Accurate Phenotypic Identification and Genetic Analysis of the Ear Leaf Veins in Maize (Zea mays L.)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Leaf Vein CT Image Acquisition and Image Reconstruction
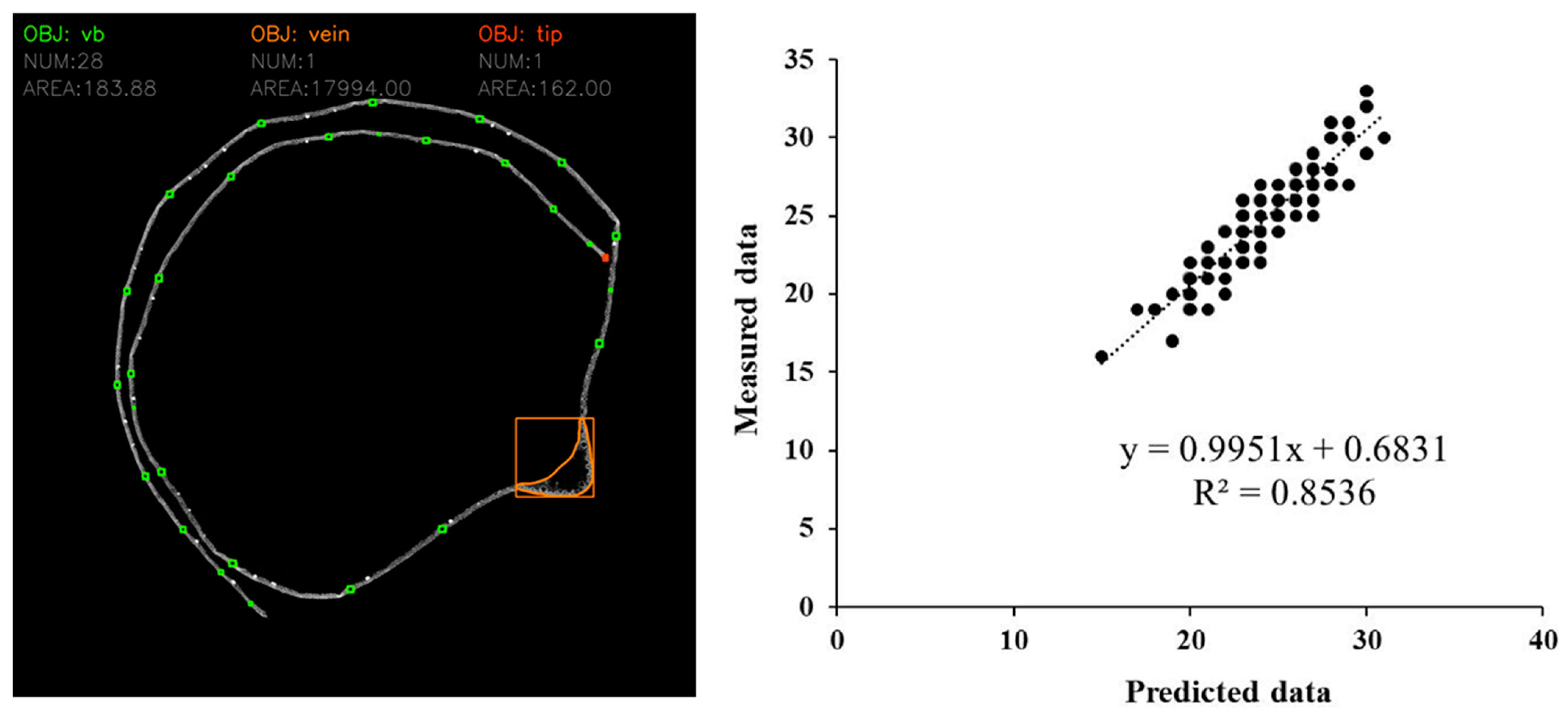
2.3. Accurate Analysis of Leaf Veins’ Microscopic Phenotypes
2.4. Phenotypic Data Analysis
2.5. Genome-Wide Association Studies
3. Results
3.1. Phenotypic Analysis of Ear Leaf Veins
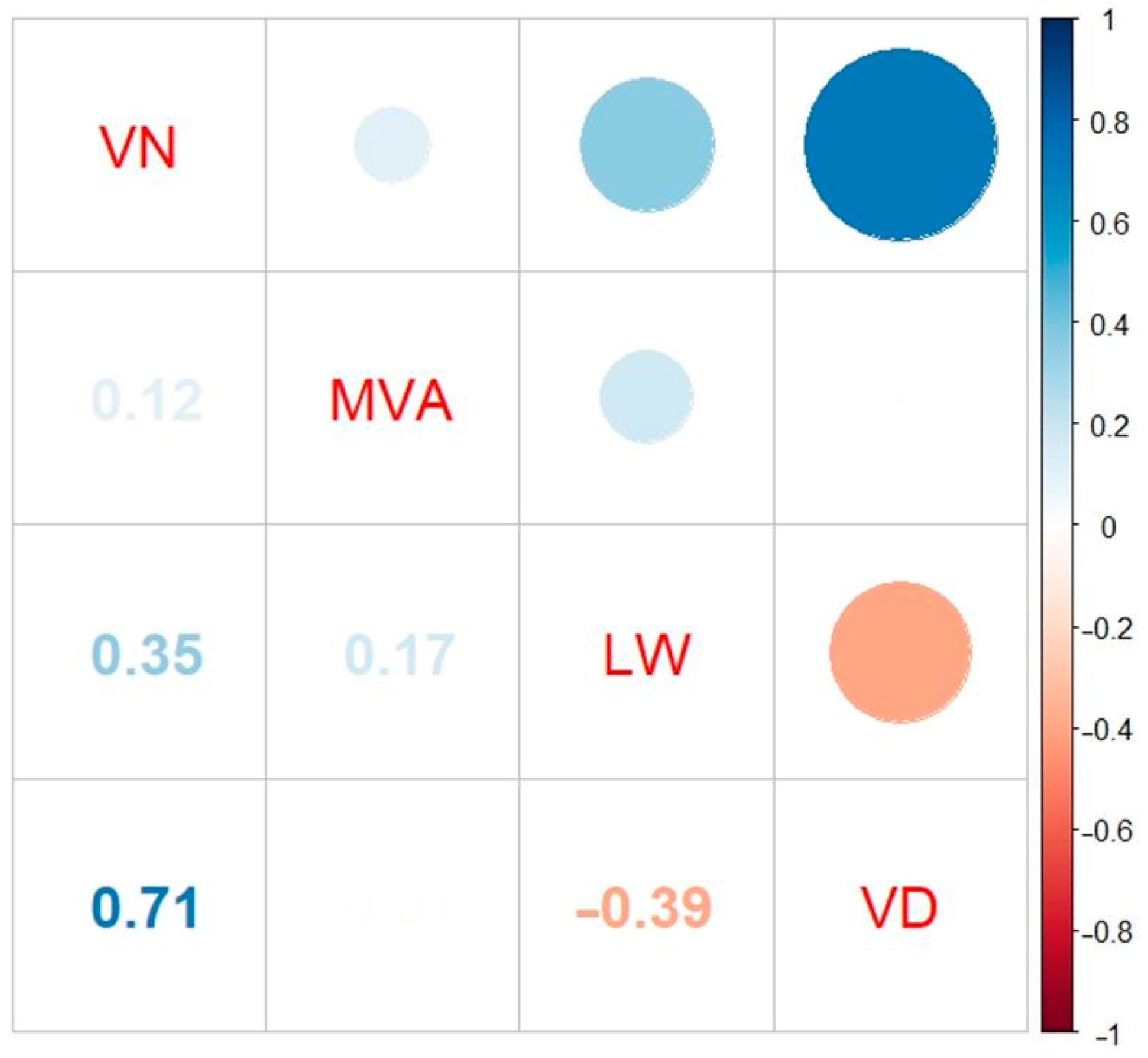
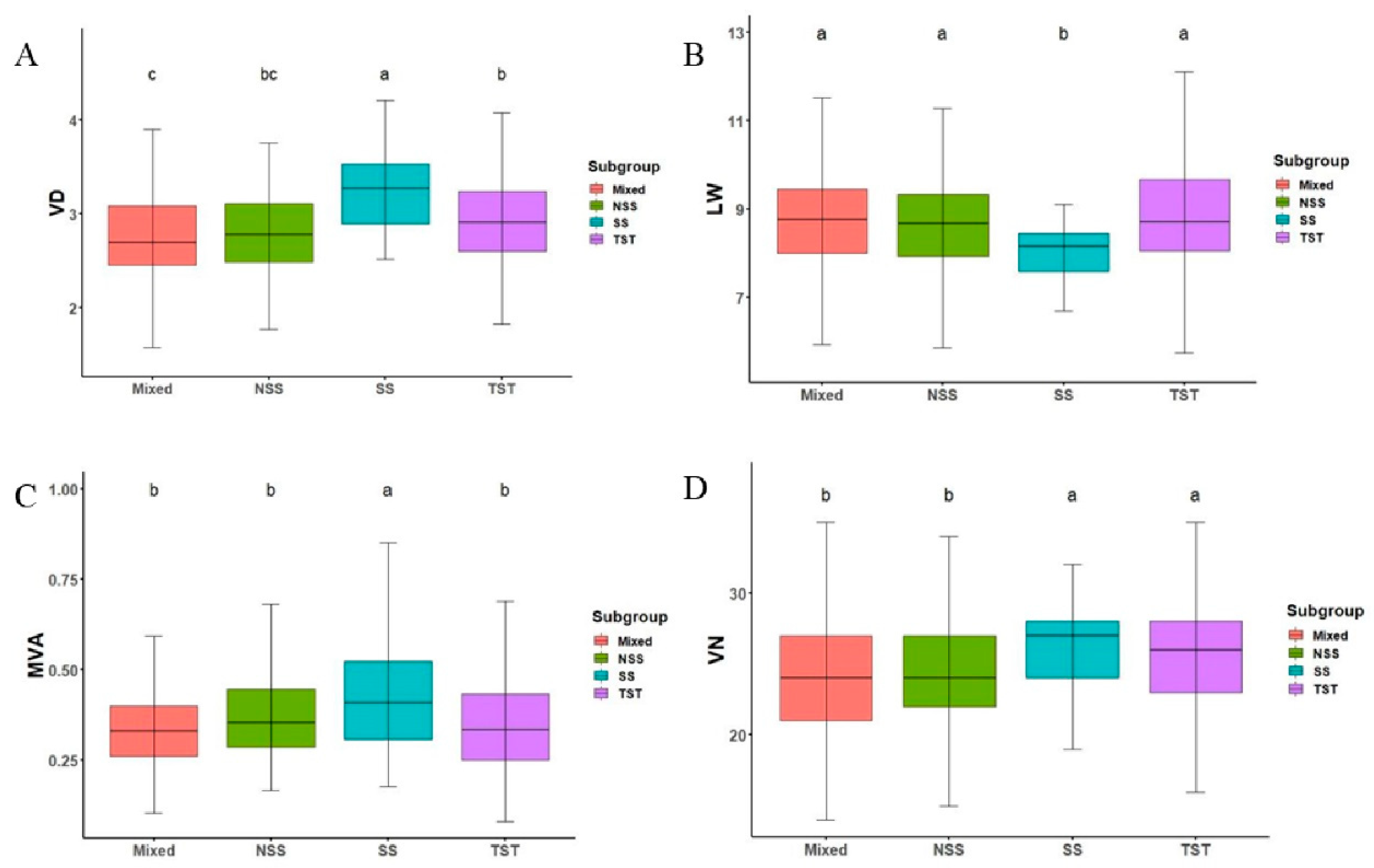
3.2. Phenotypic Variations in Maize Ear Leaf Veins among 300 Inbred Lines
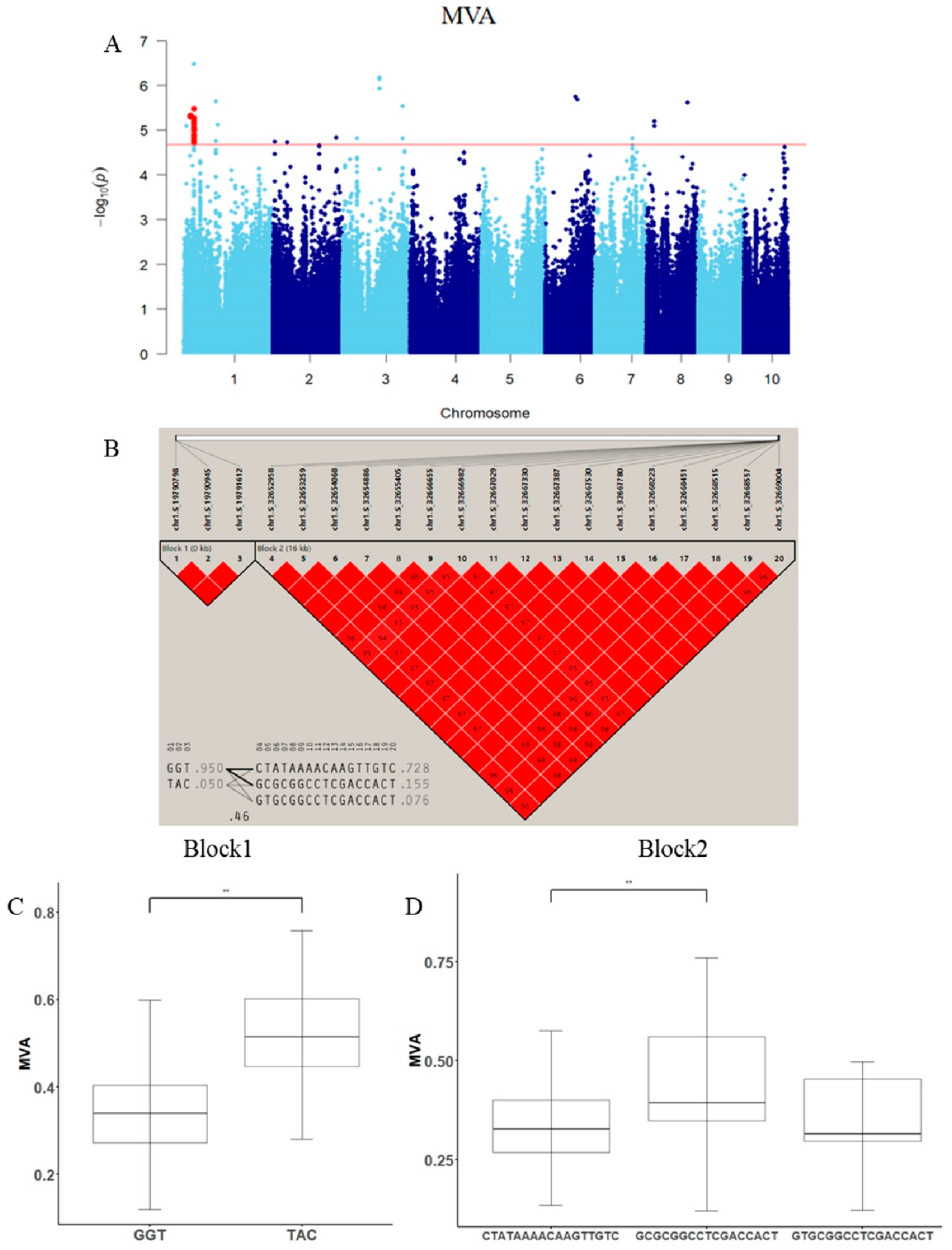
3.3. Genome-Wide Association Study of Leaf Veins’ Phenotypic Traits
4. Discussion
4.1. Advanced Methods for Acquiring and Resolving Microscopic Images of Leaf Vein Phenotypes
4.2. Phenotypic Traits of Ear Leaf Veins
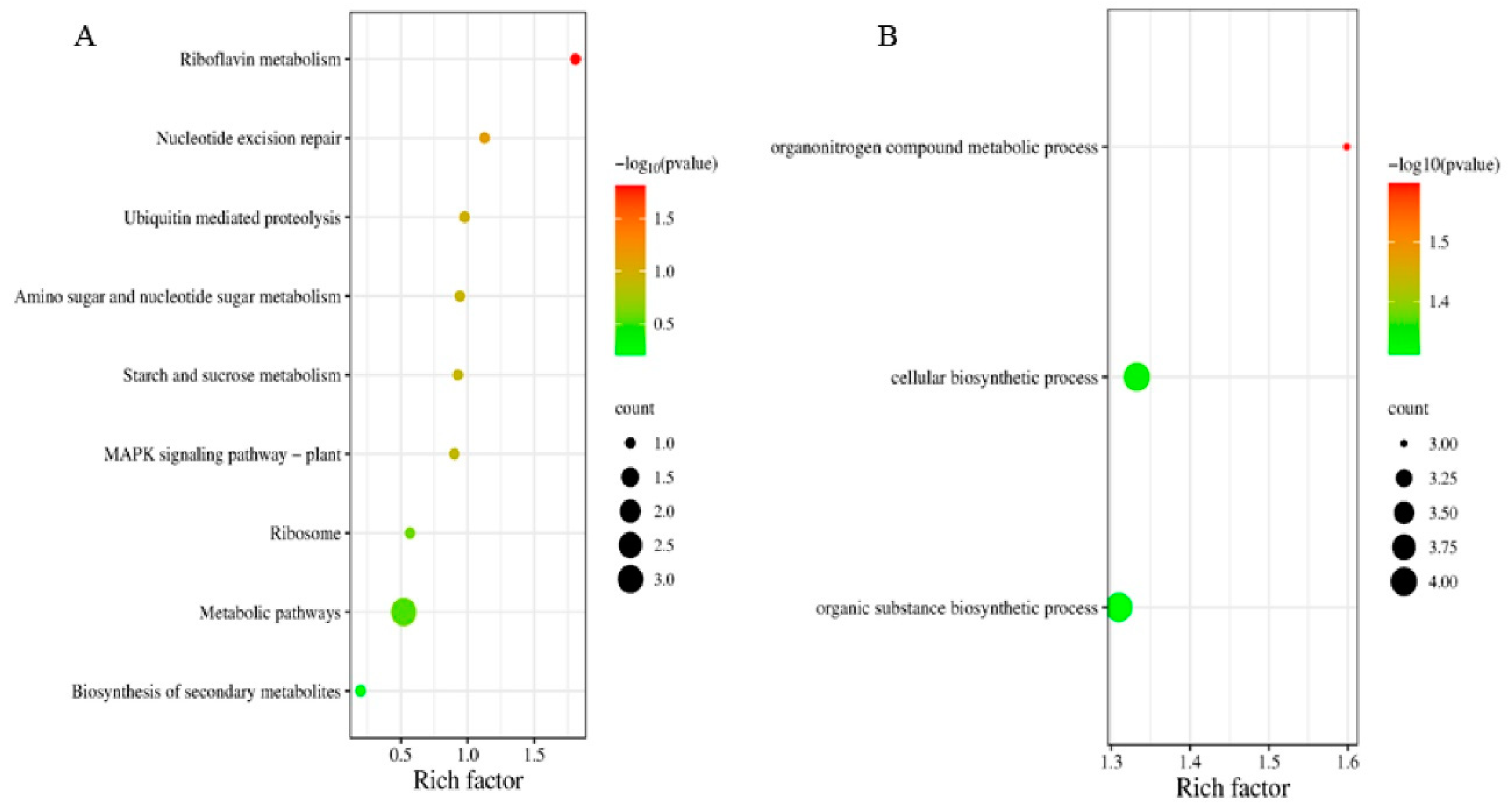
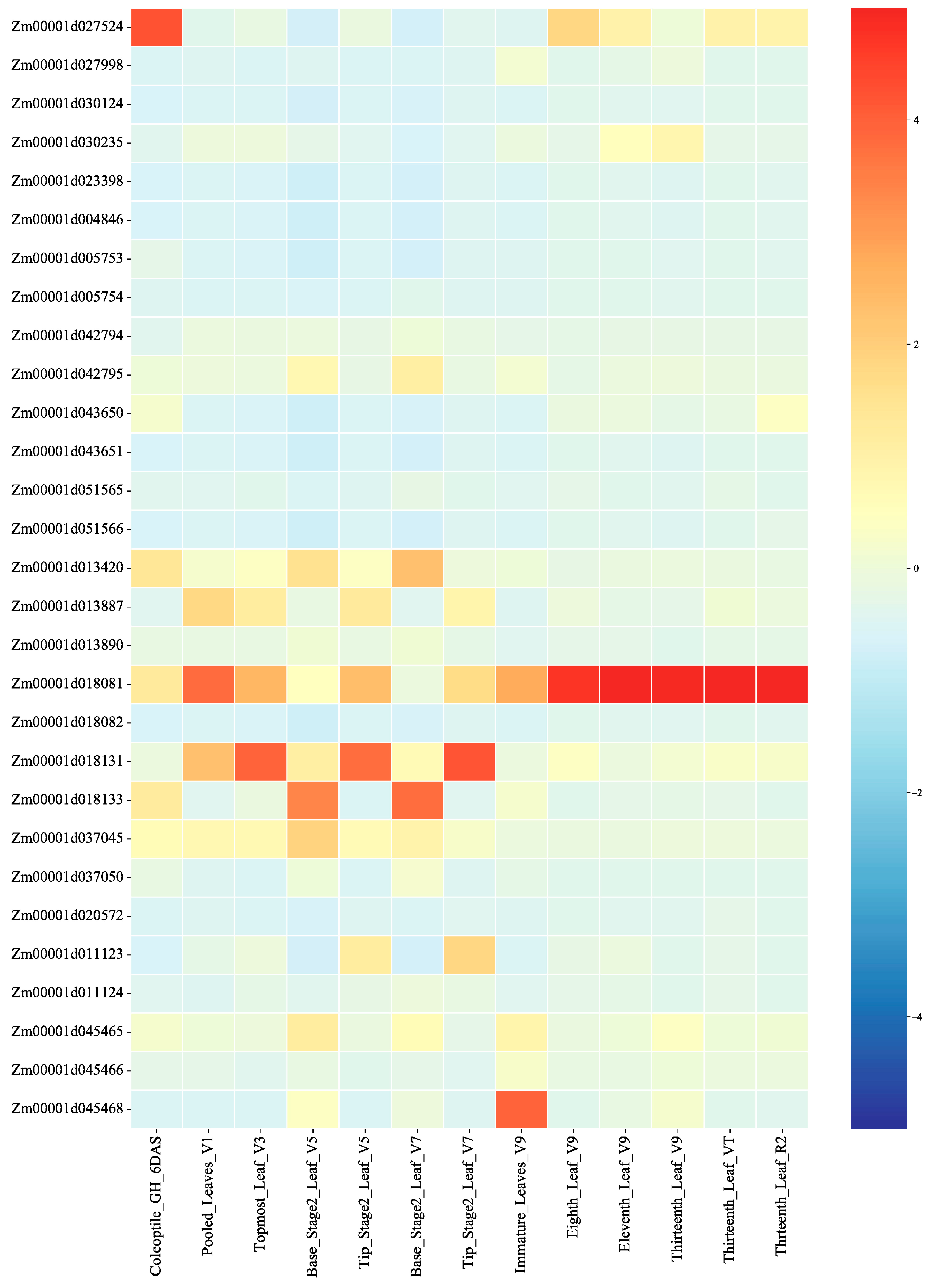
4.3. Analysis of Candidate Genes for Leaf Veins’ Phenotypic Traits
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, Y.; Wang, J.L.; Du, J.J.; Zhao, Y.X.; Lu, X.J.; Wen, W.; Gu, S.H.; Fan, J.; Wang, C.; Wu, S.; et al. Dissecting the phenotypic components and genetic architecture of maize stem vascular bundles using high-throughput phenotypic analysis. Plant Biotechnol. J. 2021, 19, 35–50. [Google Scholar] [CrossRef]
- Guo, S.; Zhou, G.; Wang, J.; Lu, X.; Zhao, H.; Zhang, M.; Guo, X.; Zhang, Y. High-Throughput Phenotyping Accelerates the Dissection of the Phenotypic Variation and Genetic Architecture of Shank Vascular Bundles in Maize (Zea mays L.). Plants 2022, 11, 1339. [Google Scholar] [CrossRef]
- Yang, H.W.; Chai, Q.; Yin, W.; Hu, F.L.; Qin, A.Z.; Fan, Z.L.; Yu, A.Z.; Zhao, C.; Fan, H. Yield photosynthesis and leaf anatomy of maize in inter- and mono-cropping systems at varying plant densities. Crop. J. 2022, 10, 893–903. [Google Scholar] [CrossRef]
- Zhang, Z.; Zheng, Y.; Ham, B.K.; Chen, J.; Lucas, W.J. Vascular-mediated signalling involved in early phosphate stress response in plants. Nat. Plants 2016, 2, 16033. [Google Scholar] [CrossRef]
- Moulia, B.; Fournier, M. Mechanics of the maize leaf: A composite beam model of the midrib. J. Mater. Sci. 1997, 32, 2771–2780. [Google Scholar] [CrossRef]
- Laguna, M.F.; Bohn, S.; Jagla, E.A. The role of elastic stresses on leaf venation morphogenesis. PLoS Comput. Biol. 2008, 4, e1000055. [Google Scholar] [CrossRef] [Green Version]
- Jonsson, K.; Hamant, O.; Bhalerao, R.P. Plant cell walls as mechanical signaling hubs for morphogenesis. Curr. Biol. 2022, 32, R334–R340. [Google Scholar] [CrossRef] [PubMed]
- Whittenberger, R.T.; Naghski, J. Separation and mounting of leaf vein skeletons and epidermis. Am. J. Bot. 1948, 35, 719–722. [Google Scholar] [CrossRef]
