Abstract
Agricultural production currently faces many challenges worldwide, mainly due to its dependence on high amounts of input for food production, which may cause many environmental issues. The present study evaluated whether the inoculation of Bacillus subtilis, Trichoderma harzianum, and rock powder into the soil would benefit soil fertility and plant growth in vase conditions. The results showed that soil fertility for some nutrients increased, such as phosphorus, iron, sulfur, calcium, and potassium. The results also showed that plant parameters related to plant growth, such as plant height, leaf area, shoot dry matter (SDM), and root dry matter (RDM) increased with the inoculation of the microorganisms coupled with rock powder into the soil, even with 50% of the chemical fertilization dose compared to their control treatments. The findings showed potential benefits to soil fertility and plants with the inoculation of B. subtilis and the fungus T. harzianum in maize plants coupled with the application of rock powder to the soil. This study concludes that there is evidence that inoculating with microorganisms and applying rock powder could reduce the amount of chemical fertilizer needed. However, many years of field research are needed to verify the real contribution of this practice to sustainable agriculture.
1. Introduction
Due to the need to produce large amounts of different types of food, many of which are traded as commodities, world agriculture is under enormous pressure to achieve high levels of production and productivity [1]. This situation requires the use of large amounts of chemical fertilizers, fungicides, insecticides, and herbicides [2]. When used in excess, many of these products pollute the soil and water. Several studies have related the use of chemical fertilizers to the increase in many diseases in humans and animals due to toxic substances and heavy metals in these fertilizers [3]. Therefore, world agriculture faces several challenges, such as maintaining high production rates, reducing production costs and environmental impacts, and decreasing dependence on fertilizer-exporting countries [4]. Therefore, it is necessary to search for new technologies that can improve the efficiency of nutrient availability for plants, reducing dependence on chemical fertilizers, which can promote environmental damage such as soil salinization and leaching with consequent contamination of groundwater and the eutrophication of rivers [5].
Recent studies have shown that rock powder called mineralizers can increase soil fertility and productivity, rehabilitate degraded areas, decontaminate waters, and sequester carbon [6]. The use of rock powder is a consequence of the deepening of knowledge due to many studies that have been conducted indirectly seeking alternatives for sustainable production through soil management [7,8,9,10].
Although using rock powder makes nutrients become available in the soil more slowly, these nutrients remain for extended periods, stimulating soil biota [11]. In addition, using rock powder may decrease the need for chemical fertilizers because of the lower nutrient losses, unlike chemical fertilization [12]. Soil biota associated with plants represent a potential solution addressing the issues in food production. Plant growth and health are modulated by microbial communities that colonize the rhizosphere and plant tissues [13] and improve the efficiency of plants in absorbing soil nutrients through different mechanisms. Among the plant-growth-promoting bacteria, Bacillus is among the most studied groups of bacteria [14]. Bacillus is also the genus most found in the rhizosphere [15], making up 95% of the Gram-positive rhizobacterial populations [16]. According to [17], this bacterium is an efficient plant-growth promoter with several abilities related to the production of antimicrobial substances [18], siderophores [19], and lytic enzymes [20]. Soil biota also include several species of fungi that exert several growth-promoting abilities, including Trichoderma harzianum. The fungus Trichoderma harzianum has broad applicability, being an excellent decomposer of organic matter and acting in biological control because it can produce secondary metabolites (glucanase and chitinase), which can degrade the cell wall of some phytopathogenic fungi, making it efficient in controlling diseases of agricultural plants [21]. The organic acids generated by fungi of the genus Trichoderma can aid in the solubilization of some micronutrients, minerals (magnesium, iron, and manganese), and phosphates. They can also make the nutrients that are available to plants more assimilable, due to their participation in the decomposition of organic matter [22].
There needs to be more data in the literature associated with the application of rock powder to the soil and the inoculation of the bacterium B. subtilis and the fungus T. harzianum in maize crops. Corn is a demanding crop in terms of soil fertility and is of enormous agricultural importance with broad applicability, with several products intended for human and animal consumption [23].
The present study hypothesizes that using rock powder together with the inoculation of the bacterium B. subtilis and the fungus T. harzianum increases the growth and development of maize plants through several mechanisms related to greater availability of nutrients and through the phytostimulating effect of microorganisms, since these microorganisms synthesize phytohormones. Thus, the present study evaluated whether the inoculation of Bacillus subtilis, Trichoderma harzianum, and rock powder into the soil would benefit soil fertility and plant growth in vase conditions.
2. Objectives
The objective of the present study was to evaluate whether the inoculation of Bacillus subtilis, Trichoderma harzianum, and rock powder into the soil would benefit soil fertility and plant growth in vase conditions.
3. Materials and Methods
3.1. Location
The experiment was conducted at the Faculty of Agricultural and Veterinary Sciences—FCAV/UNESP, in Jaboticabal—São Paulo, in a greenhouse of the Department of Agricultural Production Sciences, with a duration of 100 days, with 60 days used for the incubation of rock powder, and 40 days of experimenting in a greenhouse that were counted from the beginning of germination.
3.2. Microorganisms
The bacterium B. subtilis used in the present study is part of the Laboratory of Agricultural Microbiology collection located at the UNESP campus of Jaboticabal. This bacterium was isolated from a maize plant and identified by sequencing. The sequence deposited in GenBank is under the number MZ133755. The fungus T. harzianum belonged to Alfa Agrotec Company (Jaboticabal, Brazil) and was kindly provided for the study. The B. subtilis bacterium was grown in nutrient broth for 48 h at 28 °C. The bacterium was applied once, in amounts of 50 mL per pot, with a volumetric pipette at a concentration of 1 × 108 colony-forming units (CFU) per mL directly into the soil. The fungus T. harzianum was grown in potato dextrose broth for 14 days at 28 °C. They were inoculated once, in quantities of 50 mL per pot, with a volumetric pipette at a concentration of 1 × 109 CFU per mL directly into the soil. In the treatments of the third experiment, which received the mixture of the two microorganisms, each pot received 50 mL of each microorganism in the same volume and concentration mentioned above.
3.3. Soil Description
The soil from the experimental area was classified as clayed quartzite neosol and came from the university‘s farm [21]. The soil samples were collected according to soil fertility methods. Four soil samples were collected from the same soil for each period before and after the rock powder application. These samples were analyzed for fertility. The fertility results were analyzed according to Scott-Knott 5%.
