Response of Water-Nitrogen Distribution and Use to Water Deficit under Different Applied Nitrogen Fertilizer Rates in Bromus inermis Grassland
Abstract
1. Introduction
2. Materials and Methods
2.1. Description of the Experimental Site
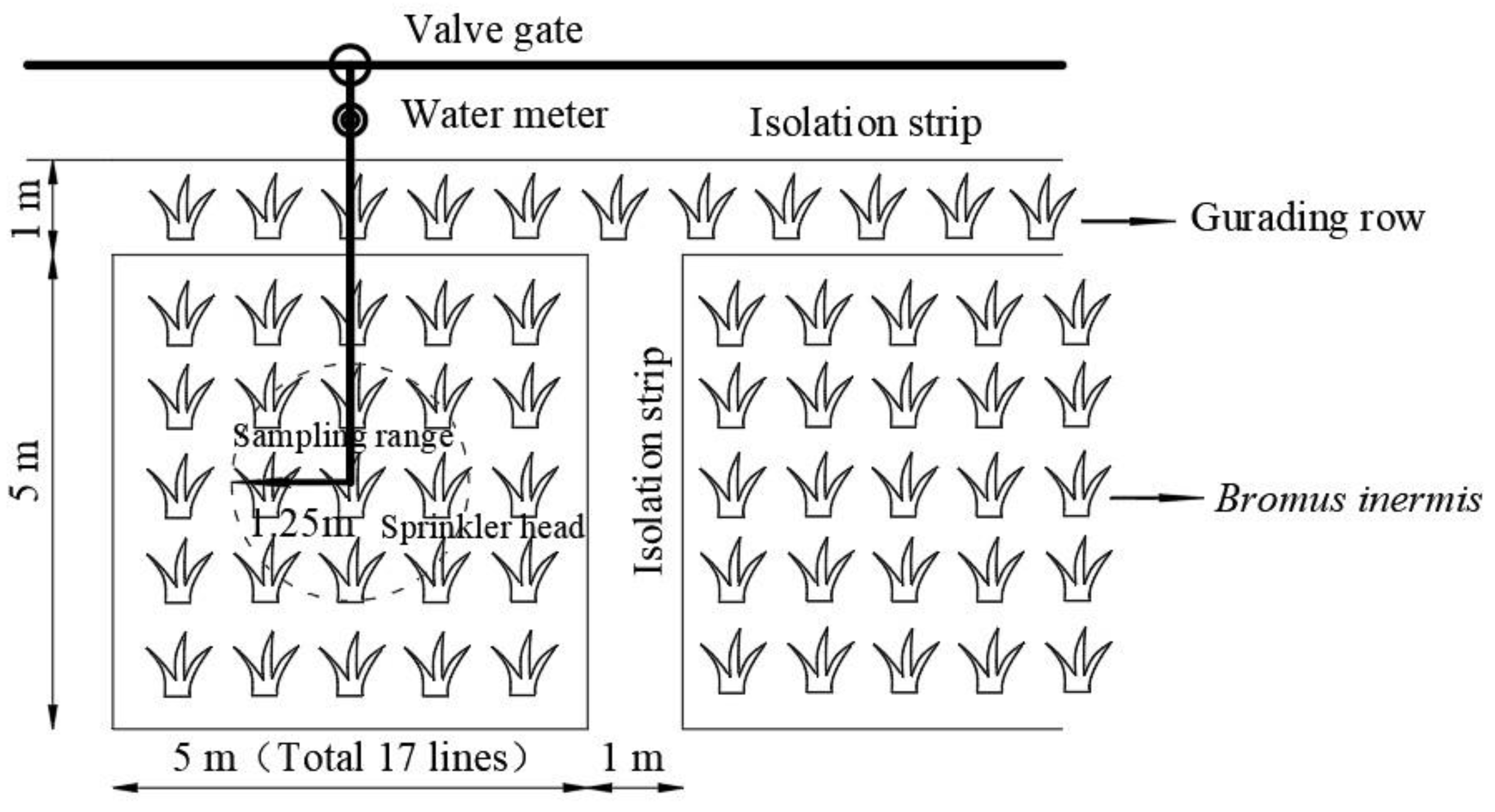
2.2. Experimental Design and Field Management
2.3. Indicators and Methods for Measurement
2.3.1. Soil Moisture Content (SWC, %)
2.3.2. Soil NO3−–N Content (mg·kg−1)
2.3.3. Plant Height (cm) and Chlorophyll Content
2.3.4. Yield (kg·ha−1)
2.3.5. Water-Nitrogen Use Efficiency
- (1)
- WUE (kg·m−3) [17]
- (2)
- PFPN (kg·kg−1) [24]
2.4. Data Analysis
3. Results
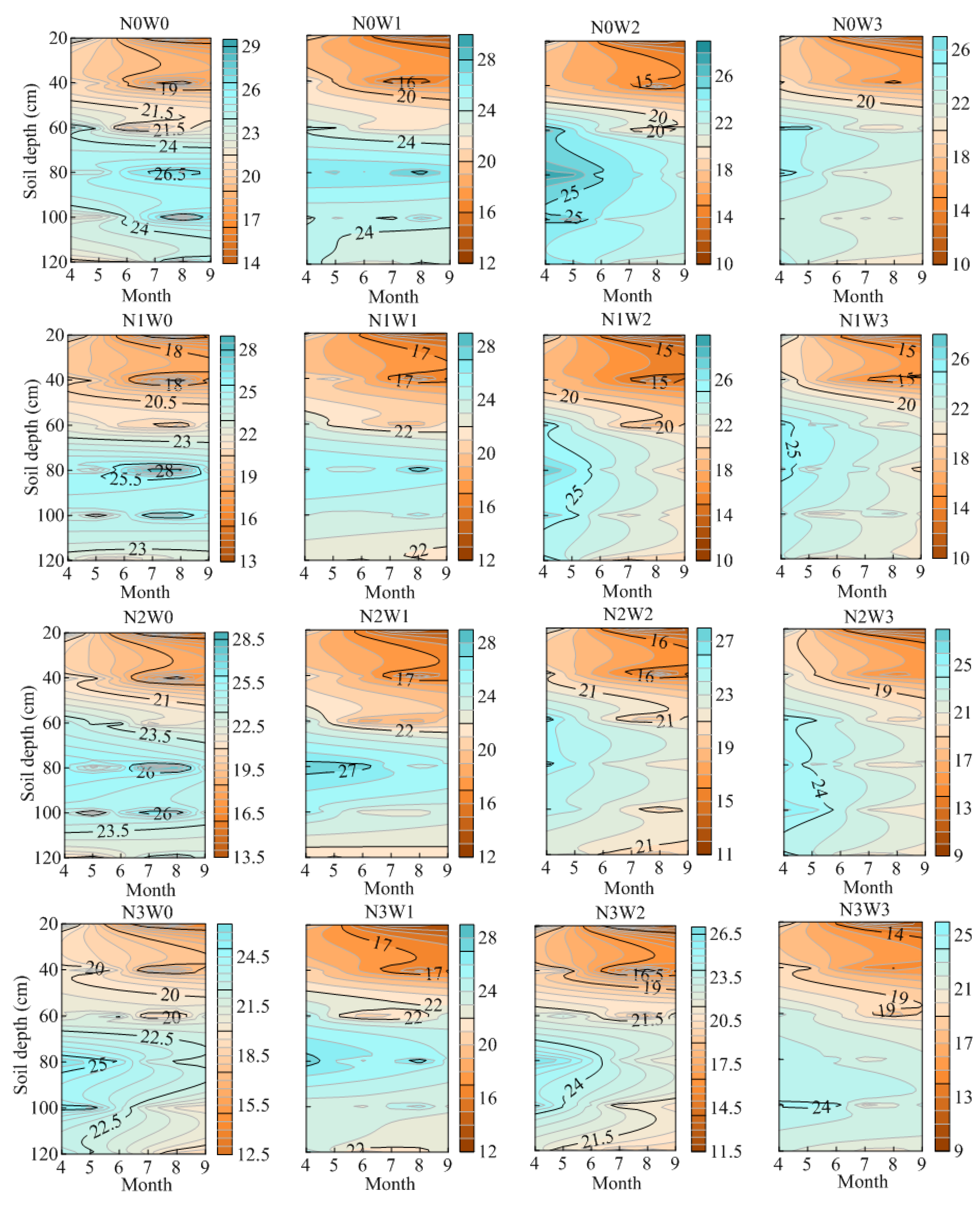
3.1. Effects of Water-Nitrogen Regulation on Soil Moisture Distribution
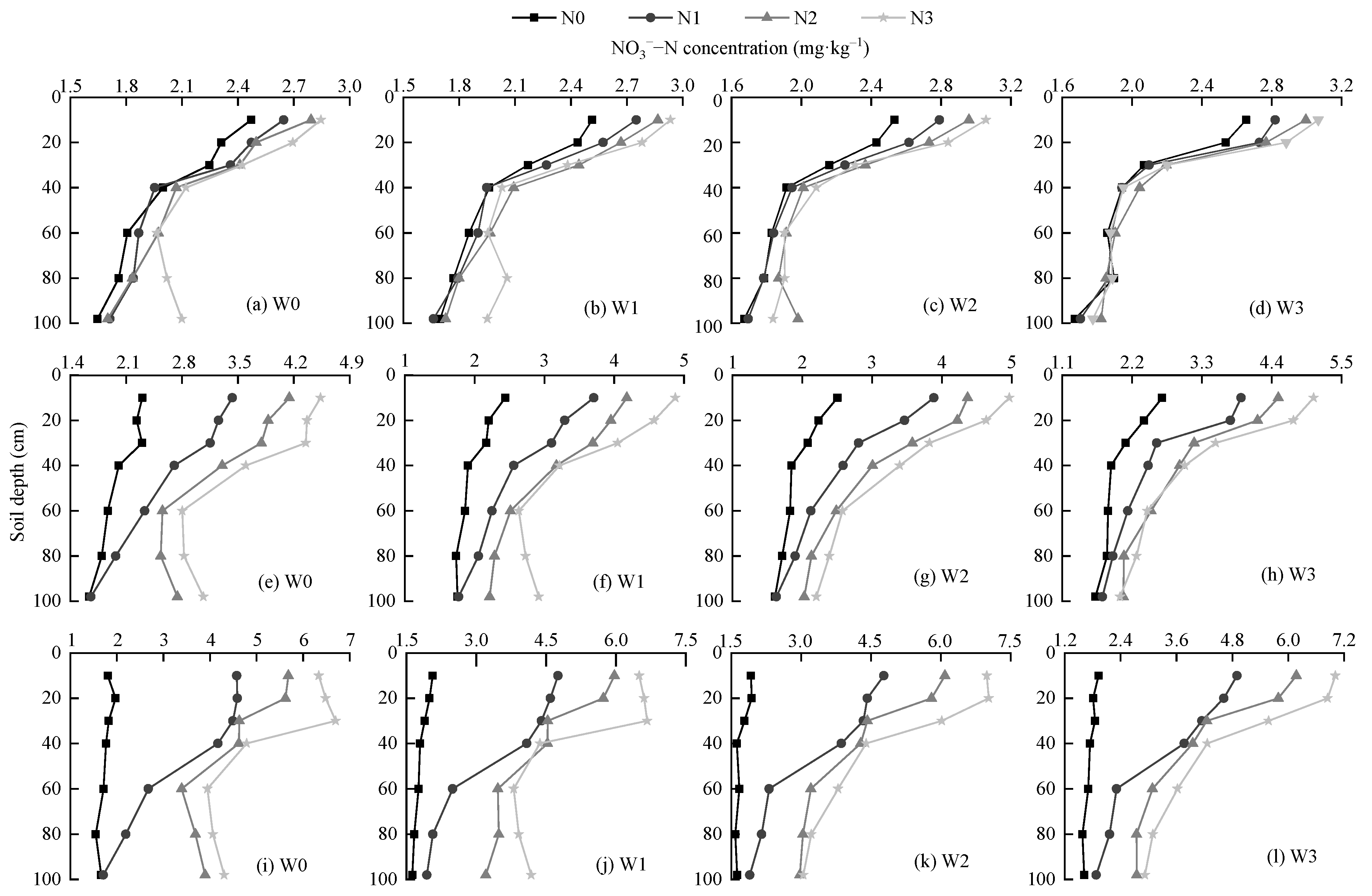
3.2. Effects of Water-Nitrogen Regulation on Distribution of Soil NO3−–N
3.3. Effects of Water-Nitrogen Regulation on Plant Height and Chlorophyll Content of Bromus inermis
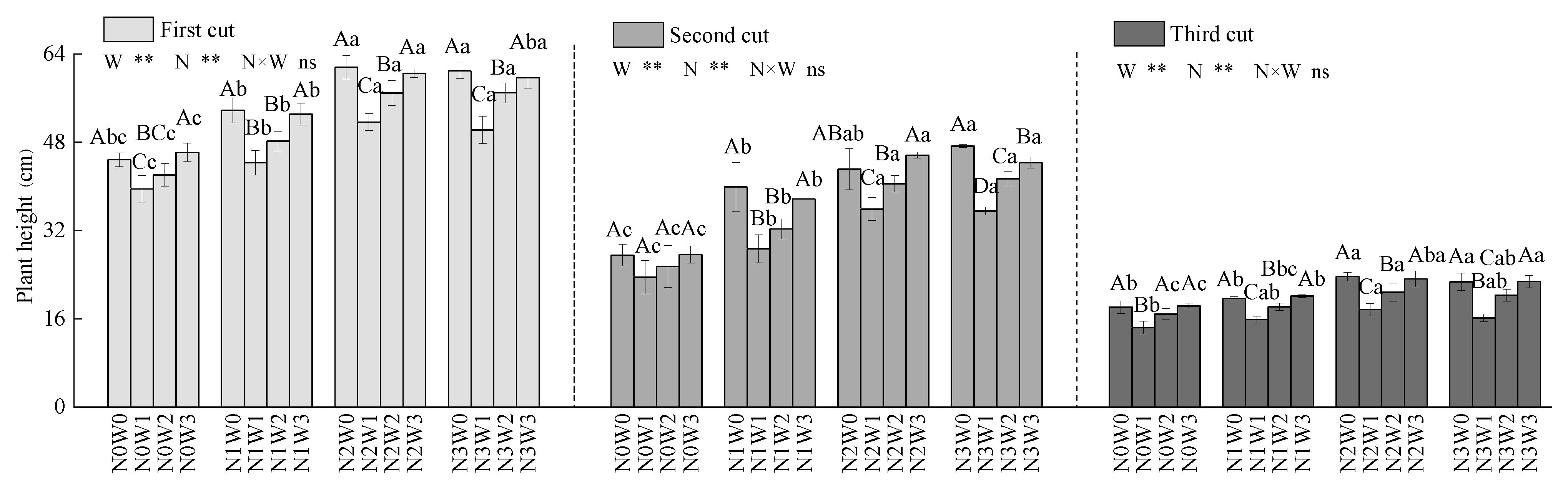
3.3.1. Plant Height
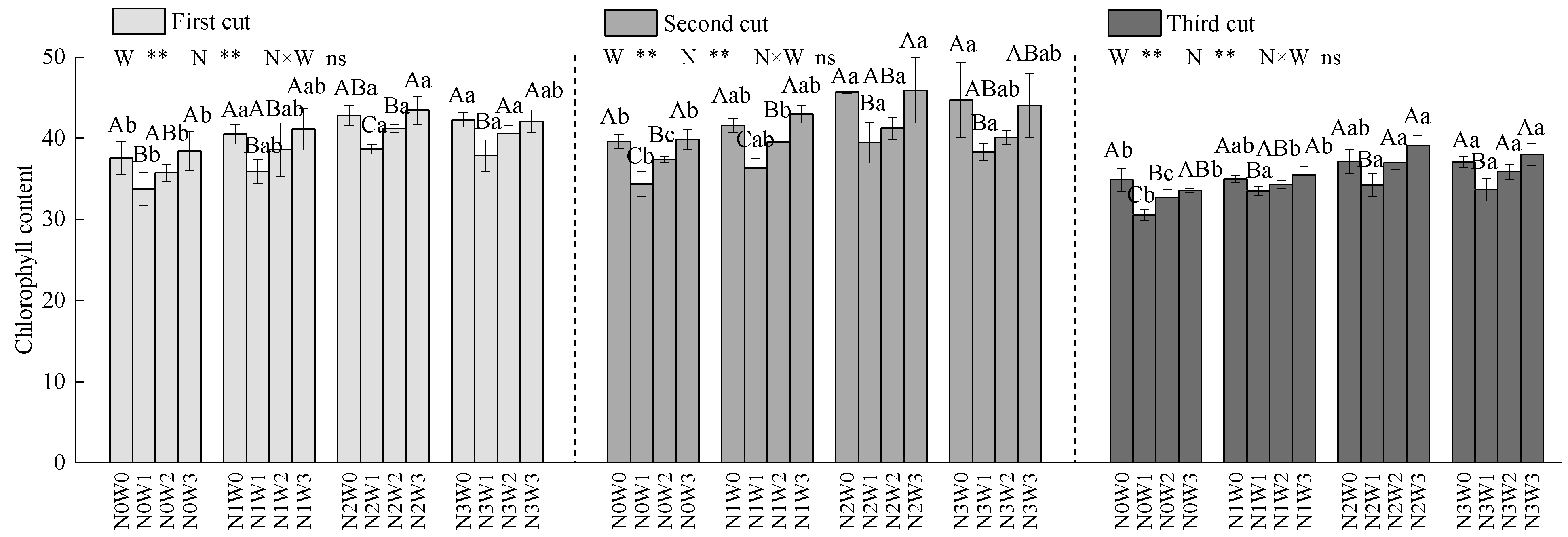
3.3.2. Chlorophyll Content
3.4. Effect of the Water-Nitrogen Regulation on Yield of Bromus inermis
3.5. Effect of Water-Nitrogen Regulation on Water-Nitrogen Use Efficiency of Bromus inermis
4. Discussion
4.1. Effects of the Water-Nitrogen Regulation on the Spatiotemporal Distribution of Water and Nitrogen in Bromus inermis
4.2. Effects of Water-Nitrogen Regulation on the Growth of Bromus inermis
4.3. Effects of Water-Nitrogen Regulation on Yield and Water-Nitrogen Use Efficiency of Bromus inermis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Paul, B.K.; Groot, J.C.; Maass, B.L.; An, M.N.; Herrero, M.; Tittonell, P.A. Improved feeding and forages at a crossroads: Farming systems approaches for sustainable livestock development in East Africa. Outlook AGR 2020, 49, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Halli, H.M.; Govindasamy, P.; Choudhary, M.; Srinivasan, R.; Prasad, M.; Wasnik, V.K.; Yadav, V.K.; Singh, A.K.; Kumar, S.; Vijay, D.; et al. Range grasses to improve soil properties, carbon sustainability, and fodder security in degraded lands of semi-arid regions. Sci. Total Environ. 2022, 851, 158211. [Google Scholar] [CrossRef]