- Lundgren, M.R.; Osborne, C.P.; Christin, P.A. Deconstructing Kranz anatomy to understand C4 evolution. J. Exp. Bot. 2014, 65, 3357–3369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Langdale, A.J. C4 cycles: Past, present, and future research on C4 photosynthesis. Plant Cell 2011, 23, 3879–3892. [Google Scholar] [CrossRef] [Green Version]
- Sage, R.F.; Roxana, K.; Sage, T.L. From proto-Kranz to C4 Kranz: Building the bridge to C4 photosynthesis. J. Exp. Bot. 2014, 65, 3341–3356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blonder, B.; Carlo, F.D.; Moore, J.; Rivers, M.; Enquist, B.J. X-ray imaging of leaf venation networks. New Phytol. 2012, 196, 1274–1282. [Google Scholar] [CrossRef]
- Kaminuma, E.; Yoshizumi, T.; Wada, T.; Matsui, M.; Toyoda, T. Quantitative analysis of heterogeneous spatial distribution of Arabidopsis leaf trichomes using micro X-ray computed tomography. Plant J. 2008, 56, 470–482. [Google Scholar] [CrossRef] [PubMed]
- Dhondt, S.; Vanhaeren, H.; Loo, D.V.; Cnudde, V.; Inzé, D. Plant structure visualization by high-resolution X-ray computed tomography. Trends Plant Sci. 2010, 15, 419–422. [Google Scholar] [CrossRef]
- Borsuk, A.M.; Roddy, A.B.; Théroux-Rancourt, G.; Brodersen, C.R. Structural organization of the spongy mesophyll. New Phytol. 2022, 234, 946–960. [Google Scholar] [CrossRef]
- Pajor, R.; Fleming, A.; Osborne, C.P.; Rolfe, S.A.; Sturrock, C.J.; Mooney, S.J. Seeing space: Visualization and quantification of plant leaf structure using X-ray micro-computed tomography. J. Exp. Bot. 2013, 64, 385–390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jankovsky, J.P.; Smith, L.G.; Nelson, T. Specification of bundle sheath cell fates during maize leaf development: Roles of lineage and positional information evaluated through analysis of the tangled1 mutant. Development 2001, 128, 2747–2753. [Google Scholar] [CrossRef] [PubMed]
- Michela, L.; Giuseppe, G.; Nicoletta, R.; Francesca, D.V.; Gabriella, C.; Silvana, D. A maize mutant with an altered vascular pattern. Ann. Bot. 2000, 85, 143–150. [Google Scholar] [CrossRef] [Green Version]
- Schneeberger, R.; Tsiantis, M.; Freeling, M.; Langdale, J.A. The rough sheath2 gene negatively regulates homeobox gene expression during maize leaf development. Development 1998, 125, 2857–2865. [Google Scholar] [CrossRef] [PubMed]
- Vollbrecht, E.; Veit, B.; Sinha, N.; Hake, S. The developmental gene Knotted-1 is a member of a maize homeobox gene family. Nature 1991, 350, 241–243. [Google Scholar] [CrossRef]
