Response of Kentucky Bluegrass Turfgrass to Plant Growth Regulators
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Measurement
2.3. Statistical Analyses
3. Results
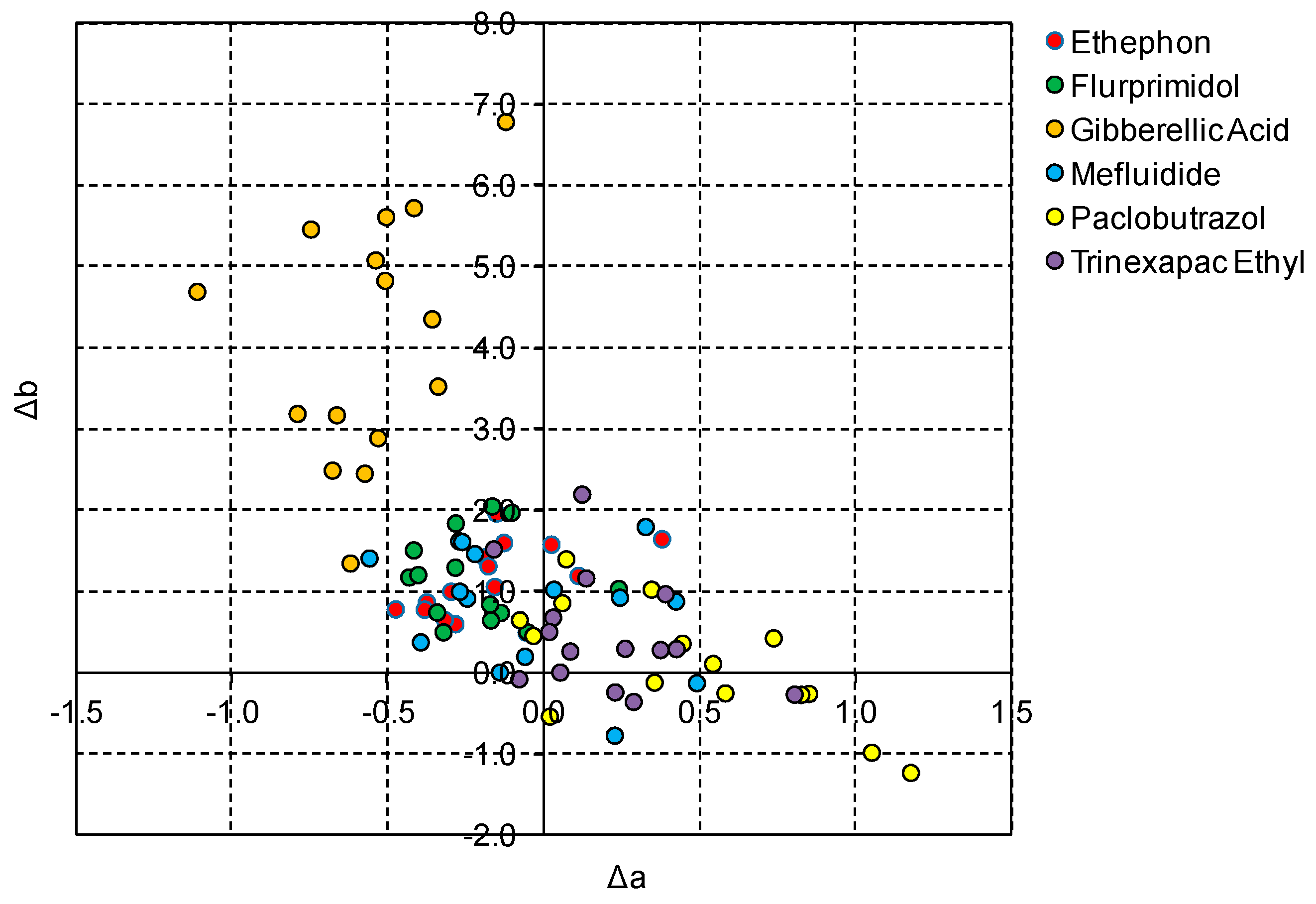
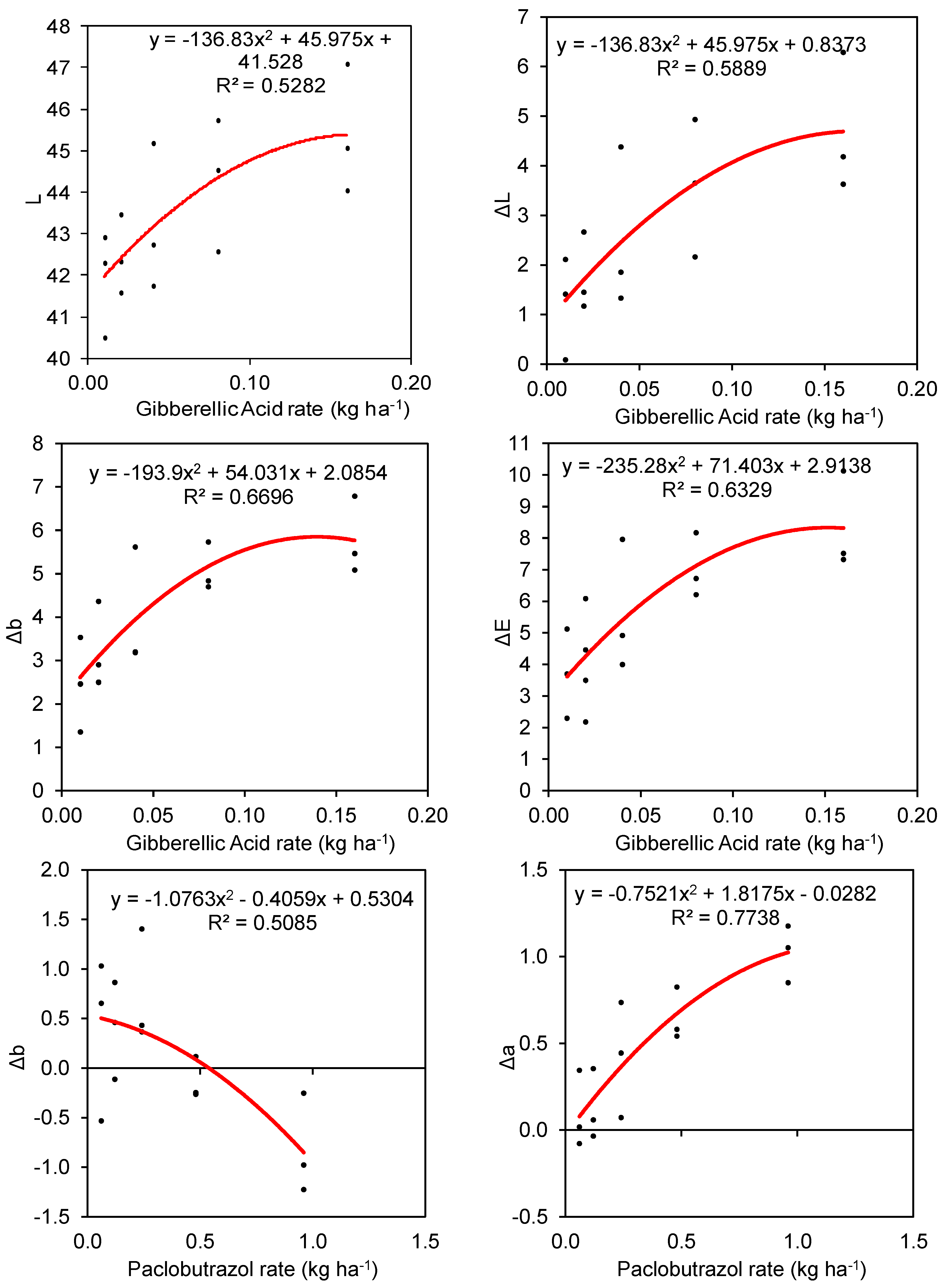
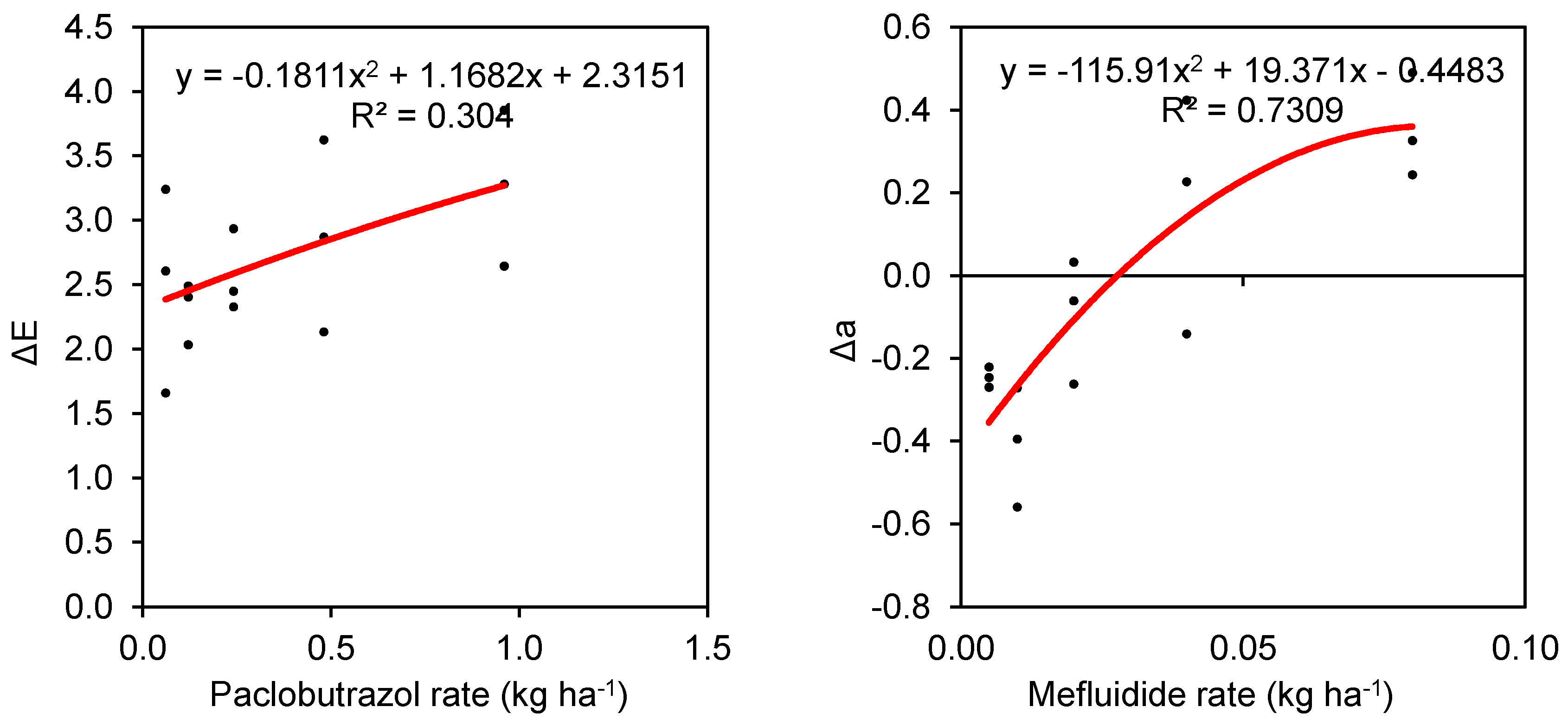
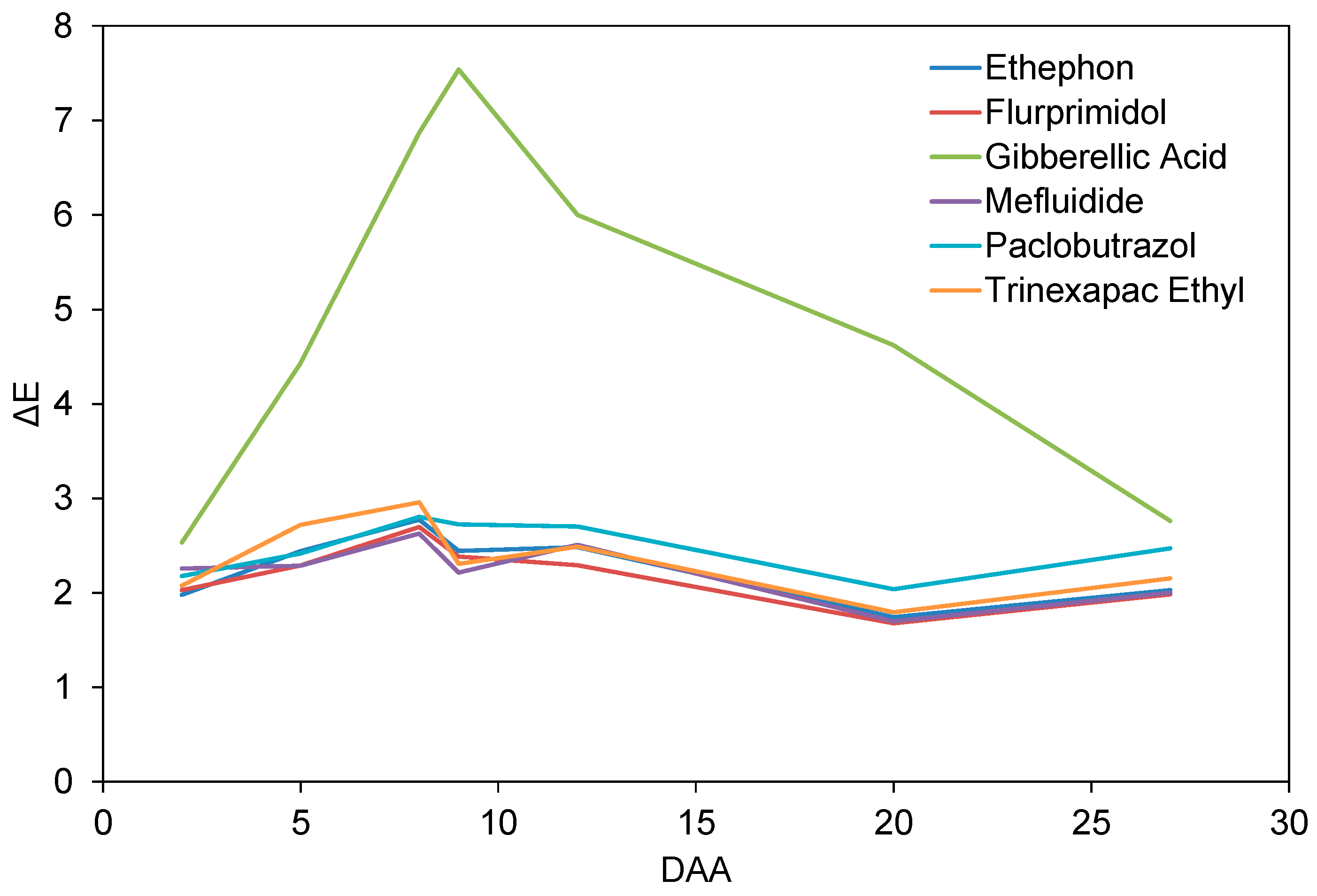
3.1. Turfgrass Color
3.2. Turfgrass Quality
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- March, S.R.; Martins, D.; McElroy, J.S. Growth inhibitors in turfgrass. Planta Daninha Viçosa-MG 2013, 31, 733–747. [Google Scholar] [CrossRef]
- Rademacher, W. Plant growth regulators: Backgrounds and uses in plant production. J. Plant Growth Regul. 2015, 34, 845–872. [Google Scholar] [CrossRef]
- DaCosta, M.; Ebdon, J.S.; Miele, K.; Bernstein, R.P.; Inguagiato, J.C. Plant growth regulator effects on winter hardiness of annual bluegrass putting green turf. Int. Turfgrass Soc. Res. J. 2022, 14, 225–235. [Google Scholar] [CrossRef]
- Henry, G.M.; Moore, M.; Tucker, K.A. Response of creeping bentgrass (Agrostis stolonifera) and weed species to plant growth regulators. Int. Turfgrass Soc. Res. J. 2022, 14, 791–796. [Google Scholar] [CrossRef]
- McCarty, L.B.; Weinbrecht, J.S.; Toler, J.E.; Miller, G.L. St. Augustinegrass response to plant growth retardants. Crop Sci. 2004, 44, 1323–1329. [Google Scholar] [CrossRef]
- Hussein, M.M.M.; Mansour, H.A.; Ashour, H.A. Response of Paspalum vaginatum Turfgrass Grown under Shade Conditions to Paclobutrazol and Trinexapac-Ethyl as Plant Growth Retardants (PGRs). J. Hortic. Sci. Ornam. Plants 2012, 4, 134–147. [Google Scholar]
- Fidanza, M.A.; Wetzel III, H.C.; Agnew, M.L.; Kaminski, J.E. Evaluation of fungicide and plant growth regulator tank-mix programmes on dollar spot severity of creeping bentgrass. Crop Prot. 2006, 25, 1032–1038. [Google Scholar] [CrossRef]
- Pannacci, E.; Covarelli, G.; Tei, F. Evaluation of trinexapac-ethyl for growth regulation of five cool-season turfgrass species. Acta Hortic. 2004, 661, 349–351. [Google Scholar] [CrossRef]
- McCann, S.E.; Huang, B.R. Effects of trinexapac-ethyl foliar application on creeping bentgrass responses to combined drought and heat stress. Crop Sci. 2007, 47, 2121–2128. [Google Scholar] [CrossRef]
