Enhancement of Morphological and Physiological Performance of Zea mays L. under Saline Stress Using ZnO Nanoparticles and 24-Epibrassinolide Seed Priming
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials, Treatments, and Experimental Design
2.2. Measurements
2.2.1. Soil Analysis
2.2.2. Root Traits
2.2.3. Physiological Traits of Maize Seedlings
2.2.4. Growth Analysis
- (i)
- Stem diameter and shoot length:
- (ii)
- Leaf area and relative leaf water contents (RLWC):
- (iii)
- Whole plant dry weight:
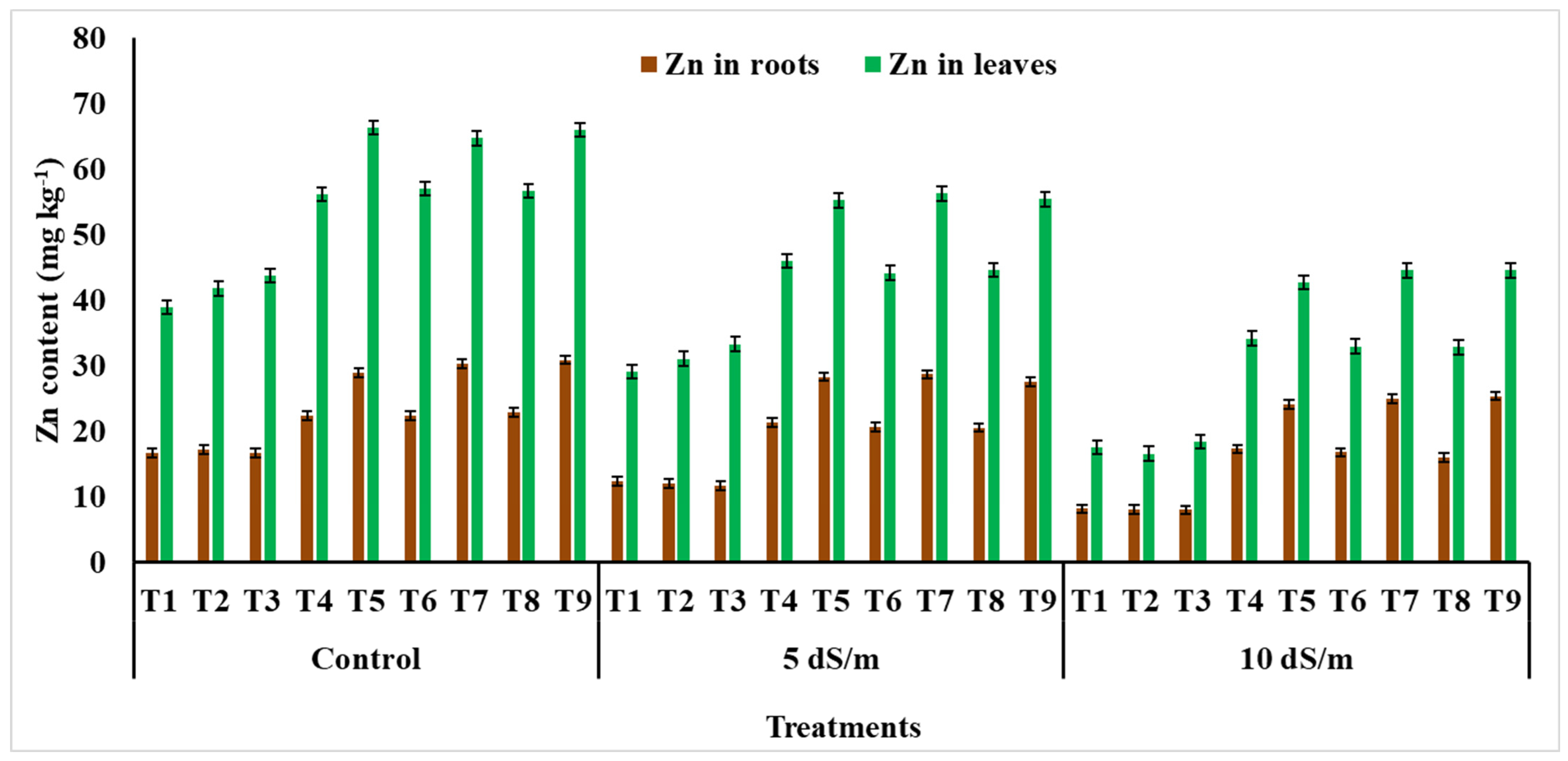
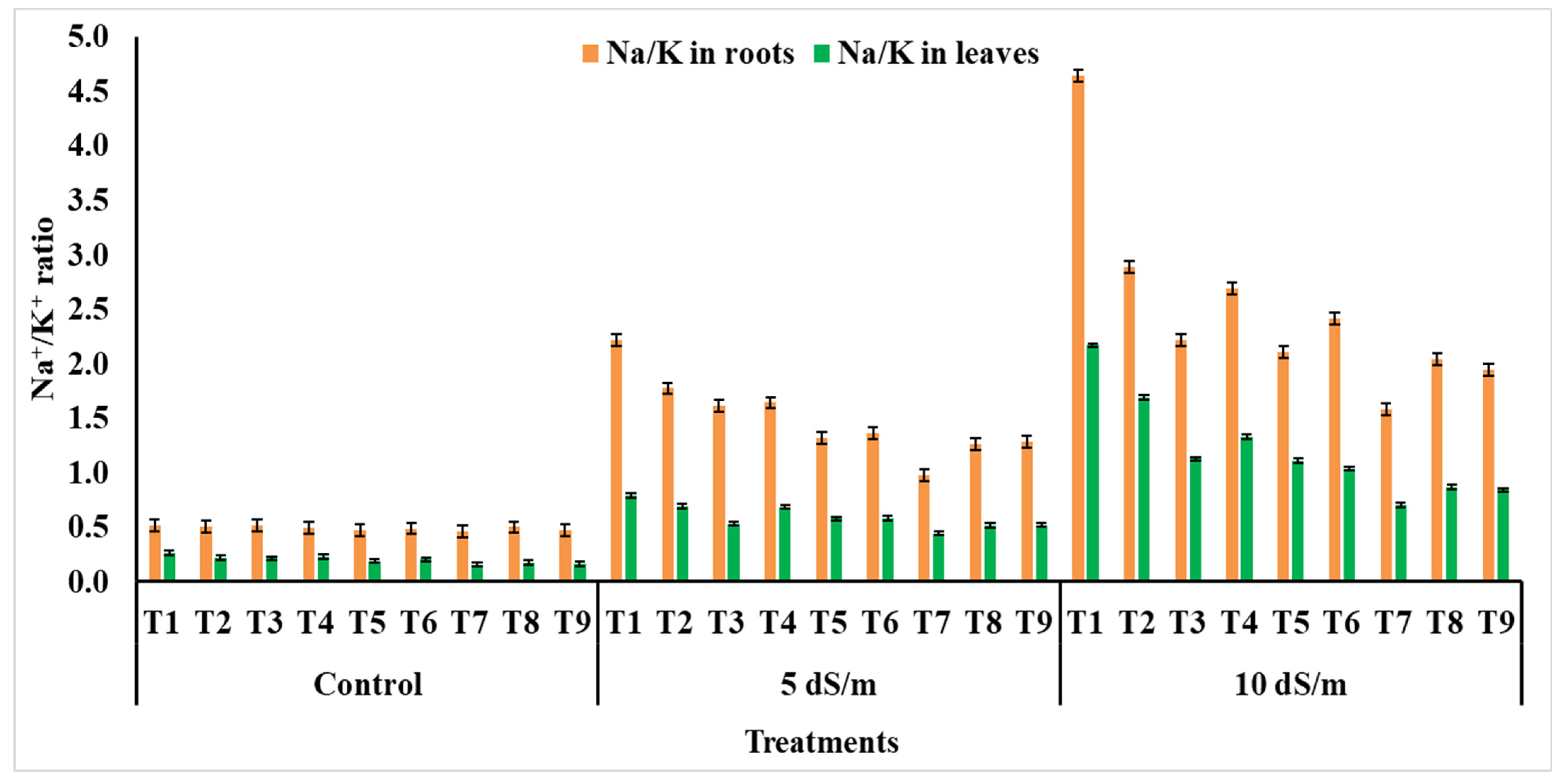
2.2.5. Mineral Profiling of Leaves and Roots
2.3. Statistical Analysis
3. Results
3.1. Root Morphological Performance
3.2. Growth Performance
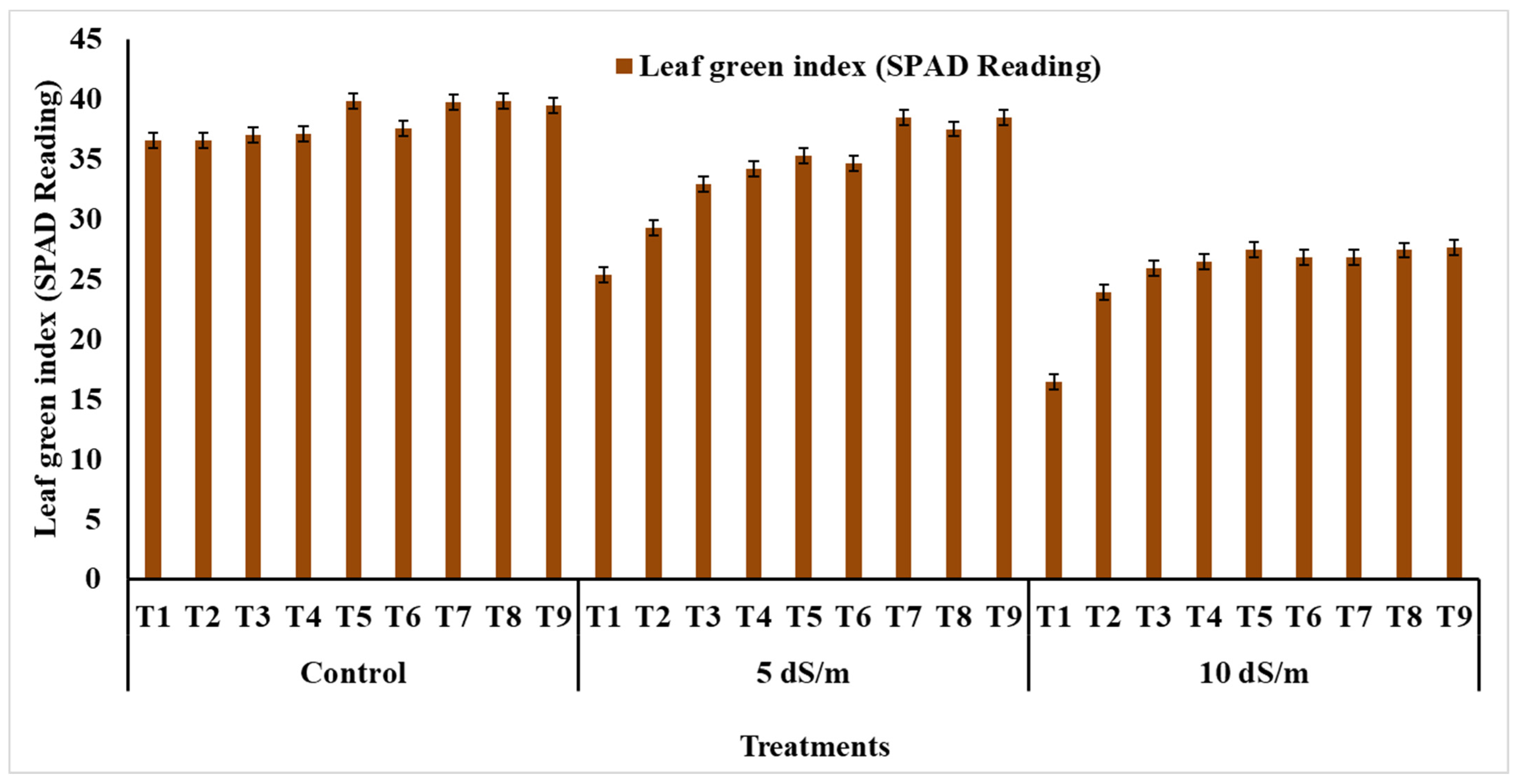
3.3. Physiological Performance
4. Discussion
4.1. Root Characteristics
4.2. Growth Characteristics
4.3. Physiological Performance
4.4. Metal Ions and Ionic Toxicity
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dustgeer, Z.M.; Seleiman, M.F.; Khan, I.; Chattha, M.U.; Ali, E.F.; Alhammad, B.A.; Jalal, R.S.; Refay, Y.; Hassan, M.U. Glycine-betaine induced salinity tolerance in maize by regulating the physiological attributes, antioxidant defense system and ionic homeostasis. Not. Bot. Horti Agrobot. Cluj Napoca 2021, 49, 12248. [Google Scholar] [CrossRef]
- Bhattarai, S.; Biswas, D.; Fu, Y.B.; Biligetu, B. Morphological, physiological, and genetic responses to salt stress in alfalfa: A Review. Agronomy 2020, 10, 577. [Google Scholar] [CrossRef] [Green Version]
- Qin, H.; Li, Y.; Huang, R. Advances and challenges in the breeding of salt-tolerant rice. Int. J. Mol. Sci. 2020, 21, 8385. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Zhang, Q.; Liu, M.; Zhou, H.; Ma, C.; Wang, P. Regulation of plant responses to salt stress. Int. J. Mol. Sci. 2021, 22, 4609. [Google Scholar] [CrossRef]
- Hao, S.; Wang, Y.; Yan, Y.; Liu, Y.; Wang, J.; Chen, S. A review on plant responses to salt stress and their mechanisms of salt resistance. Horticulturae 2021, 7, 132. [Google Scholar] [CrossRef]
- Rawat, N.; Singla-Pareek, S.L.; Pareek, A. Membrane dynamics during individual and combined abiotic stresses in plants and tools to study the same. Physiol. Plant. 2021, 171, 653–676. [Google Scholar] [CrossRef] [PubMed]
- Alkharabsheh, H.M.; Seleiman, M.F.; Hewedy, O.A.; Battaglia, M.L.; Jalal, R.S.; Alhammad, B.A.; Schillaci, C.; Ali, N.; Al-Doss, A. Field crop responses and management strategies to mitigate soil salinity in modern agriculture: A review. Agronomy 2021, 11, 2299. [Google Scholar] [CrossRef]
- Pan, T.; Liu, M.; Kreslavski, V.D.; Zharmukhamedov, S.K.; Nie, C.; Yu, M.; Kuznetsov, V.V.; Allakhverdiev, S.I.; Shabala, S. Non-stomatal limitation of photosynthesis by soil salinity. Crit. Rev. Environ. Sci. Technol. 2021, 51, 791–825. [Google Scholar] [CrossRef]
