Long-term Tillage Alters Soil Properties and Rhizosphere Bacterial Community in Lime Concretion Black Soil under Winter Wheat–Summer Maize Double-Cropping System
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Site and Design
2.2. Soil Sampling and Chemical Analysis
2.3. Yield
2.4. DNA Isolation from Soil and High-Throughput Sequencing
2.5. Statistical Analysis of Sequencing Data
2.6. Statistical Analysis
3. Results
3.1. Maize Yield and Soil Properties
3.2. The Bacterial Composition of Rhizosphere Soil under Various Tillage Practices
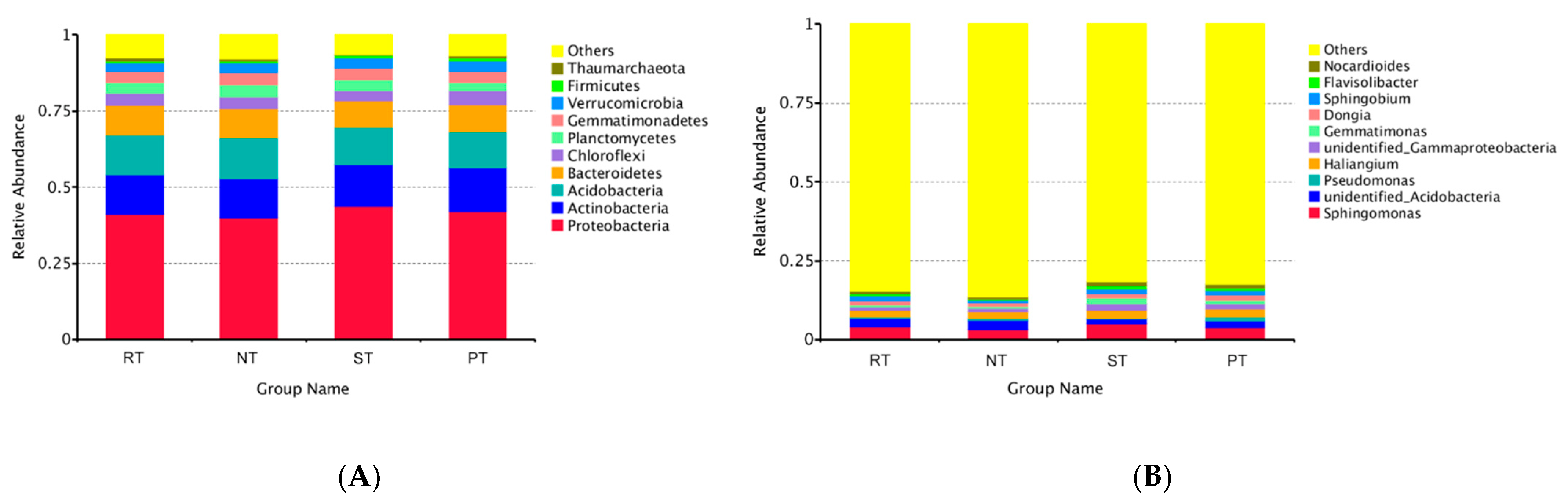
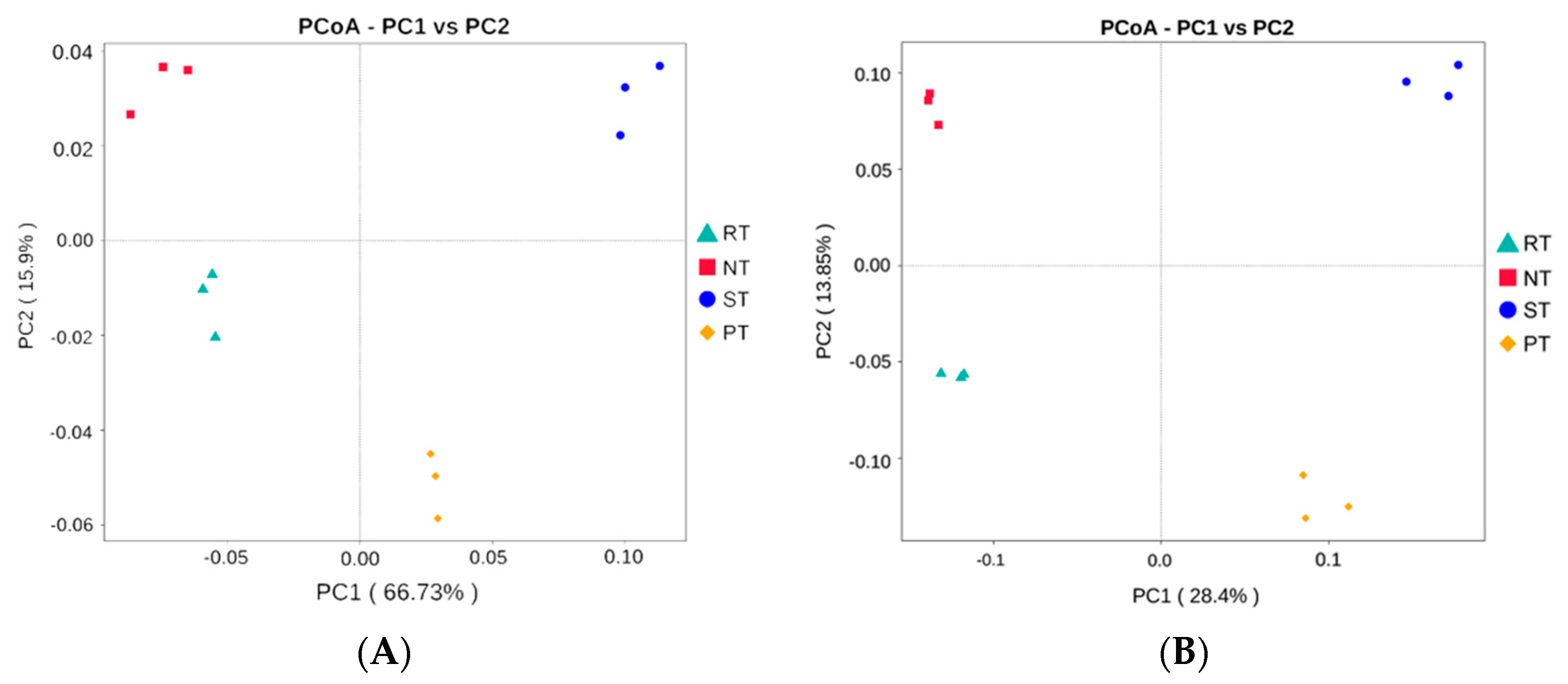
3.3. Response of Alpha and Beta Diversity to Long-Term Tillage Practices

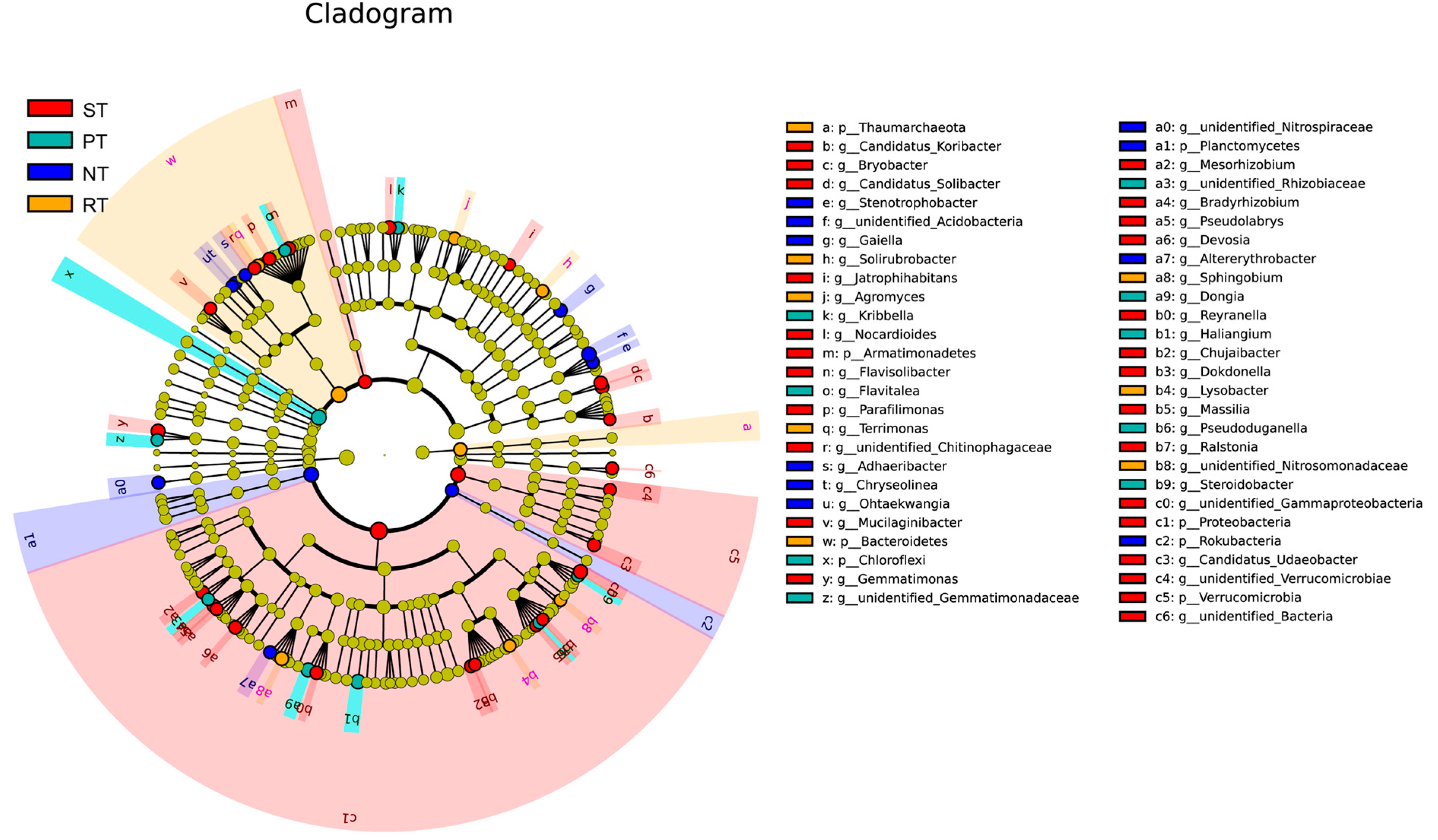
3.4. Identification of Keystone Taxa under Different Tillage Practices
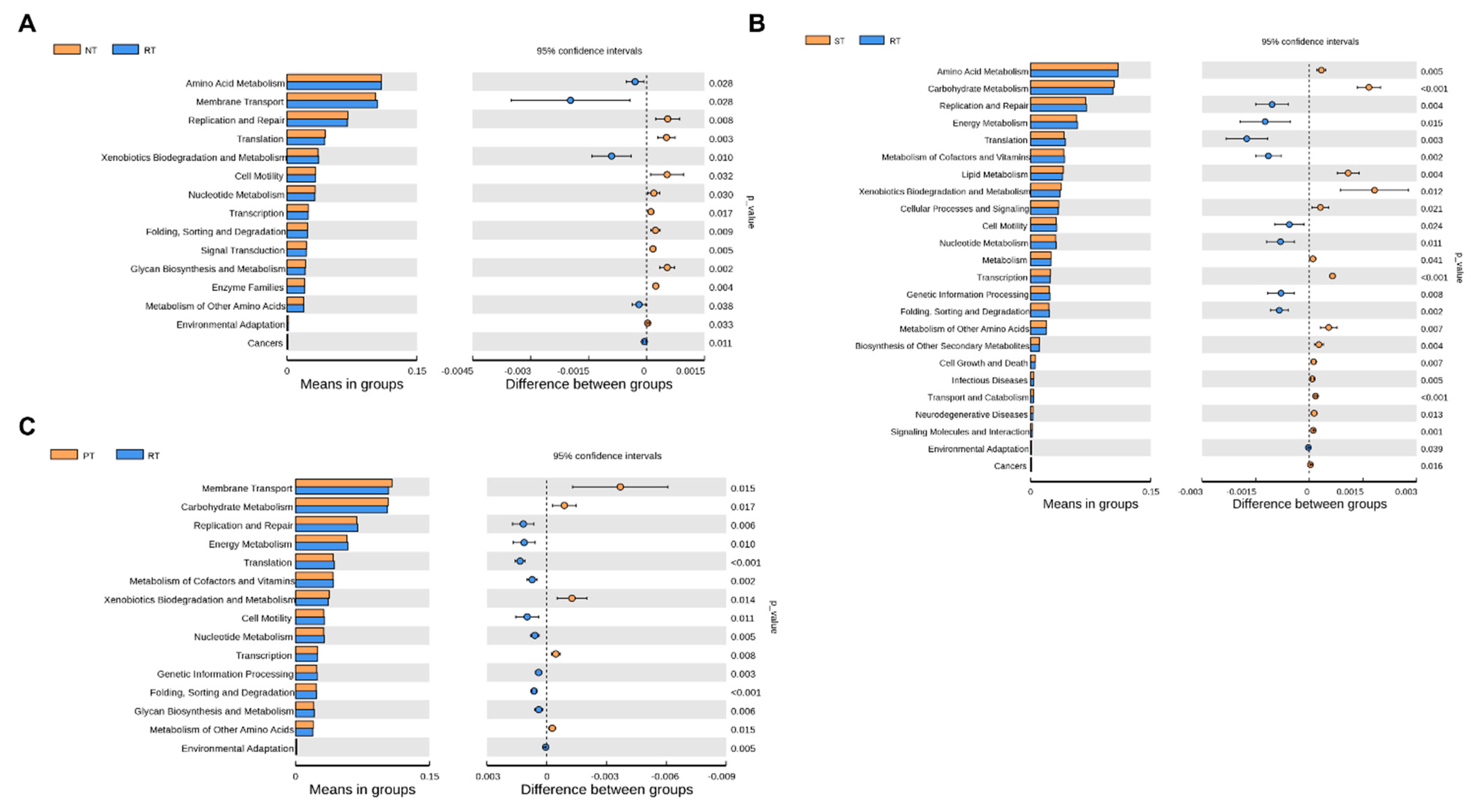
3.5. Soil Bacteria’s Metabolic Responses to Long-Term Tillage
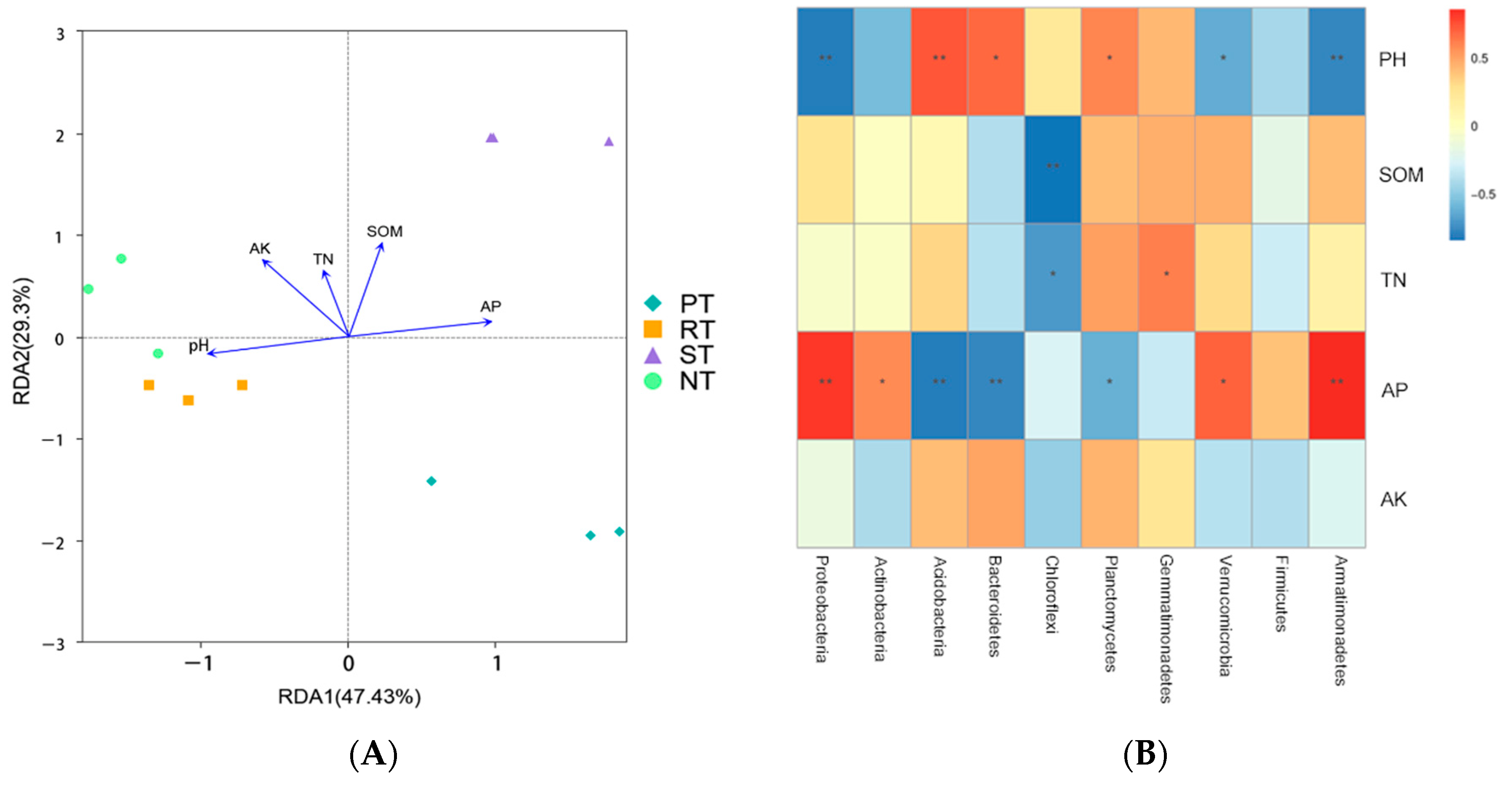
3.6. Relationships between Soil Bacterial Communities and Soil Chemical Properties
4. Discussion
4.1. Effects of Tillage Practices on Yield and Soil Properties
4.2. Response of Microbial Community Composition to Tillage Practices
4.3. Effects of Tillage on Microbial Alpha and Beta Diversity
4.4. Potential Bacterial Functions in Response to Tillage Practices
4.5. Differential Bacteria and Their Relationship with Soil Properties
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jiang, C.; Lu, D.; Zu, C.; Shen, J.; Wang, S.; Guo, Z.; Zhou, J.; Wang, H. One-time root-zone N fertilization increases maize yield, NUE and reduces soil N losses in lime concretion black soil. Sci. Rep. 2018, 8, 10258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, H.; Wu, L.; Zhu, S.; Sun, H.; Xu, C.; Fu, J.; Ning, T. Sensitivities of Physical and Chemical Attributes of Soil Quality to Different Tillage Management. Agronomy 2022, 12, 1153. [Google Scholar] [CrossRef]