- Serena, M.; Schiavon, M.; Sallenave, R.; Leinauer, B. Drought avoidance of warm-season turfgrasses affected by irrigation system, soil surfactant revolution, and plant growth regulator trinexapac-ethyl. Crop Sci. 2020, 60, 485–498. [Google Scholar] [CrossRef]
- Pornaro, C.; Fiorio, S.; Macolino, S.; Richardson, M. Growth and quality responses of low-maintenance turfgrasses to trinexapac-ethyl. Crop Prot. 2017, 98, 236–242. [Google Scholar] [CrossRef]
- Lickfeldt, D.W.; Gardner, D.S.; Branham, B.E.; Voigt, T.B. Implications of Repeated Trinexapac-Ethyl Applications on Kentucky Bluegrass. Agron. J. 2001, 93, 1164–1168. [Google Scholar] [CrossRef]
- Buchanan, B.B.; Gruissem, W.; Jones, R.L. Biochemistry and Molecular Biology of Plants; John Wiley & Sons: Hoboken, NJ, USA, 2015. [Google Scholar]
- McCullough, P.E.; Liu, H.; McCarty, L.B. Response of six dwarf-type bermudagrasses to trinexapac-ethyl. HortScience 2005, 40, 460–462. [Google Scholar] [CrossRef]
- Bigelow, C.A. Plant growth regulators in bentgrass turf areas. USGA Green Sect. Rec. 2012, 50, 1–4. [Google Scholar]
- McElroy, J.S.; Martins, D. Use of herbicides on turfgrass. Planta Daninha Viçosa-MG 2013, 31, 455–467. [Google Scholar] [CrossRef]
- Diehl, K.; Elmore, M.; Koppenhöfer, A.; Murphy, J.; Kostromytska, O. Annual bluegrass weevil (Listronotus maculicollis) and paclobutrazol control annual bluegrass (Poa annua) in creeping bentgrass fairways. Weed Technol. 2022, 36, 137–144. [Google Scholar] [CrossRef]
- Miller, G.L. Effect of watered-in demethylation-inhibitor fungicide and paclobutrazol applications on foliar disease severity and turfgrass quality of creeping bentgrass putting greens. Crop Prot. 2016, 79, 64–69. [Google Scholar] [CrossRef]
- Brosnan, J.T.; Thoms, A.W.; Breeden, G.K.; Sorochan, J.C. Effects of various plant growth regulators on the traffic tolerance of ‘Riviera’ bermudagrass (Cynodon dactylon L.). HortScience 2010, 45, 966–970. [Google Scholar] [CrossRef]
- de Lima, B.H.; dos Santos, P.L.F.; Bezerra, J.C.M.; Pagliarini, M.K.; de Castilho, R.M.M. Paclobutrazol as growth regulator in Bahiagrass. Ornam. Hortic. 2020, 26, 3. [Google Scholar] [CrossRef]
- McCullough, P.E.; Liu, H.; McCarty, L.B. Ethephon and gibberellic acid inhibitors influence creeping bentgrass putting green quality and ball roll distances. HortScience 2005, 40, 1902–1903. [Google Scholar] [CrossRef]
