Breeding Milestones Correspond with Changes to Wheat Rhizosphere Biogeochemistry That Affect P Acquisition
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Growth Conditions
2.2. Soil Collection and Analyses
2.3. Plant Digestion and Elemental Quantification
2.4. Statistical Analysis
3. Results
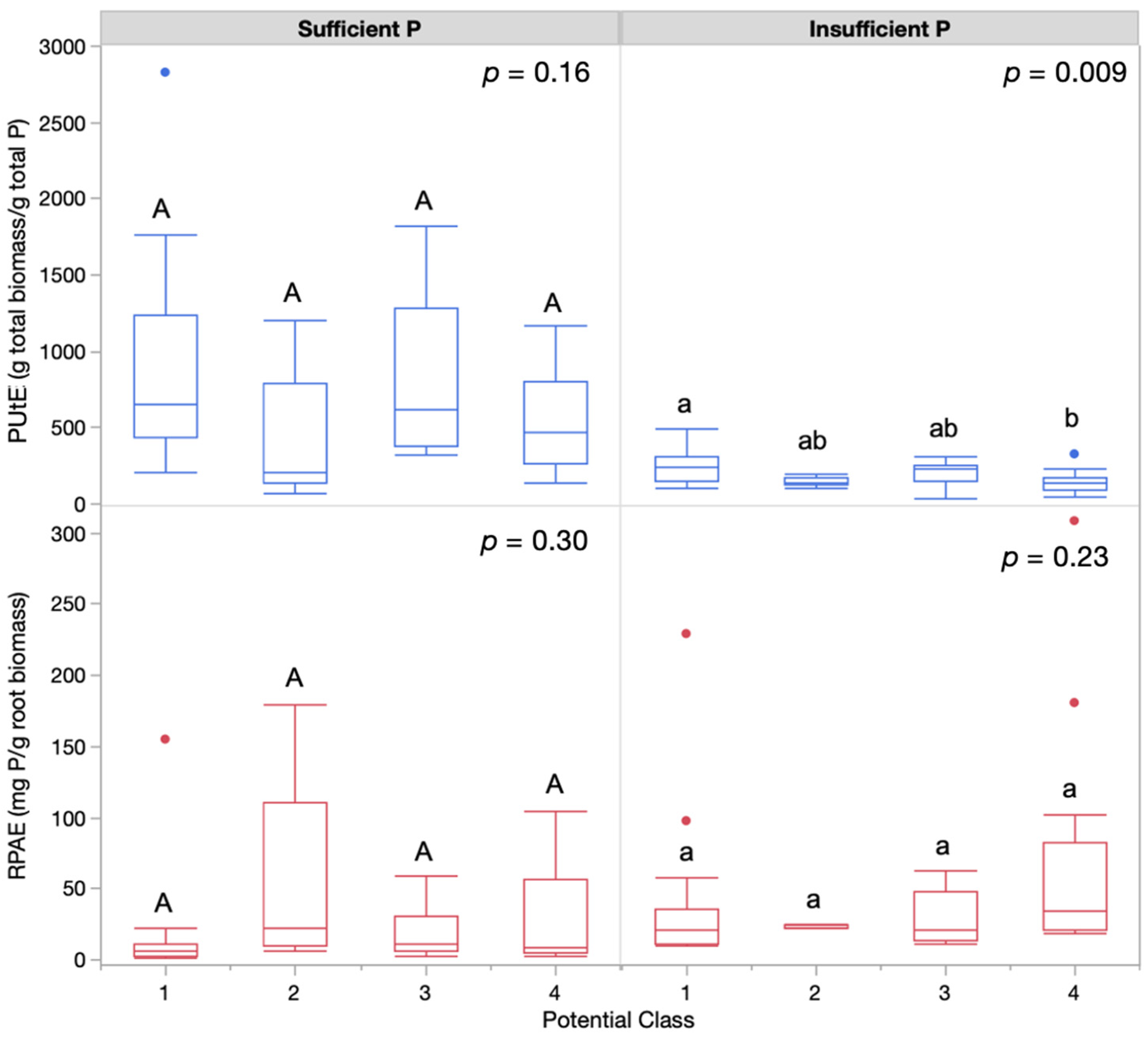
3.1. Measures of Phosphorus Efficiency
3.2. Plant Growth and Nutrient Content
3.3. Bulk and Rhizosphere Soil Chemical Variables
3.4. Rhizosphere Microbial Community Structure
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mueller, N.D.; Gerber, J.S.; Johnston, M.; Ray, D.K.; Ramankutty, N.; Foley, J.A. Closing yield gaps through nutrient and water management. Nature 2012, 490, 254–257. [Google Scholar] [CrossRef] [PubMed]
- Baligar, V.C.; Bennett, O.L. Outlook on fertilizer use efficiency in the tropics. Fertil. Res. 1986, 10, 83–96. [Google Scholar] [CrossRef]
- Batjes, N.H. A world dataset of derived soil properties by FAO-UNESCO soil unit for global modelling. Soil Use Manag. 1997, 13, 9–16. [Google Scholar] [CrossRef]
- Cordell, D.; White, S. Life’s bottleneck: Sustaining the world’s phosphorus for a food secure future. Annu. Rev. Environ. Resour. 2014, 39, 161–188. [Google Scholar] [CrossRef]
- Isherwood, K.F. Mineral Fertilizer Use and the Environment; International Fertilizer Industry Association: Paris, France, 2000. [Google Scholar]
- Lynch, J.P. Roots of the second Green Revolution. Aust. J. Bot. 2007, 55, 493–512. [Google Scholar] [CrossRef]
- Lynch, J.P. Root phenes for enhanced soil exploration and phosphorus acquisition: Tools for future crops. Plant Physiol. 2011, 156, 1041–1049. [Google Scholar] [CrossRef] [Green Version]
- Barber, S.A.; Walker, J.M.; Vasey, E.H. Mechanisms for movement of plant nutrients from soil and fertilizer to plant root. J. Agric. Food Chem. 1963, 11, 204–207. [Google Scholar] [CrossRef]
- Darrah, P.R. The rhizosphere and plant nutrition: A quantitative approach. Plant Soil 1993, 155–156, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Lynch, J.P.; Ho, M.D. Rhizoeconomics: Carbon costs of phosphorus acquisition. Plant Soil 2005, 269, 45–56. [Google Scholar] [CrossRef]
- Zhu, J.; Mickelson, S.M.; Kaeppler, S.M.; Lynch, J.P. Detection of quantitative trait loci for seminal root traits in maize (Zea mays L.) seedlings grown under differential phosphorus levels. Theor. Appl. Genet. 2006, 113, 1–10. [Google Scholar] [CrossRef]
- Sun, H.; Zhang, F.; Li, L.; Tang, C. The morphological changes of wheat genotypes as affected by the levels of localized phosphate supply. Plant Soil 2002, 245, 233–238. [Google Scholar] [CrossRef]
- Robinson, H.; Kelly, A.; Fox, G.; Franckowiak, J.; Borrell, A.; Hickey, L. Root architectural traits and yield: Exploring the relationship in barley breeding trials. Euphytica 2018, 214, 151. [Google Scholar] [CrossRef]