- Liu, Z.; Sun, K.; Liu, W.; Gao, T.; Li, G.; Han, H.; Li, Z.; Ning, T. Responses of soil carbon, nitrogen, and wheat and maize productivity to 10 years of decreased nitrogen fertilizer under contrasting tillage systems. Soil Tillage Res. 2019, 196, 104444. [Google Scholar] [CrossRef]
- Kan, Z.-R.; Ma, S.-T.; Liu, Q.-Y.; Liu, B.-Y.; Virk, A.L.; Qi, J.-Y.; Zhao, X.; Lal, R.; Zhang, H.-L. Carbon sequestration and mineralization in soil aggregates under long-term conservation tillage in the North China Plain. Catena 2020, 188, 104428. [Google Scholar] [CrossRef]
- Li, M.; He, P.; Guo, X.-L.; Zhang, X.; Li, L.-J. Fifteen-year no tillage of a Mollisol with residue retention indirectly affects topsoil bacterial community by altering soil properties. Soil Tillage Res. 2020, 205, 104804. [Google Scholar] [CrossRef]
- Liu, X.; Peng, C.; Zhang, W.; Li, S.; An, T.; Xu, Y.; Ge, Z.; Xie, N.; Wang, J. Subsoiling tillage with straw incorporation improves soil microbial community characteristics in the whole cultivated layers: A one-year study. Soil Tillage Res. 2021, 215, 105188. [Google Scholar] [CrossRef]
- Szostek, M.; Szpunar-Krok, E.; Pawlak, R.; Stanek-Tarkowska, J.; Ilek, A. Effect of Different Tillage Systems on Soil Organic Carbon and Enzymatic Activity. Agronomy 2022, 12, 208. [Google Scholar] [CrossRef]
- Zhao, H.; Qin, J.; Gao, T.; Zhang, M.; Sun, H.; Zhu, S.; Xu, C.; Ning, T. Immediate and long-term effects of tillage practices with crop residue on soil water and organic carbon storage changes under a wheat-maize cropping system. Soil Tillage Res. 2021, 218, 105309. [Google Scholar] [CrossRef]
- Wang, H.; Wang, S.; Wang, R.; Zhang, Y.; Wang, X.; Li, J. Direct and indirect linkages between soil aggregates and soil bacterial communities under tillage methods. Geoderma 2019, 354, 113879. [Google Scholar] [CrossRef]