- Becraft, P.W.; Freeling, M. Genetic analysis of Rough sheath1 developmental mutants of maize. Genetics 1994, 136, 295–311. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.H.; Yoo, S.C.; Zhang, H.; Pandeya, D.; Koh, H.J.; Hwang, J.Y.; Kim, G.T.; Paek, N.C. The rice narrow leaf2 and narrow leaf3 loci encode WUSCHEL-related homeobox 3A (OsWOX3A) and function in leaf, spikelet, tiller and lateral root development. New Phytol. 2013, 198, 1071–1084. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Douglas, R.N.; Strable, J.; Lee, M.; Buckner, B.; Janick-Buckner, D.; Schnable, P.S.; Scanlon, T.M. Punctate vascular expression1 is a novel maize gene required for leaf pattern formation that functions downstream of the trans-acting small interfering RNA pathway. Plant Physiol. 2012, 159, 1453–1462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slewinski, T.L.; Anderson, A.A.; Zhang, C.; Turgeon, R. Scarecrow plays a role in establishing Kranz anatomy in maize leaves. Plant Cell Physiol. 2012, 53, 2030–2037. [Google Scholar] [CrossRef] [Green Version]
- Slewinski, T.L.; Anderson, A.A.; Price, S.; Withee, J.R.; Gallagher, K.; Turgeon, R. Short-root1 plays a role in the development of vascular tissue and kranz anatomy in maize leaves. Mol. Plant 2014, 7, 1388–1392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dhondt, S.; Coppens, F.; Winter, F.D.; Swarup, K.; Merks, R.; Inze´, D.; Bennett, M.J.; Beemster, G. SHORT-ROOT and SCARECROW regulate leaf growth in Arabidopsis by stimulating S-phase progression of the cell cycle. Plant Physiol. 2010, 154, 1183–1195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, D.; Zhao, J.; Jing, Y.; Fan, M.; Liu, J.; Wang, Z.; Xin, W.; Hu, Y. The arabidopsis IDD14, IDD15, and IDD16 cooperatively regulate lateral organ morphogenesis and gravitropism by promoting auxin biosynthesis and transport. PLoS Genet. 2013, 9, e1003759. [Google Scholar] [CrossRef] [Green Version]
- Mir, R.R.; Reynolds, M.; Pinto, F.; Khan, M.A.; Bhat, M.A. High-throughput phenotyping for crop improvement in the genomics era. Plant Sci. 2019, 282, 60–72. [Google Scholar] [CrossRef]
- Xiao, Q.; Bai, X.; Zhang, C.; He, Y. Advanced high-throughput plant phenotyping techniques for genome-wide association studies: A review. J. Adv. Res. 2021, 35, 215–230. [Google Scholar] [CrossRef]
- Liu, L.L. Genome-Wide Association Analysis for Genetic Basis of Veins in Maize. Master’s Thesis, Shenyang Agricultural University, Shenyang, China, 2019. [Google Scholar]
- Tian, F.; Bradbury, P.J.; Brown, P.J.; Hung, H.; Sun, Q.; Flint-Garcia, S.; Rocheford, T.R.; McMullen, M.D.; Holland, J.B.; Buckler, E.S. Genome-wide association study of leaf architecture in the maize nested association mapping population. Nat. Genet. 2011, 43, 159–162. [Google Scholar] [CrossRef]
- Peng, B.; Zhao, X.; Wang, Y.; Li, C.; Wang, T. Genome-wide association studies of leaf angle in maize. Mol. Breed. 2021, 41, 1–18. [Google Scholar] [CrossRef]
