Physiological and Biochemical Changes in the Seeds of Naturally Aged Wenling Medic (Medicago polymorpha) with Its Recovery of Viability
Abstract
1. Introduction
2. Materials and Methods
2.1. Seed Material Sources and Storage Conditions
2.2. Methodology of the Experiment
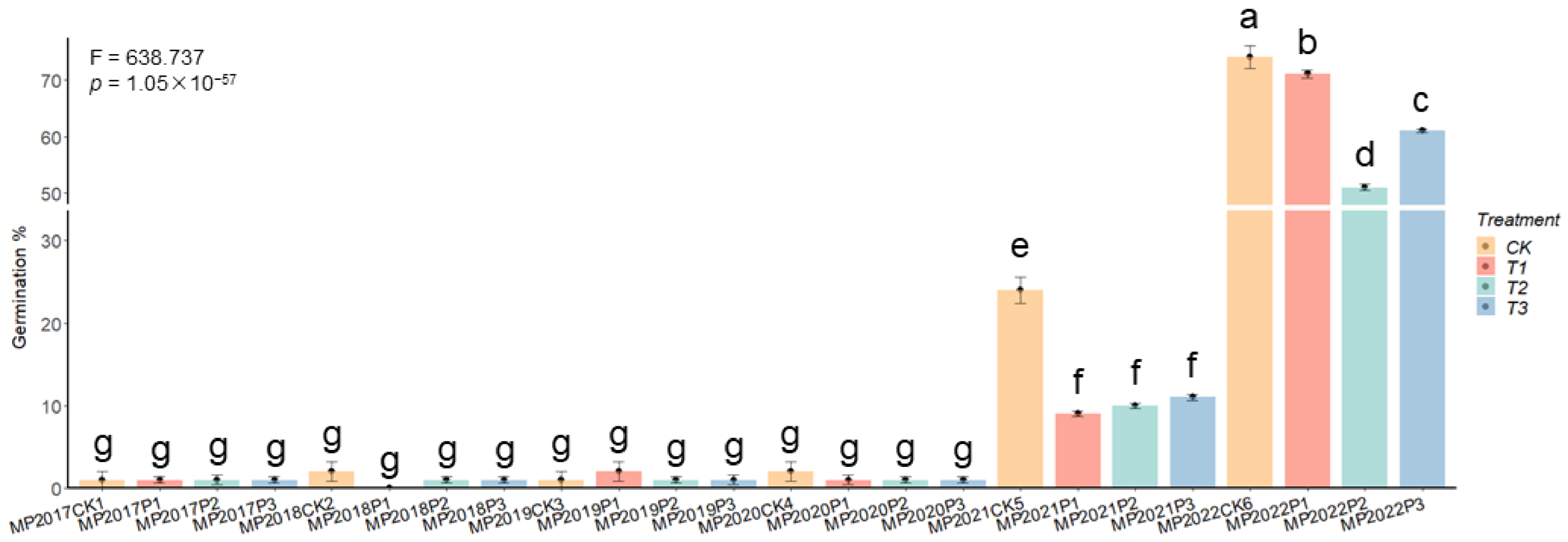
2.2.1. Germination Test and Seed Germination Capacity
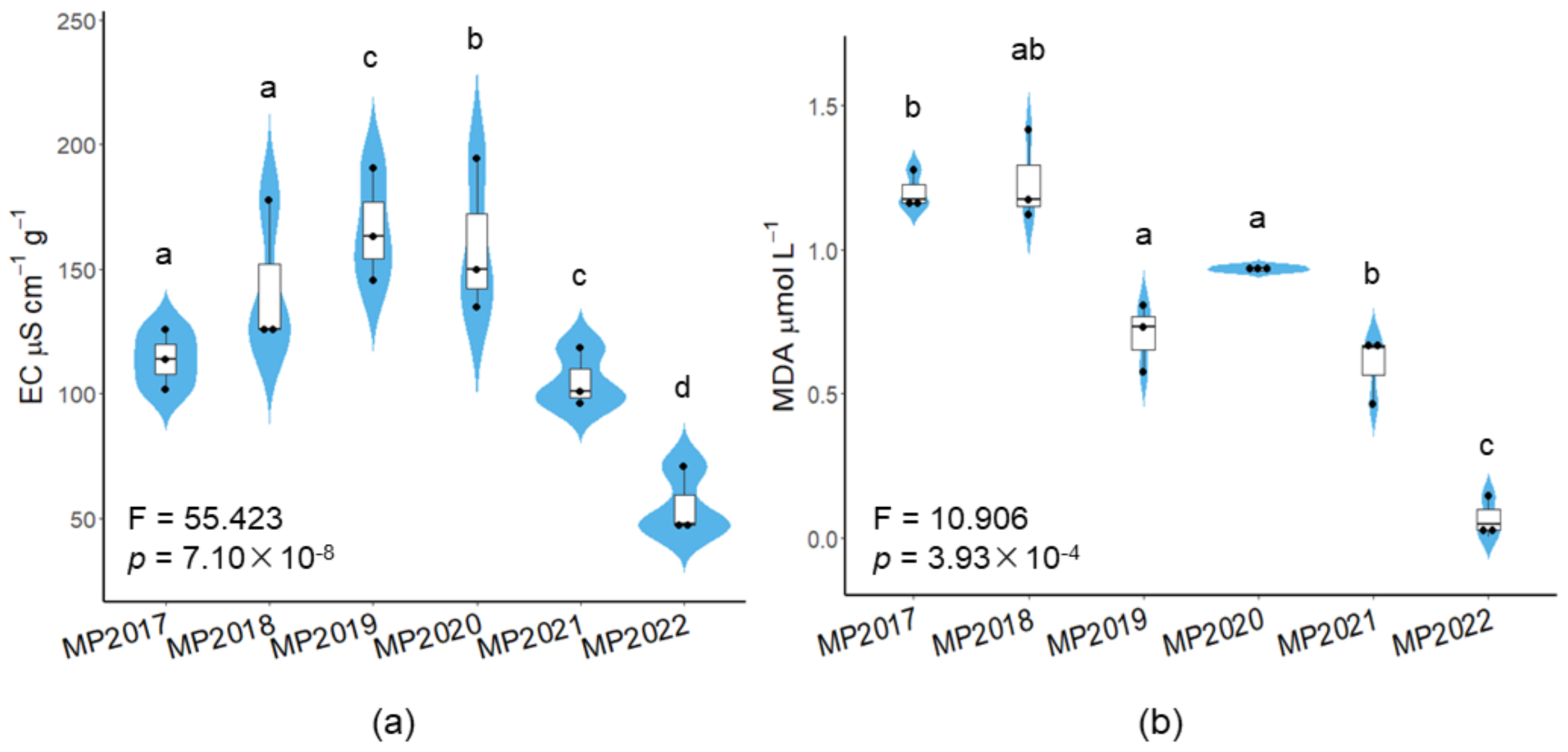
2.2.2. Seed Conductivity Test
2.2.3. Seed Viability Test (Tetrazolium Test)
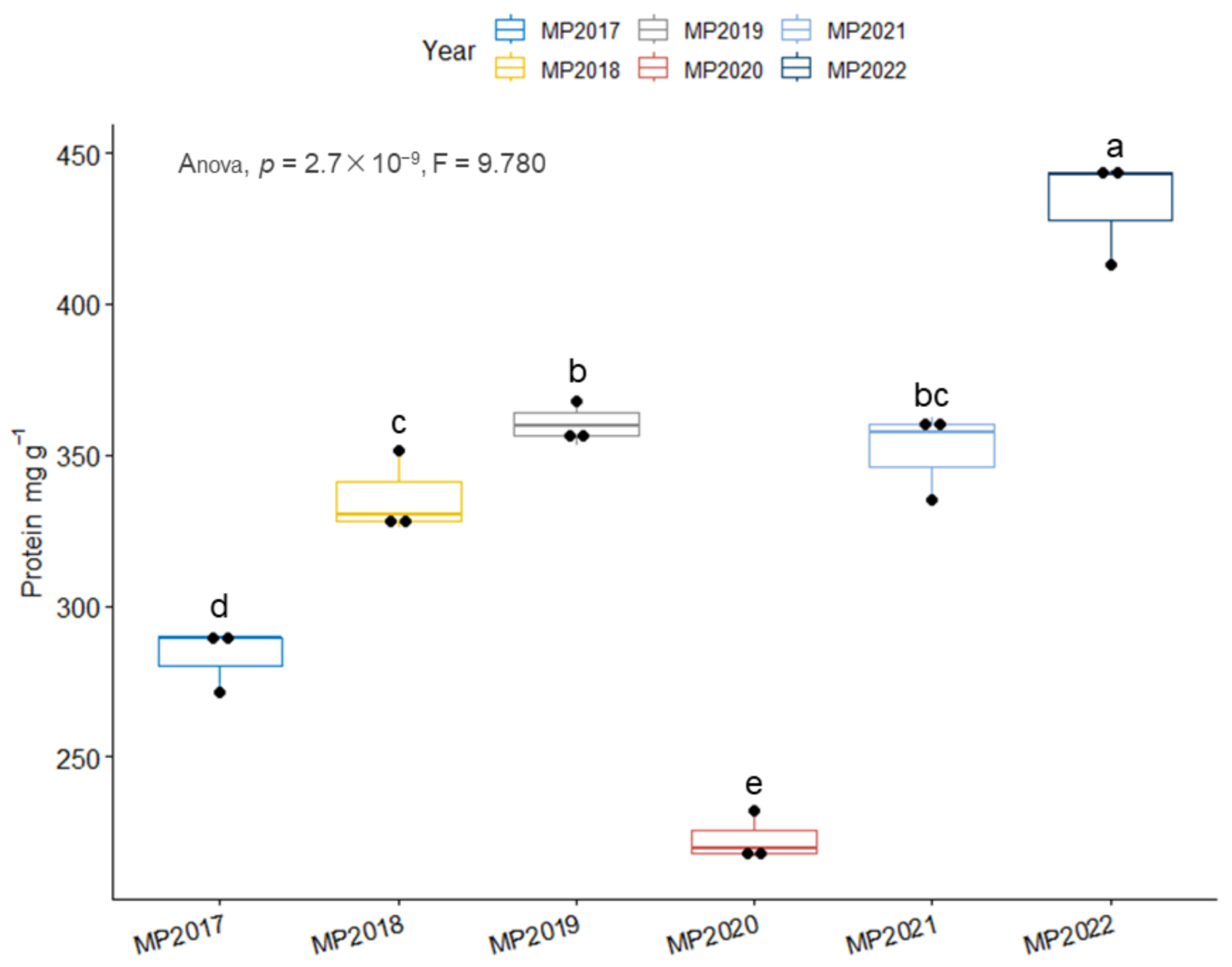
2.2.4. Determination of Seed Protein Content
2.2.5. Determination of Seed Carbohydrates Content
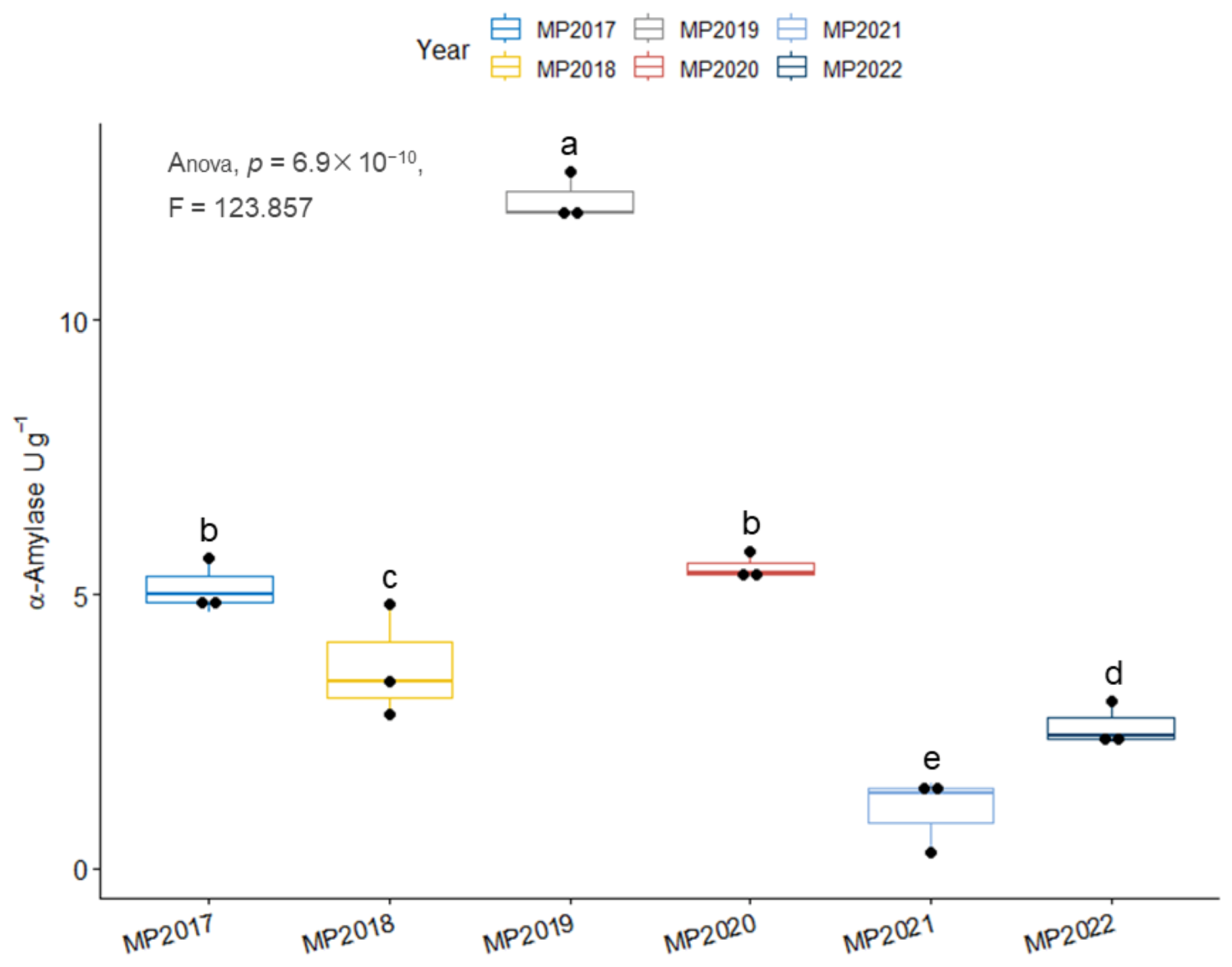
2.2.6. Determination of Seed Amylase Activity
2.2.7. Determination of Malondialdehyde Content of Seeds
2.2.8. Priming Based on Polyethylene Glycol (PEG) Osmoregulation
2.2.9. Statistical Analysis and Plotting Methods
3. Results
3.1. Age Strongly Correlated with Lipid Peroxidation and Cell Membrane Permeability
3.2. The Carbohydrate Content Decreases Significantly with Storage Age
3.3. Protein Content Was Not Significantly Correlated with Storage Time
3.4. There Is No Discernible Relationship between Storage Age and Amylase Content
3.5. PEG-6000 Seed Start Therapy Is Ineffective at Increasing Seed Viability
3.6. TTC Staining Test for Seed Viability
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cui, J.; Wang, X.; Wei, Z.; Jin, B. Medicago truncatula (Model Legume), Medicago sativa (Alfalfa), Medicago polymorpha (Bur Clover), and Medicago ruthenica. Trends Genet. 2022, 38, 782–783. [Google Scholar] [CrossRef]
