Essential Oils and Chitosan Applications to Protect Apples against Postharvest Diseases and to Extend Shelf Life
Abstract
:1. Introduction
2. Materials and Methods
2.1. Isolates of Pathogens and Apple Fruits
2.2. Essential Oils (EOs) and Edible Coatings
2.3. Experiment 1—In Vitro Tests
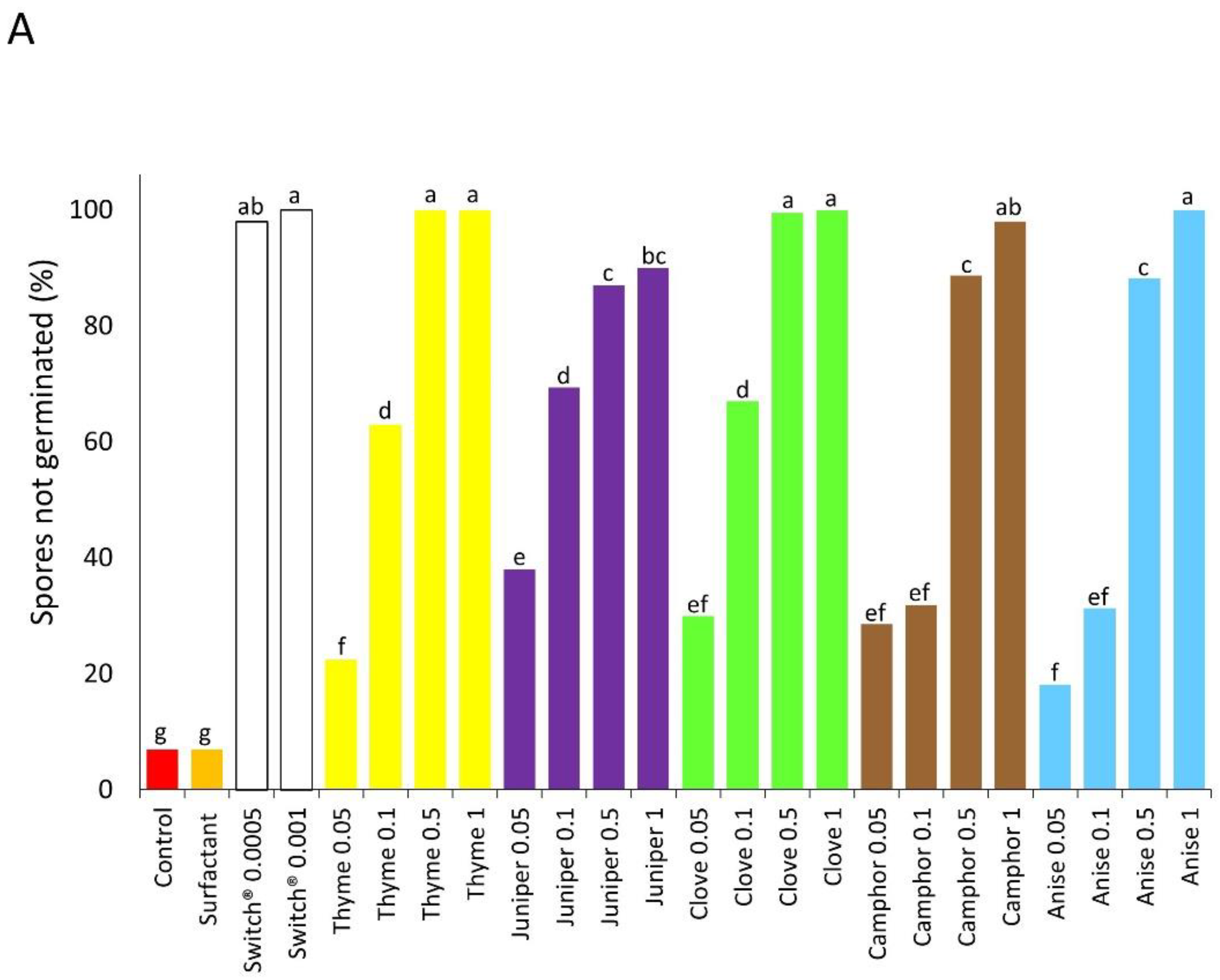
2.3.1. Spore Germination Assay
2.3.2. Mycelial Growth Assay
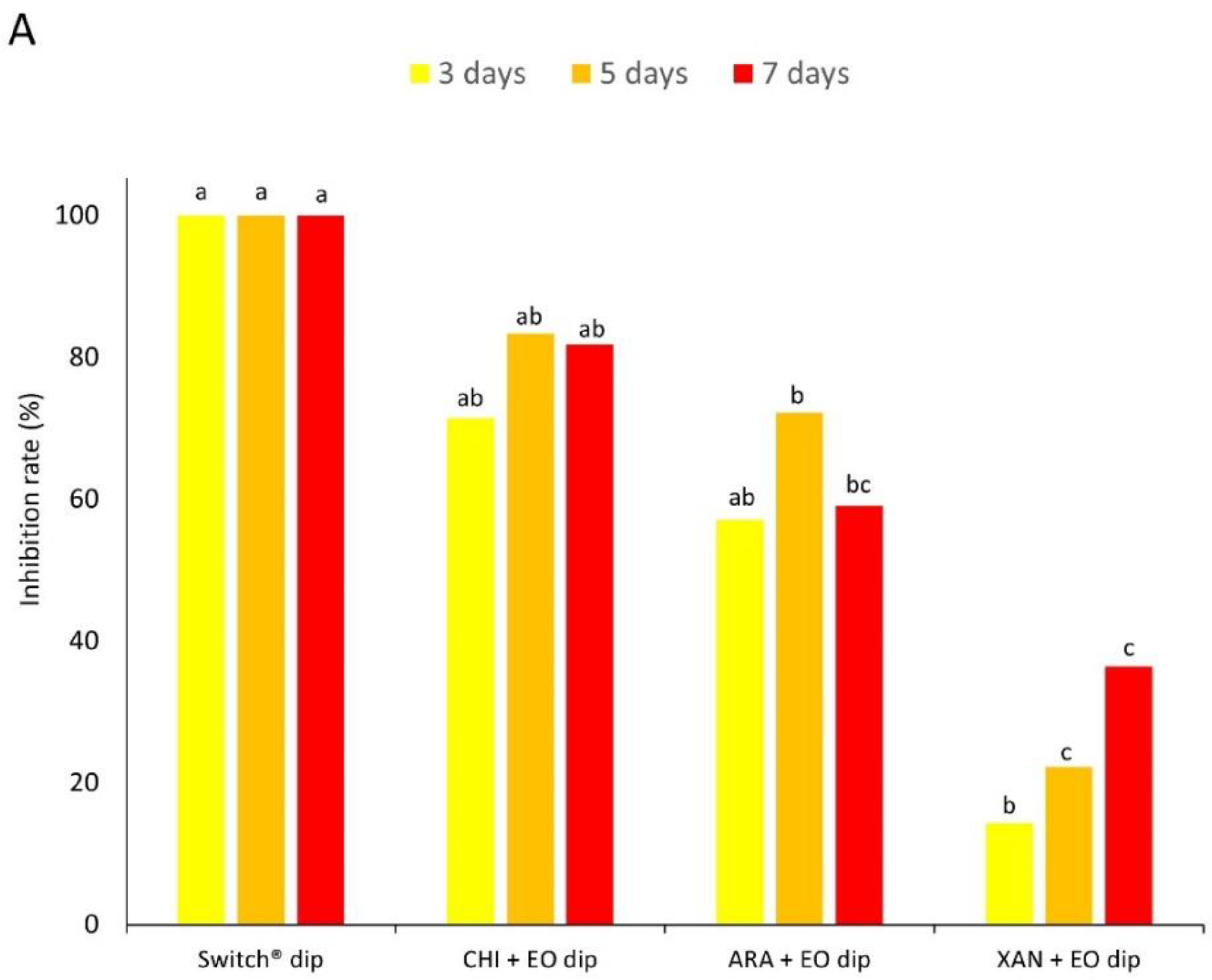
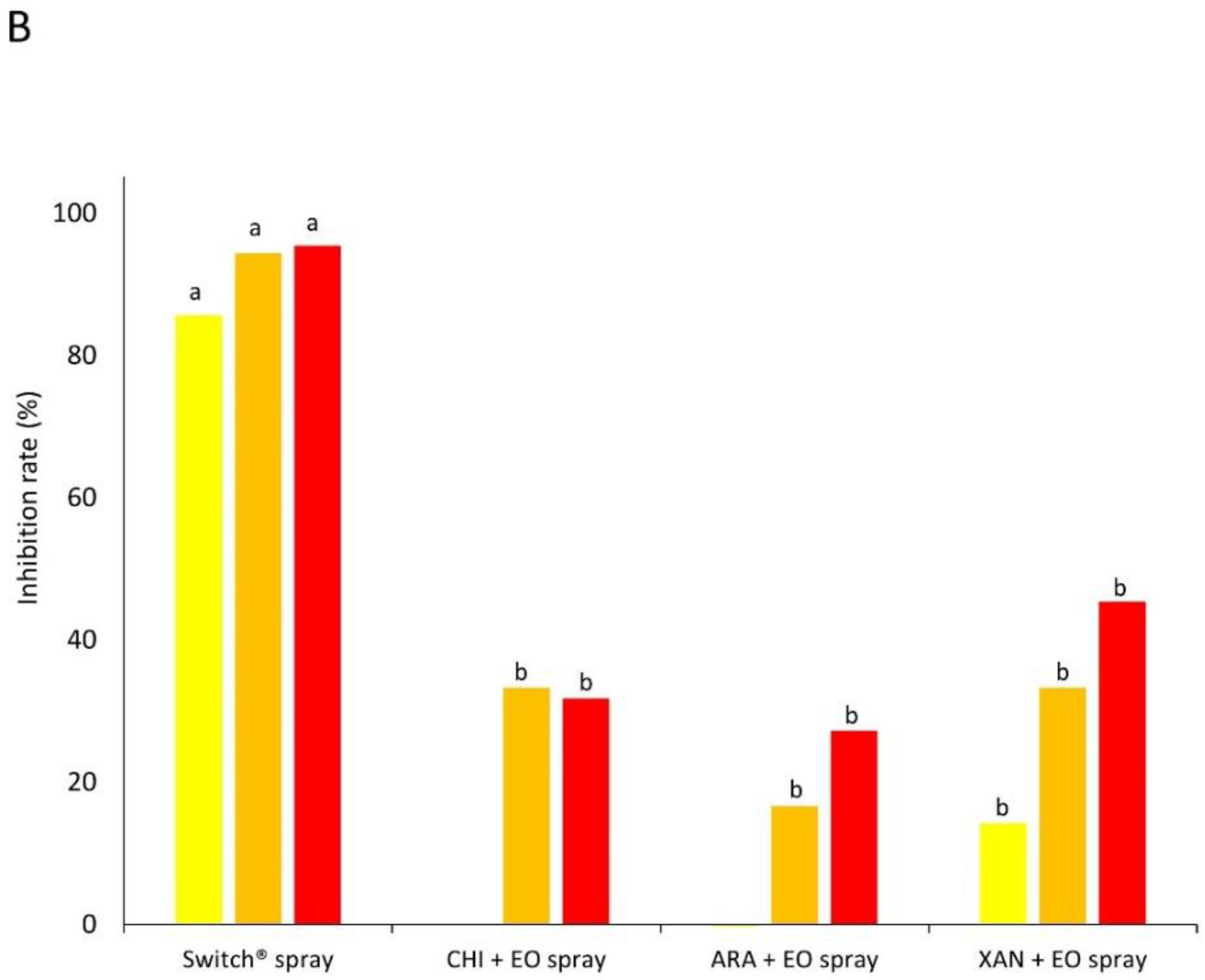
2.4. Experiment 2—In Vivo Tests
2.4.1. Preparation of Polysaccharide Matrices and EO Encapsulation
2.4.2. Preparatory Assay: Method of Application (Spraying or Dipping)
- -
- EO spray. Apple fruits were wounded at the equatorial region with a sterile tip (3 mm diameter × 3 mm deep, two wounds fruit−1). Fruits were afterward treated (see Section 2.4.1) by spraying the products on the apple surface. After 2 h, fruits were inoculated by laying on each wound 10 µL of a conidial suspension (105 conidia mL−1 for B. cinerea on ‘Braeburn’ apples and 103 conidia mL−1 for P. expansum on ‘Golden Delicious’ apples).
- -
- EO dipping. Apple fruits were wounded as described above. Fruits were then dipped in product solutions (see Section 2.4.1) for 1 min (30 sec for fungicide control). After 2 h, fruits were inoculated as described above.
- -
- Fungicide spray or dipping. Apple fruits were wounded as described above. Fruits were then sprayed or dipped in a solution of the synthetic fungicide Switch® (0.05% w/v) and after 2 h inoculated as above.
- -
- Control. Apples were wounded, treated with water, and then inoculated.
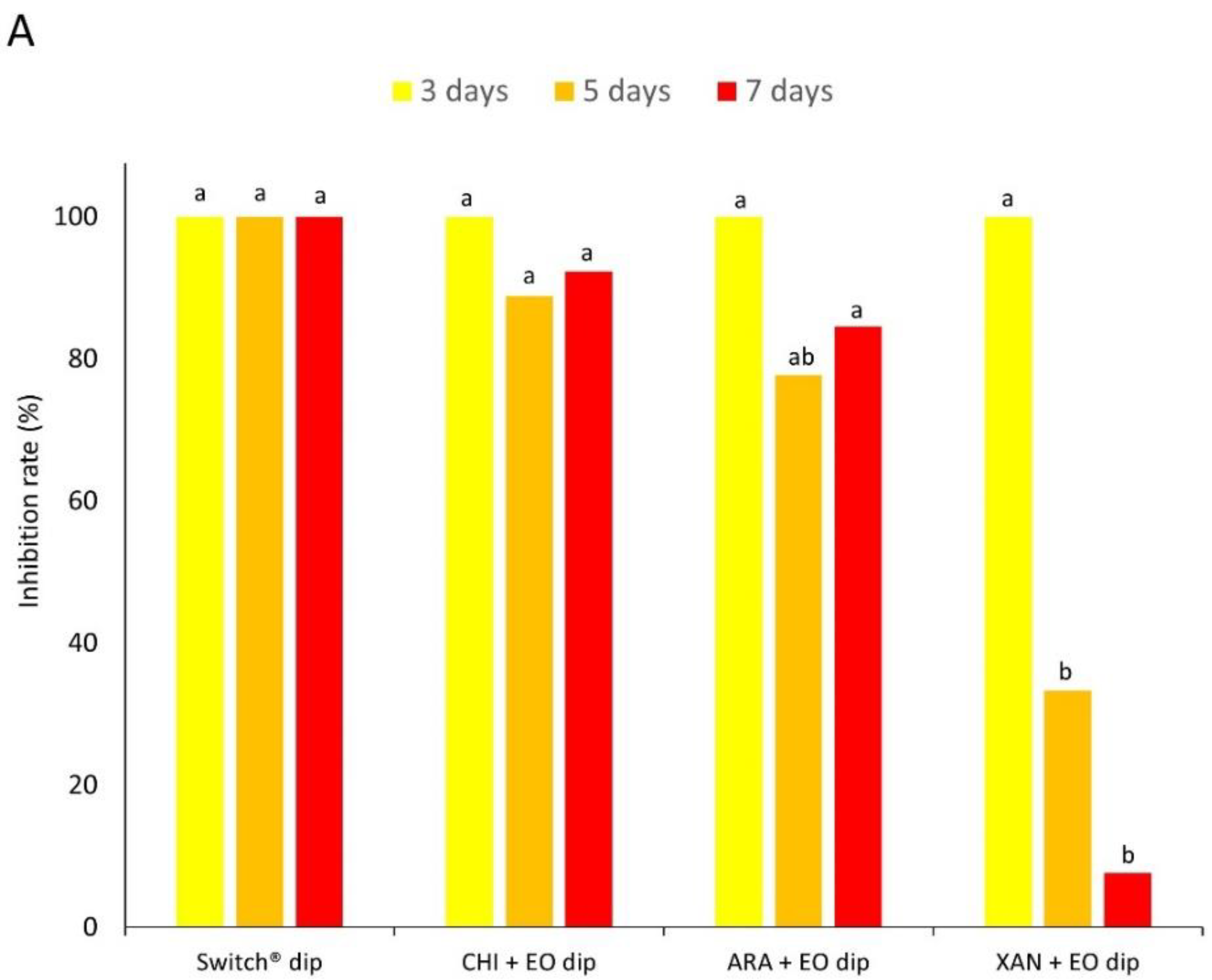
2.4.3. Extended Assay: Fruit Dipping with EOs and Chitosan as Coating Matrix
2.5. Statistical Analysis
3. Results and Discussion
3.1. Experiment 1—In Vitro Tests
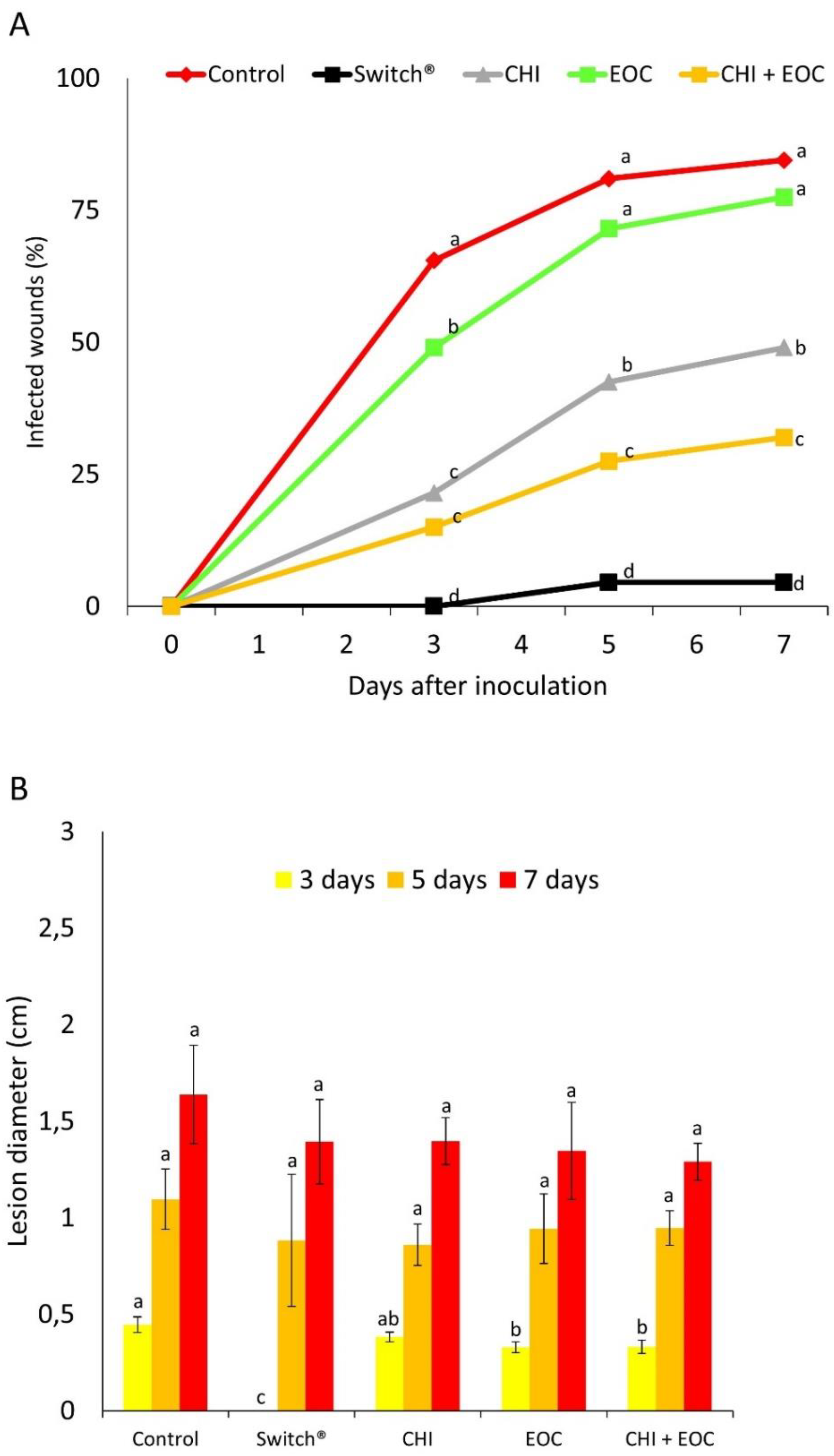
3.2. Experiment 2—In Vivo Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sivakumar, D.; Bautista-Baños, S. A review on the use of essential oils for postharvest decay control and maintenance of fruit quality during storage. Crop. Prot. 2014, 64, 27–37. [Google Scholar] [CrossRef]
- Romanazzi, G.; Sanzani, S.M.; Bi, Y.; Tian, S.; Gutiérrez Martínez, P.; Alkan, N. Induced resistance to control postharvest decay of fruit and vegetables. Postharvest Biol. Technol. 2016, 122, 82–94. [Google Scholar] [CrossRef]