- Zlobin, I.E. Current understanding of plant zinc homeostasis regulation mechanisms. Plant Physiol. Biochem. 2021, 162, 327–335. [Google Scholar] [CrossRef]
- Fatima, F.; Hashim, A.; Anees, S. Efficacy of nanoparticles as nanofertilizer production: A review. Environ. Sci. Pollut. Res. 2021, 28, 1292–1303. [Google Scholar] [CrossRef]
- Seleiman, M.F.; Almutairi, K.F.; Alotaibi, M.; Shami, A.; Alhammad, B.A.; Battaglia, M.L. Nano-fertilization as an emerging fertilization technique: Why can modern agriculture benefit from its use? Plants 2021, 10, 2. [Google Scholar] [CrossRef]
- Alhammad, B.A.; Ahmad, A.; Seleiman, M.F.; Tola, E. Seed Priming with Nanoparticles and 24-Epibrassinolide Improved Seed Germination and Enzymatic Performance of Zea mays L. in Salt-Stressed Soil. Plants 2023, 12, 690. [Google Scholar] [CrossRef] [PubMed]
- Seleiman, M.F.; Alotaibi, M.A.; Alhammad, B.A.; Alharbi, B.M.; Refay, Y.; Badawy, S.A. Effects of ZnO nanoparticles and biochar of rice straw and cow manure on characteristics of contaminated soil and sunflower productivity, oil quality, and heavy metals uptake. Agronomy 2020, 10, 790. [Google Scholar] [CrossRef]
- Srivastav, A.; Ganjewala, D.; Singhal, R.K.; Rajput, V.D.; Minkina, T.; Voloshina, M.; Srivastava, S.; Shrivastava, M. Effect of ZnO Nanoparticles on Growth and Biochemical Responses of Wheat and Maize. Plants 2021, 10, 2556. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.Y.; Bai, M.Y.; Oh, E.; Zhu, J.Y. Brassinosteroid signaling network and regulation of photomorphogenesis. Annu. Rev. Genet. 2012, 46, 701–724. [Google Scholar] [CrossRef]
- Anwar, A.; Liu, Y.; Dong, R.; Bai, L.; Yu, X.; Li, Y. The physiological and molecular mechanism of brassinosteroid in response to stress: A review. Biol. Res. 2018, 51, 46. [Google Scholar] [CrossRef] [Green Version]
- Fatichi, S.; Leuzinger, S.; Körner, C. Moving beyond photosynthesis: From carbon source to sink-driven vegetation modeling. New Phytol. 2014, 201, 1086–1095. Available online: http://www.jstor.org/stable/newphytologist.201.4.1086 (accessed on 28 July 2022). [CrossRef]
- Bajguz, A. Brassinosteroids in microalgae: Application for growth improvement and protection against abiotic stresses. In Brassinosteroids: Plant Growth and Development; Hayat, S., Yusuf, M., Bhardwaj, R., Bajguz, A., Eds.; Springer Singapore: Singapore, 2019; pp. 45–58. [Google Scholar] [CrossRef]
- Hussain, M.A.; Fahad, S.; Sharif, R.; Jan, M.F.; Mujtaba, M.; Ali, Q.; Ahmad, A.; Ahmad, H.; Amin, N.; Ajayo, B.S.; et al. Multifunctional role of brassinosteroid and its analogues in plants. Plant Growth Regul. 2020, 92, 141–156. [Google Scholar] [CrossRef]
- Azhar, N.; Su, N.; Shabala, L.; Shabala, S. Exogenously applied 24-epibrassinolide (EBL) ameliorates detrimental effects of salinity by reducing K+ efflux via depolarization-activated K+ channels. Plant Cell Physiol. 2017, 58, 802–810. [Google Scholar] [CrossRef] [Green Version]