- Cui, J.; Lu, Z.; Wang, T.; Chen, G.; Mostafa, S.; Ren, H.; Liu, S.; Fu, C.; Wang, L.; Zhu, Y.; et al. The genome of Medicago polymorpha provides insights into its edibility and nutritional value as a vegetable and forage legume. Hortic. Res. 2021, 8, 47. [Google Scholar] [CrossRef]
- Nichols, P.; Loi, A.; Nutt, B.; Evans, P.; Craig, A.; Pengelly, B.; Dear, B.; Lloyd, D.; Revell, C.; Nair, R.; et al. New annual and short-lived perennial pasture legumes for Australian agriculture—15 years of revolution. Field Crops Res. 2007, 104, 10–23. [Google Scholar] [CrossRef]
- Ren, H.; Wei, Z.; Zhihong; Chen, X.; Li, Y. Effects of Temperature and Hardseededness on Seed Germination of Medicago polymorpha. Chin. Wild Plant Resour. 2019, 38, 49–52. (In Chinese) [Google Scholar] [CrossRef]
- De Vitis, M.; Hay, F.R.; Dickie, J.B.; Trivedi, C.; Choi, J.; Fiegener, R. Seed storage: Maintaining seed viability and vigor for restoration use. Restor. Ecol. 2020, 28, S249–S255. [Google Scholar] [CrossRef]
- Kibinza, S.; Vinel, D.; Côme, D.; Bailly, C.; Corbineau, F. Sunflower seed deterioration as related to moisture content during ageing, energy metabolism and active oxygen species scavenging. Physiol. Plant. 2006, 128, 496–506. [Google Scholar] [CrossRef]