3.4. Compatibility Test
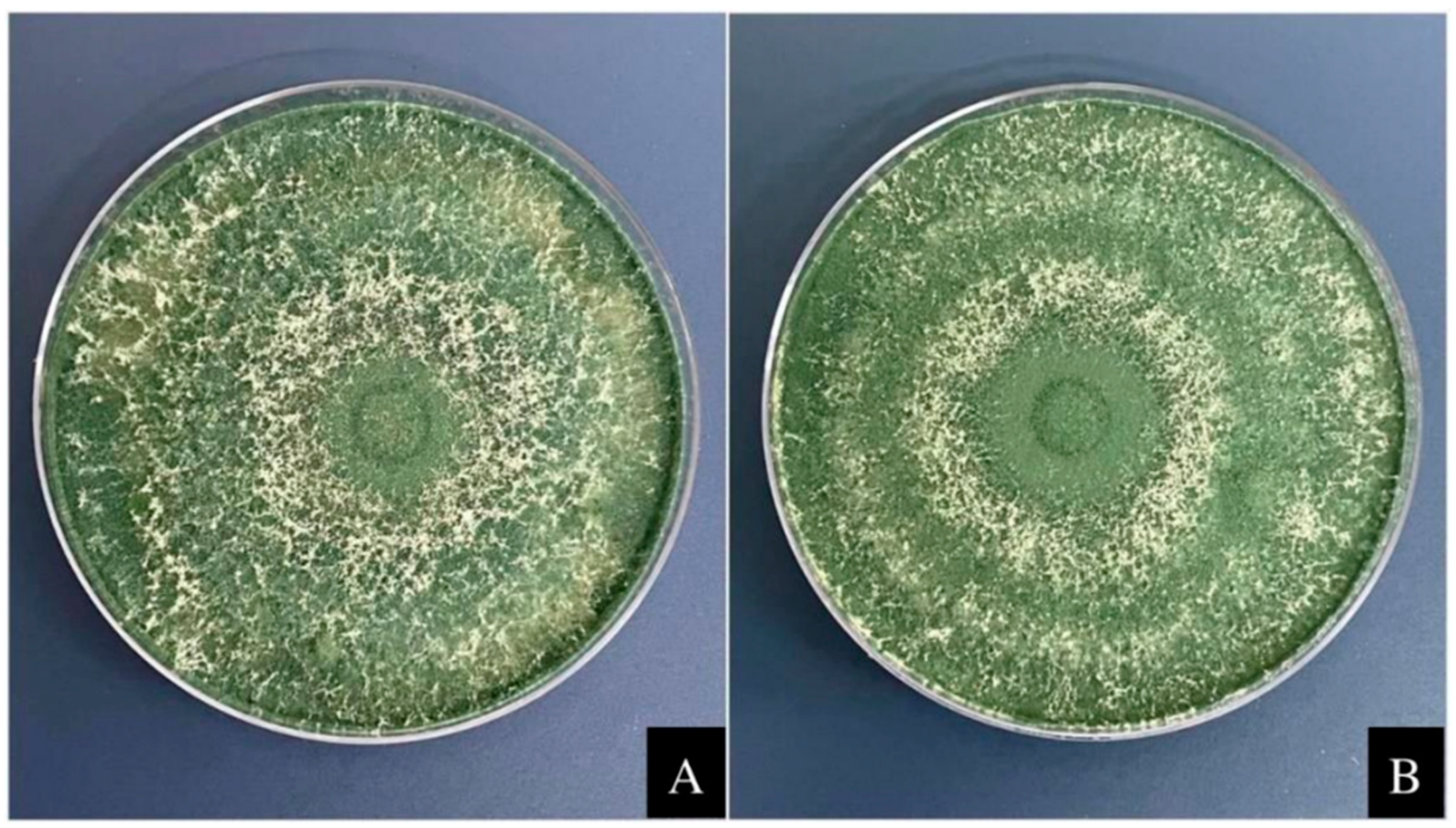
The compatibility of the microorganisms Trichoderma harzianum and Bacillus subtilis was evaluated with rock powder following a previously described methodology [22]. The fungus T. harzianum was cultivated on plates containing 20 mL of PDA culture medium (potato dextrose agar, at a concentration of 39 g L−1 deionized water) and maintained in a microbiological greenhouse oven for seven days at 26 °C, with a photoperiod of 12 h of light and 12 h of darkness. The bacterium B. subtilis was seeded in Petri dishes containing 20 mL of nutrient agar (28 g L−1) and incubated for 24 h at 28 °C. Rock powder was used at a concentration of 5 tons ha−1. The rock powder was incorporated into the culture medium after autoclaving when the medium reached 45 °C. After solidification of the medium, the fungus T. harzianum was inoculated in the center of the plate, and the bacterium B. subtilis was sown at a concentration of 1 × 108 CFU mL−1. The replicates were performed in triplicate in a completely randomized design. The controls used contained only the culture medium without rock powder. Then, the microorganism T. harzianum was placed for five days in a microbiological greenhouse at 26 °C, with a photoperiod of 12 h of light and 12 h of dark, and the mean was calculated. The fungal conidia were counted by mixing each treatment plate with 500 mL of deionized water in a blender. Then, conidia were measured in a Neubauer chamber under an optical microscope using a serial dilution of 10−3 mL−1. The bacterium B. subtilis was kept in a microbiological greenhouse for 24 h at 28 °C, and the colony-forming unit (CFU) was counted. After the results had been obtained, the average length, number of conidia, and CFUs were determined and compared using the Scott-Knott test at the 5% significance level (Figure 1).
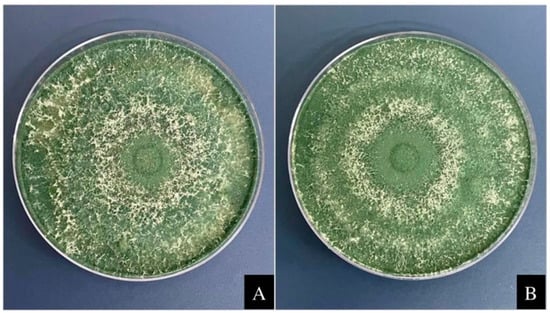
Figure 1.
Compatibility between the rock powder and the fungus T. harzianum. The fungus T. harzianum was grown in potato dextrose agar supplemented with 1 g of rock powder and incubated at 28 °C for five days (A). Control—T. harzianum was grown in potato dextrose agar medium at 28 °C for five days without rock powder (B).