- Yang, X.; Gao, S.; Xu, S.; Zhang, Z.; Prasanna, B.M.; Li, L.; Li, J.; Yan, J. Characterization of a global germplasm collectionand its potential utilization for analysis of complexquantitative traits in maize. Mol. Breed. 2011, 28, 511–526. [Google Scholar] [CrossRef]
- Sack, L.; Scoffoni, C.; McKown, A.D.; Frole, K.; Rawls, M.; Havran, J.C.; Tran, H.; Tran, T. Developmentally based scaling of leaf venation architecture explains global ecological patterns. Nat. Commun. 2012, 3, 837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Du, J.; Guo, X.; Yan, W.; Zhao, C. Three-dimensional visualization of vascular bundles in stem nodes of maize. Fresenius Environ. Bull. 2017, 26, 3395–3401. [Google Scholar]
- Marsch-Martinez, N.; Greco, R.; Becker, J.D.; Dixit, S.; Bergervoet, J.; Karaba, A.; Folter, S.; Pereira, A. BOLITA, an Arabidopsis AP2/ERF-like transcription factor that affects cell expansion and proliferation/differentiation pathways. Plant Mol. Biol. 2006, 62, 825–843. [Google Scholar] [CrossRef] [Green Version]
- Zhao, C.; Zayed, O.; Yu, Z.; Jiang, W.; Zhu, P.; Hsu, C.; Zhang, L.; Tao, W.A.; Lozano-Durán, R.; Zhu, J. Leucine-rich repeat extensin proteins regulate plant salt tolerance in Arabidopsis. Proc. Natl. Acad. Sci. USA 2018, 115, 13123–13128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tabassum, M.A.; Zhu, G.; Hafeez, A.; Wahid, M.A.; Shaban, M.; Li, Y. Influence of leaf vein density and thickness on hydraulic conductance and photosynthesis in rice (Oryza sativa L.) during water stress. Sci. Rep. 2016, 6, 36894. [Google Scholar] [CrossRef] [Green Version]
- Pierret, A.; Capowiez, Y.; Moran, C.J.; Kretzschmar, A. X-ray computed tomography to quantify tree rooting spatial distributions. Geoderma 1999, 90, 307–326. [Google Scholar] [CrossRef]
- Heeraman, D.A.; Hopmans, J.W.; Clausnitzer, V. Three dimensional imaging of plant roots in situ with X-ray Computed Tomography. Plant Soil 1997, 189, 167–179. [Google Scholar] [CrossRef]
- Du, J.; Zhang, Y.; Guo, X.; Ma, L.; Shao, M.; Pan, X.; Zhao, C. Micron-scale phenotyping quantification and three-dimensional microstructure reconstruction of vascular bundles within maize stalks based on micro-CT scanning. Funct. Plant Biol. 2016, 44, 10–22. [Google Scholar] [CrossRef]
- Huang, C.; Chen, Q.; Xu, G.; Xu, D.; Tian, J.; Tian, F. Identification and fine mapping of quantitative trait loci for the number of vascular bundle in maize stem. J. Integr. Plant Biol. 2016, 58, 81–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sack, L.; Scoffoni, C. Leaf venation: Structure, function, development, evolution, ecology and applications in the past, present and future. New Phytol. 2013, 198, 983–1000. [Google Scholar] [CrossRef] [PubMed]