- Cakmak, T.; Atici, O.; Agar, G. The natural aging-related biochemical changes in the seeds of two legume varieties stored for 40 years. Acta Agric. Scand. Sect. B Soil Plant Sci. 2010, 60, 353–360. [Google Scholar] [CrossRef]
- Min, C.W.; Lee, S.H.; Cheon, Y.E.; Han, W.Y.; Ko, J.M.; Kang, H.W.; Kim, Y.C.; Agrawal, G.K.; Rakwal, R.; Gupta, R.; et al. In-depth proteomic analysis of Glycine max seeds during controlled deterioration treatment reveals a shift in seed metabolism. J. Proteom. 2017, 169, 125–135. [Google Scholar] [CrossRef]
- Damaris, R.N.; Lin, Z.; Yang, P.; He, D. The Rice Alpha-Amylase, Conserved Regulator of Seed Maturation and Germination. Int. J. Mol. Sci. 2019, 20, 450. [Google Scholar] [CrossRef]
- Prado, J.P.D.; Krzyzanowski, F.C.; Martins, C.C.; Vieira, R.D. Physiological potential of soybean seeds and its relationshipto electrical conductivity. J. Seed Sci. 2019, 41, 407–415. [Google Scholar] [CrossRef]
- Del Egido, L.L.; Navarro-Miró, D.; Martinez-Heredia, V.; Toorop, P.E.; Iannetta, P.P.M. A Spectrophotometric Assay for Robust Viability Testing of Seed Batches Using 2,3,5-Triphenyl Tetrazolium Chloride: Using Hordeum vulgare L. as a Model. Front. Plant Sci. 2017, 8, 747. [Google Scholar] [CrossRef] [PubMed]
- Shekari, A.; Nazeri, V.; Shokrpour, M. Pollen viability and storage life in Leonurus cardiaca L. J. Appl. Res. Med. Aromat. Plants 2016, 3, 101–104. [Google Scholar] [CrossRef]