- Gahoonia, T.S.; Nielsen, N.E. Barley genotypes with long root hairs sustain high grain yields in low-P field. Plant Soil 2004, 262, 55–62. [Google Scholar] [CrossRef]
- Gahoonia, T.S.; Nielsen, N.E.; Joshi, P.A.; Jahoor, A. A root hairless barley mutant for elucidating genetic of root hairs and phosphorus uptake. Plant Soil 2001, 235, 211–219. [Google Scholar] [CrossRef] [Green Version]
- Sinclair, T.R.; Vadez, V. Physiological traits for crop yield improvement in low N and P environments. Plant Soil 2002, 245, 1–15. [Google Scholar] [CrossRef]
- Dong, D.; Peng, X.; Yan, X. Organic acid exudation induced by phosphorus deficiency and/or aluminum toxicity in two contrasting soybean genotypes. Physiol. Plant. 2004, 122, 190–199. [Google Scholar] [CrossRef]
- Iannucci, A.; Fragasso, M.; Beleggia, R.; Nigro, F.; Papa, R. Evolution of the crop rhizosphere: Impact of domestication on root exudates in tetraploid wheat (Triticum turgidum L.). Front. Plant Sci. 2017, 8, 2124. [Google Scholar] [CrossRef] [Green Version]
- McGrail, R.K.; Van Sanford, D.A.; McNear, D.H. Semi-dwarf winter wheat roots contain fewer organic acids than wild type varieties under phosphorus stress. Crop Sci. 2021, 61, 3586–3597. [Google Scholar] [CrossRef]
- Vengavasi, K.; Renu, P. Root exudation index: Screening organic acid exudation and phosphorus acquisition efficiency in soybean genotypes. Crop Pasture Sci. 2016, 67, 1096–1109. [Google Scholar] [CrossRef]
- Lipton, D.S.; Blanchar, R.W.; Blevins, D.G. Citrate, malate, and succinate concentration in exudates from P-sufficient and P-stressed Medicago sativa L. seedlings. Plant Physiol. 1987, 85, 315–317. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Krogstad, T.; Clarke, J.L.; Hallama, M.; Øgaard, A.F.; Eich-Greatorex, S.; Kandeler, E.; Clarke, N. Rhizosphere organic anions play a minor role in improving crop species’ ability to take up residual phosphorus (P) in agricultural soils low in P availability. Front. Plant Sci. 2016, 7, 1664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dakora, F.D.; Phillips, D.A. Root exudates as mediators of mineral acquisition in low-nutrient environments. Plant Soil 2002, 245, 35–47. [Google Scholar] [CrossRef]
- Ryan, P.R.; James, R.A.; Weligama, C.; Delhaize, E.; Rattey, A.; Lewis, D.C.; Bovill, W.D.; McDonald, G.; Rathjen, T.M.; Wang, E.; et al. Can citrate efflux from roots improve phosphorus uptake by plants? Testing the hypothesis with near-isogenic lines of wheat. Physiol. Plant. 2014, 151, 230–242. [Google Scholar] [CrossRef] [PubMed]
- Dotaniya, M.L.; Datta, S.C.; Biswas, D.R.; Meena, B.P. Effect of solution phosphorus concentration on the exudation of oxalate ions by wheat (Triticum aestivum L.). Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2013, 83, 305–309. [Google Scholar] [CrossRef] [Green Version]