- Oliveira, E.M.D.; Oliveira, J.D.C.; Oliveira, R.A.D.; Oliveira, R.M.D.; Cecon, P.R. Determination of xaraés grass quality submitted to irrigation water levels and nitrogen and potassium doses. Eng. Agríc. 2017, 37, 64–74. [Google Scholar] [CrossRef]
- Ma, Q.; Zhang, X.; Wu, Y.; Yang, H.; Wang, Z. Optimizing water and nitrogen strategies to improve forage oat yield and quality on the Tibetan Plateau using apsim. Agronomy 2022, 12, 933. [Google Scholar] [CrossRef]
- Brueck, H.; Erdle, K.; Gao, Y.; Giese, M.; Zhao, Y.; Peth, S.; Lin, S. Effects of N and water supply on water use-efficiency of a semiarid grassland in Inner Mongolia. Plant Soil 2010, 328, 495–505. [Google Scholar] [CrossRef]
- Martin, N.P.; Russelle, M.P.; Powell, J.M.; Sniffen, C.J.; Smith, S.I.; Tricarico, J.M.; Grant, R.J. Invited review: Sustainable forage and grain crop production for the US dairy industry. J. Dairy Sci. 2017, 100, 9479–9494. [Google Scholar] [CrossRef] [PubMed]
- Bowen, M.K.; Chudleigh, F.; Buck, S.; Hopkins, K. Productivity and profitability of forage options for beef production in the subtropics of northern Australia. Anim. Prod. Sci. 2016, 58, 332–342. [Google Scholar] [CrossRef]
- Martínez-Dalmau, J.; Berbel, J.; Ordóñez-Fernández, R. Nitrogen fertilization. A review of the risks associated with the inefficiency of its use and policy responses. Sustainability 2021, 13, 5625. [Google Scholar] [CrossRef]
- Martinez, P.; Blanco, M. Sensitivity of agricultural development to water-related drivers: The case of Andalusia (Spain). Water 2019, 11, 1854. [Google Scholar] [CrossRef]
- Tan, J.; Liu, F.; Xu, T.; Tan, X. Exploration of water–nitrogen coupling effects in paddy field based on ORYZA (v3) model. J. Sci. Food Agric. 2022, 102, 396–406. [Google Scholar] [CrossRef]
