Genome-Wide Association Study for Agronomic Traits in Wild Soybean (Glycine soja)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Experiment Field Management
2.2. Phenotypic Evaluation and Data Analysis
2.3. DNA Extraction and SNP Genotyping
2.4. Population Structure and Genetic Diversity
2.5. Linkage Disequilibrium Estimation and Candidate Gene Identification
2.6. Genome-Wide Association Study Analysis
3. Results
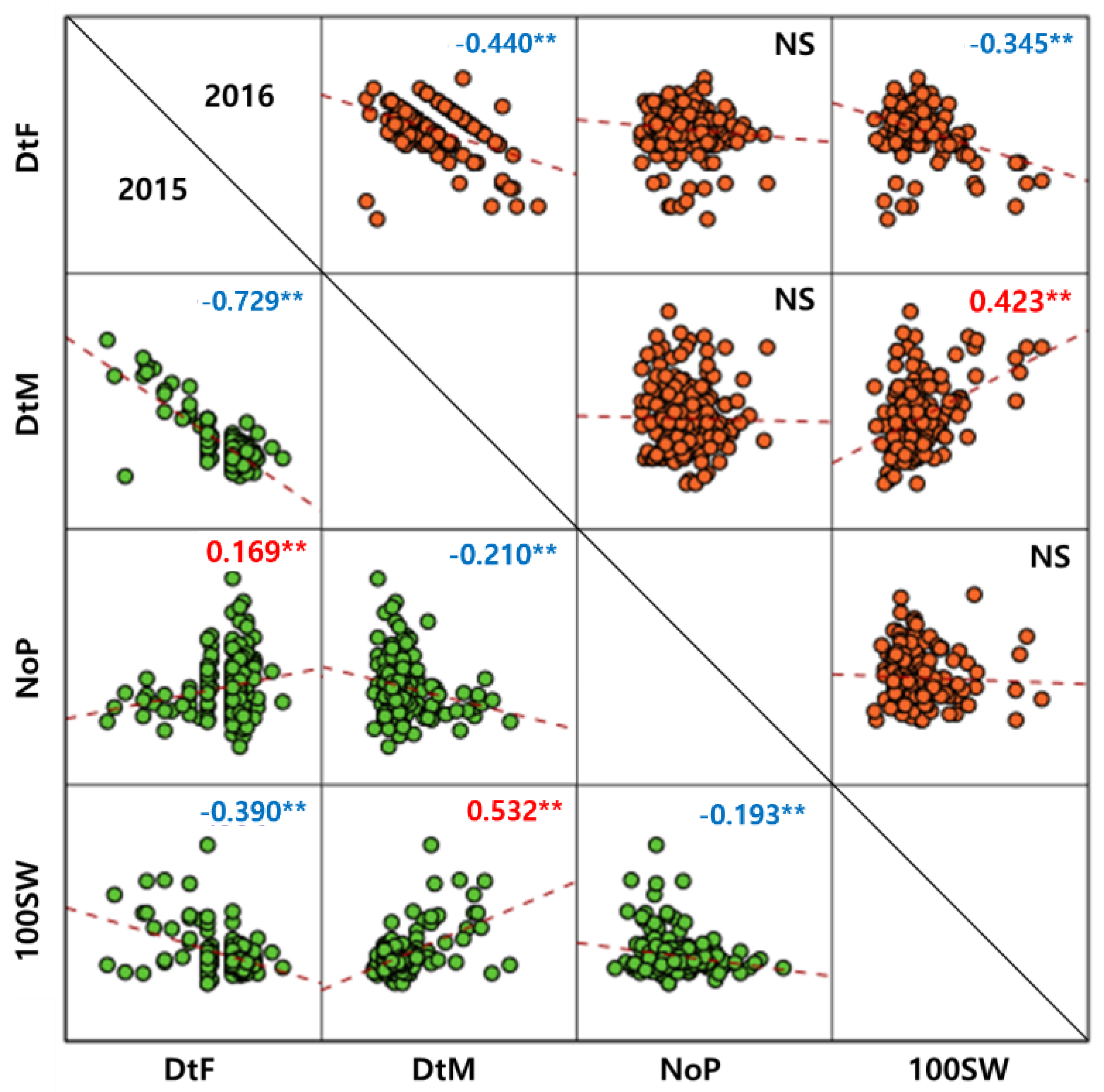
3.1. Phenotypic Variation and Correlation Analysis
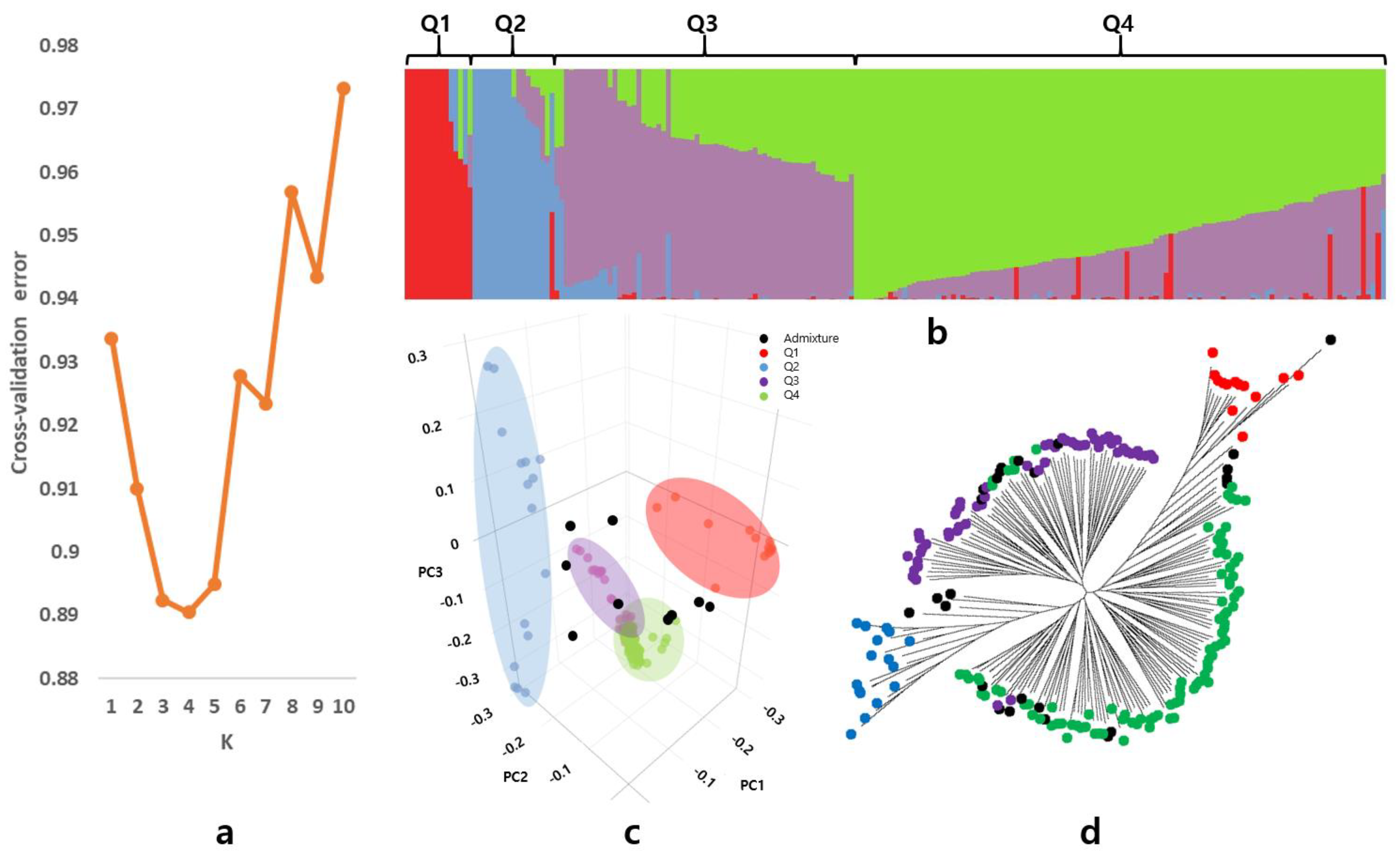
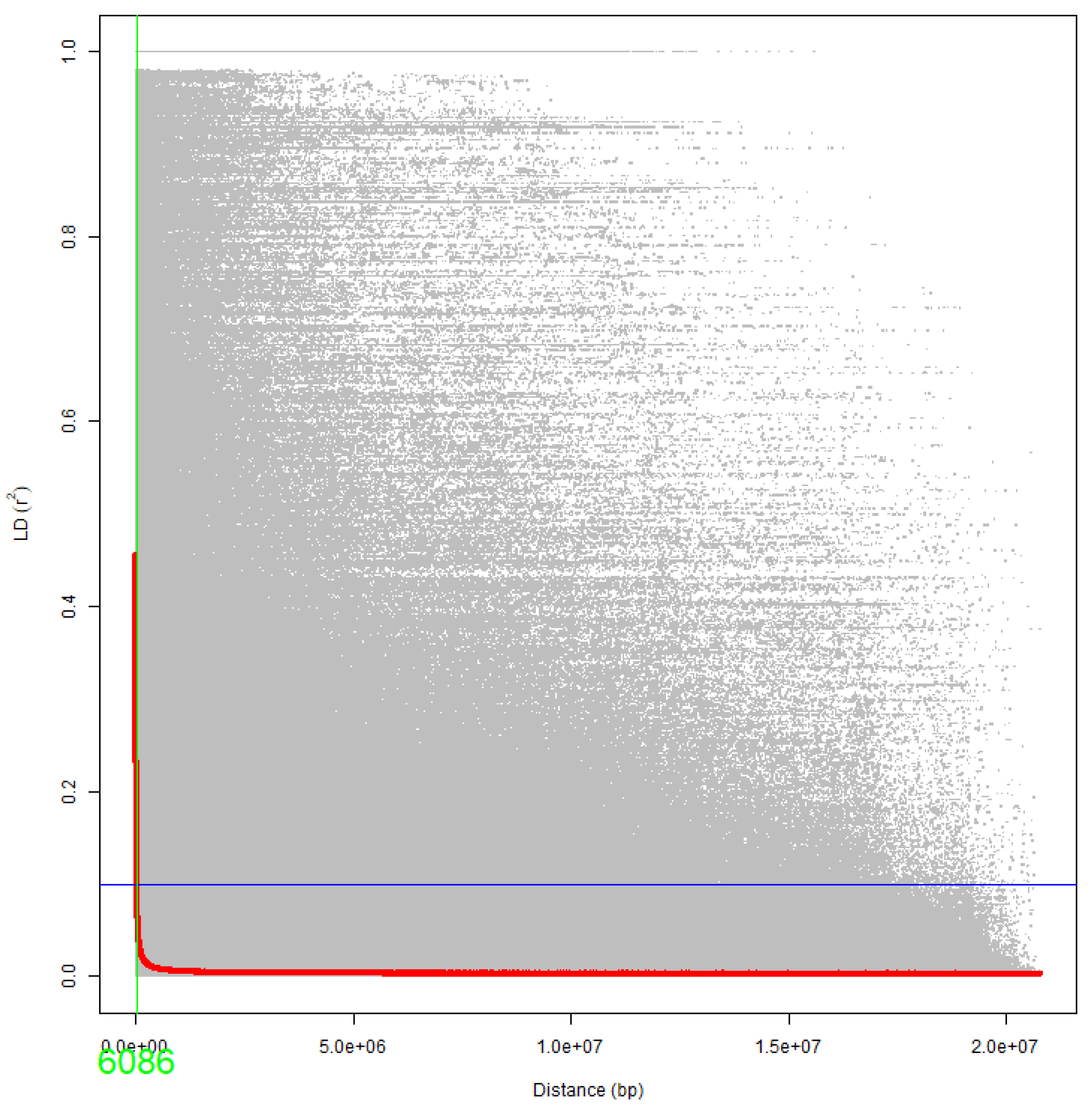
3.2. Population Structure and Linkage Disequilibrium
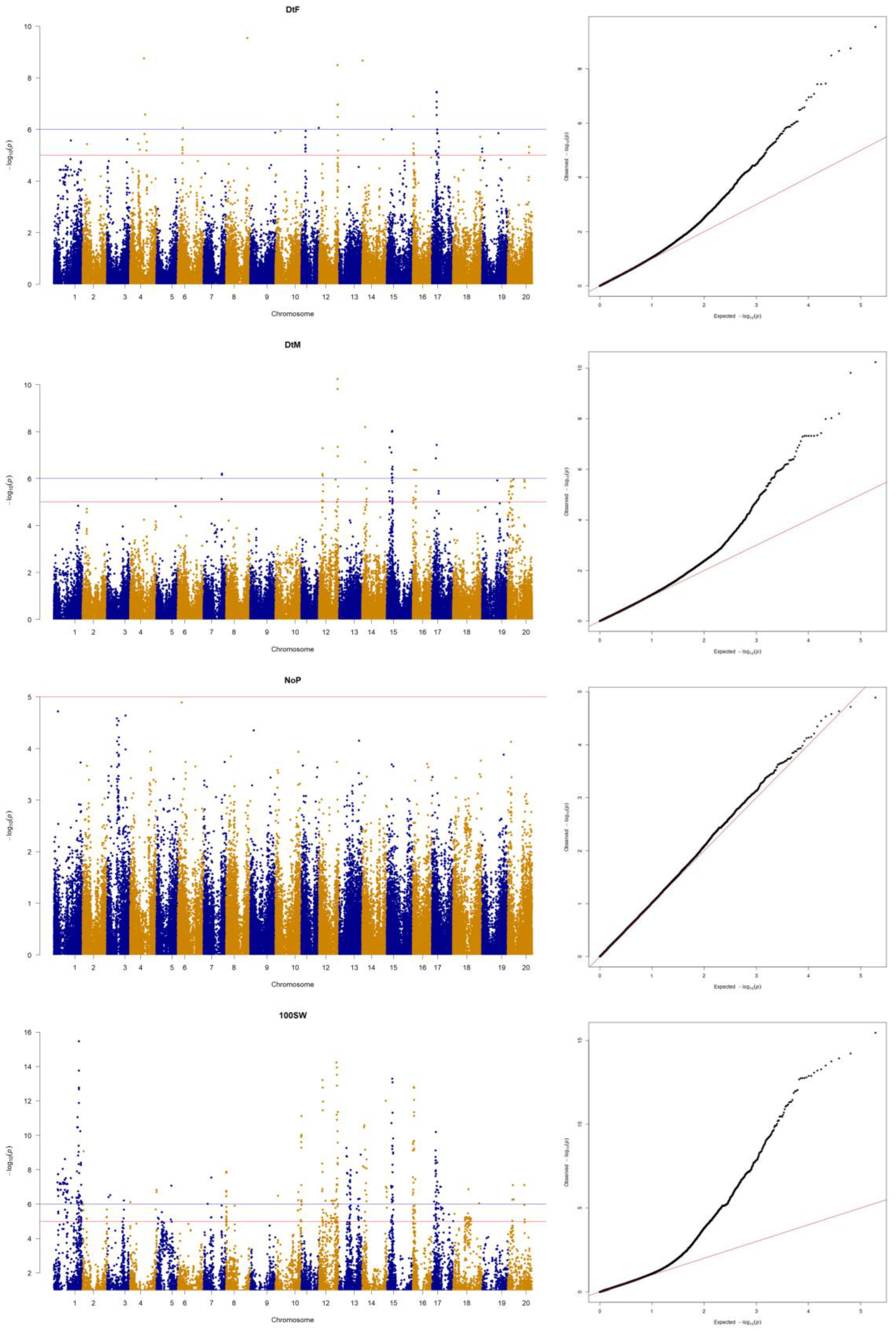
3.3. Genome-Wide Association Study for Agronomic Traits
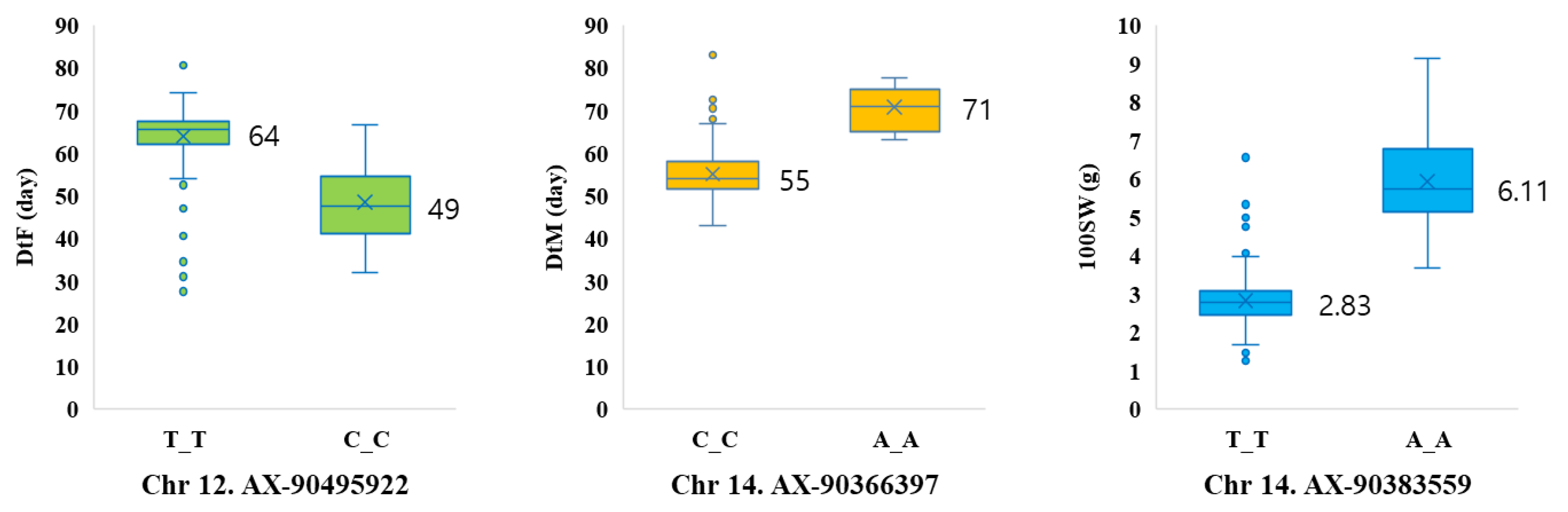
3.4. Candidate Genes for Trait-Associated SNP Markers
4. Discussion
4.1. Genetic Diversity and Origin of Wild Soybean
4.2. Correlation of Major Agronomic Traits
4.3. Candidate Genes for Major Agronomic Traits
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lam, H.-M.; Xu, X.; Liu, X.; Chen, W.; Yang, G.; Wong, F.-L.; Li, M.-W.; He, W.; Qin, N.; Wang, B.; et al. Resequencing of 31 wild and cultivated soybean genomes identifies patterns of genetic diversity and selection. Nat. Genet. 2010, 42, 1053–1059. [Google Scholar] [CrossRef] [PubMed]
- Hyten, D.L.; Song, Q.; Zhu, Y.; Choi, I.-Y.; Nelson, R.L.; Costa, J.M.; Specht, J.E.; Shoemaker, R.C.; Cregan, P.B. Impacts of genetic bottlenecks on soybean genome diversity. Proc. Natl. Acad. Sci. USA 2006, 103, 16666–16671. [Google Scholar] [CrossRef] [Green Version]
- Kofsky, J.; Zhang, H.; Song, B.-H. The untapped genetic reservoir: The past, current, and future applications of the wild soybean (Glycine soja). Front. Plant Sci. 2018, 9, 949. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carter, T.E., Jr.; Nelson, R.L.; Sneller, C.H.; Cui, Z. Genetic diversity in soybean. Soybeans Improv. Prod. Uses 2004, 16, 303–416. [Google Scholar]
- Nawaz, M.A.; Lin, X.; Chan, T.-F.; Ham, J.; Shin, T.-S.; Ercisli, S.; Golokhvast, K.S.; Lam, H.-M.; Chung, G. Korean wild soybeans (Glycine soja Sieb & Zucc.): Geographic distribution and germplasm conservation. Agronomy 2020, 10, 214. [Google Scholar]
- Kim, D.-H.; Doyle, M.R.; Sung, S.; Amasino, R.M. Vernalization: Winter and the timing of flowering in plants. Annu. Rev. Cell Dev. 2009, 25, 277–299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, W.; Kim, M.Y.; Van, K.; Lee, Y.-H.; Li, H.; Liu, X.; Lee, S.-H. QTL identification of yield-related traits and their association with flowering and maturity in soybean. J. Crop Sci. Biotechnol. 2011, 14, 65–70. [Google Scholar] [CrossRef]
- Fuller, D.Q. Contrasting patterns in crop domestication and domestication rates: Recent archaeobotanical insights from the Old World. Ann. Bot. 2007, 100, 903–924. [Google Scholar] [CrossRef] [PubMed]