3.5. Rock Powder
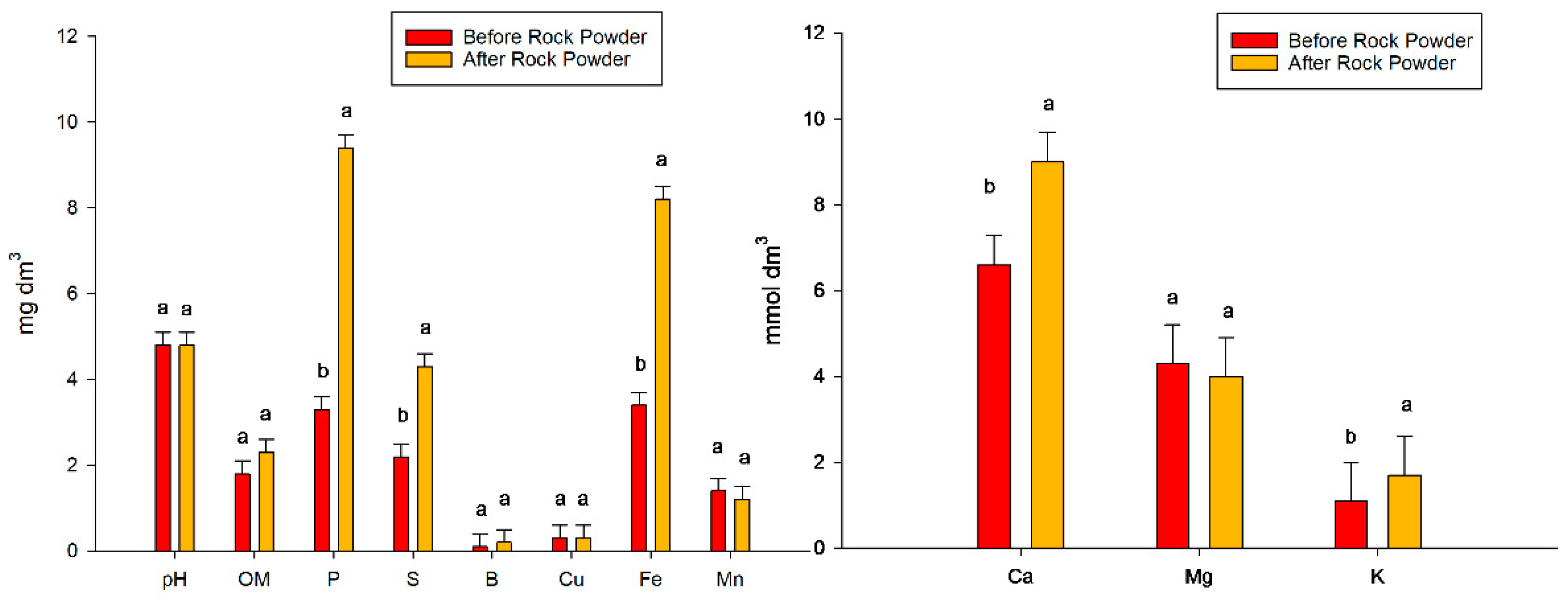
The rock powder was acquired directly from its manufacturer. The brand was Coplan, produced in the Embaúba city of Sao Paulo State, Brazil. Rock powder is a residue of basalt rock extraction and has properties that improve some soil properties [9]. The rock powder composition was SiO2 = 43.7; Al2O3 = 16.3; Fe2O3 = 9.2; Na2O = 3.4; MgO = 6.2; K2O = 3.4 and TiO2 = 3.4, and the chemical and physical attributes of the soil before rock powder application and after rock powder application are shown in Figure 2.
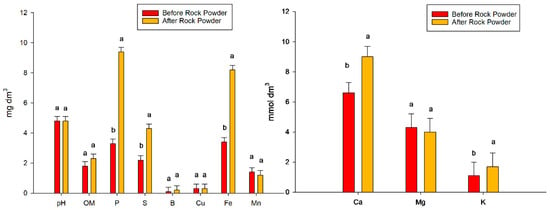
Figure 2.
Chemical and physical attributes of the soil before and after the rock powder incubation process. Bars mean = standard error. Different lowercase letters mean significant statistical differences.
3.6. Experimental Design
The maize seed cultivar RM 3700—Monsanto was acquired from Coplana in Jaboticabal—São Paulo.
The preparation of rock powder (N 0.35%; Ca 0.37%; Mg 0.20%; S 0.08%; Cu 0.01%; Fe 0.25%; Zn 0.01%; Mn 0.01% and B 0.05%) was performed by incubating the soil (quartzarenic neosol—RQ) for 60 days, with a dose of 5 tons per hectare, in pots with a volume of 5.0 dm3 (having one plant per pot). After the incubation period, the soil was turned over once a day for seven days. Then, eight soil samples were collected (four soil samples from the untreated ground and four soil samples from soil treated with rock powder), and a chemical analysis of the soil was performed according to [23].
In the second stage, basic fertilization was performed, as in [24], with applications of 80 mg dm−3 of nitrogen in the form of urea, 200 mg dm−3 of phosphorus in the form of super simple phosphate and 180 mg dm−3 of potassium in the form of potassium chloride. In addition to these nutrients, the following micronutrients were added: 0.5 mg dm−3 boron (boric acid), 1.5 mg dm−3 copper (copper sulfate), 3.0 mg dm−3 manganese (manganese) and 3.0 mg dm−3 iron (iron sulfate), as recommended by [25] Malavolta for pot tests.
The experiment was conducted in a greenhouse according to a CRD (completely randomized design) at room temperature, and complementary irrigation was maintained at 70% of field capacity with an automated sprinkler. The experimental units were constituted using rock powder and with the application of 50 mL of the microorganisms T. harzianum 1 × 108 CFU mL −1 and B. subtilis 1 × 108 CFU mL−1 in the furrow of each pot of the proposed treatments. Four replicates per treatment were performed, with a total of 60 pots. The research was divided into three experiments, and the experimental units were distributed as follows:
Experiment 1: T1 = control—conventional fertilization (100%); T2 = conventional fertilization (100%) + rock powder; T3 = conventional fertilization (100%) + T. harzianum; T4 = conventional fertilization (100%) + rock powder + T. harzianum; T5 = conventional fertilization (50%) + rock powder + T. harzianum; Experiment 2: T1 = control—conventional fertilization (100%); T2 = conventional fertilization (100%) + rock powder; T3 = conventional fertilization (100%) + B. subtilis; T4 = conventional fertilization (100%) + rock powder + B. subtilis; T5 = conventional fertilization (50%) + rock powder + B. subtilis; Experiment 3: T1 = control—conventional fertilization (100%); T2 = conventional fertilization (100%) + rock powder; T3 = conventional fertilization (100%) + T. harzianum + B. subtilis; T4 = conventional fertilization (100%) + rock powder + T. harzianum + B. subtilis; T5 = conventional fertilization (50%) + rock powder + T. harzianum + B. subtilis.
3.7. Plant Parameters
The stem diameter and plant height evaluations were performed 40 days after the beginning of the experiment. The stem diameter (SD) measurement was performed with the aid of a caliper, with the unit of measurement expressed in centimeters (cm). For the analysis of maize plant height (PH), a flexible tape measure was used to measure the plant from the stem to the apex of the leaves (cm).
The measurements of length (L) and greatest width (W) were performed on five leaves of the plant of each replicate, and the equation leaf area index estimated the area of each leaf—LAI = 0.75 × H × W. The leaf area factor was determined in all replicates and sampled 40 days after the start of each experiment [26].
The plants were removed from the pots, washed with water, and separated into shoots and roots. After separation, the plant parts were placed in paper bags and dried in an oven regulated at 70 °C for four days to obtain a constant weight of the samples. After this period, the samples were removed and cooled in a desiccator. Once at room temperature, the plants were weighed using a 0.001-g precision scale, and the result was expressed in grams (g) per plant.