- Plett, D.C.; Ranathunge, K.; Melino, V.J.; Kuya, N.; Uga, Y.; Kronzucker, H.J. The intersection of nitrogen nutrition and water use in plants: New paths toward improved crop productivity. J. Exp. Bot. 2020, 71, 4452–4468. [Google Scholar] [CrossRef] [PubMed]
- Nie, T.Z.; Huang, J.Y.; Zhang, Z.X.; Chen, P.; Li, T.C.; Dai, C.L. The inhibitory effect of a water-saving irrigation regime on CH4 emission in Mollisols under straw incorporation for 5 consecutive years. Agric. Water Manag. 2023, 278, 108163. [Google Scholar] [CrossRef]
- Crevoisier, D.; Popova, Z.; Mailhol, J.C.; Ruelle, P. Assessment and simulation of water and nitrogen transfer under furrow irrigation. Agric. Water Manag. 2008, 95, 354–366. [Google Scholar] [CrossRef]
- Qiang, S.; Zhang, F.; Zhang, Y.; Yan, S.; Fan, J.; Xiang, Y. Nitrogen application affects grain yield by altering the soil moisture and nitrate-N of maize/wheat cropping system in dryland areas of northwest China. Irrig. Drain. 2021, 70, 16–26. [Google Scholar] [CrossRef]
- Cong, X.; Pang, G.Z.; Zhang, L.Z.; Xu, Z.H.; Yang, J.L.; Mou, X.Y. Effects of nitrogen-reducing and suitable water on photosynthetic characteristics of winter wheat and distribution of soil water and nitrogen. Trans. Chin. Soc. Agric. Mach. 2021, 52, 324–332. [Google Scholar] [CrossRef]
- Ahmad, I.; Wajid, S.A.; Ahmad, A.; Cheema, M.J.M.; Judge, J. Optimizing irrigation and nitrogen requirements for maize through empirical modeling in semi-arid environment. Environ. Sci. Pollut. Res. 2019, 26, 1227–1237. [Google Scholar] [CrossRef]
- Bahrami, M.; Talebnejad, R.; Sepaskhah, A.R.; Bazile, D. Irrigation regimes and nitrogen rates as the contributing factors in quinoa yield to increase water and nitrogen efficiencies. Plants 2022, 11, 2048. [Google Scholar] [CrossRef] [PubMed]
- García-López, J.; Lorite, I.J.; García-Ruiz, R.; Ordoñez, R.; Dominguez, J. Yield response of sunflower to irrigation and fertilization under semi-arid conditions. Agric. Water Manag. 2016, 176, 151–162. [Google Scholar] [CrossRef]
- Ren, J.Z.; Zhu, X.Y. The basic pattern of grassland agriculture in Hexi Corridor of China is contrary to its system-the mechanism of grassland degradation. Acta Prataculturae Sin. 1995, 4, 69–79. [Google Scholar]