- Erenstein, O.; Chamberlin, J.; Sonder, K. Estimating the global number and distribution of maize and wheat farms. Glob. Food Secur. 2021, 30, 100558. [Google Scholar] [CrossRef]
- Grote, U.; Fasse, A.; Nguyen, T.T.; Erenstein, O. Food security and the dynamics of wheat and maize value chains in Africa and Asia. Front. Sustain. Food Syst. 2021, 4, 617009. [Google Scholar] [CrossRef]
- Wang, J.; Hu, X. Research on corn production efficiency and influencing factors of typical farms: Based on data from 12 corn-producing countries from 2012 to 2019. PLoS ONE 2021, 16, e0254423. [Google Scholar] [CrossRef] [PubMed]
- Strable, J.; Scanlon, M.J. Maize (Zea mays): A model organism for basic and applied research in plant biology. Cold Spring Harb. Protoc. 2009, 2009, pdb-emo132. [Google Scholar] [CrossRef] [Green Version]
- Bouyoucos, G.J. Hydrometer method improved for making particle size analysis of soils. Agron. J. 1962, 54, 464–465. [Google Scholar] [CrossRef]
- Richards, L.A. Diagnosis and Improvement of Saline and Alkali Soils; LWW: Philadelphia, PA, USA, 1954; Volume 78, p. 154. Available online: https://ui.adsabs.harvard.edu/link_gateway/1954SoilS..78..154R (accessed on 24 August 2022). [CrossRef]
- Yamasaki, S.; Dillenburg, L.R. Measurements of leaf relative water content in Araucaria angustifolia. Rev. Bras. De Fisiol. Veg. 1999, 11, 69–75. [Google Scholar]
- Wolf, B. The comprehensive system of leaf analysis and its use for diagnosing crop nutrient status. Commun. Soil Sci. Plant Anal. 1982, 3, 1035–1059. [Google Scholar] [CrossRef]
- Badawy, S.A.; Zayed, B.A.; Bassiouni, S.M.A.; Mahdi, A.H.A.; Majrashi, A.; Ali, E.F.; Seleiman, M.F. Influence of Nano Silicon and Nano Selenium on Root Characters, Growth, Ion Selectivity, Yield, and Yield Components of Rice (Oryza sativa L.) under Salinity Conditions. Plants 2021, 10, 1657. [Google Scholar] [CrossRef]
- Raza, M.M.; Ullah, S.; Tariq, A.Z.I.Z.; Abbas, T.; Yousaf, M.M.; Altay, V.; Ozturk, M. Alleviation of salinity stress in maize using silicon nutrition. Not. Bot. Horti Agrobot. Cluj-Napoca 2019, 47, 1340–1347. [Google Scholar] [CrossRef] [Green Version]
- Zahra, N.; Raza, Z.A.; Mahmood, S. Effect of salinity stress on various growth and physiological attributes of two contrasting maize genotypes. Braz. Arch. Biol. Technol. 2020, 63, e20200072. [Google Scholar] [CrossRef]
- Farooq, M.; Usman, M.; Nadeem, F.; ur Rehman, H.; Wahid, A.; Basra, S.M.; Siddique, K.H. Seed priming in field crops: Potential benefits, adoption and challenges. Crop Pasture Sci. 2019, 70, 731–771. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, G.; Wang, Y.; Zhou, Z.; Meng, Y.; Chen, B. Effect of soil salinity on physiological characteristics of functional leaves of cotton plants. J. Plant Res. 2013, 126, 293–304. [Google Scholar] [CrossRef]