3.8. Statistical Analysis
The data obtained through the tests were subjected to analysis of variance, and the means were compared by the Scott-Knott test at 5% significance using SISVAR v.1 software.
3.9. Chemical Analysis
Chemical analysis of plant tissues is a crucial tool for evaluating the nutritional status of plants. The presence of nutrients that the plant absorbs from the soil can be verified through it. The analyses of the other nutrients (nitrogen Kjeldahl method, nitro-perchloric digestion, and digestion of phosphorus, potassium, sulfur, calcium, magnesium, copper, iron, zinc, manganese, and boron) were performed according to the methodology of [27].
Five hundred micrograms of the dried and ground sample was weighed and transferred to 50 mL digestion tubes. The material was decoupled at room temperature for 1.5 h. The tubes were then placed in the digestion block and heated to 80 °C for 20 min, and then the temperature was adjusted to 160 °C. The tubes were carefully observed and removed from the block in case the material had climbed the walls of the digestion tube. When most of the HNO3 had evaporated and the solution was clear, i.e., (the brown gas on the tube wall had run out), the tubes were removed from the block to cool, and then 1.3 mL of concentrated HClO4 was added. The tubes were placed back in the digester block, and the temperature was increased to 210 °C. Digestion was complete when the solution became colorless and a dense, white vapor of HClO4 and H2O formed above the dissolved material inside the tube. The tubes were then cooled again, and the samples were diluted with H2O to 25 mL in the snap-cap glass.
For phosphorus measurement, 1 mL of the sample was pipetted and transferred to a test tube, where 4 mL of water and 2 mL of the reagent (a mixture of equal parts of 5% ammonium molybdate and 0.25% vanadate) were added. The tube was left to rest for 15 min to read in the UV-VIS spectrophotometer at 420 nm.
4. Results
From the compatibility analysis performed (Table 1), it can be observed that there was no reduction in the colony diameter for the fungus Trichoderma harzianum cultivated in culture medium with rock powder when compared to the culture of the fungus in medium without the addition of powder (control). Figure 1 shows that T. harzianum developed throughout the Petri dish in both treatments, thus proving that there was no significant difference in colony length between treatments. The conidial count results were also not significantly different (Table 1). The colony-forming unit (CFU) count of B. subtilis was performed on agar medium without powder (control). With the addition of rock powder, it was noted that the difference between treatments was insignificant, which indicates the compatibility of both the tested microorganisms with rock powder.

Table 1.
Compatibility analysis of rock powder with the microorganisms Trichoderma harzianum and Bacillus subtilis.
The rock stone technique involves adding a mineral raw material to the soil to aid the crop that will be implanted nutritionally. After the incubation period and the experiment was conducted, changes were observed when comparing the soil nutritional analysis before and after the procedure (adding rock powder) (Figure 2). The soil fertility was analyzed by comparing the results from four soil samples from the soil before rock powder application with soil samples after the rock powder application. The statistical test used was the Scott–Knott test at 5%.