- Sun, Q.; Sun, W.; Zhao, Z.; Jiang, W.; Zhang, P.; Sun, X.; Xue, Q. Soil Compaction and Maize Root Distribution under Subsoiling Tillage in a Wheat–Maize Double Cropping System. Agronomy 2023, 13, 394. [Google Scholar] [CrossRef]
- Lv, L.; Gao, Z.; Liao, K.; Zhu, Q.; Zhu, J. Impact of conservation tillage on the distribution of soil nutrients with depth. Soil Tillage Res. 2023, 225, 105527. [Google Scholar] [CrossRef]
- Pittelkow, C.M.; Liang, X.; Linquist, B.A.; van Groenigen, K.J.; Lee, J.; Lundy, M.E.; van Gestel, N.; Six, J.; Venterea, R.T.; van Kessel, C. Productivity limits and potentials of the principles of conservation agriculture. Nature 2015, 517, 365–368. [Google Scholar] [CrossRef]
- Yang, Y.; Wu, J.; Zhao, S.; Mao, Y.; Zhang, J.; Pan, X.; He, F.; van der Ploeg, M. Impact of long-term sub-soiling tillage on soil porosity and soil physical properties in the soil profile. Land Degrad. Dev. 2021, 32, 2892–2905. [Google Scholar] [CrossRef]
- Bilibio, C.; Uteau, D.; Horvat, M.; Rosskopf, U.; Junge, S.M.; Finckh, M.R.; Peth, S. Impact of Ten Years Conservation Tillage in Organic Farming on Soil Physical Properties in a Loess Soil—Northern Hesse, Germany. Agriculture 2023, 13, 133. [Google Scholar] [CrossRef]
- Gao, J.; Xie, Y.; Jin, H.; Liu, Y.; Bai, X.; Ma, D.; Zhu, Y.; Wang, C.; Guo, T. Nitrous Oxide Emission and Denitrifier Abundance in Two Agricultural Soils Amended with Crop Residues and Urea in the North China Plain. PLoS ONE 2016, 11, e0154773. [Google Scholar] [CrossRef] [Green Version]
- Zhai, L.; Xu, P.; Zhang, Z.; Li, S.; Xie, R.; Zhai, L.; Wei, B. Effects of Deep Vertical Rotary Tillage on Dry Matter Accumulation and Grain Yield of Summer Maize in the Huang-Huai-Hai Plain of China. Soil Tillage Res. 2017, 170, 167–174. [Google Scholar] [CrossRef]