- Dotaniya, M.L.; Datta, S.C.; Biswas, D.R.; Meena, H.M.; Kumar, K. Production of oxalic acid as influenced by the application of organic residue and its effect on phosphorus uptake by wheat (Triticum aestivum L.) in an Inceptisol of North India. Natl. Acad. Sci. Lett. 2014, 37, 401–405. [Google Scholar] [CrossRef] [Green Version]
- Hoffland, E.; Findenegg, G.; Nter, R.; Nelemans, J.A. Solubilization of rock phosphate by rape: I. Evaluation of the role of the nutrient uptake pattern. Plant Soil 1989, 113, 155–160. [Google Scholar] [CrossRef]
- Asmar, F.; Gahoonia, T.S.; Nielsen, N.E. Barley genotypes differ in activity of soluble extracellular phosphatase and depletion of organic phosphorus in the rhizosphere soil. Plant Soil 1995, 172, 117–122. [Google Scholar] [CrossRef]
- Asmar, F. Variation in activity of root extracellular phytase between genotypes of barley. Plant Soil 1997, 195, 61–64. [Google Scholar] [CrossRef]
- Richardson, A.E.; Hadobas, P.A.; Hayes, J.E. Acid phosphomonoesterase and phytase activities of wheat (Triticum aestivum L.) roots and utilization of organic phosphorus substrates by seedlings grown in sterile culture. Plant Cell Environ. 2000, 23, 397–405. [Google Scholar] [CrossRef]
- McLachlan, K.D.; Marco, D. Acid phosphatase activity of intact roots and phosphorus nutrition in plants. III. Its relation to phosphorus garnering by wheat and a comparison with leaf activity as a measure of phosphorus status. Aust. J. Agric. Res. 1982, 33, 1–11. [Google Scholar] [CrossRef]
- Brundrett, M.C. Coevolution of roots and mycorrhizas of land plants. New Phytol. 2002, 154, 275–304. [Google Scholar] [CrossRef] [PubMed]
- Schnepf, A.; Roose, T.; Schweiger, P. Growth model for arbuscular mycorrhizal fungi. J. R. Soc. Interface 2008, 5, 773–784. [Google Scholar] [CrossRef] [Green Version]
- Pii, Y.; Mimmo, T.; Tomasi, N.; Terzano, R.; Cesco, S.; Crecchio, C. Microbial interactions in the rhizosphere: Beneficial influences of plant growth-promoting rhizobacteria on nutrient acquisition process. A review. Biol. Fertil. Soils 2015, 51, 403–415. [Google Scholar] [CrossRef]
- Bakker, M.G.; Manter, D.K.; Sheflin, A.M.; Weir, T.L.; Vivanco, J.M. Harnessing the rhizosphere microbiome through plant breeding and agricultural management. Plant Soil 2012, 360, 1–13. [Google Scholar] [CrossRef]
- Koevoets, I.T.; Venema, J.H.; Elzenga, J.T.M.; Testerink, C. Roots withstanding their environment: Exploiting root system architecture responses to abiotic stress to improve crop tolerance. Front. Plant Sci. 2016, 7, 1335. [Google Scholar] [CrossRef] [Green Version]
- Lynch, J.P.; Brown, K.M. New roots for agriculture: Exploiting the root phenome. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2012, 367, 1598–1604. [Google Scholar] [CrossRef]