- EFSA (European Food Safety Authority); Medina-Pastor, P.; Triacchini, G. The 2018 European Union report on pesticide residues in food. EFSA J. 2020, 18, 103. [Google Scholar] [CrossRef] [Green Version]
- EFSA (European Food Safety Authority). 2019 Eurobarometer on Food Safety in the EU. Survey Requested by the European Food Safety Authority (EFSA) and Co-Ordinated by the European Commission, Directorate-General for Communication; EU Publications Office: Luxembourg, 2019; ISBN 978-92-9499-082-2. [Google Scholar]
- Shafie, F.A.; Rennie, D. Consumer perceptions towards organic food. Procedia Soc. Behav. Sci. 2012, 49, 360–367. [Google Scholar] [CrossRef] [Green Version]
- Prange, R.K. Pre-harvest, harvest and post-harvest strategies for organic production of fruits and vegetables. Acta Hortic. 2012, 933, 43–50. [Google Scholar] [CrossRef]
- Kopacki, M.; Pawłat, J.; Skwaryło-Bednarz, B.; Jamiołkowska, A.; Stępniak, P.M.; Kiczorowski, P.; Golan, K. Physical crop postharvest storage and protection methods. Agronomy 2021, 11, 93. [Google Scholar] [CrossRef]
- Themen, D. Food Losses and Waste in Europe and Central Asia; Food and Agriculture Organization of the United Nations: Rome, Italy, 2014; p. 81. [Google Scholar]
- Smetanska, I.; Hunaefi, D.; Barbosa-Cánovas, G.V. Nonthermal Technologies to Extend the Shelf Life of Fresh-Cut Fruits and Vegetables. In Advances in Food Process Engineering Research and Applications; Yanniotis, S., Taoukis, P., Stoforos, N.G., Karathanos, V.T., Eds.; Springer: Boston, MA, USA, 2013; pp. 375–413. ISBN 978-1-4614-7906-2. [Google Scholar]
- Dhall, R.K. Advances in edible coatings for fresh fruits and vegetables: A review. Crit. Rev. Food Sci. Nutr. 2013, 53, 435–450. [Google Scholar] [CrossRef]
- Opara, U.L.; Atukuri, J.; Fawole, O.A. Application of physical and chemical postharvest treatments to enhance storage and shelf life of pomegranate fruit—A review. Sci. Hortic. 2015, 197, 41–49. [Google Scholar] [CrossRef]
- Boubaker, H.; Karim, H.; El Hamdaoui, A.; Msanda, F.; Leach, D.; Bombarda, I.; Vanloot, P.; Abbad, A.; Boudyach, E.H.; Ait Ben Aoumar, A. Chemical characterization and antifungal activities of four Thymus species essential oils against postharvest fungal pathogens of citrus. Ind. Crops Prod. 2016, 86, 95–101. [Google Scholar] [CrossRef]