- Liang, H.; Xiong, Y.; Guo, B.; Yan, H.; Jian, S.; Ren, H.; Zeng, S.; Wu, K.; da Silva, J.A.T.; Xiong, Y.; et al. Effective breaking of dormancy of Scaevola sericea seeds with seawater, improved germination, and reliable viability testing with 2,3,5-triphenyl-tetrazolium chloride. South Afr. J. Bot. 2020, 132, 73–78. [Google Scholar] [CrossRef]
- Bortolin, G.S.; Teixeira, S.B.; Pinheiro, R.D.M.; Ávila, G.E.; Carlos, F.; Pedroso, C.E.D.S.; Deuner, S. Seed Priming with Salicylic Acid Minimizes Oxidative Effects of Aluminum on Trifolium Seedlings. J. Soil Sci. Plant Nutr. 2020, 20, 2502–2511. [Google Scholar] [CrossRef]
- Colete, J.C.F.; Vieira, R.D.; Dutra, A.S. Electrical conductivity and soybean seedling emergence. Sci. Agric. 2004, 61, 386–391. [Google Scholar] [CrossRef]
- Hopper, N.W.; Hinton, H.R. Electrical Conductivity as a Measure of Planting Seed Quality in Cotton. Agron. J. 1987, 79, 147–152. [Google Scholar] [CrossRef]
- Li, H.; Guo, X. Principles and Methods of Biochemistry Experimental Techniques, 2nd ed.; China Agriculture Press: Beijing, China, 2013; ISBN 978-7-109-18403-9. (In Chinese) [Google Scholar]
- Li, H.; Guo, X. Chemistry of Carbohydrate. In Principles and Techniques of Biochemistry Experiment; China Agriculture Press: Beijing, China, 2013; pp. 64–77. ISBN 978-7-109-18403-9. (In Chinese) [Google Scholar]
- John, A.; Barnett, G.; Miller, T.B. The determination of soluble carbohydrate in dried samples of grass silage by the anthrone method. J. Sci. Food Agric. 1950, 1, 336–339. [Google Scholar] [CrossRef]