- Peiffer, J.A.; Spor, A.; Koren, O.; Jin, Z.; Tringe, S.G.; Dangl, J.L.; Buckler, E.S.; Ley, R.E. Diversity and heritability of the maize rhizosphere microbiome under field conditions. Proc. Natl. Acad. Sci. USA 2013, 110, 6548–6553. [Google Scholar] [CrossRef] [Green Version]
- Smith, C.R.; Blair, P.L.; Boyd, C.; Cody, B.; Hazel, A.; Hedrick, A.; Kathuria, H.; Khurana, P.; Kramer, B.; Muterspaw, K.; et al. Microbial community responses to soil tillage and crop rotation in a corn/soybean agroecosystem. Ecol. Evol. 2016, 6, 8075–8084. [Google Scholar] [CrossRef]
- Liu, X.; Liu, H.; Ren, D.; Liu, C.; Zhang, Y.; Wang, S.; Li, Z.; Zhang, M. Interlinkages between soil properties and keystone taxa under different tillage practices on the North China Plain. Appl. Soil Ecol. 2022, 178, 104551. [Google Scholar] [CrossRef]
- Sun, R.; Li, W.; Dong, W.; Tian, Y.; Hu, C.; Liu, B. Tillage changes vertical distribution of soil bacterial and fungal communities. Front. Microbiol. 2018, 9, 699. [Google Scholar] [CrossRef]
- Dong, W.; Liu, E.; Yan, C.; Tian, J.; Zhang, H.; Zhang, Y. Impact of no tillage vs. conventional tillage on the soil bacterial community structure in a winter wheat cropping succession in northern China. Eur. J. Soil Biol. 2017, 80, 35–42. [Google Scholar] [CrossRef]
- Hariharan, J.; Sengupta, A.; Grewal, P.; Dick, W.A. Functional Predictions of Microbial Communities in Soil as Affected by Long-term Tillage Practices. Agric. Environ. Lett. 2017, 2, 170031. [Google Scholar] [CrossRef] [Green Version]
- Sun, Q.; Zhang, P.; Zhao, Z.; Li, X.; Sun, X.; Jiang, W. Continuous Wheat/Soybean Cropping Influences Soybean Yield and Rhizosphere Microbial Community Structure and Function. Agronomy 2022, 13, 28. [Google Scholar] [CrossRef]