- Blonder, B.; Violle, C.; Bentley, L.P.; Enquist, B.J. Venation networks and the origin of the leaf economics spectrum. Ecol. Lett. 2011, 14, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Vermerris, W.; Sherman, D.M.; Mcintyre, L.M. Phenotypic plasticity in cell walls of maize brown midrib mutants is limited by lignin composition. J. Exp. Bot. 2010, 61, 2479–2490. [Google Scholar] [CrossRef]
- Westgate, M.E.; Steudle, E. Water transport in the midrib tissue of maize leaves: Direct measurement of the propagation of changes in cell turgor across a plant tissue. Plant Physiol. 2019, 78, 183–191. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.; Yu, C.; Wu, Y.; Lu, M.J.; Tu, S.; Wu, S.; Shiu, S.; Ku, M.; Li, W. Elevated auxin biosynthesis and transport underlie high vein density in C₄ leaves. Proc. Natl. Acad. Sci. USA 2017, 114, E6884–E6891. [Google Scholar] [CrossRef] [Green Version]
- Kumar, D.; Kellogg, E.A. Getting closer: Vein density in C4 leaves. New Phytol. 2019, 221, 1260–1267. [Google Scholar] [CrossRef] [Green Version]
- Nelson, T.; Dengler, N. Leaf Vascular Pattern Formation. Plant Cell 1997, 9, 1121–1135. [Google Scholar] [CrossRef] [Green Version]
- Linh, N.M.; Verna, C.; Scarpella, E. Coordination of cell polarity and the patterning of leaf vein networks. Curr. Opin. Plant Biol. 2018, 41, 116–124. [Google Scholar] [CrossRef]
- Evert, R.F.; Eschrich, W.; Heyser, W. Distribution and structure of the plasmodesmata in mesophyll and bundle-sheath cells of Zea mays L. Planta 1977, 136, 77–89. [Google Scholar] [CrossRef]
- Evert, R.F.; Eschrich, W.; Heyser, W. Leaf structure in relation to solute transport and phloem loading in Zea mays L. Planta 1978, 138, 279–294. [Google Scholar] [CrossRef]
- Heyser, W.; Evert, R.F.; Eschrich, F.W. Sucrose in the free space of translocating maize leaf bundles. Plant Physiol. 1978, 62, 491–494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, G.; Zhang, X.; Duan, H.; Gao, J.; Li, N.; Su, P.; Xie, H.; Li, W.; Fu, Z.; Huang, Y.; et al. Dissection of the genetic architecture of peduncle vascular bundle-related traits in maize by a genome-wide association study. Plant Biotechnol. J. 2022, 20, 1042–1053. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Lu, Y.; Yang, X.; Huang, J.; Zhou, Y.; Ali, F.; Wen, W.; Liu, J.; Li, J.; Yan, J. Genome wide association studies using a new nonparametric model reveal the genetic architecture of 17 agronomic traits in an enlarged maize association panel. PLoS Genet. 2014, 10, e1004573. [Google Scholar] [CrossRef] [Green Version]
- Macovei, A.; Vaid, N.; Tula, S.; Tuteja, N. A new DEAD-box helicase ATP-binding protein (OsABP) from rice is responsive to abiotic stress. Plant Signal. Behav. 2012, 7, 1138–1143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, G.; Li, X.; Ye, N.; Huang, M.; Feng, L.; Li, H.; Zhang, J. OsTPP1 regulates seed germination through the crosstalk with abscisic acid in rice. New Phytol. 2021, 230, 1925–1939. [Google Scholar] [CrossRef]