- Li, H. Principles and Techniques of Plant Physiological Biochemical Experiment; Higher Education Press: Beijing, China, 2000; ISBN 7-04-008076-1. (In Chinese) [Google Scholar]
- Fu, B.; Ma, L.; Ma, H.; He, R.; Yang, X. Effects of PEG priming on seed germination and physiological characteristics of seedlings of Coronilla varia. Grassl. Turf 2021, 41, 126–131. (In Chinese) [Google Scholar] [CrossRef]
- Wang, D.; Wang, Y.; Pan, T.; Gao, M.; Miao, X. Effect of PEG Priming on Seed Vigor of Foxtail Millet at Germination Period and Comprehensive Evaluation. Seed 2022, 41, 106–110+120. (In Chinese) [Google Scholar] [CrossRef]
- Zhu, Y.; Chen, D.; Huang, Y.; Fu, F. Effects of PEG Priming on seed vigor during artificial aging of Chrysanthemum. J. Chin. Med. Mater. 2020, 43, 802–806. (In Chinese) [Google Scholar] [CrossRef]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis, 2nd ed.; Springer: New York, NY, USA, 2016; ISBN 978-3-319-24277-4. [Google Scholar]
- Kassambara, A. Ggpubr: “ggplot2” Based Publication Ready Plots, version 0.4.0. Available online: https://cran.r-project.org/package=ggpubr (accessed on 2 January 2023).
- Xu, S.; Chen, M.; Feng, T.; Zhan, L.; Zhou, L.; Yu, G. Use ggbreak to Effectively Utilize Plotting Space to Deal With Large Datasets and Outliers. Front Genet. 2021, 12, 774846. [Google Scholar] [CrossRef]
- Silva, L.; Medeiros, A.D.; Oliveira, A.M.S. SeedCalc: Seed Germination and Seedling Growth Indexes, version 1.0.0; Available online: https://cran.r-project.org/package=SeedCalc (accessed on 2 January 2023).