- Cindi, M.D.; Soundy, P.; Romanazzi, G.; Sivakumar, D. Different defense responses and brown rot control in two Prunus persica cultivars to essential oil vapours after storage. Postharvest Biol. Technol. 2016, 119, 9–17. [Google Scholar] [CrossRef]
- Wińska, K.; Mączka, W.; Łyczko, J.; Grabarczyk, M.; Czubaszek, A.; Szumny, A. Essential oils as antimicrobial agents-myth or real alternative? Molecules 2019, 24, 2130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El Khetabi, A.; Lahlali, R.; Ezrari, S.; Radouane, N.; Lyousfi, N.; Banani, H.; Askarne, L.; Tahiri, A.; El Ghadraoui, L.; Belmalha, S.; et al. Role of plant extracts and essential oils in fighting against postharvest fruit pathogens and extending fruit shelf life: A review. Trends Food Sci. Technol. 2022, 120, 402–417. [Google Scholar] [CrossRef]
- Nazzaro, F.; Fratianni, F.; Coppola, R.; Feo, V.D. Essential oils and antifungal activity. Pharmaceuticals 2017, 10, 86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carson, C.F.; Hammer, K.A. Chemistry and Bioactivity of Essential Oils. In Lipids and Essential Oils as Antimicrobial Agents; Thormar, H., Ed.; John Wiley & Sons, Ltd.: Chichester West Sussex, UK, 2010. [Google Scholar]
- Farzaneh, M.; Kiani, H.; Sharifi, R.; Reisi, M.; Hadian, J. Chemical composition and antifungal effects of three species of Satureja (S. hortensis, S. spicigera, and S. khuzistanica) essential oils on the main pathogens of strawberry fruit. Postharvest Biol. Technol. 2015, 109, 145–151. [Google Scholar] [CrossRef]
- Mohammadi, S.; Aroiee, H.; Aminifard, M.H.; Tehranifar, A.; Jahanbakhsh, V. Effect of fungicidal essential oils against Botrytis cinerea and Rhizopus stolonifer rot fungus in vitro conditions. Arch. Phytopathol. Plant Prot. 2014, 47, 1603–1610. [Google Scholar] [CrossRef]
- Venturini, M.E.; Blanco, D.; Oria, R. In vitro antifungal activity of several antimicrobial compounds against Penicillium expansum. J. Food Prot. 2002, 65, 834–839. [Google Scholar] [CrossRef]
- Vitoratos, A.; Bilalis, D.; Karkanis, A.; Efthimiadou, A. Antifungal activity of plant essential oils against Botrytis cinerea, Penicillium italicum and Penicillium digitatum. Not. Bot. Horti Agrobot. Cluj Napoca 2013, 41, 86. [Google Scholar] [CrossRef] [Green Version]