- Kanno, Y.; Kamiya, Y.; Seo, M. Nitrate does not compete with abscisic acid as a substrate of AtNPF4.6/NRT1.2/AIT1 in Arabidopsis. Plant Signal. Behav. 2013, 8, e26624. [Google Scholar] [CrossRef] [Green Version]
- Shao, A.; Ma, W.; Zhao, X.; Hu, M.; He, X. The auxin biosynthetic tryptophan aminotransferase related tatar2.1-3a increases grain yield of wheat. Plant Physiol. 2017, 174, 2274–2288. [Google Scholar] [CrossRef] [Green Version]
- Liang, X.; Qin, L.; Liu, P.; Wang, M.; Ye, H. Genes for iron-sulphur cluster assembly are targets of abiotic stress in rice, Oryza sativa. Plant Cell Environ. 2014, 37, 780–794. [Google Scholar] [CrossRef]
- Bouard, W.; Houde, M. The C2H2 zinc finger protein TaZFP13D increases drought stress tolerance in wheat. Plant Stress 2022, 6, 100119. [Google Scholar] [CrossRef]
- Chen, F.; Ji, X.; Bai, M.; Zhuang, Z.; Peng, Y. Network analysis of different exogenous hormones on the regulation of deep sowing tolerance in maize seedlings. Front. Plant Sci. 2021, 12, 2843. [Google Scholar] [CrossRef] [PubMed]
- Xia, S.; Xiao, L.; Gannon, P.; Li, X. RFC3 regulates cell proliferation and pathogen resistance in Arabidopsis. Plant Signal. Behav. 2010, 5, 168–170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Breton, C.; Fournel-Gigleux, S.; Palcic, M.M. Recent structures, evolution and mechanisms of glycosyltransferases. Curr. Opin. Struct. Biol. 2012, 22, 540–549. [Google Scholar] [CrossRef]
- Moriyama, E.N.; Strope, P.K.; Opiyo, S.O.; Chen, Z.; Jones, A.M. Mining the Arabidopsis thaliana genome for highly-divergent seven transmembrane receptors. Genome Biol. 2006, 7, R96. [Google Scholar] [CrossRef] [Green Version]
- Anitha, V.; Panneerselvam, V.; Arun Kuma, V.; Ram, R. The Arabidopsis ABHD11 mutant accumulates polar lipids in leaves as a consequence of absent acylhydrolase activity. Plant Physiol. 2016, 170, 180–193. [Google Scholar] [CrossRef] [Green Version]
- Mizoi, J.; Shinozaki, K.; Yamaguchi-Shinozaki, K. AP2/ERF family transcription factors in plant abiotic stress responses. BBA—Gene Regul. Mech. 2012, 1819, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liao, J.; Ling, Q.; Xi, Y.; Qian, Y. Genome-wide identification and expression profiling analysis of maize AP2/ERF superfamily genes reveal essential roles in abiotic stress tolerance. BMC Genom. 2022, 23, 125. [Google Scholar] [CrossRef] [PubMed]
- Yaish, M.W.; Peng, M.; Rothstein, S.J. AtMBD9 modulates Arabidopsis development through the dual epigenetic pathways of DNA methylation and histone acetylation. Plant J. Cell Mol. Biol. 2009, 59, 123–135. [Google Scholar] [CrossRef] [PubMed]