- Tan, Z.G.; Qian, Y.L. Light Intensity Affects Gibberellic Acid Content in Kentucky Bluegrass. HortScience 2003, 38, 113–116. [Google Scholar] [CrossRef]
- Whitehead, D.; Edwards, G.R. Assessment of the application of gibberellins to increase productivity and reduce nitrous oxide emissions in grazed grassland. Agric. Ecosyst. Environ. 2015, 207, 40–50. [Google Scholar] [CrossRef]
- Hofmann, W.A.; Osborne, M.A. Pasture response to gibberellins: A review and recommendations. N. Z. J. Agric. Res. 2009, 52, 213–225. [Google Scholar] [CrossRef]
- Głąb, T.; Szewczyk, W.; Gondek, K.; Knaga, J.; Tomasik, M.; Kowalik, K. Effect of plant growth regulators on visual quality of turfgrass. Sci. Hortic. 2020, 267, 109314. [Google Scholar] [CrossRef]
- Whitman, B.; Iannone, B.V.; Kruse, J.K.; Unruh, J.B.; Dale, A.G. Cultivar blends: A strategy for creating more resilient warm season turfgrass lawns. Urban Ecosyst. 2022, 25, 797–810. [Google Scholar] [CrossRef]
- Knot, P.; Hrabe, F.; Hejduk, S.; Skladanka, J.; Kvasnovsky, M.; Hodulikova, L.; Caslavova, I.; Horky, P. The impacts of different management practices on botanical composition, quality, colour and growth of urban lawns. Urban For. Urban Green. 2017, 26, 178–183. [Google Scholar] [CrossRef]
- Martiniello, P. Effect of traffic stress on cool-season turfgrass under a Mediterranean climate. Agron. Sustain. Dev. 2007, 27, 293–301. [Google Scholar] [CrossRef]
- Park, B.S.; Lawson, T.J.; Samaranayake, H.; Murphy, J.A. Tolerance and recovery of Kentucky bluegrass subjected to seasonal wear. Crop Sci. 2010, 50, 1526–1536. [Google Scholar] [CrossRef]
- Karcher, D.E.; Richardson, M.D. Quantifying turfgrass color using digital image analysis. Crop Sci. 2003, 43, 943–951. [Google Scholar] [CrossRef]
- Kazemi, F.; Golzarian, M.R.; Nematollahi, F. Quality Assessment of Turfgrasses Using NTEP Method Compared to an Image-Based Scoring System. J. Ornam. Plants 2020, 10, 167–178. [Google Scholar]
- Głąb, T.; Szewczyk, W.; Dubas, E.; Kowalik, K.; Jezierski, T. Anatomical and morphological factors affecting wear tolerance of turfgrass. Sci. Hortic. 2015, 185, 1–13. [Google Scholar] [CrossRef]
- Ghalia, I.E.; Miller, G.L.; Grabowa, G.L.; Huffmana, R.L. Using variability within digital images to improve tall fescue color characterization. Crop Sci. 2012, 52, 2365–2374. [Google Scholar] [CrossRef]