- Chaieb, K.; Hajlaoui, H.; Zmantar, T.; Kahla-Nakbi, A.B.; Rouabhia, M.; Mahdouani, K.; Bakhrouf, A. The chemical composition and biological activity of clove essential oil, Eugenia caryophyllata (Syzigium aromaticum L. Myrtaceae): A short review. Phytother. Res. 2007, 21, 501–506. [Google Scholar] [CrossRef]
- Aguilar-González, A.E.; Palou, E.; López-Malo, A. Antifungal activity of essential oils of clove (Syzygium aromaticum) and/or mustard (Brassica nigra) in vapor phase against gray mold (Botrytis cinerea) in strawberries. Innov. Food Sci. Emerg. Technol. 2015, 32, 181–185. [Google Scholar] [CrossRef]
- Amiri, A.; Dugas, R.; Pichot, A.; Bompeix, G. In vitro and in vitro activity of eugenol oil (Eugenia caryophylata) against four important postharvest apple pathogens. Int. J. Food Microbiol. 2008, 126, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Jhalegar, M.D.J.; Sharma, R.R.; Singh, D. In vitro and in vivo activity of essential oils against major postharvest pathogens of Kinnow (Citrus nobilis × C. deliciosa) mandarin. J. Food Sci. Technol. 2015, 52, 2229–2237. [Google Scholar] [CrossRef]
- Antunes, M.D.C.; Cavaco, A.M. The use of essential oils for postharvest decay control. A review. Flavour Fragr. J. 2010, 25, 351–366. [Google Scholar] [CrossRef]
- Mani-López, E.; Palou, E.; López-Malo, A. Chapter 8—Biopreservatives as Agents to Prevent Food Spoilage. In Microbial Contamination and Food Degradation; Holban, A.M., Grumezescu, A.M., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 235–270. ISBN 978-0-12-811515-2. [Google Scholar]
- Sánchez-González, L.; Vargas, M.; González-Martínez, C.; Chiralt, A.; Cháfer, M. Use of essential oils in bioactive edible coatings: A review. Food Eng. Rev. 2011, 3, 1–16. [Google Scholar] [CrossRef]
- Hyldgaard, M.; Mygind, T.; Meyer, R. Essential Oils in Food Preservation: Mode of Action, Synergies, and Interactions with Food Matrix Components. Front. Microbiol. 2012, 3, 12. [Google Scholar] [CrossRef] [Green Version]
- Turek, C.; Stintzing, F.C. Stability of essential oils: A review. Compr. Rev. Food Sci. Food Saf. 2013, 12, 40–53. [Google Scholar] [CrossRef]