- Kiss, R.; Deák, B.; Tóthmérész, B.; Miglécz, T.; Tóth, K.; Török, P.; Lukács, K.; Godó, L.; Körmöczi, Z.; Radócz, S.; et al. Establishment gaps in species-poor grasslands: Artificial biodiversity hotspots to support the colonization of target species. Restor. Ecol. 2021, 29, e13135. [Google Scholar] [CrossRef]
- Bao, G.; Lu, G.; Guo, J.; Bao, J. Effects of grazing, cutting and decapitating on grass populations on the artificial grassland in subtropical zone of China. J. Appl. Ecol. 2003, 14, 1327–1331. [Google Scholar] [PubMed]
- Tang, Z.X.; Yin, M.H.; Qi, G.P.; Kang, Y.X.; Ma, Y.L.; Wang, J.H.; Jia, Q.; Jiang, Y.B.; Wang, A.X. Effects of optimal irrigation and nitrogen coupling on Bromus inermis yield, nitrogen, phosphorus, and potassium stoichiometric characteristics. J. Plant Nutr. Fertil. 2022, 28, 532–545. [Google Scholar]
- Saeidnia, F.; Majidi, M.M.; Dehghani, M.R.; Mirlohi, A.; Araghi, B. Multi environmental evaluation of persistence and drought tolerance in smooth bromegrass (Bromus inermis): Genetic analysis for stability in combining ability. Crop Pasture Sci. 2021, 72, 565–574. [Google Scholar] [CrossRef]
- Wang, A.X.; Ma, Y.L.; Qi, G.P.; Kang, Y.X.; Yin, M.H.; Wang, J.H.; Jia, Q.; Tang, Z.X.; Jiang, Y.B. Water and nitrogen regulation patterns for productivity improvement of Bromus inermis and alfalfa mixed grassland. J. Soil Water Conserv. 2022, 36, 322–330. [Google Scholar] [CrossRef]
- Kang, W.Y.; Qi, G.P.; Kang, Y.X.; Yin, M.H.; Ma, Y.L.; Zhang, H.B. Influence of various irrigation schedules on yield and quality of grass-legume mixed artificial grassland. Water Resour. Plan. Des. 2020, 198, 103–109. [Google Scholar]
- Wang, L.; D’Odorico, P.; O’Halloran, L.R.; Caylor, K.; Macko, S. Combined effects of soil moisture and nitrogen availability variations on grass productivity in African savannas. Plant Soil 2010, 328, 95–108. [Google Scholar] [CrossRef]