- West, G.; Inzé, D.; Beemster, G.T. Cell cycle modulation in the response of the primary root of Arabidopsis to salt stress. Plant Physiol. 2004, 135, 1050–1058. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Li, J.L.; Liu, L.N.; Xie, Q.; Sui, N. Photosynthetic regulation under salt stress and salt-tolerance mechanism of sweet sorghum. Front. Plant Sci. 2020, 10, 1722. [Google Scholar] [CrossRef] [Green Version]
- Paramo, L.A.; Feregrino-Pérez, A.A.; Guevara, R.; Mendoza, S.; Esquivel, K. Nanoparticles in agroindustry: Applications, toxicity, challenges, and trends. Nanomaterials 2020, 10, 1654. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Zhou, Y.; Ma, C.; Feng, Y.; Hao, Y.; Rui, Y.; Wu, W.; Gui, X.; Le, V.N.; Han, Y.; et al. Jointed toxicity of TiO2 NPs and Cd to rice seedlings: NPs alleviated Cd toxicity and Cd promoted NPs uptake. Plant Physiol. Biochem. 2017, 110, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Faizan, M.; Bhat, J.A.; Hessini, K.; Yu, F.; Ahmad, P. Zinc oxide nanoparticles alleviates the adverse effects of cadmium stress on Oryza sativa via modulation of the photosynthesis and antioxidant defense system. Ecotoxicol. Environ. Saf. 2021, 220, 112401. [Google Scholar] [CrossRef]
- Savassa, S.M.; Duran, N.M.; Rodrigues, E.S.; De Almeida, E.; Van Gestel, C.A.; Bompadre, T.F.; de Carvalho, H.W.P. Effects of ZnO nanoparticles on Phaseolus vulgaris germination and seedling development determined by X-ray spectroscopy. ACS Appl. Nano Mater. 2018, 1, 6414–6426. [Google Scholar] [CrossRef]
- Zafar, S.; Perveen, S.; Kamran Khan, M.; Shaheen, M.R.; Hussain, R.; Sarwar, N.; Rashid, S.; Nafees, M.; Farid, G.; Alamri, S.; et al. Effect of zinc nanoparticles seed priming and foliar application on the growth and physio-biochemical indices of spinach (Spinacia oleracea L.) under salt stress. PLoS ONE 2022, 17, e0263194. [Google Scholar] [CrossRef]
- Chaiwanon, J.; Wang, Z.Y. Spatiotemporal brassinosteroid signaling and antagonism with auxin pattern stem cell dynamics in Arabidopsis roots. Curr. Biol. 2015, 25, 1031–1042. [Google Scholar] [CrossRef] [Green Version]
- Gerona, M.E.B.; Deocampo, M.P.; Egdane, J.A.; Ismail, A.M.; Dionisio-Sese, M.L. Physiological responses of contrasting rice genotypes to salt stress at reproductive stage. Rice Sci. 2019, 26, 207–219. [Google Scholar] [CrossRef]
- Guo, Q.; Liu, L.; Barkla, B.J. Membrane lipid remodeling in response to salinity. Int. J. Mol. Sci. 2019, 20, 4264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Almeida, D.M.; Oliveira, M.M.; Saibo, N.J. Regulation of Na+ and K+ homeostasis in plants: Towards improved salt stress tolerance in crop plants. Genet. Mol. Biol. 2017, 40, 326–345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shabala, S.; Shabala, S.; Cuin, T.A.; Pang, J.; Percey, W.; Chen, Z.; Conn, S.; Eing, C.; Wegner, L.H. Xylem ionic relations and salinity tolerance in barley. Plant J. 2010, 61, 839–853. [Google Scholar] [CrossRef] [PubMed]