- Ribeiro-Santos, R.; Andrade, M.; Sanches-Silva, A. Application of encapsulated essential oils as antimicrobial agents in food packaging. Curr. Opin. Food Sci. 2017, 14, 78–84. [Google Scholar] [CrossRef]
- Palou, L.; Valencia-Chamorro, S.; Pérez-Gago, M. Antifungal edible coatings for fresh citrus fruit: A review. Coatings 2015, 5, 962–986. [Google Scholar] [CrossRef] [Green Version]
- Daniel, C.K.; Lennox, C.L.; Vries, F.A. In vivo application of garlic extracts in combination with clove oil to prevent postharvest decay caused by Botrytis cinerea, Penicillium expansum and Neofabraea alba on apples. Postharvest Biol. Technol. 2015, 99, 88–92. [Google Scholar] [CrossRef]
- Morkeliūnė, A.; Rasiukevičiūtė, N.; Šernaitė, L.; Valiuškaitė, A. The use of essential oils from thyme, sage and peppermint against Colletotrichum acutatum. Plants 2021, 10, 114. [Google Scholar] [CrossRef]
- Perdones, A.; Sánchez-González, L.; Chiralt, A.; Vargas, M. Effect of chitosan–lemon essential oil coatings on storage-keeping quality of strawberry. Postharvest Biol. Technol. 2012, 70, 32–41. [Google Scholar] [CrossRef]
- Maqbool, M.; Ali, A.; Alderson, P.G.; Mohamed, M.T.M.; Siddiqui, Y.; Zahid, N. Postharvest application of gum arabic and essential oils for controlling anthracnose and quality of banana and papaya during cold storage. Postharvest Biol. Technol. 2011, 62, 71–76. [Google Scholar] [CrossRef]
- Sharma, S.; Rao, T.V.R. Xanthan gum based edible coating enriched with cinnamic acid prevents browning and extends the shelf-life of fresh-cut pears. LWT Food Sci. Technol. 2015, 62, 791–800. [Google Scholar] [CrossRef]
- Antunes, M.D.; Custodia, M.G.; Ana, M.C.; Miguel, G.M. Edible coatings enriched with essential oils and their compounds for fresh and fresh-cut fruit. Recent Pat. Food Nutr. Agric. 2012, 4, 114–122. [Google Scholar] [CrossRef] [Green Version]
- Fatemi, H.; Aminifard, M.H.; Mohammadi, S. Efficacy of plant essential oils on post-harvest control of rot caused by Botrytis cinerea on kiwi fruits. Arch. Phytopathol. Plant Prot. 2013, 46, 536–547. [Google Scholar] [CrossRef]
- Fathi, Z.; Hassani, A.; Ghosta, Y.; Abdollahi, A.; Meshkatalsadat, M.H. The potential of thyme, clove, cinnamon and ajowan essential oils in inhibiting the growth of Botrytis cinerea and Monilinia fructicola. J. Essent. Oil Bear. Plants 2012, 15, 38–47. [Google Scholar] [CrossRef]
