bhattacharjee, R.B.; Jourand, P.; Chaintreuil, C.; Dreyfus, B.; Singh, A.; Mukhopadhyay, S.N. Indole acetic acid and ACC deaminase-producing Rhizobium leguminosarum bv. trifolii SN10 promote rice growth, and in the process undergo colonization and chemotaxis. Biol. Fertil. Soils 2012, 48, 173–182. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).