- Yun, P.; Xu, L.; Wang, S.S.; Shabala, L.; Shabala, S.; Zhang, W.Y. Piriformospora indica improves salinity stress tolerance in Zea mays L. plants by regulating Na+ and K+ loading in root and allocating K+ in shoot. Plant Growth Regul. 2018, 86, 323–331. [Google Scholar] [CrossRef]
- Seleiman, M.F.; Al-Selwey, W.A.; Ibrahim, A.A.; Shady, M.; Alsadon, A.A. Foliar Applications of ZnO and SiO2 Nanoparticles Mitigate Water Deficit and Enhance Potato Yield and Quality Traits. Agronomy 2023, 13, 466. [Google Scholar] [CrossRef]
- Cakmak, I. Enrichment of cereal grains with zinc: Agronomic or genetic biofortification? Plant Soil 2008, 302, 1–17. [Google Scholar] [CrossRef]
- El-Badri, A.M.; Batool, M.; Mohamed, I.A.; Khatab, A.; Sherif, A.; Wang, Z.K.; Salah, A.; Nishawy, E.; Ayaad, M.; Kuai, J.; et al. Modulation of salinity impact on early seedling stage via nano-priming application of zinc oxide on rapeseed (Brassica napus L.). Plant Physiol. Biochem. 2021, 166, 376–392. [Google Scholar] [CrossRef]
- Ahmad, P.; Ahanger, M.A.; Egamberdieva, D.; Alam, P.; Alyemeni, M.N.; Ashraf, M. Modification of osmolytes and antioxidant enzymes by 24-epibrassinolide in chickpea seedlings under mercury (Hg) toxicity. J. Plant Growth Regul. 2018, 37, 309–322. [Google Scholar] [CrossRef]
- Nolan, T.M.; Vukašinović, N.; Liu, D.; Russinova, E.; Yin, Y. Brassinosteroids: Multidimensional regulators of plant growth, development, and stress responses. Plant Cell 2020, 32, 295–318. [Google Scholar] [CrossRef] [Green Version]
- Desoky, E.S.M.; Mansour, E.; Ali, M.M.; Yasin, M.A.; Abdul-Hamid, M.I.; Rady, M.M.; Ali, E.F. Exogenously used 24-epibrassinolide promotes drought tolerance in maize hybrids by improving plant and water productivity in an arid environment. Plants 2021, 10, 354. [Google Scholar] [CrossRef]
- Khan, I.; Awan, S.A.; Ikram, R.; Rizwan, M.; Akhtar, N.; Yasmin, H.; Sayyed, R.Z.; Ali, S.; Ilyas, N. Effects of 24-epibrassinolide on plant growth, antioxidants defense system, and endogenous hormones in two wheat varieties under drought stress. Physiol. Plant. 2021, 172, 696–706. [Google Scholar] [CrossRef]
- Yue, J.; Fu, Z.; Zhang, L.; Zhang, Z.; Zhang, J. The positive effect of different 24-epiBL pretreatments on salinity tolerance in Robinia pseudoacacia L. seedlings. Forests 2018, 10, 4. [Google Scholar] [CrossRef] [Green Version]
- Shin, Y.K.; Bhandari, S.R.; Jo, J.S.; Song, J.W.; Cho, M.C.; Yang, E.Y.; Lee, J.G. Response to salt stress in lettuce: Changes in chlorophyll fluorescence parameters, phytochemical contents, and antioxidant activities. Agronomy 2020, 10, 1627. [Google Scholar] [CrossRef]