- Wang, Y.; Bian, W.; Ren, X.; Song, X.; He, S. Microencapsulation of clove essential oil improves its antifungal activity against Penicillium digitatum in vitro and green mould on Navel oranges. J. Hortic. Sci. Biotechnol. 2018, 93, 159–166. [Google Scholar] [CrossRef]
- Grande-Tovar, C.D.; Chaves-Lopez, C.; Serio, A.; Rossi, C.; Paparella, A. Chitosan coatings enriched with essential oils: Effects on fungi involved in fruit decay and mechanisms of action. Trends Food Sci. Technol. 2018, 78, 61–71. [Google Scholar] [CrossRef]
- Latifah-Munirah, B.; Himratul-Aznita, W.H.; Mohd Zain, N. Eugenol, an essential oil of clove, causes disruption to the cell wall of Candida albicans (ATCC 14053). Front. Life Sci. 2015, 8, 231–240. [Google Scholar] [CrossRef] [Green Version]
- Soković, M.; Ristić, M.; Grubišić, D. Chemical composition and antifungal activity of the essential oil from Juniperus excelsa berries. Pharm. Biol. 2004, 42, 328–331. [Google Scholar] [CrossRef]
- Coyotl-Pérez, W.A.; Rubio-Rosas, E.; Morales-Rabanales, Q.N.; Ramírez-García, S.A.; Pacheco-Hernández, Y.; Pérez-España, V.H.; Romero-Arenas, O.; Villa-Ruano, N. Improving the Shelf Life of Avocado Fruit against Clonostachys rosea with Chitosan Hybrid Films Containing Thyme Essential Oil. Polymers 2022, 14, 2050. [Google Scholar] [CrossRef]
- Cháfer, M.; Sánchez-González, L.; González-Martínez, C.; Chiralt, A. Fungal decay and shelf life of oranges coated with chitosan and bergamot, thyme, and tea tree essential oils. J. Food Sci. 2012, 77, E182–E187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garrido Assis, O.B.; de Britto, D. Evaluation of the antifungal properties of chitosan coating on cut apples using a non-invasive image analysis technique. Polym. Int. 2011, 60, 932–936. [Google Scholar] [CrossRef]
- Shao, X.; Cao, B.; Xu, F.; Xie, S.; Yu, D.; Wang, H. Effect of postharvest application of chitosan combined with clove oil against citrus green mold. Postharvest Biol. Technol. 2015, 99, 37–43. [Google Scholar] [CrossRef]
- Romanazzi, G.; Moumni, M. Chitosan and other edible coatings to extend shelf life, manage postharvest decay, and reduce loss and waste of fresh fruits and vegetables. Curr. Opin. Biotechnol. 2022, 78, 102834. [Google Scholar] [CrossRef] [PubMed]