- Lozano-Parra, J.; Schnabel, S.; Pulido, M.; Gómez-Gutiérrez, Á.; Lavado-Contador, F. Effects of soil moisture and vegetation cover on biomass growth in water-limited environments. Land Degrad. Dev. 2018, 29, 4405–4414. [Google Scholar] [CrossRef]
- von Haden, A.C.; Dornbush, M.E. Patterns of root decomposition in response to soil moisture best explain high soil organic carbon heterogeneity within a mesic, restored prairie. Agric. Ecosyst. Environ. 2014, 185, 188–196. [Google Scholar] [CrossRef]
- Tu, N.L.; Ba-Yin, Q.K.L.; Mo, R.G.; Cao, X.S. Soil moisture characteristics of different vegetation types in the Mu Us Sandyland. Yellow River 2020, 42, 63–64, 72. [Google Scholar] [CrossRef]
- Hou, Q.; Miao, B.L.; Wang, Y.S.; Dong, C.L. Effects of water stress on soil moisture in semiarid typical steppe. J. Arid. Meteorol. 2020, 38, 987–993. [Google Scholar] [CrossRef]
- Chen, J.; Song, N.P.; Chen, L.; Wang, X.; Wang, Q.X. Soil moisture dynamics and its response to precipitation in different cover types of desert steppe. J. Soil Water Conserv. 2021, 35, 198–206. [Google Scholar] [CrossRef]
- Lin, P.F.; Zhu, X.; He, Z.B.; Du, J.; Chen, L.F. Research progress on soil moisture temporal stability. Acta Ecol. Sin. 2018, 38, 3403–3413. [Google Scholar] [CrossRef]
- Yin, M.; Li, Y.; Li, H.; Xu, Y.; Zhou, C.; Zhang, T. Effects of nitrogen application rates on root growth and nitrogen use of summer maize. Trans. Chin. Soc. Agric. Mach. 2016, 47, 129–138. [Google Scholar] [CrossRef]
- Sahoo, S.; Mukhopadhyay, P.; Sinha, A.K.; Bhattacharya, P.M.; Rakesh, S.; Kumar, R.; Padbhushan, R.; Bijay, S.; Parmar, B.; Vishwakerma, A.; et al. Yield, nitrogen-use efficiency, and distribution of nitrate-nitrogen in the soil profile as influenced by irrigation and fertilizer nitrogen levels under zero-till wheat in the eastern Indo-Gangetic plains of India. Front. Environ. Sci. 2022, 10, 1329. [Google Scholar] [CrossRef]
- Rushimisha, I.E.; Wang, W.; Li, Y.; Li, X. Translocation of nitrate in rice rhizosphere and total nitrogen uptake improvement under interactive effect of water and nitrogen supply. Commun. Soil Sci. Plant Anal. 2023, 54, 378–391. [Google Scholar] [CrossRef]