- McGrail, R.K.; McNear, D.H. Two centuries of breeding has altered root system architecture of winter wheat. Rhizosphere 2021, 19, 100411. [Google Scholar] [CrossRef]
- Van Sanford, D.A.; Clark, A.J.; Bradley, C.; Brown-Guedira, G.L.; Cowger, C.; Dong, Y.; Baik, B.-K. Registration of ‘Pembroke 2016′ soft red winter wheat. J. Plant Regist. 2018, 12, 373–378. [Google Scholar] [CrossRef]
- Natural Resource Conservation Service. Crider—Kentucky State Soil. United States Department of Agriculture. Available online: https://mikhailova.org/documents/poster_015.pdf (accessed on 5 May 2021).
- Ritchey, E.L.; McGrath, J.M. AGR-1-Lime and Fertilizer Recommendation, 2019–2020; University of Kentucky Agricultural Communications Service: Lexington, KY, USA, 2019; p. 24. [Google Scholar]
- Poorter, H.; Bühler, J.; van Dusschoten, D.; Climent, J.; Postma, J.A. Pot size matters: A meta-analysis of the effects of rooting volume on plant growth. Funct. Plant Biol. 2012, 39, 839–850. [Google Scholar] [CrossRef] [Green Version]
- Page, M.B.; Smalley, J.L.; Talibudeen, O. The growth and nutrient uptake of winter wheat. Plant Soil 1978, 49, 149–160. [Google Scholar] [CrossRef]
- Malhi, S.S.; Johnston, A.M.; Schoenau, J.J.; Wang, Z.H.; Vera, C.L. Seasonal biomass accumulation and nutrient uptake of wheat, barley and oat on a Black Chernozern soil in Saskatchewan. Can. J. Plant Sci. 2006, 86, 1005–1014. [Google Scholar] [CrossRef]
- Jones, C.; Olson-Rutz, K.; Dinkins, C.P. Nutrient Uptake Timing by Crops to Assist with Fertilizing Decisions; Montana State University Extension: Bozeman, MT, USA, 2015. [Google Scholar]
- Buyer, J.S.; Sasser, M. High throughput phospholipid fatty acid analysis of soils. Appl. Soil Ecol. 2012, 61, 127–130. [Google Scholar] [CrossRef]
- Tiessen, H.; Stewart, J.W.B.; Cole, C.V. Pathways of phosphorus transformations in soils of differing pedogenesis. Soil Sci. Soc. Am. J. 1984, 48, 853–858. [Google Scholar] [CrossRef]
- Pan, X.; Li, W.; Zhang, Q.; Li, Y.; Liu, M. Assessment on phosphorus efficiency characteristics of soybean genotypes in phosphorus-deficient soils. Agric. Sci. China 2008, 7, 958–969. [Google Scholar] [CrossRef]
- Ramette, A. Multivariate analyses in microbial ecology. FEMS Microbiol. Ecol. 2007, 62, 142–160. [Google Scholar] [CrossRef] [Green Version]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Manske, G.G.B.; Ortiz-Monasterio, J.I.; Van Ginkel, M.; González, R.M.; Rajaram, S.; Molina, E.; Vlek, P.L.G. Traits associated with improved P-uptake efficiency in CIMMYT’s semidwarf spring bread wheat grown on an acid Andisol in Mexico. Plant Soil 2000, 221, 189–204. [Google Scholar] [CrossRef]
- Jones, G.P.D.; Jessop, R.S.; Blair, G.J. Alternative methods for the selection of phosphorus efficiency in wheat. Field Crops Res. 1992, 30, 29–40. [Google Scholar] [CrossRef]