- Li, Y.-M.; Duan, Y.; Wang, G.-L.; Wang, A.-Q.; Shao, G.-Z.; Meng, X.-H.; Hu, H.-Y.; Zhang, D.-M. Straw alters the soil organic carbon composition and microbial community under different tillage practices in a meadow soil in Northeast China. Soil Tillage Res. 2021, 208, 104879. [Google Scholar] [CrossRef]
- Xu, J.; Han, H.; Ning, T.; Li, Z.; Lal, R. Long-term effects of tillage and straw management on soil organic carbon, crop yield, and yield stability in a wheat-maize system. Field Crop. Res. 2019, 233, 33–40. [Google Scholar] [CrossRef]
- Liu, W.-S.; Kan, Z.-R.; Chen, J.-S.; Zhao, X.; Zhang, H.-L. Effects of tillage and straw management on grain yield and SOC storage in a wheat-maize cropping system. Eur. J. Agron. 2022, 137, 126530. [Google Scholar] [CrossRef]
- Zhang, H.; Shi, Y.; Dong, Y.; Lapen, D.R.; Liu, J.; Chen, W. Subsoiling and conversion to conservation tillage enriched nitrogen cycling bacterial communities in sandy soils under long-term maize monoculture. Soil Tillage Res. 2021, 215, 105197. [Google Scholar] [CrossRef]
- Jackson, L.; Calderon, F.; Steenwerth, K.; Scow, K.; Rolston, D. Responses of soil microbial processes and community structure to tillage events and implications for soil quality. Geoderma 2003, 114, 305–317. [Google Scholar] [CrossRef]
- Prasuhn, V. On-farm effects of tillage and crops on soil erosion measured over 10 years in Switzerland. Soil Tillage Res. 2012, 120, 137–146. [Google Scholar] [CrossRef]
- Shipitalo, M.J.; Owens, L.B.; Bonta, J.V.; Edwards, W.M. Effect of No-Till and Extended Rotation on Nutrient Losses in Surface Runoff. Soil Sci. Soc. Am. J. 2013, 77, 1329–1337. [Google Scholar] [CrossRef]
- Wang, H.; Li, X.; Li, X.; Wang, J.; Li, X.; Guo, Q.; Yu, Z.; Yang, T.; Zhang, H. Long-term no-tillage and different residue amounts alter soil microbial community composition and increase the risk of maize root rot in northeast China. Soil Tillage Res. 2019, 196, 104452. [Google Scholar] [CrossRef]