- Cockram, J.; Jones, H.; Leigh, F.; O’Sullivan, D.; Powell, W.; Laurie, D.A.; Greenland, A.J. Control of flowering time in temperate cereals: Genes, domestication, and sustainable productivity. J. Exp. Bot. 2007, 58, 1231–1244. [Google Scholar] [CrossRef]
- Machado, B.; Nogueira, A.; Hamawaki, O.; Rezende, G.; Jorge, G.; Silveira, I.; Medeiros, L.; Hamawaki, R.; Hamawaki, C. Phenotypic and genotypic correlations between soybean agronomic traits and path analysis. Genet. Mol. Res. 2017, 16, gmr16029696. [Google Scholar] [CrossRef]
- Hartman, G.L.; West, E.D.; Herman, T. Crops that feed the World 2. Soybean—Worldwide production, use, and constraints caused by pathogens and pests. Food Secur. 2011, 3, 5–17. [Google Scholar] [CrossRef]
- Bernard, R. Two major genes for time of flowering and maturity in soybeans 1. Crop Sci. 1971, 11, 242–244. [Google Scholar] [CrossRef]
- Cober, E.R.; Voldeng, H.D. A new soybean maturity and photoperiod-sensitivity locus linked to E1 and T. Crop Sci. 2001, 41, 698–701. [Google Scholar] [CrossRef]
- Buzzell, R. Inheritance of a soybean flowering response to fluorescent-daylength conditions. Can. J. Genet. Cytol. 1971, 13, 703–707. [Google Scholar] [CrossRef]
- Buzzell, R.; Voldeng, H. Inheritance of insensitivity to long daylength. Soybean Genet. Newsl. 1980, 7, 26–29. [Google Scholar]
- McBlain, B.; Bernard, R. A new gene affecting the time of flowering and maturity in soybeans. J. Hered. 1987, 78, 160–162. [Google Scholar] [CrossRef]
- Bonato, E.R.; Vello, N.A. E6, a dominant gene conditioning early flowering and maturity in soybeans. Genet. Mol. Biol. 1999, 22, 229–232. [Google Scholar] [CrossRef] [Green Version]
- Cober, E.R.; Molnar, S.J.; Charette, M.; Voldeng, H.D. A new locus for early maturity in soybean. Crop Sci. 2010, 50, 524–527. [Google Scholar] [CrossRef]
- Kong, F.; Nan, H.; Cao, D.; Li, Y.; Wu, F.; Wang, J.; Lu, S.; Yuan, X.; Cober, E.R.; Abe, J.; et al. A new dominant gene E9 conditions early flowering and maturity in soybean. Crop Sci. 2014, 54, 2529–2535. [Google Scholar] [CrossRef]
- Cober, E.; Tanner, J.; Voldeng, H. Genetic control of photoperiod response in early-maturing, near-isogenic soybean lines. Crop Sci. 1996, 36, 601–605. [Google Scholar] [CrossRef]
- Cober, E.; Tanner, J.; Voldeng, H. Soybean photoperiod-sensitivity loci respond differentially to light quality. Crop Sci. 1996, 36, 606–610. [Google Scholar] [CrossRef]
- Kong, F.; Liu, B.; Xia, Z.; Sato, S.; Kim, B.M.; Watanabe, S.; Yamada, T.; Tabata, S.; Kanazawa, A.; Harada, K.; et al. Two coordinately regulated homologs of FLOWERING LOCUS T are involved in the control of photoperiodic flowering in soybean. Plant Physiol. 2010, 154, 1220–1231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thakare, D.; Kumudini, S.; Dinkins, R. The alleles at the E1 locus impact the expression pattern of two soybean FT-like genes shown to induce flowering in Arabidopsis. Planta 2011, 234, 933–943. [Google Scholar] [CrossRef]