- Ozturk, L.; Eker, S.; Torun, B.; Cakmak, I. Variation in phosphorus efficiency among 73 bread and durum wheat genotypes grown in a phosphorus-deficient calcareous soil. Plant Soil 2005, 269, 69–80. [Google Scholar] [CrossRef]
- Hetrick, B.A.D.; Wilson, G.W.T.; Cox, T.S. Mycorrhizal dependence of modern wheat varieties, landraces, and ancestors. Can. J. Bot. 1992, 70, 2032–2040. [Google Scholar] [CrossRef]
- Tang, X.; Placella, S.A.; Daydé, F.; Bernard, L.; Robin, A.; Journet, E.-P.; Justes, E.; Hinsinger, P. Phosphorus availability and microbial community in the rhizosphere of intercropped cereal and legume along a P-fertilizer gradient. Plant Soil 2016, 407, 119–134. [Google Scholar] [CrossRef]
- Shen, P.; Murphy, D.V.; George, S.J.; Lapis-Gaza, H.; Xu, M.; Gleeson, D.B. Increasing the size of the microbial biomass altered bacterial community structure which enhances plant phosphorus uptake. PLoS ONE 2016, 11, e0166062. [Google Scholar] [CrossRef] [Green Version]
- Shi, S.; Richardson, A.E.; O’Callaghan, M.; DeAngelis, K.M.; Jones, E.E.; Stewart, A.; Firestone, M.K.; Condron, L.M. Effects of selected root exudate components on soil bacterial communities. FEMS Microbiol. Ecol. 2011, 77, 600–610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhalnina, K.; Louie, K.B.; Hao, Z.; Mansoori, N.; Da Rocha, U.N.; Shi, S.; Cho, H.; Karaoz, U.; Loqué, D.; Bowen, B.P.; et al. Dynamic root exudate chemistry and microbial substrate preferences drive patterns in rhizosphere microbial community assembly. Nat. Microbiol. 2018, 3, 470–480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wen, T.; Yu, G.-H.; Hong, W.-D.; Yuan, J.; Niu, G.-Q.; Xie, P.-H.; Sun, F.-S.; Guo, L.-D.; Kuzyakov, Y.; Shen, Q.-R. Root exudate chemistry affects soil carbon mobilization via microbial community reassembly. Fundam. Res. 2022, 2, 697–707. [Google Scholar] [CrossRef]
- Gadd, G.M. Fungal production of citric and oxalic acid: Importance in metal speciation, physiology and biogeochemical processes. Adv. Microb. Physiol. 1999, 41, 47–92. [Google Scholar] [CrossRef]
- Bonkowski, M. Protozoa and plant growth: The microbial loop in soil revisited. New Phytol. 2004, 162, 617–631. [Google Scholar] [CrossRef]
- Bonkowski, M.; Clarholm, M. Stimulation of plant growth through interactions of bacteria and protozoa: Testing the auxiliary microbial loop hypothesis. Acta Protozool. 2012, 51, 237–247. [Google Scholar] [CrossRef]
- Bonkowski, M.; Griffiths, B.; Scrimgeour, C. Substrate heterogeneity and microfauna in soil organic ‘hotspots’ as determinants of nitrogen capture and growth of ryegrass. Appl. Soil Ecol. 2000, 14, 37–53. [Google Scholar] [CrossRef]
- Oliverio, A.M.; Geisen, S.; Delgado-Baquerizo, M.; Maestre, F.T.; Turner, B.L.; Fierer, N. The global-scale distributions of soil protists and their contributions to belowground systems. Sci. Adv. 2020, 6, e8787. [Google Scholar] [CrossRef] [Green Version]