- Acosta-Motos, J.R.; Ortuño, M.F.; Bernal-Vicente, A.; Diaz-Vivancos, P.; Sanchez-Blanco, M.J.; Hernandez, J.A. Plant responses to salt stress: Adaptive mechanisms. Agronomy 2017, 7, 18. [Google Scholar] [CrossRef] [Green Version]
- Faizan, M.; Hayat, S.; Pichtel, J. Effects of zinc oxide nanoparticles on crop plants: A perspective analysis. In Sustainable Agriculture Reviews 41; Hayat, S., Pichtel, J., Faizan, M., Fariduddin, Q., Eds.; Springer: Cham, Switzerland, 2020; pp. 83–99. [Google Scholar] [CrossRef]
- Rizwan, M.; Ali, S.; Ali, B.; Adrees, M.; Arshad, M.; Hussain, A.; ur Rehman, M.Z.; Waris, A.A. Zinc and iron oxide nanoparticles improved the plant growth and reduced the oxidative stress and cadmium concentration in wheat. Chemosphere 2019, 214, 269–277. [Google Scholar] [CrossRef]
- Abou-Zeid, H.M.; Ismail, G.S.M.; Abdel-Latif, S.A. Influence of seed priming with ZnO nanoparticles on the salt-induced damages in wheat (Triticum aestivum L.) plants. J. Plant Nutr. 2021, 44, 629–643. [Google Scholar] [CrossRef]
- Zhu, J.K. Regulation of ion homeostasis under salt stress. Curr. Opin. Plant Biol. 2003, 6, 441–445. [Google Scholar] [CrossRef]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdelaziz, M.E.; Kim, D.; Ali, S.; Fedoroff, N.V.; Al-Babili, S. The endophytic fungus Piriformospora indica enhances Arabidopsis thaliana growth and modulates Na+/K+ homeostasis under salt stress conditions. Plant Sci. 2017, 263, 107–115. [Google Scholar] [CrossRef] [Green Version]
- Heikal, Y.M.; El-Esawi, M.A.; El-Ballat, E.M.; Abdel-Aziz, H.M. Applications of nanoparticles for mitigating salinity and drought stress in plants: An overview on the physiological, biochemical and molecular genetic aspects. N. Z. J. Crop. Hortic. Sci. 2022, 1–31. [Google Scholar] [CrossRef]
- Noohpisheh, Z.; Amiri, H.; Mohammadi, A.; Farhadi, S. Effect of the foliar application of zinc oxide nanoparticles on some biochemical and physiological parameters of Trigonella foenum-graecum under salinity stress. Plant Biosyst. -Int. J. Deal. All Asp. Plant Biol. 2021, 155, 267–280. [Google Scholar] [CrossRef]
- Dey, A.; Somaiah, S. Green synthesis and characterization of zinc oxide nanoparticles using leaf extract of Thryallis glauca (Cav.) Kuntze and their role as antioxidant and antibacterial. Microsc. Res. Technol. 2022, 85, 2835–2847. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, D.; Rattan, A.; Gautam, V.; Bhardwaj, R. Mercury-induced changes in growth, metal & ions uptake, photosynthetic pigments, osmoprotectants and antioxidant defence system in Raphanus sativus L. seedlings and role of steroid hormone in stress amelioration. J. Pharmacogn. Phytochem. 2016, 5, 259. Available online: https://www.phytojournal.com/archives?year=2016&vol=5&issue=4&ArticleId=921&si=false (accessed on 16 February 2023).