- Watanabe, S.; Harada, K.; Abe, J. Genetic and molecular bases of photoperiod responses of flowering in soybean. Breed. Sci. 2012, 61, 531–543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Li, Y.; Zhang, H.; Sun, G.; Zhang, W.; Qiu, L. Evolution and association analysis of GmCYP78A10 gene with seed size/weight and pod number in soybean. Mol. Biol. Rep. 2015, 42, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Josephs, E.B.; Stinchcombe, J.; Wright, S. What can genome-wide association studies tell us about the evolutionary forces maintaining genetic variation for quantitative traits? New Phytol. 2017, 214, 21–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, J.; Zhao, W.; Tong, W.; He, Q.; Yoon, M.-Y.; Li, F.-P.; Choi, B.; Heo, E.-B.; Kim, K.-W.; Park, Y.-J. A genome-wide association study reveals candidate genes related to salt tolerance in rice (Oryza sativa) at the germination stage. Int. J. Mol. Sci. 2018, 19, 3145. [Google Scholar] [CrossRef] [Green Version]
- Sheoran, S.; Jaiswal, S.; Kumar, D.; Raghav, N.; Sharma, R.; Pawar, S.; Paul, S.; Iquebal, M.A.; Jaiswar, A.; Sharma, P.; et al. Uncovering genomic regions associated with 36 agro-morphological traits in Indian spring wheat using GWAS. Front. Plant Sci. 2019, 10, 527. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Yan, J.; Zhao, J.; Song, W.; Zhang, X.; Xiao, Y.; Zheng, Y. Genome-wide association study (GWAS) of resistance to head smut in maize. Plant Sci. 2012, 196, 125–131. [Google Scholar] [CrossRef]
- Zeng, A.; Chen, P.; Korth, K.; Hancock, F.; Pereira, A.; Brye, K.; Wu, C.; Shi, A. Genome-wide association study (GWAS) of salt tolerance in worldwide soybean germplasm lines. Mol. Breed. 2017, 37, 30. [Google Scholar] [CrossRef]
- Song, J.; Sun, X.; Zhang, K.; Liu, S.; Wang, J.; Yang, C.; Jiang, S.; Siyal, M.; Li, X.; Qi, Z.; et al. Identification of QTL and genes for pod number in soybean by linkage analysis and genome-wide association studies. Mol. Breed. 2020, 40, 60. [Google Scholar] [CrossRef]
- Sonah, H.; O’Donoughue, L.; Cober, E.; Rajcan, I.; Belzile, F. Identification of loci governing eight agronomic traits using a GBS-GWAS approach and validation by QTL mapping in soya bean. Plant Biotechnol. J. 2015, 13, 211–221. [Google Scholar] [CrossRef]
- Hwang, E.-Y.; Song, Q.; Jia, G.; Specht, J.E.; Hyten, D.L.; Costa, J.; Cregan, P.B. A genome-wide association study of seed protein and oil content in soybean. BMC Genom. 2014, 15, 1. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.-G.; Jeong, N.; Kim, J.H.; Lee, K.; Kim, K.H.; Pirani, A.; Ha, B.-K.; Kang, S.-T.; Park, B.-S.; Moon, J.-K.; et al. Development, validation and genetic analysis of a large soybean SNP genotyping array. Plant J. 2015, 81, 625–636. [Google Scholar] [CrossRef] [PubMed]