- Kahlon, M.S.; Lal, R.; Ann-Varughese, M. Twenty two years of tillage and mulching impacts on soil physical characteristics and carbon sequestration in Central Ohio. Soil Tillage Res. 2013, 126, 151–158. [Google Scholar] [CrossRef]
- Liu, E.; Teclemariam, S.G.; Yan, C.; Yu, J.; Gu, R.; Liu, S.; He, W.; Liu, Q. Long-term effects of no-tillage management practice on soil organic carbon and its fractions in the northern China. Geoderma 2014, 213, 379–384. [Google Scholar] [CrossRef]
- Mishra, U.; Ussiri, D.A.; Lal, R. Tillage effects on soil organic carbon storage and dynamics in Corn Belt of Ohio USA. Soil Tillage Res. 2010, 107, 88–96. [Google Scholar] [CrossRef]
- Srour, A.Y.; Ammar, H.A.; Subedi, A.; Pimentel, M.; Cook, R.L.; Bond, J.; Fakhoury, A.M. Microbial Communities Associated with Long-Term Tillage and Fertility Treatments in a Corn-Soybean Cropping System. Front. Microbiol. 2020, 11, 1363. [Google Scholar] [CrossRef]
- Xie, B.; Chen, Y.; Cheng, C.; Ma, R.; Zhao, D.; Li, Z.; Li, Y.; An, X.; Yang, X. Long-term soil management practices influence the rhizosphere microbial community structure and bacterial function of hilly apple orchard soil. Appl. Soil Ecol. 2022, 180, 104627. [Google Scholar] [CrossRef]
- Delgado-Baquerizo, M.; Oliverio, A.M.; Brewer, T.E.; Benavent-González, A.; Eldridge, D.J.; Bardgett, R.D.; Maestre, F.T.; Singh, B.K.; Fierer, N. A global atlas of the dominant bacteria found in soil. Science 2018, 359, 320–325. [Google Scholar] [CrossRef] [Green Version]
- Bryant, D.A.; Costas, A.M.G.; Maresca, J.A.; Chew, A.G.M.; Klatt, C.G.; Bateson, M.M.; Tallon, L.J.; Hostetler, J.; Nelson, W.C.; Heidelberg, J.F.; et al. Candidatus Chloracidobacterium thermophilum: An Aerobic Phototrophic Acidobacterium. Science 2007, 317, 523–526. [Google Scholar] [CrossRef] [Green Version]