- Oliveira, H.C.; Sodek, L. Effect of oxygen deficiency on nitrogen assimilation and amino acid metabolism of soybean root segments. Amino Acids 2013, 44, 743–755. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.L.; Tian, C.; Zhang, L.; Huang, J.; Zhu, L.F.; Zhang, J.H.; Cao, X.C.; Jin, Q.Y. Research advance in the roles of water-nitrogen-oxygen factors in mediating rice growth, photosynthesis and nitrogen utilization in paddy soils. Chin. J. Appl. Ecol. 2021, 32, 1498–1508. [Google Scholar] [CrossRef]
- Li, Z.P.; Song, M.D.; Fen, H. Dynamic characteristics of leaf area index and plant height of winter wheat influenced by irrigation and nitrogen coupling and their relationships with yield. Trans. Chin. Soc. Agric. Eng. 2017, 33, 195–202. [Google Scholar] [CrossRef]
- Wang, J.H.; Li, G.; Yin, M.H.; Qi, G.P.; Kang, Y.X.; Ma, Y.L. Effects of regulated deficit irrigation on the soil environment and forage growth of mixed-species forage plantings in China’s high-cold desert area. Acta Prataculturae Sin. 2022, 31, 95–106. [Google Scholar] [CrossRef]
- Fessehazion, M.K.; Annandale, J.G.; Everson, C.S.; Stirzaker, R.J.; Tesfamariam, E.H. Evaluating of soil water balance (SWB-Sci) model for water and nitrogen interactions in pasture: Example using annual ryegrass. Agric. Water Manag. 2014, 146, 238–248. [Google Scholar] [CrossRef]
- Sha, B.P.; Xie, Y.Z.; Gao, X.Q.; Cai, W.; Fu, B.Z. Effects of coupling of drip irrigation water and fertilizer on yield and quality of alfalfa in the yellow river irrigation district. Acta Prataculturae Sin. 2021, 30, 102–114. [Google Scholar] [CrossRef]
- Zheng, Y.M.; Zhou, L.M.; Zheng, Y.P.; Wu, Z.F.; Sun, X.W.; Ding, T.Y.; Shen, P.; Wang, C.B. Relationship between carbon metabolism indices of peanut leaves and nitrogen fixation ability of nodules. J. Plant Nutr. Fertil. 2021, 27, 75–86. [Google Scholar] [CrossRef]
- Muhammad, I.; Yang, L.; Ahmad, S.; Farooq, S.; Al-Ghamdi, A.A.; Khan, A.; Zeeshan, M.; Elshikh, M.S.; Abbasi, A.M.; Zhou, X.B. Nitrogen fertilizer modulates plant growth, chlorophyll pigments and enzymatic activities under different irrigation regimes. Agronomy 2022, 12, 845. [Google Scholar] [CrossRef]