- David, S.; Christopher, W.; Philippe, L.; Berardini, T.Z.; Margarita, G.H.; Hartmut, F.; Li, D.; Tom, M.; Robert, M.; Larry, P. The Arabidopsis Information Resource (TAIR): Gene structure and function annotation. Nucleic Acids Res. 2008, 36, D1009–D1014. [Google Scholar] [CrossRef]
- Weber, H.; Hellmann, H. Arabidopsis thaliana BTB/ POZ-MATH proteins interact with members of the ERF/AP2 transcription factor family. FEBS J. 2009, 276, 6624–6635. [Google Scholar] [CrossRef]
- Jiang, C.; Iu, B.; Singh, J. Requirement of a CCGAC cis-acting element for cold induction of the BN115 gene from winter Brassica napus. Plant Mol. Biol. 1996, 30, 679–684. [Google Scholar] [CrossRef]
- Borassi, C.; Sede, A.; Mecchia, M.; Salgado Salter, J.; Marzol, E.; Muschietti, J.; Estevez, J. An update on cell surface proteins containing extensin-motifs. J. Exp. Bot. 2016, 67, 477–487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Draeger, C.; Fabrice, T.N.; Gineau, E.; Mouille, G.; Kuhn, B.M.; Moller, I.; Abdou, M.; Frey, B.; Pauly, M.; Bacic, A.; et al. Arabidopsis leucine-rich repeat extensin (LRX) proteins modify cell wall composition and influence plant growth. BMC Plant Biol. 2015, 15, 155. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Wang, Z.Y.; Li, J.; Zhu, Q.; Lamb, C.; Ronald, P.; Chory, J. Perception of brassinosteroids by the extracellular domain of the receptor kinase BRI1. Science 2000, 288, 2360–2363. [Google Scholar] [CrossRef] [Green Version]
- Dharmasiri, N.; Dharmasiri, S.; Estelle, M. The F-box protein TIR1 is an auxin receptor. Nature 2005, 435, 441–445. [Google Scholar] [CrossRef]
- Cannon, M.C.; Terneus, K.; Hall, Q.; Tan, L.; Wang, Y.; Wegenhart, B.L.; Chen, L.; Lamport, D.; Chen, Y.; Kieliszewski, M.J. Self-assembly of the plant cell wall requires an extensin scaffold. Proc. Natl. Acad. Sci. USA 2008, 105, 2226–2231. [Google Scholar] [CrossRef] [Green Version]
- Baumberger, N.; Doesseger, B.; Guyot, R.; Diet, A.; Parsons, R.L.; Clark, M.A.; Simmons, M.P.; Bedinger, P.; Goff, S.A.; Ringli, C.; et al. Whole-genome comparison of leucine-rich repeat extensins in Arabidopsis and rice. a conserved family of cell wall proteins form a vegetative and a reproductive clade. Plant Physiol. 2003, 131, 1313–1326. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Method | Pretreatment | Image Scanning | Image Accuracy | Phenotype Analysis | |
|---|---|---|---|---|---|
| Paraffin section + manual/semi-automatic analysis method | Paraffin sectioning About 2–3 weeks | The scanning of slide scanner can only obtain local microscopic images, and the scanning range is 0.5–20 µm | Up to 0.5 µm resolution | Manual or semi-automatic detecting, the standard varies from person to person, and the detection efficiency is low | Analysis of local characteristics of leaves |