- Popescu, P.-A.; Palade, L.M.; Nicolae, I.-C.; Popa, E.E.; Miteluț, A.C.; Drăghici, M.C.; Matei, F.; Popa, M.E. Chitosan-Based Edible Coatings Containing Essential Oils to Preserve the Shelf Life and Postharvest Quality Parameters of Organic Strawberries and Apples during Cold Storage. Foods 2022, 11, 3317. [Google Scholar] [CrossRef] [PubMed]
- Hadwiger, L.A.; Kendra, D.F.; Fristensky, B.W.; Wagoner, W. Chitosan Both Activates Genes in Plants and Inhibits RNA Synthesis in Fungi. In Chitin in Nature and Technology; Muzzarelli, R., Jeuniaux, C., Gooday, G.W., Eds.; Springer: Boston, MA, USA, 1986; pp. 209–214. ISBN 978-1-4613-2167-5. [Google Scholar]
- Verlee, A.; Mincke, S.; Stevens, C.V. Recent developments in antibacterial and antifungal chitosan and its derivatives. Carbohydr. Polym. 2017, 164, 268–283. [Google Scholar] [CrossRef]
- Almasaudi, N.M.; Al-Qurashi, A.D.; Elsayed, M.I.; Abo-Elyousr, K.A.M. Essential oils of oregano and cinnamon as an alternative method for control of gray mold disease of table grapes caused by Botrytis cinerea. J. Plant Pathol. 2022, 104, 317–328. [Google Scholar] [CrossRef]
- Elsayed, M.I.; Al-Qurashi, A.D.; Almasaudi, N.M.; Abo-Elyousr, K.A.M. Efficacy of essential oils against gray mold and effect on fruit quality during cold storage in table grapes. S. Afr. J. Bot. 2022, 146, 481–490. [Google Scholar] [CrossRef]
- Leite, B.S.F.; Borges, C.D.; Carvalho, P.G.B.; Botrel, N. Xanthan gum based edible coating combined with oleic acid or peppermint essential oil in the preservation of strawberries (Fragaria × ananassa). Rev. Bras. Frutic. 2015, 37, 1027–1036. [Google Scholar] [CrossRef]
- Lopez-Reyes, J.G.; Spadaro, D.; Gullino, M.L.; Garibaldi, A. Efficacy of plant essential oils on postharvest control of rot caused by fungi on four cultivars of apples in vivo. Flavour Fragr. J. 2010, 25, 171–177. [Google Scholar] [CrossRef]




| Substance | Origin | Description | Commercial Name |
|---|---|---|---|
| Thyme EO | Thymus vulgaris (Fam. Lamiaceae) | EO obtained after distillation of thyme leaves/flowers (main component: thymol) | Essential oil, Vitalis Dr. Joseph s.r.l., Italy |