- Carlos, F.S.; Schaffer, N.; Marcolin, E.; Fernandes, R.S.; Mariot, R.; Mazzurana, M.; Roesch, L.F.W.; Levandoski, B.; Camargo, F.A.D.O. A long-term no-tillage system can increase enzymatic activity and maintain bacterial richness in paddy fields. Land Degrad. Dev. 2021, 32, 2257–2268. [Google Scholar] [CrossRef]
- Sun, Y.F.; Liu, Z.; Zhang, Y.Q.; Lai, Z.R.; She, W.W.; Bai, Y.X.; Feng, W.; Qin, S.G. Microbial communities and their genetic repertoire mediate the decomposition of soil organic carbon pools in revegetation shrublands in a desert in northern China. Eur. J. Soil Sci. 2019, 71, 93–105. [Google Scholar] [CrossRef]
- Duan, N.; Li, L.; Liang, X.; Fine, A.; Zhuang, J.; Radosevich, M.; Schaeffer, S.M. Variation in Bacterial Community Structure Under Long-Term Fertilization, Tillage, and Cover Cropping in Continuous Cotton Production. Front. Microbiol. 2022, 13. [Google Scholar] [CrossRef] [PubMed]
- Rao, M.P.N.; Luo, Z.-H.; Dong, Z.-Y.; Li, Q.; Liu, B.-B.; Guo, S.-X.; Nie, G.-X.; Li, W.-J. Metagenomic analysis further extends the role of Chloroflexi in fundamental biogeochemical cycles. Environ. Res. 2022, 209, 112888. [Google Scholar] [CrossRef]
- Wang, Z.; Li, Y.; Li, T.; Zhao, D.; Liao, Y. Tillage practices with different soil disturbance shape the rhizosphere bacterial community throughout crop growth. Soil Tillage Res. 2019, 197, 104501. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, L.; Chen, Q.; Wen, X.; Liao, Y. Conservation tillage increases soil bacterial diversity in the dryland of northern China. Agron. Sustain. Dev. 2016, 36, 28. [Google Scholar] [CrossRef] [Green Version]
- Hu, X.; Liu, J.; Liang, A.; Li, L.; Yao, Q.; Yu, Z.; Li, Y.; Jin, J.; Liu, X.; Wang, G. Conventional and conservation tillage practices affect soil microbial co-occurrence patterns and are associated with crop yields. Agric. Ecosyst. Environ. 2021, 319, 107534. [Google Scholar] [CrossRef]