The pH value was 4.8 and did not change after the use of the rock powder. Noticeably, after this input, the pH level approached what was considered optimal for the crop, even though this was not one of the purposes of using rock. The use of powder also did not increase the organic matter. After basaltic rock powder incubation, significant increases in the macronutrients phosphorus, sulfur, calcium, and potassium were observed. The increases for each nutrient were 6.1 mg dm−3 for phosphorus, 2.1 mg dm−3 for sulfur, 2.4 mmol dm−3 for calcium, and 0.60 mmol dm−3 for potassium, which were noted in the second analysis of soil. For the micronutrient boron, there was no difference between the values. In contrast, an increase of 4.8 mg dm−3 was observed for iron. There was no difference in manganese between the two values (Figure 2).
4.1. Experiment 1: Trichoderma harzianum + Rock Powder
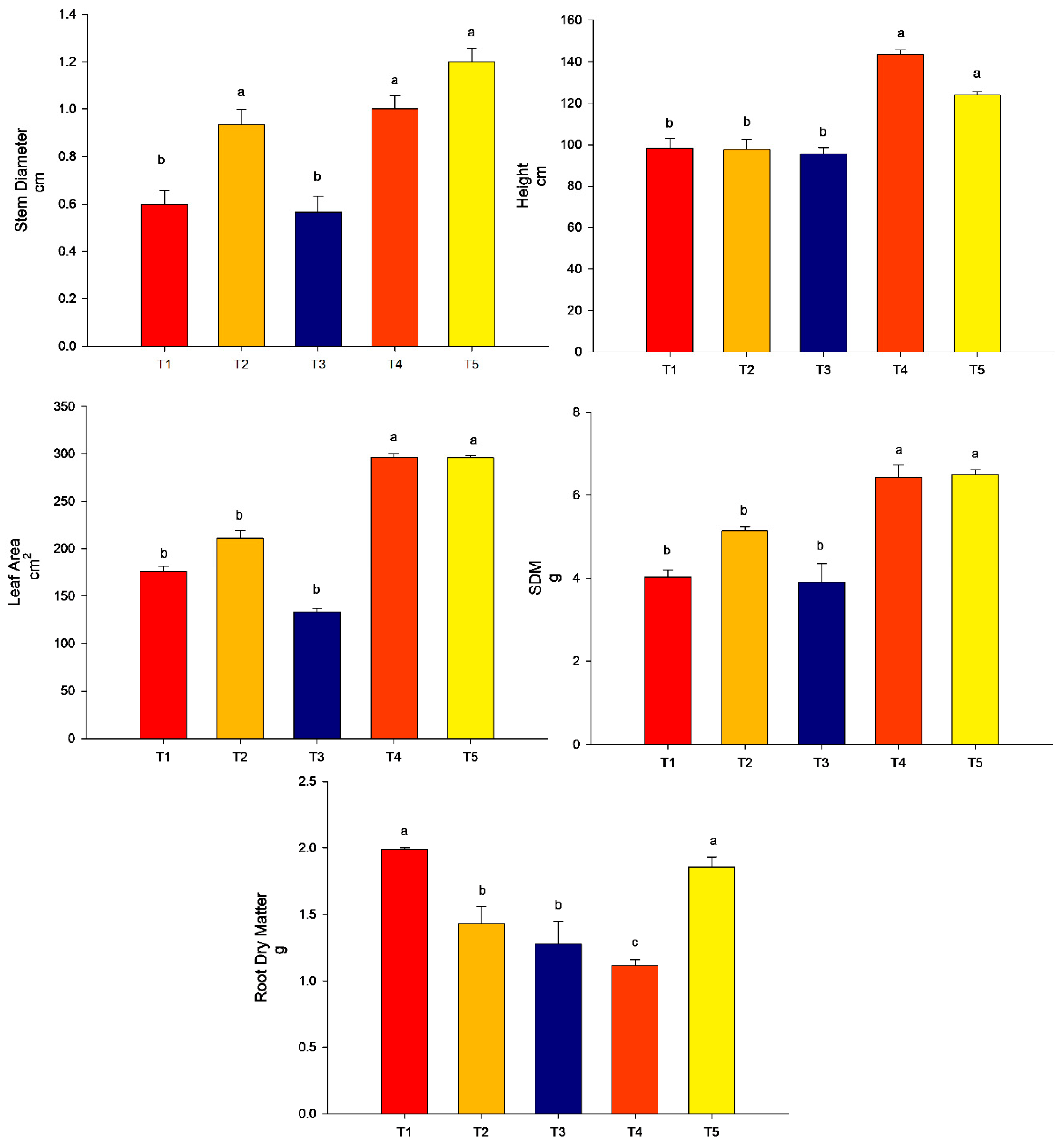
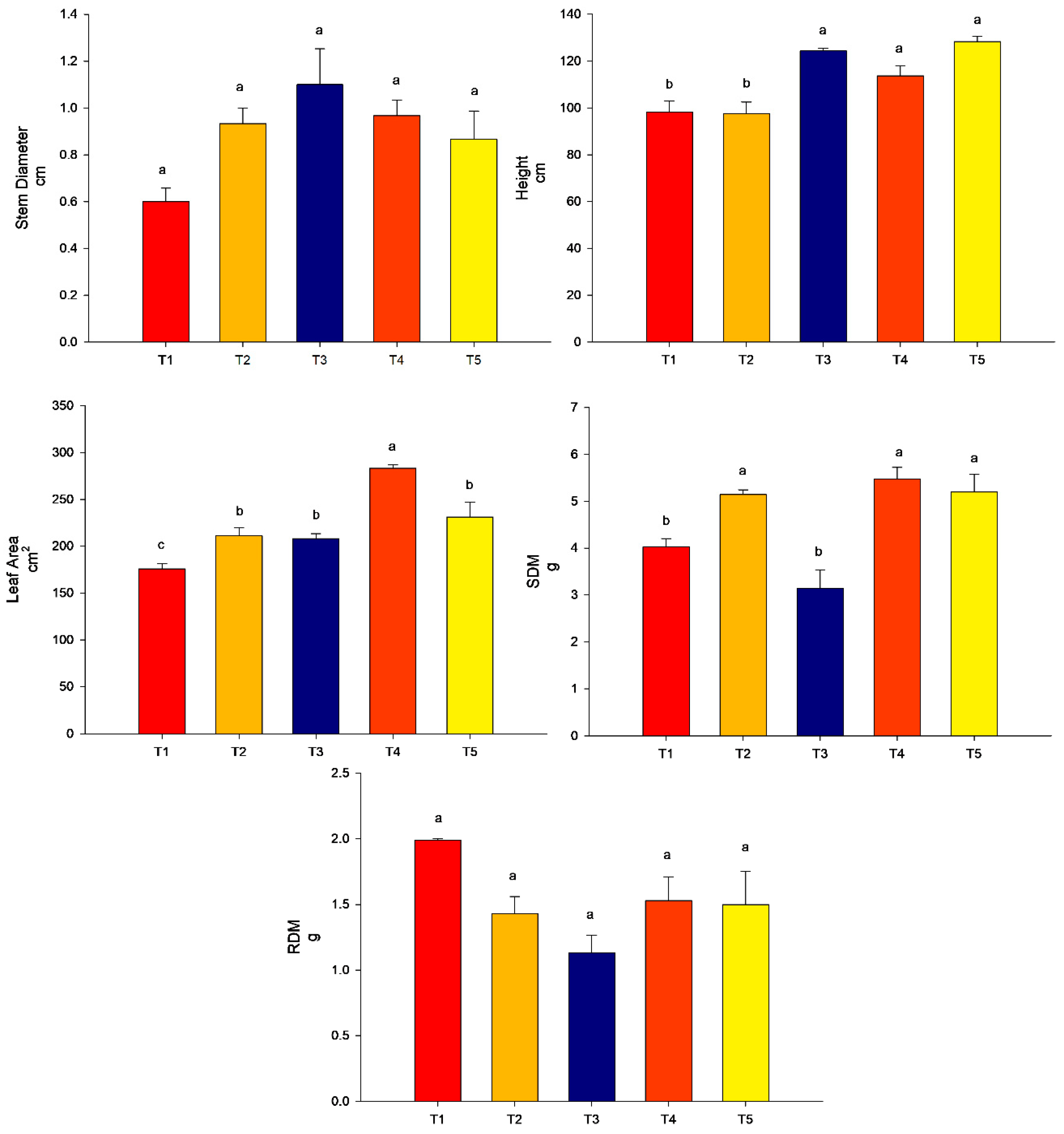
From the analyses performed, it can be observed that in experiment 1, the highest value for stem diameter was found for T5 (conventional fertilization (50%) + rock powder + T. harzianum), followed by T4 (conventional fertilization (100%) + rock powder + T. harzianum) and T2 (conventional fertilization (100%) + rock powder) (p < 0.05). T3 did not differ from the control (p > 0.05). For plant height, the highest value was found for T4 (conventional fertilization (100%) + rock powder + B. subtilis) followed by T5 (conventional fertilization (50%) + rock powder + T. harzianum. The treatments T2 (conventional fertilization (100%) + rock powder) and T3 (conventional fertilization (100%) did not differ from the control T1 (conventional fertilization 100%) (p > 0.05). Regarding the results of the leaf area, treatment 4 (conventional fertilization (100%) + rock powder + T. harzianum) and T5 (conventional fertilization (100%) + rock powder + T. harzianum) promoted the highest values compared to the control (conventional fertilization 100%) (p < 0.05). The treatments T2 (conventional fertilization (100%) + rock powder) and T3 (conventional fertilization (100%) + T. harzianum) did not differ from the control (p > 0.05). For shoot dry matter (SDM), the highest values were found for T4 (conventional fertilization (100%) + rock powder + T. harzianum) and T5 (conventional fertilization (50%) + rock powder + T. harzianum) compared to the control (conventional fertilization 100%) (p < 0.05). The treatments T2 (conventional fertilization (100%) + rock powder) and T3 (conventional fertilization (100%) + T. harzianum) did not differ from the control (p > 0.05). Interestingly, for the root dry matter, the lowest value was found for T4 (conventional fertilization (100%) + rock powder + T. harzianum), followed by T2 (conventional fertilization (100%) + rock powder) and T3 (conventional fertilization (100%) + T. harzianum) compared to the control (p < 0.05). The only treatment that did not differ from the control was T5 (conventional fertilization (50%) + rock powder + T. harzianum) (p > 0.05) (Figure 3).