- Ruggeri, R.; Provenzano, M.E.; Rossini, F. Effect of mulch on initial coverage of four groundcover species for low input landscaping in a Mediterranean climate. Urban For. Urban Green. 2016, 19, 176–183. [Google Scholar] [CrossRef]
- Ntoulas, N.; Nektarios, P.A.; Kotopoulis, G.; Ilia, P.; Ttooulou, T. Quality assessment of three warm-season turfgrasses growing in different substrate depths on shallow green roof systems. Urban For. Urban Green. 2017, 26, 163–168. [Google Scholar] [CrossRef]
- Taiz, L.; Zeiger, E. Plant Physiology, 4th ed.; Sinauer Associates, Inc.: Sunderland, MA, USA, 2006. [Google Scholar]
- Dunne, J.C.; Miller, G.L.; Arellano, C.; Brandenburg, R.L.; Schoeman, A.; Milla-Lewis, S.R. Shade response of bermudagrass accessions under different management practices. Urban For. Urban Green. 2017, 26, 169–177. [Google Scholar] [CrossRef]
- Agwa, M.H.; Reade, J.H.; Hare, M. Leaf physiological and water soluble carbohydrate content responses to trinexapac-ethyl application of sports turf grasses exposed to water stress. Asian Plant Res. J. 2021, 7, 36–49. [Google Scholar] [CrossRef]
- Ervin, E.H.; Koski, A.J. Trinexapac-ethyl Increases Kentucky Bluegrass Leaf Cell Density and Chlorophyll Concentration. HortScience 2001, 36, 787–789. [Google Scholar] [CrossRef]
- Stier, J.; Rogers, J. Trinexapac-Ethyl and iron effects on Supina and Kentucky bluegrasses under low irradiance. Crop Sci. 2001, 41, 457–465. [Google Scholar] [CrossRef]
- Cohen, I.; Netzer, Y.; Sthein, I.; Gilichinsky, M.; Tel-Or, E. Plant growth regulators improve drought tolerance, reduce growth and evapotranspiration in deficit irrigated Zoysia japonica under field conditions. Plant Growth Regul. 2019, 88, 9–17. [Google Scholar] [CrossRef]
- Melero, M.M.; Ferreira dos Santos, P.L.; Bezerra, C.M.; Horschut de Lima, B.; Pagliarini, M.K.; Monteiro de Castilho, R.M. Paclobutrazol and phenoxaprope-P-ethyl potential as growth regulator in Carpet grass Plus. Ornam. Hortic. 2020, 26, 432–439. [Google Scholar] [CrossRef]
- Kopec, D.M.; Gilbert, J.; Pesserakli, M.; Nolan, S. Repeat Applications of Paclobutrazole (Tgr) Plant Growth Regulator on Overseeded Bermudagrass Turf: Weed Control and Bermudagrass Transition; Turfgrass, Landscape and Urban IPM Research Summary; College of Agriculture and Life Sciences, University of Arizona: Tucson, AZ, USA, 2009; pp. 174–196. [Google Scholar]