| (A) Saline Stress (Main Factor) | |
| Control | Control, normal soil |
| Moderate | 5 dS m−1 |
| High | 10 dS m−1 |
| (B) Seed-Priming Treatments (Sub-Factor) | |
| Treatment | Treatment Details: Concentrations and Combinations of ZnO NPs and EBL |
| T1 | Control (zero EBL and zero ZnO NPs) |
| T2 | EBL (0.2 µM) |
| T3 | EBL (0.4 µM) |
| T4 | ZnO NPs (50.0 mg L−1) |
| T5 | ZnO NPs (100.0 mg L−1) |
| T6 | ZnO NPs (50.0 mg L−1) + EBL (0.2 µM) |
| T7 | ZnO NPs (100.0 mg L−1) + EBL (0.2 µM) |
| T8 | ZnO NPs (50.0 mg L−1) + EBL (0.4 µM) |
| T9 | ZnO NPs (100.0 mg L−1) + EBL (0.4 µM) |
| Treatment | Root Length (cm) | Total Root Length (cm) | Root Total Surface Area (cm2) | Root Average Diameter (mm) | Root Total Volume (cm3) | Number of Root Tips |
|---|---|---|---|---|---|---|
| Saline Stress | ||||||
| Control | 36.55 A | 414.32 A | 319.48 A | 2.67 | 24.76 A | 2487.94 A |
| 5 dS m−1 | 27.46 B | 286.95 B | 198.68 B | 2.65 | 14.21 B | 1590.06 B |
| 10 dS m−1 | 24.67 C | 238.32 C | 158.08 C | 2.35 | 10.31 B | 1391.11 B |
| SEM0.05 | 0.438 | 15.624 | 13.512 | 0.203 | 2.083 | 114.162 |
| Significance | *** | *** | *** | NS | *** | *** |
| Seed-Priming Treatments | ||||||
| T1 | 24.28 e | 234.84 d | 133.12 d | 2.35 bc | 7.37 c | 850.17 d |
| T2 | 26.41 de | 301.86 cd | 182.08 cd | 2.44 bc | 13.49 c | 1506.00 c |
| T3 | 27.13 cd | 263.95 d | 172.18 cd | 2.24 c | 10.64 c | 1686.67 bc |
| T4 | 29.27 c | 306.85 cd | 181.92 cd | 2.01 c | 9.78 c | 1450.17 c |
| T5 | 31.81 b | 243.01 d | 259.91 ab | 3.37 ab | 27.71 ab | 2031.67 abc |
| T6 | 26.54 d | 395.16 ab | 266.52 ab | 2.31 bc | 16.89 c | 2229.17 ab |
| T7 | 33.10 ab | 463.19 a | 326.06 a | 2.27 bc | 18.88 bc | 2303.17 ab |
| T8 | 32.44 b | 251.54 d | 274.96 ab | 3.88 a | 30.02 a | 2432.83 a |
| T9 | 35.08 a | 358.38 bc | 231.96 bc | 2.14 c | 13.06 c | 1917.50 abc |
| SEM0.05 | 0.758 | 27.061 | 23.403 | 0.352 | 3.608 | 197.735 |
| Significance | *** | *** | *** | ** | *** | *** |
| Salinity | Treatment | Root Length (cm) | Total Root Length (cm) | Root Surface Area (cm2) | Root Average Diameter (mm) | Root Total Volume (cm3) | Number of Root Tips (Number) |
|---|---|---|---|---|---|---|---|
| Control | T1 | 32.53 | 326.32 | 164.99 | 1.61 | 6.64 | 870.50 |
| T2 | 32.97 | 472.80 | 234.18 | 1.67 | 11.57 | 2085.50 | |
| T3 | 39.00 | 372.64 | 198.90 | 1.89 | 11.32 | 2250.50 | |
| T4 | 36.13 | 359.27 | 236.76 | 2.36 | 14.84 | 2764.00 | |
| T5 | 40.30 | 353.60 | 487.02 | 4.36 | 58.07 | 2871.00 | |
| T6 | 37.17 | 463.63 | 384.86 | 3.01 | 29.17 | 3507.50 | |
| T7 | 34.50 | 729.16 | 487.62 | 2.07 | 26.45 | 2687.00 | |
| T8 | 42.33 | 306.21 | 412.96 | 4.37 | 46.32 | 3044.50 | |
| T9 | 34.03 | 345.28 | 268.04 | 2.67 | 18.45 | 2311.00 | |
| 5 dS m−1 | T1 | 22.13 | 220.81 | 130.24 | 3.24 | 9.62 | 1008.00 |
| T2 | 27.47 | 204.11 | 192.61 | 3.97 | 23.73 | 1485.00 | |
| T3 | 24.43 | 252.04 | 200.91 | 2.52 | 12.75 | 1450.50 | |
| T4 | 29.10 | 351.23 | 186.39 | 1.63 | 8.02 | 851.50 | |
| T5 | 31.37 | 169.04 | 190.64 | 3.86 | 20.09 | 2197.00 | |
| T6 | 23.40 | 487.13 | 250.12 | 1.57 | 11.28 | 2237.00 | |
| T7 | 28.57 | 293.97 | 271.86 | 2.86 | 20.13 | 2216.50 | |
| T8 | 28.80 | 297.52 | 197.74 | 2.54 | 14.66 | 1324.50 | |
| T9 | 31.90 | 306.71 | 167.65 | 1.67 | 7.62 | 1540.50 | |
| 10 dS m−1 | T1 | 18.17 | 157.39 | 104.14 | 2.21 | 5.83 | 672.00 |
| T2 | 18.80 | 228.67 | 119.46 | 1.67 | 5.17 | 947.50 | |
| T3 | 17.97 | 167.17 | 116.73 | 2.31 | 7.86 | 1359.00 | |
| T4 | 22.57 | 210.06 | 122.62 | 2.04 | 6.48 | 735.00 | |
| T5 | 23.77 | 206.40 | 102.06 | 1.89 | 4.96 | 1027.00 | |
| T6 | 19.07 | 234.71 | 164.59 | 2.36 | 10.23 | 943.00 | |
| T7 | 36.23 | 366.46 | 218.72 | 1.88 | 10.07 | 2006.00 | |
| T8 | 26.20 | 150.88 | 214.19 | 4.73 | 29.07 | 2929.50 | |