- Lauber, C.L.; Hamady, M.; Knight, R.; Fierer, N. Pyrosequencing-Based Assessment of Soil pH as a Predictor of Soil Bacterial Community Structure at the Continental Scale. Appl. Environ. Microbiol. 2009, 75, 5111–5120. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Pang, X.; Chen, X.; Ye, J.; Lin, S.; Jia, X. Rain-shelter cultivation influence rhizosphere bacterial community structure in pear and its relationship with fruit quality of pear and soil chemical properties. Sci. Hortic. 2020, 269, 109419. [Google Scholar] [CrossRef]

| Treatment | pH | SOM (g/kg) | TN (g/kg) | AP (mg/kg) | AK (mg/kg) |
|---|---|---|---|---|---|
| RT | 7.37 b | 18.88 c | 1.53 b | 23.83 b | 144.92 a |
| NT | 7.83 a | 23.26 b | 2.10 a | 21.99 b | 144.85 a |
| ST | 6.39 d | 28.67 a | 1.97 a | 41.54 a | 144.86 a |
| PT | 6.71 c | 18.96 c | 1.55 b | 44.31 a | 121.57 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Q.; Zhang, P.; Liu, X.; Zhang, H.; Liu, S.; Sun, X.; Jiang, W. Long-term Tillage Alters Soil Properties and Rhizosphere Bacterial Community in Lime Concretion Black Soil under Winter Wheat–Summer Maize Double-Cropping System. Agronomy 2023, 13, 790. https://doi.org/10.3390/agronomy13030790
Sun Q, Zhang P, Liu X, Zhang H, Liu S, Sun X, Jiang W. Long-term Tillage Alters Soil Properties and Rhizosphere Bacterial Community in Lime Concretion Black Soil under Winter Wheat–Summer Maize Double-Cropping System. Agronomy. 2023; 13(3):790. https://doi.org/10.3390/agronomy13030790
Chicago/Turabian StyleSun, Qing, Peiyu Zhang, Xiang Liu, Hongsheng Zhang, Shutang Liu, Xuefang Sun, and Wen Jiang. 2023. "Long-term Tillage Alters Soil Properties and Rhizosphere Bacterial Community in Lime Concretion Black Soil under Winter Wheat–Summer Maize Double-Cropping System" Agronomy 13, no. 3: 790. https://doi.org/10.3390/agronomy13030790