- Abdullah, B.S.; Abdulrahman, Y.A. Effect of different concentrations of gibberellic acid on seeds germination and growth in different turf grass genera. Kufa J. Agric. Sci. 2017, 9, 226–247. [Google Scholar]
- Christians, N.E.; Patton, A.J.; Law, Q.D. Fundamentals of Turfgrass Management; John Wiley & Sons: Hoboken, NJ, USA, 2016; p. 480. [Google Scholar]
- McCullough, P.E.; Liu, H.; McCarty, L.B.; Whitwell, T. Response of ‘TifEagle’ bermudagrass to paclobutrazol. HortScience 2005, 40, 224–226. [Google Scholar] [CrossRef]
- Carrow, R.N.; Johnson, B.J. Response of centipedegrass to plant growth regulator and iron treatment combinations. Appl. Agric. Res. 1990, 5, 21–26. [Google Scholar]
- Dernoeden, P.H. Creeping Bentgrass Management; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Kumar, M.; Chaudhary, V.; Sirohi, U. Plant Growth Regulators and their Implication in Ornamental Horticulture: An Overview. Int. J. Agric. Environ. Biotechnol. 2021, 14, 417–445. [Google Scholar] [CrossRef]
- Akdeniz, H.; Hosaflioglu, I. Effects of nitrogen fertilization on some turfgrass characteristics of perennial ryegrass (Lolium perenne L.). J. Agric. Sci. Technol. B 2016, 6, 226–237. [Google Scholar] [CrossRef]
- Zhang, X.; Schmidt, R.E.; Ervin, E.H.; Doak, S. Creeping Bentgrass Physiological Responses to Natural Plant Growth Regulators and Iron Under Two Regimes. HortScience 2002, 37, 898–902. [Google Scholar] [CrossRef]
- Caturegli, L.; Grossi, N.; Saltari, M.; Gaetani, M.; Magni, S.; Nikolopoulou, A.E.; Bonari, E.; Volterrani, M. Spectral reflectance of tall fescue (Festuca arundinacea Schreb.) under different irrigation and nitrogen conditions. Agric. Agric. Sci. Procedia 2015, 4, 59–67. [Google Scholar] [CrossRef]

| PGR | Rate (kg PGR ha−1) | ||||
|---|---|---|---|---|---|
| R1 | R2 | R3 | R4 | R5 | |
| Trinexapac Ethyl | 0.02 | 0.04 | 0.08 | 0.16 | 0.32 |
| Paclobutrazol | 0.06 | 0.12 | 0.24 | 0.48 | 0.96 |
| Flurprimidol | 0.0005 | 0.0010 | 0.0020 | 0.0040 | 0.0080 |
| Mefluidide | 0.005 | 0.010 | 0.020 | 0.040 | 0.080 |
| Ethephon | 1.0 | 2.0 | 4.0 | 8.0 | 16.0 |
| Gibberellic Acid | 0.01 | 0.02 | 0.04 | 0.08 | 0.16 |