| Juniper EO | Juniperus communis (Fam. Cupressaceae) | EO obtained after distillation of juniper berries (main component: α-pinene) | Essential oil, Vitalis Dr. Joseph s.r.l., Italy |
| Clove EO | Syzygium aromaticum (Fam. Myrtaceae) | EO obtained after distillation of clove flower buds (main component: eugenol) | Essential oil, Vitalis Dr. Joseph s.r.l., Italy |
| Camphor EO | Cinnamomum camphora (Fam. Lauraceae) | EO obtained after distillation of wood/leaves (main component: camphor) | Essential oil, Vitalis Dr. Joseph s.r.l., Italy |
| Anise EO | Pimpinella anisum (Fam. Apiaceae) | EO obtained after distillation of fruits (main component: anethole) | Essential oil, Vitalis Dr. Joseph s.r.l., Italy |
| Chitosan | Crustaceans shell | Obtained from chitin, component of the shells of crustaceans (main component: polysaccharides) | Molekula Ltd., Shaftesbury, Dorset, UK |
| Arabic gum | Acacia tree | Obtained from the sap of the acacia trees (main component: polysaccharides) | MP Biomedicals, LLC. Solon, OH, USA |
| Xanthan gum | Xanthomonas campestris | Obtained after fermentation of sugars (i.e., sucrose) by X. campestris (main components: polysaccharides) | Doves Farm Foods Ltd. Company, Berkshire, UK |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soppelsa, S.; Van Hemelrijck, W.; Bylemans, D.; Andreotti, C. Essential Oils and Chitosan Applications to Protect Apples against Postharvest Diseases and to Extend Shelf Life. Agronomy 2023, 13, 822. https://doi.org/10.3390/agronomy13030822
Soppelsa S, Van Hemelrijck W, Bylemans D, Andreotti C. Essential Oils and Chitosan Applications to Protect Apples against Postharvest Diseases and to Extend Shelf Life. Agronomy. 2023; 13(3):822. https://doi.org/10.3390/agronomy13030822
Chicago/Turabian StyleSoppelsa, Sebastian, Wendy Van Hemelrijck, Dany Bylemans, and Carlo Andreotti. 2023. "Essential Oils and Chitosan Applications to Protect Apples against Postharvest Diseases and to Extend Shelf Life" Agronomy 13, no. 3: 822. https://doi.org/10.3390/agronomy13030822
APA StyleSoppelsa, S., Van Hemelrijck, W., Bylemans, D., & Andreotti, C. (2023). Essential Oils and Chitosan Applications to Protect Apples against Postharvest Diseases and to Extend Shelf Life. Agronomy, 13(3), 822. https://doi.org/10.3390/agronomy13030822