| T9 | 39.30 | 423.16 | 260.20 | 2.09 | 13.11 | 1901.00 | |
| SEM0.05 | 1.313 | 46.871 | 40.536 | 0.609 | 6.249 | 342.486 | |
| Significance | *** | *** | ** | * | ** | ** |
| Treatment | Stem Diameter (mm) | Shoot Length (cm) | Whole Plant Dry Weight (g) | Average Leaf Area (cm2) | RLWC (%) |
|---|---|---|---|---|---|
| Saline Stress | |||||
| Control | 7.62 A | 92.02 A | 2.35 A | 117.29 A | 81.63 A |
| 5 dS m−1 | 5.38 B | 65.59 B | 1.02 B | 53.19 B | 73.53 B |
| 10 dS m−1 | 4.77 C | 47.76 C | 0.84 C | 42.80 C | 65.01 C |
| SEM0.05 | 0.048 | 0.625 | 0.009 | 0.654 | 0.188 |
| Significance | *** | *** | *** | *** | *** |
| Seed-Priming Treatments | |||||
| T1 | 4.69 e | 54.96 f | 0.79 g | 53.01 f | 70.32 d |
| T2 | 4.68 e | 60.02 e | 1.20 e | 63.11 e | 72.58 bc |
| T3 | 5.59 c | 77.98 a | 1.19 e | 61.09 e | 72.46 bc |
| T4 | 6.64 a | 67.98 cd | 1.54 d | 82.32 ab | 74.66 a |
| T5 | 6.62 a | 73.97 b | 1.65 c | 73.07 d | 75.11 a |
| T6 | 6.82 a | 72.68 b | 1.81 a | 85.28 a | 75.32 a |
| T7 | 6.82 a | 68.87 c | 1.72 b | 81.47 b | 75.26 a |
| T8 | 6.19 b | 74.60 b | 1.68 bc | 78.25 c | 73.19 b |
| T9 | 5.27 d | 65.07 d | 1.01 f | 62.24 e | 71.63 c |
| SEM0.05 | 0.083 | 1.086 | 0.015 | 1.133 | 0.325 |
| Significance | *** | *** | *** | *** | *** |
| Salinity | Treatment | Stem Diameter (mm) | Shoot Length (cm) | Whole Plant Dry Weight (g) | Average Leaf Area (cm2) | RLWC (%) |
|---|---|---|---|---|---|---|
| Control | T1 | 6.10 | 74.47 | 1.14 | 88.21 | 79.30 |
| T2 | 5.97 | 85.07 | 2.34 | 107.55 | 80.67 | |
| T3 | 7.63 | 111.37 | 2.08 | 116.69 | 78.58 | |
| T4 | 8.13 | 92.50 | 2.47 | 132.95 | 82.36 | |
| T5 | 7.83 | 104.03 | 2.82 | 115.34 | 84.42 | |
| T6 | 8.87 | 94.80 | 2.92 | 145.71 | 81.12 | |
| T7 | 8.03 | 89.47 | 2.65 | 114.96 | 84.24 | |
| T8 | 9.07 | 97.17 | 3.25 | 130.04 | 83.76 | |
| T9 | 6.97 | 79.30 | 1.48 | 104.18 | 80.21 | |
| 5 dS m−1 | T1 | 4.90 | 54.83 | 0.69 | 42.90 | 69.33 |
| T2 | 4.90 | 54.43 | 0.73 | 50.81 | 72.82 | |
| T3 | 4.77 | 70.70 | 0.92 | 40.95 | 74.41 | |
| T4 | 5.97 | 68.87 | 1.23 | 72.99 | 75.52 | |
| T5 | 7.00 | 72.83 | 1.35 | 60.76 | 76.89 | |
| T6 | 5.67 | 70.53 | 1.42 | 53.46 | 78.17 | |
| T7 | 6.03 | 62.57 | 1.29 | 74.73 | 73.70 | |
| T8 | 4.97 | 70.87 | 0.90 | 42.13 | 70.65 | |
| T9 | 4.20 | 64.70 | 0.64 | 39.95 | 70.29 | |
| 10 dS m−1 | T1 | 3.07 | 35.57 | 0.55 | 27.93 | 62.31 |
| T2 | 3.17 | 40.57 | 0.53 | 30.97 | 64.25 | |
| T3 | 4.37 | 51.87 | 0.60 | 25.61 | 64.40 | |
| T4 | 5.83 | 42.57 | 0.93 | 41.02 | 66.11 | |
| T5 | 5.03 | 45.03 | 0.79 | 43.13 | 64.01 | |
| T6 | 5.93 | 52.70 | 1.08 | 56.69 | 66.65 | |
| T7 | 6.40 | 54.57 | 1.21 | 54.71 | 67.83 | |
| T8 | 4.53 | 55.77 | 0.91 | 62.59 | 65.15 | |
| T9 | 4.63 | 51.20 | 0.91 | 42.58 | 64.41 | |
| SEM0.05 | 0.144 | 1.881 | 0.026 | 1.963 | 0.564 | |
| Significance | *** | *** | *** | *** | *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmad, A.; Tola, E.; Alshahrani, T.S.; Seleiman, M.F. Enhancement of Morphological and Physiological Performance of Zea mays L. under Saline Stress Using ZnO Nanoparticles and 24-Epibrassinolide Seed Priming. Agronomy 2023, 13, 771. https://doi.org/10.3390/agronomy13030771
Ahmad A, Tola E, Alshahrani TS, Seleiman MF. Enhancement of Morphological and Physiological Performance of Zea mays L. under Saline Stress Using ZnO Nanoparticles and 24-Epibrassinolide Seed Priming. Agronomy. 2023; 13(3):771. https://doi.org/10.3390/agronomy13030771
Chicago/Turabian StyleAhmad, Awais, ElKamil Tola, Thobayet S. Alshahrani, and Mahmoud F. Seleiman. 2023. "Enhancement of Morphological and Physiological Performance of Zea mays L. under Saline Stress Using ZnO Nanoparticles and 24-Epibrassinolide Seed Priming" Agronomy 13, no. 3: 771. https://doi.org/10.3390/agronomy13030771