| Micro-CT + automatic detection based on deep learning | Dehydration, tertbutyl alcohol replacement, drying, etc. About 1 week | Micro-CT can obtain a complete cross-sectional microscopic image of leaves, with a scanning range of 2.0 µm–30 mm | Up to 2 µm resolution | Automatic analysis, unified standard, average efficiency of about 2–3 s/CT image | Complete cross section of leaves or analysis of local characteristics can be realized |
| Phenotypic Traits | Abbreviation | Unit | Median | Maximum | Minimum |
|---|---|---|---|---|---|
| Vein number | VN | pcs | 25.000 ± 0.163 | 38.000 ± 0.164 | 14.000 ± 0.020 |
| Midvein area | MVA | mm2 | 0.341 ± 0.005 | 0.849 ± 0.005 | 0.080 ± 0.005 |
| Leaf width | LW | cm | 8.665 ± 0.043 | 12.101 ± 0.042 | 5.447 ± 0.042 |
| Vein density | VD | pcs/cm | 2.855 ± 0.019 | 4.804 ± 0.019 | 1.432 ± 0.019 |
| Trait | p-Value | Mixed | NSS | TST | SS |
|---|---|---|---|---|---|
| VN | 9.28 × 10−7 *** | 23.785 ± 0.303b | 24.190 ± 0.334b | 25.735 ± 0.328a | 26.043 ± 0.246a |
| MVA | 0.000303 *** | 0.339 ± 0.008b | 0.371 ± 0.009b | 0.360 ± 0.011b | 0.434 ± 0.012a |
| LW | 0.000195 *** | 8.696 ± 0.083a | 8.642 ± 0.087a | 8.816 ± 0.084a | 8.059 ± 0.058b |
| VD | 1.09 × 10−8 *** | 2.766 ± 0.039c | 2.821 ± 0.038bc | 2.942 ± 0.037b | 3.246 ± 0.031a |
| Trait | No. of Significant SNPs | No. of Annotated Genes | No. of Functionally Annotated Genes |
|---|---|---|---|
| VN | 14 | 26 | 7 |
| MVA | 32 | 59 | 10 |
| LW | 5 | 10 | 6 |
| VD | 10 | 19 | 6 |
| Total | 61 | 114 | 29 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, S.; Zhu, M.; Du, J.; Wang, J.; Lu, X.; Jin, Y.; Zhang, M.; Guo, X.; Zhang, Y. Accurate Phenotypic Identification and Genetic Analysis of the Ear Leaf Veins in Maize (Zea mays L.). Agronomy 2023, 13, 753. https://doi.org/10.3390/agronomy13030753
Guo S, Zhu M, Du J, Wang J, Lu X, Jin Y, Zhang M, Guo X, Zhang Y. Accurate Phenotypic Identification and Genetic Analysis of the Ear Leaf Veins in Maize (Zea mays L.). Agronomy. 2023; 13(3):753. https://doi.org/10.3390/agronomy13030753
Chicago/Turabian StyleGuo, Shangjing, Mingyi Zhu, Jianjun Du, Jinglu Wang, Xianju Lu, Yu Jin, Minggang Zhang, Xinyu Guo, and Ying Zhang. 2023. "Accurate Phenotypic Identification and Genetic Analysis of the Ear Leaf Veins in Maize (Zea mays L.)" Agronomy 13, no. 3: 753. https://doi.org/10.3390/agronomy13030753
APA StyleGuo, S., Zhu, M., Du, J., Wang, J., Lu, X., Jin, Y., Zhang, M., Guo, X., & Zhang, Y. (2023). Accurate Phenotypic Identification and Genetic Analysis of the Ear Leaf Veins in Maize (Zea mays L.). Agronomy, 13(3), 753. https://doi.org/10.3390/agronomy13030753