- Kochsiek, A.; Ciganda, V.; Bryan, N.; Hite, L.; Awada, T. Ecophysiological responses of Schizachyrium scoparium to water and nitrogen manipulations. Great Plains Res. 2006, 16, 29–36. [Google Scholar]
- Ge, Y.Q.; Yan, Y.L.; Liang, Y.; Gan-Zhu, Z.B.; Hu, G.Z.; Yang, J.; Gao, Z.Q.; He, S.C.; Dan-Jiu, L.B. The effects of nitrogen deposition on soil respiration in an Alpine Meadow in Northern Tibet. Chin. J. Agrometeorol. 2019, 40, 214–221. [Google Scholar] [CrossRef]
- Kumar, R.; Pareek, N.K.; Kumar, U.; Javed, T.; Al-Huqail, A.A.; Rathore, V.S.; Nangia, V.; Choudhary, A.; Nanda, G.; Ali, H.M.; et al. Coupling effects of nitrogen and irrigation levels on growth attributes, nitrogen use efficiency, and economics of cotton. Front. Plant Sci. 2022, 13, 890181. [Google Scholar] [CrossRef]
- Al-Solaimani, S.G.; Alghabari, F.; Ihsan, M.Z.; Fahad, S. Water deficit irrigation and nitrogen response of Sudan grass under arid land drip irrigation conditions. Irrig. Drain. 2017, 66, 365–376. [Google Scholar] [CrossRef]
- Saravia, D.; Farfán-Vignolo, E.R.; Gutiérrez, R.; De Mendiburu, F.; Schafleitner, R.; Bonierbale, M.; Khan, M.A. Yield and physiological response of potatoes indicate different strategies to cope with drought stress and nitrogen fertilization. Am. J. Potato Res. 2016, 93, 288–295. [Google Scholar] [CrossRef]
- Dell, E.A.; Carley, D.S.; Rufty, T.; Shi, W. Heat stress and N fertilization affect soil microbial and enzyme activities in the creeping bentgrass (Agrostis stolonifera L.) rhizosphere. Appl. Soil Ecol. 2012, 56, 19–26. [Google Scholar] [CrossRef]



| Treatment | Water Use Efficiency (WUE, kg·m−3) | Partial-Factor Productivity of Applied Nitrogen (PFPN, kg·kg−1) | Treatment | Water Use Efficiency (WUE, kg·m−3) | Partial-Factor Productivity of Applied Nitrogen (PFPN, kg·kg−1) | |||
|---|---|---|---|---|---|---|---|---|
| N0W0 | 1.06 ± 0.03 Cc | - | N2W0 | 1.72 ± 0.10 Ca | 105.03 ± 4.74 Ab | |||