- Deng, Y.; Teng, W.; Tong, Y.-P.; Chen, X.-P.; Zou, C.-Q. Phosphorus efficiency mechanisms of two wheat cultivars as affected by a range of phosphorus levels in the field. Front. Plant Sci. 2018, 9, 1614. [Google Scholar] [CrossRef] [PubMed]
- Hedley, M.J.; Stewart, J.W.B. Method to measure microbial phosphate in soils. Soil Biol. Biochem. 1982, 14, 377–385. [Google Scholar] [CrossRef]
- Ström, L.; Owen, A.G.; Godbold, D.L.; Jones, D.L. Organic acid behaviour in a calcareous soil implications for rhizosphere nutrient cycling. Soil Biol. Biochem. 2005, 37, 2046–2054. [Google Scholar] [CrossRef]
- Keiluweit, M.; Bougoure, J.J.; Nico, P.S.; Pett-Ridge, J.; Weber, P.K.; Kleber, M. Mineral protection of soil carbon counteracted by root exudates. Nat. Clim. Change 2015, 5, 588–595. [Google Scholar] [CrossRef] [Green Version]
- Sokol, N.W.; Sanderman, J.; Bradford, M.A. Pathways of mineral-associated soil organic matter formation: Integrating the role of plant carbon source, chemistry, and point of entry. Glob. Change Biol. 2019, 25, 12–24. [Google Scholar] [CrossRef] [Green Version]
- Cross, A.F.; Schlesinger, W.H. A literature review and evaluation of the Hedley fractionation: Applications to the biogeochemical cycle of soil phosphorus in natural ecosystems. Geoderma 1995, 64, 197–214. [Google Scholar] [CrossRef]
- Gérard, F. Clay minerals, iron/aluminum oxides, and their contribution to phosphate sorption in soils—A myth revisited. Geoderma 2016, 262, 213–226. [Google Scholar] [CrossRef]
- Klotzbücher, A.; Kaiser, K.; Klotzbücher, T.; Wolff, M.; Mikutta, R. Testing mechanisms underlying the Hedley sequential phosphorus extraction of soils. J. Plant Nutr. Soil Sci. 2019, 182, 570–577. [Google Scholar] [CrossRef]
- Boudiar, R.; González, J.M.; Mekhlouf, A.; Casas, A.M.; Igartua, E. Durum wheat seminal root traits within modern and landrace germplasm in Algeria. Agronomy 2020, 10, 713. [Google Scholar] [CrossRef]
- Figueroa-Bustos, V.; Palta, J.A.; Chen, Y.; Siddique, K.H.M. Characterization of root and shoot traits in wheat cultivars with putative differences in root system size. Agronomy 2018, 8, 109. [Google Scholar] [CrossRef] [Green Version]
- Qin, X.; Feng, F.; Wen, X.; Siddique, K.H.M.; Liao, Y. Historical genetic responses of yield and root traits in winter wheat in the yellow-Huai-Hai River valley region of China due to modern breeding (1948–2012). Plant Soil 2019, 439, 7–18. [Google Scholar] [CrossRef]
- Libert, B.; Franceschi, V.R. Oxalate in crop plants. J. Agric. Food Chem. 1987, 35, 926–938. [Google Scholar] [CrossRef]
- Tu, S.-X.; Guo, Z.-F.; Sun, J.-H. Effect of oxalic acid on potassium release from typical Chinese soils and minerals. Pedosphere 2007, 17, 457–466. [Google Scholar] [CrossRef]
- Wang, J.G.; Zhang, F.S.; Zhang, X.L.; Cao, Y.P. Release of potassium from K-bearing minerals: Effect of plant roots under P deficiency. Nutr. Cycl. Agroecosyst. 2000, 56, 45–52. [Google Scholar] [CrossRef]