- Jeong, N.; Kim, K.-S.; Jeong, S.; Kim, J.-Y.; Park, S.-K.; Lee, J.S.; Jeong, S.-C.; Kang, S.-T.; Ha, B.-K.; Kim, D.-Y.; et al. Korean soybean core collection: Genotypic and phenotypic diversity population structure and genome-wide association study. PLoS ONE 2019, 14, e0224074. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Kim, J.-Y.; Lim, W.-J.; Jeong, S.; Lee, H.-Y.; Cho, Y.; Moon, J.-K.; Kim, N. Genome-wide association and epistatic interactions of flowering time in soybean cultivar. PLoS ONE 2020, 15, e0228114. [Google Scholar] [CrossRef]
- Lee, T.; Kim, K.; Kim, J.-M.; Shin, I.; Heo, J.; Jung, J.; Lee, J.; Moon, J.-K.; Kang, S. Genome-Wide Association Study for Ultraviolet-B Resistance in Soybean (Glycine max L.). Plants 2021, 10, 1335. [Google Scholar] [CrossRef]
- Lee, S.-B.; Lee, K.-S.; Kim, H.-Y.; Kim, D.-Y.; Seo, M.-S.; Jeong, S.-C.; Moon, J.-K.; Park, S.-K.; Choi, M.-S. The discovery of novel SNPs associated with group a soyasaponin biosynthesis from Korea soybean core collection. Genomics 2022, 114, 110432. [Google Scholar] [CrossRef]
- Kim, B.; Dai, X.; Zhang, W.; Zhuang, Z.; Sanchez, D.L.; Lübberstedt, T.; Kang, Y.; Udvardi, M.K.; Beavis, W.D.; Xu, S.; et al. GWASpro: A high-performance genome-wide association analysis server. Bioinformatics 2019, 35, 2512–2514. [Google Scholar] [CrossRef] [Green Version]
- Wickham, H.; François, R.; Henry, L.; Müller, K.; Vaughan, D. dplyr: A Grammar of Data Manipulation. R Package Version 0.7. 6. Comput. Softw. 2018. Available online: https://CRAN.R-project.org/package=dplyr (accessed on 19 January 2023).
- Browning, B.L.; Browning, S.R. A unified approach to genotype imputation and haplotype-phase inference for large data sets of trios and unrelated individuals. Am. J. Hum. Genet. 2009, 84, 210–223. [Google Scholar] [CrossRef] [Green Version]
- Alexander, D.H.; Lange, K. Enhancements to the ADMIXTURE algorithm for individual ancestry estimation. BMC Bioinform. 2011, 12, 246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular evolutionary genetics analysis version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- Purcell, S.; Neale, B.; Todd-Brown, K.; Thomas, L.; Ferreira, M.A.R.; Bender, D.; Maller, J.; Sklar, P.; de Bakker, P.I.W.; Daly, M.J.; et al. PLINK: A tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 2007, 81, 559–575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bradbury, P.J.; Zhang, Z.; Kroon, D.E.; Casstevens, T.M.; Ramdoss, Y.; Buckler, E.S. TASSEL: Software for association mapping of complex traits in diverse samples. Bioinformatics 2007, 23, 2633–2635. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Huang, Y.; Hou, L.; Ma, J.; Chen, C.; Ai, H.; Huang, L.; Ren, J. Genome-wide detection of genetic markers associated with growth and fatness in four pig populations using four approaches. Genet. Sel. Evol. 2017, 49, 21. [Google Scholar] [CrossRef] [Green Version]
- Kuroda, Y.; Tomooka, N.; Kaga, A.; Wanigadeva, S.M.S.W.; Vaughan, D.A. Genetic diversity of wild soybean (Glycine soja Sieb. et Zucc.) and Japanese cultivated soybeans [G. max (L.) Merr.] based on microsatellite (SSR) analysis and the selection of a core collection. Genet. Resour. Crop Evol. 2009, 56, 1045–1055. [Google Scholar] [CrossRef]