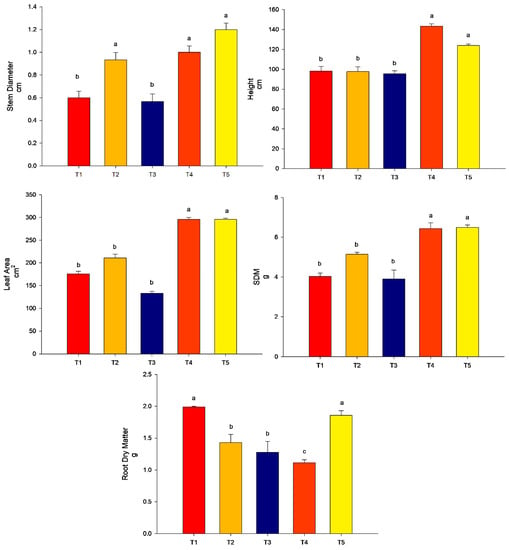
Figure 3.
Agronomic analyses were performed on maize plants (40 DAE) in association with Trichoderma harzianum, rock powder, and conventional fertilization (experiment 1). Means followed by the same letter on the line do not differ from each other according to the Scott-Knott test at 5%. T1 = control—conventional fertilization (100%); T2 = conventional fertilization (100%) + rock powder; T3 = conventional fertilization (100%) + T. harzianum; T4 = conventional fertilization (100%) + rock powder + T. harzianum; T5 = conventional fertilization (50%) + rock powder + T. harzianum. Bars mean = standard error. SDM = shoot dry matter. Different lowercase letters mean significant statistical differences.
In Table 2, it is possible to observe the amount of macronutrients found from the chemical analysis in the maize leaves. Nitrogen, a crucial element for photosynthesis, was found in more significant amounts in treatments 4, 3, and 2, which contained 100% fertilization, with the accompaniment of rock powder and T. harzianum, unlike T1, which used only 100% fertilization, and for T5, with 50% of the recommended fertilization.

Table 2.
Chemical analyses of the macronutrients nitrogen (N), phosphorus (P), potassium (K), calcium (Ca), magnesium (Mg), and sulfur (S) expressed in g/kg of dry matter, performed on maize leaves (40 DAE) with the association of Trichoderma harzianum, rock powder, and conventional fertilization (experiment 1).
When the results of phosphorus and sulfur were evaluated (Table 2), treatment 1 differed from all the others with the highest amount, even though it consisted of only fertilization (100%), and the other treatments did not differ from each other. Regarding potassium, treatment two also stood out, having the highest mean.
For calcium (Table 2), treatments 3 and 4 obtained the lowest means, even with 100% fertilization and the use of the microorganism. However, treatment 2 with rock powder and 100% fertilization showed the best result among the treatments tested, while treatments 1 and 4 expressed intermediate results. Regarding magnesium, there was no significant difference between treatments.
4.2. Experiment 2: Bacillus subtilis + Rock Powder
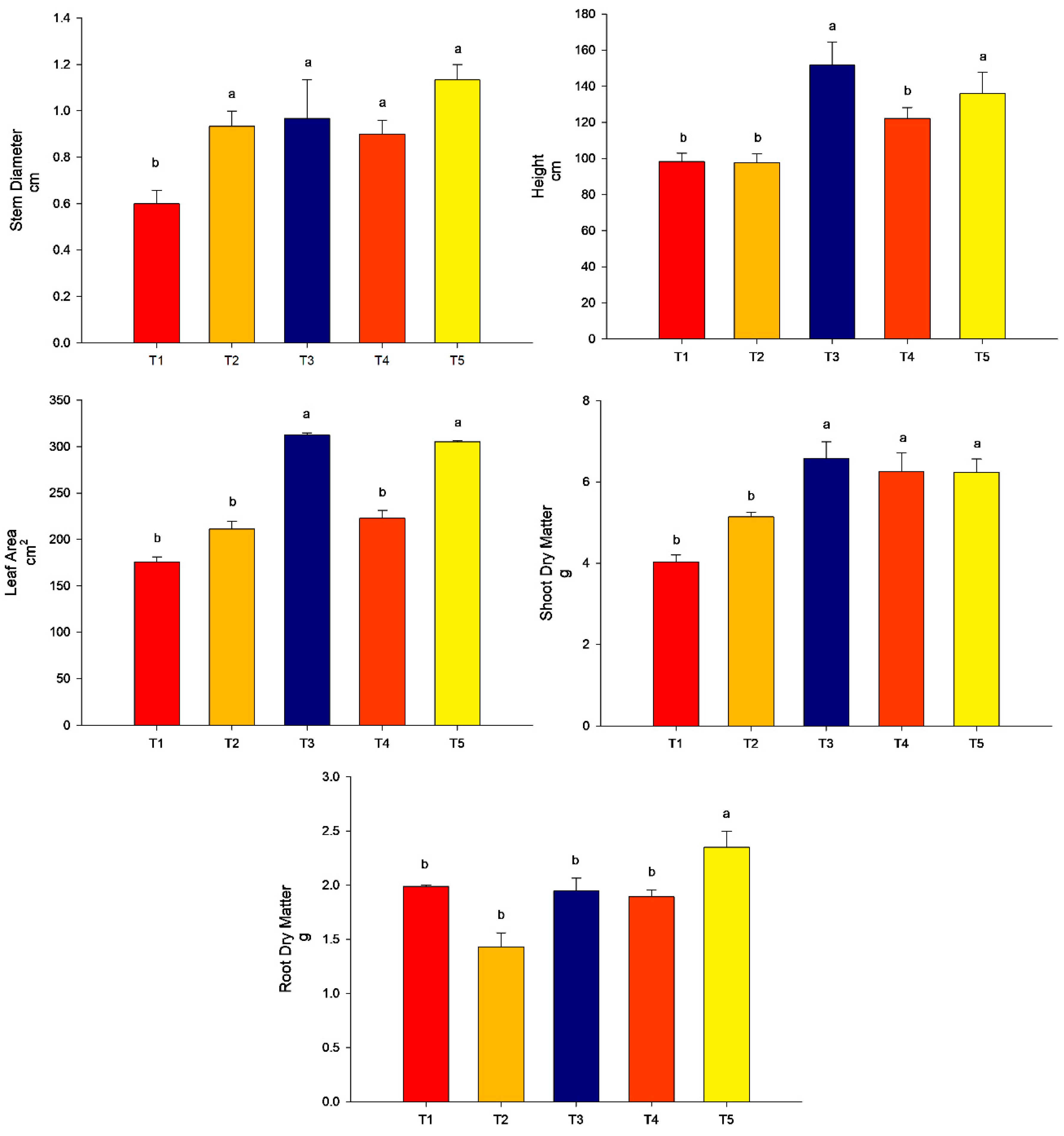
All the treatments promoted stem diameter more than the control (p < 0.05). For the height of plants and leaf area, the highest values were found for treatment T3 (conventional fertilization (100%) + B. subtilis) and T5 (conventional fertilization (50%) + rock powder + B. subtilis) (p < 0.05). The treatments T2 (conventional fertilization (100%) + rock powder) and T4 (conventional fertilization (100%) + rock powder + B. subtilis) did not differ from the control (p > 0.05). For the SDM, the lowest treatment was found for T2 (conventional fertilization (100%) + rock powder) and did not differ from that of the control (p > 0.05). The other treatments, T3 (conventional fertilization (100%) + B. subtilis), T4 (conventional fertilization (100%) + rock powder + B. subtilis), and T5 (conventional fertilization (50%) + rock powder + B. subtilis), were higher than the control (p < 0.05). For the RDM, the highest value was found for T5 (conventional fertilization (50%) + rock powder + B. subtilis). The other treatments did not differ from the control (p > 0.05) (Figure 4).