- Wang, H.-Y.; Shen, Q.-H.; Zhou, J.-M.; Wang, J.; Du, C.-W.; Chen, X.-Q. Plants use alternative strategies to utilize nonexchangeable potassium in minerals. Plant Soil 2011, 343, 209–220. [Google Scholar] [CrossRef]
- Karathanasis, A.D.; Wells, K.L. Conservation tillage effects on the potassium status of some Kentucky soils. Soil Sci. Soc. Am. J. 1990, 54, 800–806. [Google Scholar] [CrossRef]
- McGrail, R.K.; Van Sanford, D.A.; McNear, D.H. Trait-based root phenotyping as a necessary tool for crop selection and improvement. Agronomy 2020, 10, 1328. [Google Scholar] [CrossRef]


| Cultivar | Year Released | Era | Dwarfing Status | OA Potential Class | RSA Potential Class | PEP Class |
|---|---|---|---|---|---|---|
| Goens | 1808 | old | wild-type | high | large | 1 |
| Red May | 1830 | old | wild-type | high | large | 1 |
| Blackhull | 1917 | old | wild-type | high | large | 1 |
| Norin-10 | 1935 | old | semi-dwarf | low | large | 2 |
| Nicoma | 1971 | modern | wild-type | high | small | 3 |
| Baca | 1973 | modern | wild-type | high | small | 3 |
| Prowers | 1997 | modern | wild-type | high | small | 3 |
| Lindon | 1975 | modern | semi-dwarf | low | small | 4 |
| Akron | 1994 | modern | semi-dwarf | low | small | 4 |
| Ankor | 2002 | modern | semi-dwarf | low | small | 4 |
| Sufficient P | Insufficient P | |||
|---|---|---|---|---|
| PEP Class | PUtE | RPAE | PUtE | RPAE |
| Class 1 | 897 ± 136 A | 9 ± 13 A | 259 ± 23 a | 41 ± 14 a |
| Class 2 | 412 ± 235 A | 44 ± 19 A | 148 ± 41 ab | 24 ± 25 a |
| Class 3 | 818 ± 140 A | 13 ± 13 A | 204 ± 24 ab | 29 ± 15 a |
| Class 4 | 539 ± 146 A | 23 ± 13 A | 147 ± 23 b | 68 ± 14 a |
| Sufficient P | Insufficient P | |||||||
|---|---|---|---|---|---|---|---|---|
| Dwarfing Status | Era | Dwarfing Status | Era | |||||
| F-Value | p-Value | F-Value | p-Value | F-Value | p-Value | F-Value | p-Value | |
| Plant Growth Metrics | ||||||||
| Growth Stage | 0.53 | 0.4702 | 3.01 | 0.0892 | 4.29 | 0.0440 | 11.8 | 0.0017 |
| Main Stem Height | 12.53 | 0.0009 | 0.50 | 0.4810 | 8.85 | 0.0047 | 1.77 | 0.1902 |
| Number of Heads | 0.06 | 0.8085 | 3.72 | 0.0601 | 1.49 | 0.2286 | 5.86 | 0.0196 |
| Shoot Mass | 5.70 | 0.0212 | 0.20 | 0.6562 | 1.15 | 0.2894 | 0.96 | 0.3327 |
| Nutrient Concentration (mg kg−1) | ||||||||
| Shoot K | 0.15 | 0.6970 | 2.58 | 0.1151 | 4.59 | 0.0374 | 5.52 | 0.0231 |
| Shoot Mg | 0.96 | 0.3322 | 12.82 | 0.0008 | 5.76 | 0.0205 | 6.41 | 0.0148 |
| Shoot P | 11.27 | 0.0016 | 0.01 | 0.9275 | 11.62 | 0.0014 | 2.58 | 0.1147 |
| Root Al | 4.87 | 0.0328 | 2.93 | 0.0940 | 0.65 | 0.4228 | 0.09 | 0.7675 |
| Root Fe | 5.57 | 0.0229 | 4.75 | 0.0349 | 0.54 | 0.4663 | 0.00 | 0.9905 |
| Total Nutrient Content (mg) | ||||||||
| Shoot Ca | 7.39 | 0.0092 | 0.00 | 0.9795 | 2.76 | 0.1037 | 1.42 | 0.2402 |