| PGR | Rate | L | a | b |
|---|---|---|---|---|
| Ethephon | R1 | 40.60 fg | −6.48 | 17.46 |
| R2 | 40.27 fg | −6.65 | 17.54 | |
| R3 | 40.74 fg | −6.56 | 17.70 | |
| R4 | 40.67 fg | −6.59 | 17.88 | |
| R5 | 40.42 fg | −6.42 | 17.91 | |
| Flurprimidol | R1 | 40.87 fg | −6.68 | 17.52 |
| R2 | 40.88 fg | −6.54 | 17.72 | |
| R3 | 40.53 fg | −6.65 | 17.65 | |
| R4 | 40.88 fg | −6.44 | 17.82 | |
| R5 | 40.74 fg | −6.63 | 17.74 | |
| Gibberellic Acid | R1 | 41.90 efg | −6.88 | 19.01 |
| R2 | 42.45 de | −6.90 | 19.81 | |
| R3 | 43.21 cd | −7.03 | 20.56 | |
| R4 | 44.27 bc | −7.05 | 21.65 | |
| R5 | 45.39 a | −6.84 | 22.34 | |
| Mefluidide | R1 | 40.98 fg | −6.62 | 17.70 |
| R2 | 40.41 fg | −6.78 | 17.71 | |
| R3 | 40.94 fg | −6.47 | 17.51 | |
| R4 | 40.28 fg | −6.20 | 16.61 | |
| R5 | 40.76 fg | −6.02 | 17.43 | |
| Paclobutrazol | R1 | 40.36 fg | −6.28 | 16.95 |
| R2 | 40.63 fg | −6.25 | 16.97 | |
| R3 | 40.63 fg | −5.96 | 17.30 | |
| R4 | 40.43 fg | −5.72 | 16.43 | |
| R5 | 39.91 f | −5.35 | 15.74 | |
| Trinexapac Ethyl | R1 | 41.19 fg | −6.35 | 17.50 |
| R2 | 40.63 fg | −6.20 | 16.75 | |
| R3 | 40.72 fg | −6.33 | 17.34 | |
| R4 | 40.57 fg | −6.07 | 16.69 | |
| R5 | 40.80 fg | −5.92 | 16.97 | |
| Control | 40.69 | −6.37 | 16.56 | |
| Means for PGR | ||||
| Ethephon | 40.54 b | −6.54 b | 17.70 b | |
| Flurprimidol | 40.78 b | −6.59 b | 17.69 b | |
| Gibberellic Acid | 43.45 a | −6.94 a | 20.68 a | |
| Mefluidide | 40.67 b | −6.42 bc | 17.39 b | |
| Paclobutrazol | 40.39 b | −5.91 d | 16.68 b | |
| Trinexapac Ethyl | 40.78 b | −6.18 cd | 17.05 b | |
| Means for Cultivars | ||||
| Limousine | 40.44 c | −6.30 b | 16.74 b | |
| Niweta | 41.20 b | −6.69 a | 18.22 a | |
| Baronial | 41.62 a | −6.29 b | 18.50 a | |
| PGR | Rate | ΔL | Δa | Δb | ΔE |
|---|---|---|---|---|---|
| Ethephon | R1 | −0.086 e | −0.105 | 0.898 fg | 2.565 d |
| R2 | −0.426 e | −0.277 | 0.982 fg | 2.726 d | |
| R3 | 0.048 e | −0.185 | 1.140 fg | 2.587 d | |
| R4 | −0.023 e | −0.216 | 1.315 fg | 2.789 d | |
| R5 | −0.270 e | −0.044 | 1.348 fg | 2.550 d | |
| Flurprimidol | R1 | 0.174 e | −0.304 | 0.957 fg | 2.426 d |
| R2 | 0.185 e | −0.167 | 1.161 fg | 2.388 d | |
| R3 | −0.159 e | −0.275 | 1.087 fg | 2.403 d | |
| R4 | 0.193 e | −0.069 | 1.256 fg | 2.503 d | |
| R5 | 0.046 e | −0.256 | 1.179 fg | 2.396 d | |