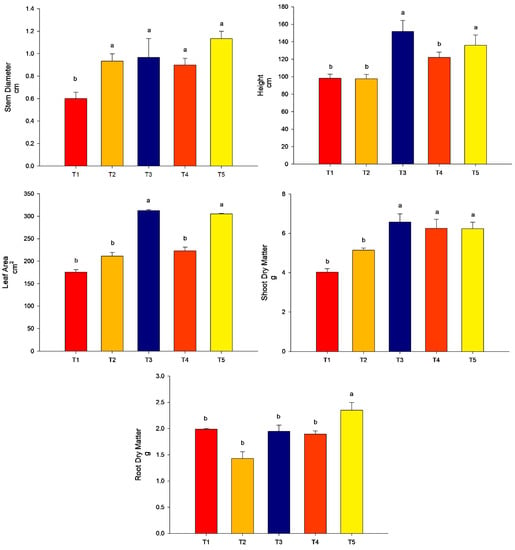
Figure 4.
Agronomic analyses were performed on maize plants (40 DAE) with the combination of B. subtilis, rock powder, and conventional fertilization (experiment 2). Means followed by the same letter on the line do not differ from each other by the Scott-Knott test at 5%. T1 = control—conventional fertilization (100%); T2 = conventional fertilization (100%) + rock powder; T3 = conventional fertilization (100%) + B. subtilis; T4 = conventional fertilization (100%) + rock powder + B. subtilis; T5 = conventional fertilization (50%) + rock powder + B. subtilis. Bars mean = standard error. Different lowercase letters mean significant statistical differences.
For the chemical analysis of corn leaf nitrogen (Table 3), it was observed that treatments 2, 3, 4, and 5 differed only from the control (100% fertilization), which reached the lowest average. This result was similar when the potassium analysis was observed, where all treatments obtained were higher than treatment 1.

Table 3.
Chemical analyses of the macronutrients nitrogen (N), phosphorus (P), potassium (K), calcium (Ca), magnesium (Mg), and sulfur (S) expressed in g/kg of dry matter, performed on maize leaves (40 DAE) with the association of Bacillus subtilis, rock powder, and conventional fertilization (experiment 2).
Regarding the phosphorus analysis (Table 3), treatments 1, 5, and 3 reached the highest means, significantly differentiating from treatments 2 and 4, which had less than 2 g/kg of the macronutrient. Analyzing the results for sulfur, treatments 1 and 3 reached the highest means, differing from 2, 4, and 5, while for the calcium and magnesium analyses, the results did not differ statistically.
4.3. Experiment 3: Trichoderma harzianum + Bacillus subtilis + Rock Powder
For stem diameter, there was no significant difference (p > 0.05) among the treatments. For plant height, the highest values were found for treatments T3 (conventional fertilization (100%) + T. harzianum + B. subtilis), T4 (conventional fertilization (100%) + rock powder + T. harzianum + B. subtilis) and T5 (conventional fertilization (50%) + rock powder + T. harzianum + B. subtilis). For the leaf area, the highest value was found for T4 ((Conventional fertilization (100%) + rock powder + T. harzianum + B. subtilis) (p < 0.05) followed by T2 (conventional fertilization (100%) + rock powder), T3 (conventional fertilization (100%) + T. harzianum + B. subtilis) and T5 (conventional fertilization (50%) + rock powder + T. harzianum + B. subtilis) compared to the control. The lowest value was found for the control. For the SDM, the highest values were found for T2 (conventional fertilization (100%) + rock powder), T4 (conventional fertilization (100%) + rock powder + T. harzianum + B. subtilis), and T5 (conventional fertilization (50%) + rock powder + T. harzianum + B. subtilis). Treatment T3 did not differ from the control (p > 0.05). For the RDM, there was no significant difference among the treatments (Figure 5).
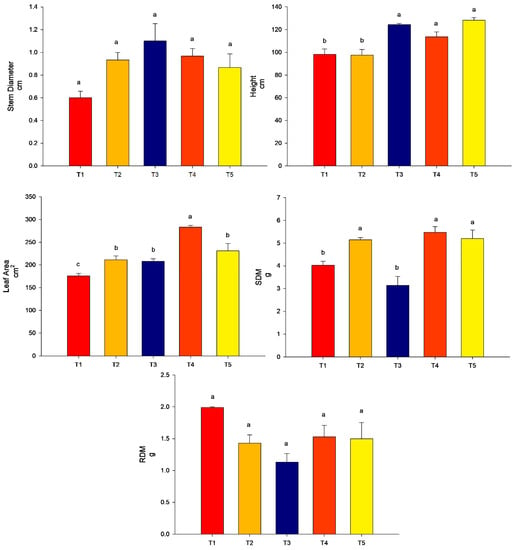
Figure 5.
Agronomic analyses were performed on maize plants (40 DAE) associated with Trichoderma harzianum + Bacillus subtilis, rock powder, and conventional fertilization (experiment 3). Means followed by the same letter on the line do not differ from each other according to the Scott-Knott test at 5%. T1 = control—conventional fertilization (100%); T2 = conventional fertilization (100%) + rock powder; T3 = conventional fertilization (100%) + T. harzianum + B. subtilis; T4 = conventional fertilization (100%) + rock powder + T. harzianum + B. subtilis; T5 = conventional fertilization (50%) + rock powder + T. harzianum + B. subtilis. Bars mean = standard error. Shoot dry matter. Different lowercase letters mean significant statistical differences.
In experiment three, for nitrogen (Table 4), treatments 1 and 5 had lower means, differing from 2, 3, and 4, treatments that exceeded 30 g/kg of the macronutrient in the analysis performed. In contrast, when analyzing the results for phosphorus, none of the treatments exceeded the value of 3.23 g/kg, a value of the average of the control that differed from the others.

Table 4.
Chemical analyses of the macronutrients nitrogen (N), phosphorus (P), potassium (K), calcium (Ca), magnesium (Mg), and sulfur (S) expressed in g/kg of dry matter, performed on maize leaves (40 DAE) with the association of Trichoderma harzianum, Bacillus subtilis, rock powder and conventional fertilization (experiment 3).