| Shoot K | 5.85 | 0.0195 | 0.09 | 0.7674 | 2.77 | 0.1029 | 3.26 | 0.0776 |
| Shoot Al | 4.60 | 0.0373 | 0.45 | 0.5037 | 3.28 | 0.0767 | 0.67 | 0.4156 |
| Shoot Fe | 7.67 | 0.0081 | 0.61 | 0.4398 | 3.09 | 0.0853 | 5.29 | 0.0261 |
| Sufficient P | Insufficient P | |||||||
| Dwarfing Status | Era | Dwarfing Status | Era | |||||
| F-value | p-value | F-value | p-value | F-value | p-value | F-value | p-value | |
| Shoot Mg | 4.41 | 0.0413 | 0.67 | 0.4165 | 0.30 | 0.5847 | 3.06 | 0.0867 |
| Shoot Mn | 4.62 | 0.0370 | 0.03 | 0.8585 | 0.92 | 0.3417 | 2.27 | 0.1391 |
| Root K | 0.81 | 0.3718 | 2.55 | 0.1176 | 0.09 | 7.598 | 4.34 | 0.0428 |
| Bulk Soil Physicochemical Parameters | ||||||||
| P | 5.17 | 0.0278 | 0.01 | 0.9223 | 1.45 | 0.2344 | 1.86 | 0.1798 |
| K | 7.64 | 0.0082 | 0.96 | 0.3317 | 1.06 | 0.3087 | 1.91 | 0.1734 |
| Mg | 0.10 | 0.7535 | 0.91 | 0.3442 | 4.21 | 0.0460 | 0.06 | 0.8071 |
| Mn | 1.19 | 0.2817 | 10.83 | 0.0019 | 0.06 | 0.8070 | 2.93 | 0.0939 |
| Rhizosphere Soil Inorganic P Fractions | ||||||||
| Resin P | 0.13 | 0.7251 | 0.02 | 0.8798 | 0.51 | 0.4798 | 5.42 | 0.0243 |
| Bicarbonate P | 0.44 | 0.5093 | 0.15 | 0.7009 | 6.48 | 0.0143 | 0.04 | 0.8452 |
| Rhizosphere Soil Microbial Biomass | ||||||||
| G+ Bacteria | 10.90 | 0.0019 | 0.13 | 0.7222 | 5.05 | 0.0295 | 0.06 | 0.8035 |
| G- Bacteria | 0.08 | 0.7735 | 7.09 | 0.0106 | 0.09 | 0.7892 | 2.48 | 0.1218 |
| Actinobacteria | 2.76 | 0.1035 | 6.51 | 0.0141 | 0.19 | 0.6634 | 3.43 | 0.0706 |
| Protists | 5.43 | 0.0242 | 1.63 | 0.2083 | 6.96 | 0.0113 | 0.09 | 0.7694 |
| Sufficient P | Insufficient P | |||
|---|---|---|---|---|
| Wild-Type | Semi-Dwarf | Wild-Type | Semi-Dwarf | |
| Mg (mg kg−1) | 117 ± 2 A | 118 ± 3 A | 119 ± 1 a | 114 ± 2 b |
| Fe (mg kg−1) | 84 ± 5 A | 77 ± 6 A | 103 ± 6 a | 97 ± 7 a |
| Al (mg kg−1) | 716 ± 9 A | 723 ± 12 A | 762 ± 9 a | 743 ± 12 a |
| Old | Modern | Old | Modern | |
| Mg (mg kg−1) | 119 ± 3 A | 115 ± 2 A | 117 ± 2 a | 116 ± 1 a |
| Fe (mg kg−1) | 75 ± 6 A | 87 ± 5 A | 95 ± 7 a | 104 ± 6 a |
| Al (mg kg−1) | 714 ± 12 A | 725 ± 9 A | 741 ± 12 a | 764 ± 9 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
McGrail, R.K.; Van Sanford, D.A.; McNear, D.H., Jr. Breeding Milestones Correspond with Changes to Wheat Rhizosphere Biogeochemistry That Affect P Acquisition. Agronomy 2023, 13, 813. https://doi.org/10.3390/agronomy13030813
McGrail RK, Van Sanford DA, McNear DH Jr. Breeding Milestones Correspond with Changes to Wheat Rhizosphere Biogeochemistry That Affect P Acquisition. Agronomy. 2023; 13(3):813. https://doi.org/10.3390/agronomy13030813
Chicago/Turabian StyleMcGrail, Rebecca K., David A. Van Sanford, and David H. McNear, Jr. 2023. "Breeding Milestones Correspond with Changes to Wheat Rhizosphere Biogeochemistry That Affect P Acquisition" Agronomy 13, no. 3: 813. https://doi.org/10.3390/agronomy13030813