| Gibberellic Acid | R1 | 1.206 cde | −0.511 | 2.448 ef | 3.705 cd |
| R2 | 1.762 cd | −0.522 | 3.251 de | 4.679 bc | |
| R3 | 2.523 bc | −0.653 | 3.997 cd | 5.626 b | |
| R4 | 3.582 ab | −0.679 | 5.087 bc | 7.036 a | |
| R5 | 4.700 a | −0.469 | 5.781 ab | 8.325 a | |
| Mefluidide | R1 | 0.290 e | −0.245 | 1.133 fg | 2.573 d |
| R2 | −0.286 e | −0.408 | 1.143 fg | 2.659 d | |
| R3 | 0.251 e | −0.097 | 0.951 fg | 2.377 d | |
| R4 | −0.411 e | 0.170 | 0.044 fg | 2.309 d | |
| R5 | 0.068 e | 0.353 | 0.871 fg | 2.623 d | |
| Paclobutrazol | R1 | −0.336 e | 0.096 | 0.385 fg | 2.504 d |
| R2 | −0.059 e | 0.127 | 0.405 fg | 2.311 d | |
| R3 | −0.061 e | 0.418 | 0.735 fg | 2.572 d | |
| R4 | −0.260 e | 0.650 | −0.131 gh | 2.878 d | |
| R5 | −0.780 e | 1.026 | −0.818 h | 3.261 d | |
| Trinexapac Ethyl | R1 | 0.500 e | 0.024 | 0.939 fg | 2.711 d |
| R2 | −0.065 e | 0.170 | 0.190 fg | 2.311 d | |
| R3 | 0.028 e | 0.039 | 0.781 fg | 2.854 d | |
| R4 | −0.126 e | 0.302 | 0.130 fg | 2.527 d | |
| R5 | 0.105 e | 0.455 | 0.403 fg | 2.318 d | |
| Means for PGRs | |||||
| Ethephon | −0.151 b | −0.165 d | 1.137 b | 2.643 b | |
| Flurprimidol | 0.088 b | −0.214 d | 1.128 b | 2.423 b | |
| Gibberellic Acid | 2.755 a | −0.567 e | 4.113 a | 5.874 a | |
| Mefluidide | −0.018 b | −0.045 cd | 0.828 bc | 2.508 b | |
| Paclobutrazol | −0.299 b | 0.463 a | 0.115 d | 2.705 b | |
| Trinexapac Ethyl | 0.088 b | 0.198 b | 0.489 cd | 2.544 b | |
| Means for Cultivars | |||||
| Limousine | 0.041 b | −0.111 | 0.841 c | 2.405 c | |
| Niweta | 0.335 b | −0.029 | 1.147 b | 3.222 b | |
| Baronial | 0.856 a | −0.025 | 1.917 a | 3.722 a | |
| PGR Rate | L | a | b | ΔL | Δa | Δb | ΔE |
|---|---|---|---|---|---|---|---|
| Flurprimidol | −0.008 | 0.007 | 0.010 | −0.011 | 0.012 | 0.034 | 0.002 |
| Ethephon | −0.008 | 0.032 | 0.026 | −0.011 | 0.050 | 0.084 | −0.012 |
| Trinexapac Ethyl | −0.024 | 0.107 | −0.020 | −0.028 | 0.177 | −0.065 | −0.072 |
| Paclobutrazol | −0.090 | 0.232 | −0.086 | −0.105 | 0.337 * | −0.240 * | 0.197 * |
| Gibberellic Acid | 0.339 * | 0.011 | 0.183 | 0.337 * | 0.017 | 0.313 * | 0.378 * |
| Mefluidide | −0.015 | 0.171 | −0.026 | −0.018 | 0.303 * | −0.083 | 0.002 |
| PGR | Rate | Overall Appearance | Turf Color | Leaf Texture |
|---|---|---|---|---|
| Ethephon | R1 | 7.86 ab | 7.77 | 7.46 |