- Sulistyo, A.; Sari, K. Correlation, path analysis and heritability estimation for agronomic traits contribute to yield on soybean. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2018. [Google Scholar]
- Balla, M.Y.; Ibrahim, S.E. Genotypic correlation and path coefficient analysis of soybean [Glycine max (L.) Merr.] for yield and its components. Agric. Res. Technol. 2017, 7, 5557. [Google Scholar]
- Li, M.; Liu, Y.; Wang, C.; Yang, X.; Li, D.; Zhang, X.; Xu, C.; Zhang, Y.; Li, W.; Zhao, L. Identification of traits contributing to high and stable yields in different soybean varieties across three Chinese latitudes. Front. Plant Sci. 2020, 10, 1642. [Google Scholar] [CrossRef]
- Zhang, J.; Song, Q.; Cregan, P.B.; Nelson, R.L.; Wang, X.; Wu, J.; Jiang, G.-L. Genome-wide association study for flowering time, maturity dates and plant height in early maturing soybean (Glycine max) germplasm. BMC Genom. 2015, 16, 217. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Z.; Jiang, Y.; Wang, Z.; Gou, Z.; Lyu, J.; Li, W.; Yu, Y.; Shu, L.; Zhao, Y.; Ma, Y.; et al. Resequencing 302 wild and cultivated accessions identifies genes related to domestication and improvement in soybean. Nat. Biotechnol. 2015, 33, 408–414. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.-S.; Lozano, R.; Kim, J.H.; Bae, D.N.; Kim, S.-T.; Park, J.-H.; Choi, M.S.; Kim, J.; Ok, H.-C.; Park, S.-K.; et al. The patterns of deleterious mutations during the domestication of soybean. Nat. Commun. 2021, 12, 97. [Google Scholar] [CrossRef]
- Taylor, C.M.; Garg, G.; Berger, J.D.; Ribalta, F.M.; Croser, J.S.; Singh, K.B.; Cowling, W.A.; Kamphuis, L.G.; Nelson, M.N. A Trimethylguanosine Synthase1-like (TGS1) homologue is implicated in vernalisation and flowering time control. Theor. Appl. Genet. 2021, 134, 3411–3426. [Google Scholar] [CrossRef]
- Shi, S.; Duan, G.; Li, D.; Wu, J.; Liu, X.; Hong, B.; Yi, M.; Zhang, Z. Two-dimensional analysis provides molecular insight into flower scent of Lilium ‘Siberia’. Sci. Rep. 2018, 8, 5352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campobenedetto, C.; Mannino, G.; Agliassa, C.; Acquadro, A.; Contartese, V.; Garabello, C.; Bertea, C.M. Transcriptome analyses and antioxidant activity profiling reveal the role of a lignin-derived biostimulant seed treatment in enhancing heat stress tolerance in soybean. Plants 2020, 9, 1308. [Google Scholar] [CrossRef] [PubMed]
- Spanudakis, E.; Jackson, S. The role of microRNAs in the control of flowering time. J. Exp. Bot. 2014, 65, 365–380. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Ruan, H.; Yu, L.; Li, Q.; Song, Q.; Yang, S.; Gai, J. miR156b from soybean CMS line modulates floral organ development. J. Plant Biol. 2020, 63, 141–153. [Google Scholar] [CrossRef]