- Aravind, J.; Devi, S.V.; Radhamani, J.; Jacob, S.R.; Srinivasan, K. Germinationmetrics: Seed Germination Indices and Curve Fitting, version 0.1.7.9000; Available online: https://github.com/aravind-j/germinationmetrics (accessed on 2 January 2023).
- Chen, C.; Wang, R.; Dong, S.; Wang, J.; Ren, C.X.; Chen, C.P.; Yan, J.; Zhou, T.; Wu, Q.H.; Pei, J.; et al. Integrated proteome and lipidome analysis of naturally aged safflower seeds varying in vitality. Plant Biol. 2022, 24, 266–277. [Google Scholar] [CrossRef] [PubMed]
- Rajjou, L.; Duval, M.; Gallardo, K.; Catusse, J.; Bally, J.; Job, C.; Job, D. Seed Germination and Vigor. Annu. Rev. Plant Biol. 2012, 63, 507–533. [Google Scholar] [CrossRef]
- Sung, J.; Chiu, C. Lipid peroxidation and peroxide-scavenging enzymes of naturally aged soybean seed. Plant Sci. 1995, 110, 45–52. [Google Scholar] [CrossRef]
- Tsikas, D. Assessment of lipid peroxidation by measuring malondialdehyde (MDA) and relatives in biological samples: Analytical and biological challenges. Anal. Biochem. 2017, 524, 13–30. [Google Scholar] [CrossRef]
- Thornton, J.M.; Powell, A.A.; Matthews, S. Investigation of the relationship between seed leachate conductivity and the germination of Brassica seed. Ann. Appl. Biol. 1990, 117, 129–135. [Google Scholar] [CrossRef]
- Sen, A.; Puthur, J.T. Chapter 18—Seed Priming-Induced Physiochemical and Molecular Events in Plants Coupled to Abiotic Stress Tolerance: An Overview. In Priming-Mediated Stress and Cross-Stress Tolerance in Crop Plants; Hossain, M.A., Liu, F., Burritt, D.J., Fujita, M., Huang, B., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 303–316. ISBN 978-0-12-817892-8. [Google Scholar]
- Huang, P.; Li, C.; Che, P.; Liu, H.; Zhao, Z.; Feng, L.; Liu, X.; Liao, W. Hydrogen Gas Enhanced Seed Germination by Increasing Trehalose Biosynthesis in Cucumber. J. Plant Growth Regul. 2022, 1–15. [Google Scholar] [CrossRef]
- Zhang, W.; Mace, W.J.; Matthew, C.; Card, S. The Impact of Endophyte Infection, Seed Aging, and Imbibition on Selected Sugar Metabolite Concentrations in Seed. J. Agric. Food Chem. 2019, 67, 6921–6929. [Google Scholar] [CrossRef]
- Iseki, K.; Olaleye, O.; Ishikawa, H. Intra-plant variation in seed weight and seed protein content of cowpea. Plant Prod. Sci. 2020, 23, 103–113. [Google Scholar] [CrossRef]
- Wang, B.; Yang, R.; Ji, Z.; Zhang, H.; Zheng, W.; Zhang, H.; Feng, F. Evaluation of Biochemical and Physiological Changes in Sweet Corn Seeds under Natural Aging and Artificial Accelerated Aging. Agronomy 2022, 12, 1028. [Google Scholar] [CrossRef]
- Das, G.; Sen-Mandi, S. Scutellar Amylase Activity in Naturally Aged and Accelerated Aged Wheat Seeds. Ann. Bot. 1992, 69, 497–501. [Google Scholar] [CrossRef]
- Livesley, M.A.; Bray, C.M. The Effects of Ageing upon α-Amylase Production and Protein Synthesis by Wheat Aleurone Layers. Ann. Bot. 1991, 68, 69–73. [Google Scholar] [CrossRef]
- Petruzzelli, L.; Taranto, G. Amylase Activity and Loss of Viability in Wheat. Ann. Bot. 1990, 66, 375–378. [Google Scholar] [CrossRef]