| R2 | 7.62 bc | 7.79 | 7.50 | |
| R3 | 7.60 bc | 7.79 | 7.50 | |
| R4 | 7.84 ab | 7.77 | 7.46 | |
| R5 | 7.76 b | 7.79 | 7.50 | |
| Flurprimidol | R1 | 7.45 c | 7.88 | 7.50 |
| R2 | 7.74 b | 7.79 | 7.50 | |
| R3 | 7.88 ab | 7.85 | 7.50 | |
| R4 | 7.88 ab | 7.79 | 7.50 | |
| R5 | 8.02 a | 7.85 | 7.50 | |
| Gibberellic Acid | R1 | 7.79 b | 7.77 | 7.46 |
| R2 | 7.82 abc | 7.73 | 7.43 | |
| R3 | 7.71 b | 7.73 | 7.79 | |
| R4 | 7.57 bc | 7.55 | 7.83 | |
| R5 | 7.05 d | 7.48 | 7.83 | |
| Mefluidide | R1 | 7.88 ab | 7.73 | 7.50 |
| R2 | 7.79 b | 7.79 | 7.50 | |
| R3 | 7.86 ab | 7.79 | 7.50 | |
| R4 | 7.50 bc | 7.70 | 7.50 | |
| R5 | 7.69 b | 7.70 | 7.50 | |
| Paclobutrazol | R1 | 7.86 ab | 7.96 | 7.46 |
| R2 | 7.55 bc | 7.97 | 7.50 | |
| R3 | 7.64 bc | 8.00 | 7.50 | |
| R4 | 7.33 cd | 8.03 | 7.50 | |
| R5 | 7.14 cd | 8.00 | 7.42 | |
| Trinexapac Ethyl | R1 | 7.88 ab | 7.79 | 7.50 |
| R2 | 7.79 b | 7.79 | 7.25 | |
| R3 | 7.83 ab | 7.67 | 7.25 | |
| R4 | 7.67 bc | 7.82 | 7.42 | |
| R5 | 7.81 b | 7.85 | 7.50 | |
| Control | 7.62 | 7.67 | 7.29 | |
| Means for PGR | ||||
| Ethephon | 7.73 ab | 7.78 ab | 7.49 ab | |
| Flurprimidol | 7.80 a | 7.83 ab | 7.50 ab | |
| Gibberellic Acid | 7.59 ab | 7.65 b | 7.67 a | |
| Mefluidide | 7.74 ab | 7.74 ab | 7.50 ab | |
| Paclobutrazol | 7.51 b | 7.99 a | 7.48 b | |
| Trinexapac Ethyl | 7.80 a | 7.78 ab | 7.38 b | |
| Means for Cultivars | ||||
| Limousine | 8.26 a | 7.99 a | 8.64 a | |
| Niweta | 7.71 b | 7.93 a | 7.64 b | |
| Baronial | 7.11 c | 7.47 b | 6.24 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Głąb, T.; Szewczyk, W.; Gondek, K. Response of Kentucky Bluegrass Turfgrass to Plant Growth Regulators. Agronomy 2023, 13, 799. https://doi.org/10.3390/agronomy13030799
Głąb T, Szewczyk W, Gondek K. Response of Kentucky Bluegrass Turfgrass to Plant Growth Regulators. Agronomy. 2023; 13(3):799. https://doi.org/10.3390/agronomy13030799
Chicago/Turabian StyleGłąb, Tomasz, Wojciech Szewczyk, and Krzysztof Gondek. 2023. "Response of Kentucky Bluegrass Turfgrass to Plant Growth Regulators" Agronomy 13, no. 3: 799. https://doi.org/10.3390/agronomy13030799
APA StyleGłąb, T., Szewczyk, W., & Gondek, K. (2023). Response of Kentucky Bluegrass Turfgrass to Plant Growth Regulators. Agronomy, 13(3), 799. https://doi.org/10.3390/agronomy13030799