| N0W1 | 1.41 ± 0.06 Ad | - | N2W1 | 2.20 ± 0.04 Aa | 107.69 ± 2.51 Ab | |||
| N0W2 | 1.43 ± 0.08 Ad | - | N2W2 | 2.31 ± 0.05 Aa | 96.81 ± 1.69 Bb | |||
| N0W3 | 1.26 ± 0.10 Bc | - | N2W3 | 1.89 ± 0.09 Ba | 69.97 ± 1.92 Cb | |||
| N1W0 | 1.34 ± 0.12 Cb | 158.92 ± 9.43 Ba | N3W0 | 1.62 ± 0.01 Ba | 68.11 ± 0.87 Ac | |||
| N1W1 | 1.81 ± 0.09 Ac | 171.52 ± 5.42 Aa | N3W1 | 2.06 ± 0.06 Ab | 69.20 ± 1.28 Ac | |||
| N1W2 | 1.79 ± 0.03 Ac | 139.14 ± 3.56 Ca | N3W2 | 2.08 ± 0.05 Ab | 59.33 ± 1.18 Bc | |||
| N1W3 | 1.58 ± 0.09 Bb | 110.52 ± 6.27 Da | N3W3 | 1.73 ± 0.10 Bab | 43.77 ± 2.80 Cc | |||
| Test of variance of significance | ||||||||
| Index | N | W | N × W | |||||
| WUE | 108.021 ** | 35.696 ** | 0.956 ns | |||||
| PFPN | 282.156 ** | 38.345 ** | 3.274 * | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, Y.; Yin, M.; Ma, Y.; Tang, Z.; Jia, Q.; Qi, G.; Wang, J.; Jiang, Y.; Wang, A. Response of Water-Nitrogen Distribution and Use to Water Deficit under Different Applied Nitrogen Fertilizer Rates in Bromus inermis Grassland. Agronomy 2023, 13, 745. https://doi.org/10.3390/agronomy13030745
Kang Y, Yin M, Ma Y, Tang Z, Jia Q, Qi G, Wang J, Jiang Y, Wang A. Response of Water-Nitrogen Distribution and Use to Water Deficit under Different Applied Nitrogen Fertilizer Rates in Bromus inermis Grassland. Agronomy. 2023; 13(3):745. https://doi.org/10.3390/agronomy13030745
Chicago/Turabian StyleKang, Yanxia, Minhua Yin, Yanlin Ma, Zhongxia Tang, Qiong Jia, Guangping Qi, Jinghai Wang, Yuanbo Jiang, and Aixia Wang. 2023. "Response of Water-Nitrogen Distribution and Use to Water Deficit under Different Applied Nitrogen Fertilizer Rates in Bromus inermis Grassland" Agronomy 13, no. 3: 745. https://doi.org/10.3390/agronomy13030745
APA StyleKang, Y., Yin, M., Ma, Y., Tang, Z., Jia, Q., Qi, G., Wang, J., Jiang, Y., & Wang, A. (2023). Response of Water-Nitrogen Distribution and Use to Water Deficit under Different Applied Nitrogen Fertilizer Rates in Bromus inermis Grassland. Agronomy, 13(3), 745. https://doi.org/10.3390/agronomy13030745