- Jaspert, N.; Throm, C.; Oecking, C. Arabidopsis 14-3-3 proteins: Fascinating and less fascinating aspects. Front. Plant Sci. 2011, 2, 96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pnueli, L.; Gutfinger, T.; Hareven, D.; Ben-Naim, O.; Ron, N.; Adir, N.; Lifschitz, E. Tomato SP-interacting proteins define a conserved signaling system that regulates shoot architecture and flowering. Plant Cell 2001, 13, 2687–2702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lifschitz, E.; Eviatar, T.; Rozman, A.; Shalit, A.; Goldshmidt, A.; Amsellem, Z.; Alvarez, J.P.; Eshed, Y. The tomato FT ortholog triggers systemic signals that regulate growth and flowering and substitute for diverse environmental stimuli. Proc. Natl. Acad. Sci. USA 2006, 103, 6398–6403. [Google Scholar] [CrossRef] [Green Version]
- Purwestri, Y.A.; Ogaki, Y.; Tamaki, S.; Tsuji, H.; Shimamoto, K. The 14-3-3 protein GF14c acts as a negative regulator of flowering in rice by interacting with the florigen Hd3a. Plant Cell Physiol. 2009, 50, 429–438. [Google Scholar] [CrossRef] [Green Version]
- Han, G.; Qiao, Z.; Li, Y.; Wang, C.; Wang, B. The roles of CCCH zinc-finger proteins in plant abiotic stress tolerance. Int. J. Mol. Sci. 2021, 22, 8327. [Google Scholar] [CrossRef]
- Kazan, K.; Lyons, R. The link between flowering time and stress tolerance. J. Exp. Bot. 2016, 67, 47–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Almagro, A.; Lin, S.; Tsay, Y. Characterization of the Arabidopsis nitrate transporter NRT1. 6 reveals a role of nitrate in early embryo development. Plant Cell 2008, 20, 3289–3299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samanfar, B.; Molnar, S.J.; Charette, M.; Schoenrock, A.; Dehne, F.; Golshani, A.; Belzile, F.; Cober, E.R. Mapping and identification of a potential candidate gene for a novel maturity locus, E10, in soybean. Theor. Appl. Genet. 2017, 130, 377–390. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Cheng, Z.; Qin, R.; Qiu, Y.; Heng, Y.; Yang, H.; Ren, Y.; Wang, X.; Bi, J.; Ma, X.; et al. O s ARG encodes an arginase that plays critical roles in panicle development and grain production in rice. Plant J. 2013, 73, 190–200. [Google Scholar] [CrossRef] [PubMed]
- Baldoni, A.; Von Pinho, E.; Fernandes, J.; Abreu, V.; Carvalho, M. Gene expression in the lignin biosynthesis pathway during soybean seed development. Genet. Mol. Res. 2013, 12, 2618–2624. [Google Scholar] [CrossRef]
- Yan, Q.; Si, J.; Cui, X.; Peng, H.; Chen, X.; Xing, H.; Dou, D. The soybean cinnamate 4-hydroxylase gene GmC4H1 contributes positively to plant defense via increasing lignin content. Plant Growth Regul. 2019, 88, 139–149. [Google Scholar] [CrossRef]
- Bhadouria, J.; Singh, A.P.; Mehra, P.; Verma, L.; Srivastawa, R.; Parida, S.K.; Giri, J. Identification of purple acid phosphatases in chickpea and potential roles of CaPAP7 in seed phytate accumulation. Sci. Rep. 2017, 7, 11012. [Google Scholar] [CrossRef] [Green Version]
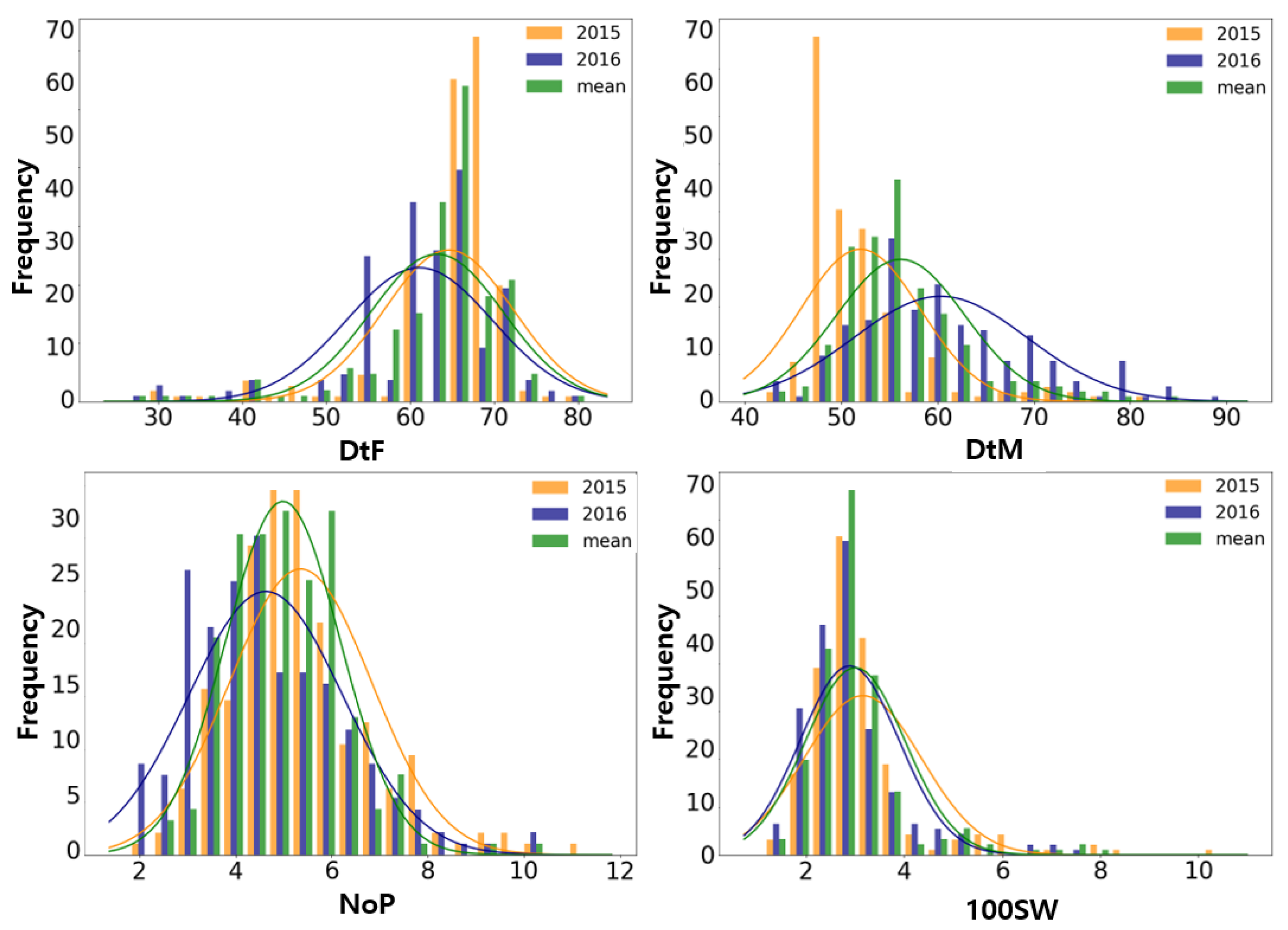
| Trait | Year | Min | Max | SD | Mean | CV (%) | Skew | Kur | h2 |
|---|---|---|---|---|---|---|---|---|---|
| DtF | 2015 | 29 | 80 | 7.69 | 64.61 | 11.90 | −2.33 | 6.39 | 0.89 |