- Chen, L.T.; Sun, A.Q.; Yang, M.; Chen, L.L.; Ma, X.L.; Li, M.L.; Yin, Y.P. Relationships of wheat seed vigor with enzyme activities and gene expression related to seed germination under stress conditions. J. Appl. Ecol. 2017, 28, 609–619. (In Chinese) [Google Scholar] [CrossRef]
- Lakon, G. The topographical tetrazolium method for determining the germinating capacity of seeds. Plant Physiol. 1949, 24, 389–394. [Google Scholar] [CrossRef]
- Banerjee, P.; Venugopalan, V.K.; Nath, R.; Chakraborty, P.K.; Gaber, A.; Alsanie, W.F.; Raafat, B.M.; Hossain, A. Seed Priming and Foliar Application of Nutrients Influence the Productivity of Relay Grass Pea (Lathyrus sativus L.) through Accelerating the Photosynthetically Active Radiation (PAR) Use Efficiency. Agronomy 2022, 12, 1125. [Google Scholar] [CrossRef]
- Jisha, K.C.; Vijayakumari, K.; Puthur, J.T. Seed priming for abiotic stress tolerance: An overview. Acta Physiol. Plant. 2013, 35, 1381–1396. [Google Scholar] [CrossRef]
- Ibrahim, E.A. Seed priming to alleviate salinity stress in germinating seeds. J. Plant Physiol. 2016, 192, 38–46. [Google Scholar] [CrossRef]
- Lei, C.; Bagavathiannan, M.; Wang, H.; Sharpe, S.M.; Meng, W.; Yu, J. Osmopriming with Polyethylene Glycol (PEG) for Abiotic Stress Tolerance in Germinating Crop Seeds: A Review. Agronomy 2021, 11, 2194. [Google Scholar] [CrossRef]
- Liu, G.; Li, Y.; Yang, L.; Wei, Y.; Wang, X.; Wang, Z.; Tao, L. Cytotoxicity study of polyethylene glycol derivatives. RSC Adv. 2017, 7, 18252–18259. [Google Scholar] [CrossRef]
- Chen, H.; Zeng, Y.; Yang, Y.; Huang, L.; Tang, B.; Zhang, H.; Hao, F.; Liu, W.; Li, Y.; Liu, Y.; et al. Allele-aware chromosome-level genome assembly and efficient transgene-free genome editing for the autotetraploid cultivated alfalfa. Nat. Commun. 2020, 11, 2494. [Google Scholar] [CrossRef] [PubMed]
- Arif, M.; Jan, M.; Marwat, K.; Khan, M. Seed Priming Improves Emergence and Yield of Soybean. Can. J. Bot. 2008, 40, 1169–1177. [Google Scholar]
- Guo, H. Seed Biology; Beijing United Publishing: Beijing, China, 2019; ISBN 978-7-5596-3428-3. [Google Scholar]
- Zhang, Y.; Tao, M.; Guo, X.; Xin, P. Study on Optimal Moisture Contents for Seed Storage of Millet, Mung Bean, Pea and Adzuki Bean. Seed 2001, 16–20. (In Chinese) [Google Scholar] [CrossRef]
- Dobiesz, M.; Piotrowicz-Cieślak, A.I. Proteins in Relation to Vigor and Viability of White Lupin (Lupinus albus L.) Seed Stored for 26 Years. Front. Plant Sci. 2017, 8, 1392. [Google Scholar] [CrossRef] [PubMed]
- Porsild, A.E.; Harington, C.R.; Mulligan, G.A. Lupinus arcticus Wats. Grown from Seeds of Pleistocene Age. Science 1967, 158, 113–114. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Ren, H.; Wu, Z.; Liu, G.; Chen, X.; Qiao, Z. A New Medicago polymorpha Cultivar “Huaiyang Jinhuacai”. Acta Hortic. Sin. 2015, 42, 2335. (In Chinese) [Google Scholar] [CrossRef]


| Harvested Year | Storage Age (Years) | Test Number | Location |
|---|---|---|---|
| 2017 | 6 | MP2017 | Wenling, Zhejiang, China |
| 2018 | 5 | MP2018 | Wenling, Zhejiang, China |
| 2019 | 4 | MP2019 | Wenling, Zhejiang, China |
| 2020 | 3 | MP2020 | Wenling, Zhejiang, China |
| 2021 | 2 | MP2021 | Wenling, Zhejiang, China |
| 2022 | 1 | MP2022 | Wenling, Zhejiang, China |