| 2016 | 26 | 81 | 8.71 | 61.11 | 14.26 | −1.34 | 3.11 | ||
| Mean | 28 | 81 | 7.90 | 63.20 | 12.50 | −1.91 | 5.03 | ||
| DtM | 2015 | 44 | 82 | 6.22 | 51.98 | 11.98 | 2.28 | 6.04 | 0.55 |
| 2016 | 42 | 90 | 9.02 | 60.29 | 14.96 | 0.71 | 0.33 | ||
| Mean | 43 | 83 | 6.67 | 56.15 | 11.87 | 1.33 | 2.23 | ||
| NoP | 2015 | 2.0 | 11.4 | 1.47 | 5.36 | 27.51 | 0.91 | 1.58 | 0.39 |
| 2016 | 1.8 | 10.2 | 1.60 | 4.63 | 34.55 | 0.76 | 0.61 | ||
| Mean | 2.3 | 10.2 | 1.19 | 4.99 | 23.88 | 0.69 | 1.53 | ||
| 100SW | 2015 | 1.30 | 10.57 | 1.20 | 3.15 | 38.00 | 2.80 | 11.01 | 0.75 |
| 2016 | 1.15 | 7.69 | 1.01 | 2.88 | 35.00 | 2.01 | 5.85 | ||
| Mean | 1.26 | 7.83 | 1.02 | 3.00 | 33.99 | 2.33 | 7.17 |
| SNP | Chr | Position | −log10(p) | Reference + | Minor | Major | MAF |
|---|---|---|---|---|---|---|---|
| AX-90393598 | 6 | 9,723,612 | 6.05 | G | T | G | 0.09 |
| AX-90507114 | 11 | 34,284,790 | 6.06 | G | A | G | 0.07 |
| AX-90495922 | 12 | 36,991,550 | 8.49 | C | C | T | 0.08 |
| AX-90386690 | 16 | 1,150,092 | 6.50 | G | G | A | 0.16 |
| AX-90395214 | 17 | 9,164,086 | 7.43 | T | T | C | 0.08 |
| SNP | Chr | Position | −log10(p) | Reference + | Minor | Major | MAF |
|---|---|---|---|---|---|---|---|
| AX-90440044 | 12 | 7,550,988 | 7.29 | T | T | C | 0.08 |
| AX-90495922 | 12 | 36,991,550 | 9.82 | C | C | T | 0.08 |
| AX-90366397 | 14 | 5,987,400 | 8.2 | A | A | C | 0.06 |
| AX-90472604 | 15 | 10,746,176 | 8.03 | G | G | T | 0.07 |
| AX-90351904 | 16 | 2,017,593 | 6.37 | T | T | C | 0.09 |
| AX-90338412 | 17 | 9,141,000 | 7.43 | A | A | T | 0.07 |
| SNP | Chr | Position | −log10(p) | Reference + | Minor | Major | MAF |
|---|---|---|---|---|---|---|---|
| AX-90454597 | 1 | 46,804,555 | 11.05 | C | C | T | 0.07 |
| AX-90377215 | 8 | 213,783 | 7.87 | T | T | C | 0.09 |
| AX-90374196 | 10 | 51,266,958 | 11.10 | C | C | A | 0.09 |
| AX-90408186 | 12 | 6,713,247 | 13.20 | A | A | C | 0.06 |
| AX-90370905 | 12 | 34,609,739 | 14.22 | T | T | C | 0.09 |
| AX-90318417 | 13 | 14,296,524 | 9.26 | C | C | T | 0.07 |
| AX-90483232 | 14 | 3,979,096 | 10.57 | C | C | G | 0.06 |
| AX-90383559 | 14 | 47,044,439 | 11.99 | A | A | T | 0.07 |
| AX-90472604 | 15 | 10,746,176 | 13.28 | G | G | T | 0.07 |
| AX-90416982 | 16 | 1,413,306 | 12.80 | G | G | A | 0.05 |
| AX-90375042 | 17 | 7,394,535 | 10.18 | G | G | A | 0.09 |
| SNP | Chr | Position | Gene + | Location (bp) | Gene Description |
|---|---|---|---|---|---|
| AX-90393598 | 6 | 9,723,612 | Glyma.06g119400 | 9719172..9726230 | S-adenosyl-L-methionine-dependent methyltransferases superfamily protein |
| AX-90507114 | 11 | 34,284,790 | Glyma.11g251500 | 34283168..34289333 | squamosa promoter binding protein-like 2 |
| AX-90495922 | 12 | 36,991,550 | Glyma.12g210400 | 36943077..36946491 | 14-3-3 protein |
| AX-90395214 | 17 | 9,164,086 | Glyma.17g116200 | 9181066..9183991 | CCCH-type zinc finger family protein |
| SNP | Chr | Position | Gene + | Location (bp) | Gene Description |
|---|---|---|---|---|---|
| AX-90440044 | 12 | 7,550,988 | Glyma.12g091600 | 7494222..7499978 | OTUBAIN-LIKE DEUBIQUITINASE 1 |
| AX-90495922 | 12 | 36,991,550 | Glyma.12g210400 | 36943077..36946491 | 14-3-3 protein |
| AX-90366397 | 14 | 5,987,400 | Glyma.14g071300 | 5986366..5990379 | RING/U-box superfamily protein |
| AX-90472604 | 15 | 10,746,176 | Glyma.15g133700 | 10744186..10748830 | Glycosyl hydrolases family 31 protein |
| AX-90351904 | 16 | 2,017,593 | Glyma.16g021200 | 1996084..1998423 | Cytochrome P450, Family 78 |
| AX-90338412 | 17 | 9,141,000 | Glyma.17g115300 | 9122203..9127019 | NRT1 |
| SNP | Chr | Position | Gene + | Location (bp) | Gene Description |
|---|---|---|---|---|---|
| AX-90454597 | 1 | 46,804,555 | Glyma.01g140200 | 46825221..46841112 | arginase |
| AX-90377215 | 8 | 213,783 | Glyma.08g002900 | 211294..221624 | cyclin-dependent kinase E;1 |
| AX-90374196 | 10 | 51,266,958 | Glyma.10g295400 | 51258630..51267380 | 2-isopropylmalate synthase 1 |
| AX-90370905 | 12 | 34,609,739 | Glyma.12g184700 | 34608320..34611560 | Homeodomain-like superfamily protein |
| AX-90318417 | 13 | 14,296,524 | Glyma.13g047500 | 14291927..14296667 | NagB/RpiA/CoA transferase-like superfamily protein |
| AX-90483232 | 14 | 3,979,096 | Glyma.14g050900 | 3977944..3980627 | GDSL-like Lipase/Acylhydrolase superfamily protein |
| AX-90383559 | 14 | 47,044,439 | Glyma.14g205200 | 47041931..47046048 | cinnamate-4-hydroxylase |
| AX-90472604 | 15 | 10,746,176 | Glyma.15g133700 | 10744186..10748830 | Glycosyl hydrolases family 31 protein |
| AX-90416982 | 16 | 1,413,306 | Glyma.16g016000 | 1400064..1404488 | Purple acid phosphatase 29 |
| AX-90375042 | 17 | 7,394,535 | Glyma.17g094400 | 7393359..7395553 | Homeodomain-like superfamily protein |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, W.J.; Kang, B.H.; Moon, C.Y.; Kang, S.; Shin, S.; Chowdhury, S.; Jeong, S.-C.; Choi, M.-S.; Park, S.-K.; Moon, J.-K.; et al. Genome-Wide Association Study for Agronomic Traits in Wild Soybean (Glycine soja). Agronomy 2023, 13, 739. https://doi.org/10.3390/agronomy13030739
Kim WJ, Kang BH, Moon CY, Kang S, Shin S, Chowdhury S, Jeong S-C, Choi M-S, Park S-K, Moon J-K, et al. Genome-Wide Association Study for Agronomic Traits in Wild Soybean (Glycine soja). Agronomy. 2023; 13(3):739. https://doi.org/10.3390/agronomy13030739
Chicago/Turabian StyleKim, Woon Ji, Byeong Hee Kang, Chang Yeok Moon, Sehee Kang, Seoyoung Shin, Sreeparna Chowdhury, Soon-Chun Jeong, Man-Soo Choi, Soo-Kwon Park, Jung-Kyung Moon, and et al. 2023. "Genome-Wide Association Study for Agronomic Traits in Wild Soybean (Glycine soja)" Agronomy 13, no. 3: 739. https://doi.org/10.3390/agronomy13030739
APA StyleKim, W. J., Kang, B. H., Moon, C. Y., Kang, S., Shin, S., Chowdhury, S., Jeong, S.-C., Choi, M.-S., Park, S.-K., Moon, J.-K., & Ha, B.-K. (2023). Genome-Wide Association Study for Agronomic Traits in Wild Soybean (Glycine soja). Agronomy, 13(3), 739. https://doi.org/10.3390/agronomy13030739