| Samples | N | Mean ± SE * (p < 0.01) | 95%CI † | |
|---|---|---|---|---|
| Upper | Lower | |||
| MP2017P1 | 3 | 0.33 ± 0.33 g | −1.10 | 1.77 |
| MP2017P2 | 3 | 1.00 ± 0.58 g | −1.48 | 3.48 |
| MP2017P3 | 3 | 0.33 ± 0.33 g | −1.10 | 1.77 |
| MP2017CK1 | 4 | 1.00 ± 1.00 g | −2.18 | 4.18 |
| MP2018P1 | 3 | 0.00 ± 0.00 g | 0.00 | 0.00 |
| MP2018P2 | 3 | 0.67 ± 0.33 g | −0.77 | 2.10 |
| MP2018P3 | 3 | 0.33 ± 0.33 g | −1.10 | 1.77 |
| MP2018CK2 | 4 | 2.00 ± 1.15 g | −1.67 | 5.67 |
| MP2019P1 | 3 | 2.00 ± 1.15 g | −2.97 | 6.97 |
| MP2019P2 | 3 | 0.33 ± 0.33 g | −1.10 | 1.77 |
| MP2019P3 | 3 | 1.00 ± 0.58 g | −1.48 | 3.48 |
| MP2019CK3 | 4 | 1.00 ± 1.00 g | −2.18 | 4.18 |
| MP2020P1 | 3 | 1.00 ± 0.58 g | −1.48 | 3.48 |
| MP2020P2 | 3 | 0.67 ± 0.33 g | −0.77 | 2.10 |
| MP2020P3 | 3 | 0.33 ± 0.33 g | −1.10 | 1.77 |
| MP2020CK4 | 4 | 2.00 ± 1.15 g | −1.67 | 5.67 |
| MP2021P1 | 3 | 8.67 ± 0.33 f | 7.23 | 10.10 |
| MP2021P2 | 3 | 9.33 ± 0.33 f | 7.90 | 10.77 |
| MP2021P3 | 3 | 10.67 ± 0.33 f | 9.23 | 12.10 |
| MP2021CK5 | 4 | 24.00 ± 1.63 e | 18.80 | 29.20 |
| MP2022P1 | 3 | 70.67 ± 0.67 b | 67.80 | 73.54 |
| MP2022P2 | 3 | 51.00 ± 0.58 d | 48.52 | 53.48 |
| MP2022P3 | 3 | 60.33 ± 0.33 c | 58.90 | 61.77 |
| MP2022CK6 | 4 | 74.00 ± 2.00 a | 67.64 | 80.36 |
| Samples | GSI * ± SE | CVG ‡ ± SE | CUG † ± SE |
|---|---|---|---|
| MP2017P | 0.38 ± 0.20 | 35.00 ± 15.00 | 0.07 ± 0.03 |
| MP2017 | 0.04 ± 0.00 | 100.00 ± 0.00 | 0.25 ± 0.25 |
| MP2018P | 0.11 ± 0.11 | 33.33 ± 0.00 | 0.06 ± 0.00 |
| MP2018 | 0.20 ± 0.09 | 17.14 ± 2.86 | 0.09 ± 0.05 |
| MP2019P | 0.66 ± 0.37 | 25.40 ± 3.17 | 0.09 ± 0.02 |
| MP2019 | 0.04 ± 0.00 | 50.00 ± 0.00 | 0.13 ± 0.13 |
| MP2020P | 0.46 ± 0.14 | 34.44 ± 8.68 | 0.07 ± 0.02 |
| MP2020 | 0.04 ± 0.00 | 75.00 ± 25.00 | 0.38 ± 0.24 |
| MP2021P | 3.65 ± 0.38 | 37.79 ± 4.34 | 0.07 ± 0.02 |
| MP2021 | 0.04 ± 0.00 | 49.36 ± 3.45 | 3.63 ± 0.25 |
| MP2022P | 25.56 ± 2.54 | 38.09 ± 0.15 | 0.05 ± 0.00 |
| MP2022 | 0.06 ± 0.00 | 36.66 ± 2.58 | 10.82 ± 1.09 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Wei, Z.; Min, X.; Zhao, P.; Yang, L.; Liu, N. Physiological and Biochemical Changes in the Seeds of Naturally Aged Wenling Medic (Medicago polymorpha) with Its Recovery of Viability. Agronomy 2023, 13, 787. https://doi.org/10.3390/agronomy13030787
Li J, Wei Z, Min X, Zhao P, Yang L, Liu N. Physiological and Biochemical Changes in the Seeds of Naturally Aged Wenling Medic (Medicago polymorpha) with Its Recovery of Viability. Agronomy. 2023; 13(3):787. https://doi.org/10.3390/agronomy13030787
Chicago/Turabian StyleLi, Jiaqing, Zhenwu Wei, Xueyang Min, Peizhou Zhao, Linghua Yang, and Nana Liu. 2023. "Physiological and Biochemical Changes in the Seeds of Naturally Aged Wenling Medic (Medicago polymorpha) with Its Recovery of Viability" Agronomy 13, no. 3: 787. https://doi.org/10.3390/agronomy13030787
APA StyleLi, J., Wei, Z., Min, X., Zhao, P., Yang, L., & Liu, N. (2023). Physiological and Biochemical Changes in the Seeds of Naturally Aged Wenling Medic (Medicago polymorpha) with Its Recovery of Viability. Agronomy, 13(3), 787. https://doi.org/10.3390/agronomy13030787