The combination of microorganisms and 100% of the recommended fertilization T3 (Table 4) reached the highest average for calcium, 1.55 g/kg dry matter, among the treatments evaluated. Treatment 4, which, in addition to the microorganisms and 100% of the fertilization and composed of the presence of rock powder, obtained the second highest mean, with 1.13 g/kg of calcium, followed by treatments 2 and 1, which presented 0.55 and 0.79 g/kg of the macronutrient, respectively. The control (fertilization (100%) reached the highest mean (2.26 g/kg) for sulfur of the evaluated treatments, differing statistically from all treatments. The treatments did not result in a significant difference in potassium and magnesium.
5. Discussion
The results show that the application of rock powder together with the fungus T. harzianum promoted increases in essential parameters related to maize plant growth, such as leaf area, shoot dry matter, and root dry matter, compared to treatments that received only conventional fertilization and conventional fertilization with the application of rock powder.
Ramos et al. [28] investigated the potential use of rock powder application as soil amendment in agriculture. The nutrient potentially available to plants were evaluated through leaching laboratory tests. According to the results obtained in that study, it is concluded that the volcanic rock powder can be used as a source of macro- and micro-nutrients to the soil because it presents in its composition a relevant proportion of easily weathered glassy amorphous matrix as well as many silicate minerals, such as plagioclase, pyroxene, and iron–magnesium minerals that are easily amendable. Leaching tests in an acidic medium influenced the release speed of these minerals, making the process faster and consequently releasing the elements/nutrients to the soil. Cabalar and Alosman [29] evaluated rock powder mixed with organic soil at different contents of 10%, 20%, 35%, and 50% by dry weight of the mixture. The results showed a marked effect of rock powder inclusion on compaction tests, with a substantial increase in dry unit weight and a decrease in optimum moisture content. Rock-forming minerals of igneous and metamorphic rocks contain most of the nutrients higher plants require for growth and development. Ground rock fertilizers may provide a source of nutrients to depleted topsoils where bulk soil solutions are not in equilibrium with fresh primary minerals [30]. Hasan et al. [31] evaluated different levels of 25%, 50%, and 75% of the recommended dose of potassium sulfate with feldspar rock application along with Bacillus circulans inoculation. The authors showed that adding 50% from the recommended dose of potassium sulfate plus 50% feldspar rock with inoculation by Bacillus circulans bacteria yielded the highest values of vegetative growth (plant length per plant, number of branches per plant, leaf area, and chlorophyll content), total yield, yield properties (tuber root diameter, tuber root weight, tuber root length, and tuber root dry matter), and chemical content (leaves potassium, total carbohydrate, and tuber root potassium). Ramos et al. [23] evaluated many doses of rock powder as a by-product in maize in greenhouse conditions, and verified that the by-product comprised plagioclase, K-feldspar, quartz, clinopyroxene, smectites, and opaque minerals, with apatite as an accessory mineral and adding 3625 and 7251 kg ha−1 doses of the by-product substantially increased the dry matter yield in maize leaves.
Rock powder may have stimulated the mineralization effect, in contrast to the readily available fertilizer that decreases this fungal action. Interestingly, the plants that received the application of the fungus T. harzianum and only 50% of the dose of conventional fertilization provided similar results in shoots and a more significant increase and roots compared to plants that received 100% of the dose of conventional fertilization. Inoculation with the bacterium B. subtilis promoted the highest values for the parameters of plant height, leaf area, and shoot dry matter in the treatments that received the inoculation of the bacterium B. subtilis with 100% and 50% of the fertilizer dose. Interestingly, the highest value for the root dry matter was found for the treatment, which received 50% of the fertilization with B. subtilis inoculation dose, compared to the treatment, which received 100%. This significant result shows the potential of using a reduced dose of fertilizers with B. subtilis inoculation and rock powder application. In the third experiment, in which the mixture of B. subtilis and the fungus T. harzianum was used, there was no difference between the values of stem diameter and root dry matter. Nevertheless, while there were some increases in some parameters, in general, the application of rock powder and the mixture of B. subtilis and T. harzianum did not present higher values than the treatments that received these same microorganisms separately.
In this sense, applying a mixture of the two microorganisms B. subtilis and T. harzianum, as was undertaken in the present study, would increase the chances of success in microbial colonization and interaction with the plant [32,33]. However, as seen in the present study, these mixtures of microorganisms do not necessarily potentiate each microorganism’s effects or growth-promoting abilities.
6. Conclusions
The findings showed potential benefits to soil fertility and plant growth with the inoculation of B. subtilis and the fungus T. harzianum in maize plants coupled with rock powder applied to the soil. This study concludes that there is evidence that could reduce the amount of chemical fertilizer inoculating microorganisms and applying rock powder. However, this study was carried out in vase conditions, and many years of field research are needed to verify the real contribution of this practice to a sustainable agricultural system.
Author Contributions
Conceptualization, E.C.R. and P.H.V.S.; methodology, P.H.V.S., A.G.V.S., L.D.d.A. and G.V.L.d.S.; software, P.H.V.S. and E.T.F.; validation, P.H.V.S. and G.V.L.d.S.; formal analysis, P.H.V.S.; investigation, P.H.V.S.; resources, P.H.V.S.; data curation, P.H.V.S. and E.C.R.; writing—original draft preparation, P.H.V.S. and E.C.R.; writing—review and editing, E.C.R.; visualization, P.H.V.S.; supervision, E.C.R. and C.M.d.S.; project administration, P.H.V.S.; funding acquisition, E.C.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Not applicable.
Acknowledgments
Agricultural and Livestock Microbiology Graduation Program, School of Agricultural and Veterinary Sciences, São Paulo State University (UNESP), São Paulo, Brazil.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Devika, O.S.; Singh, S.; Sarkar, D.; Barnwal, P.; Suman, J.; Rakshit, A. Seed Priming: A Potential Supplement in Integrated Resource Management Under Fragile Intensive Ecosystems. Front. Sustain. Food Syst. 2021, 5, 654001. [Google Scholar] [CrossRef]
- Adeleke, B.S.; Ayangbenro, A.S.; Babalola, O.O. Genomic analysis of endophytic Bacillus cereus T4S and its plant growth-promoting traits. Plants 2021, 10, 1776. [Google Scholar] [CrossRef]
- Babalola, O.O. Beneficial bacteria of agricultural importance. Biotechnol. Lett. 2010, 32, 1559–1570. [Google Scholar] [CrossRef]
- Aloo, B.; Makumba, B.; Mbega, E. The potential of bacilli rhizobacteria for sustainable crop production and environmental sustainability. Microbiol. Res. 2019, 219, 26–39. [Google Scholar] [CrossRef]
- Kafle, A.; Cope, K.R.; Raths, R.; Krishna Yakha, J.; Subramanian, S.; Bücking, H.; Garcia, K. Harnessing soil microbes to improve plant phosphate efficiency in cropping systems. Agronomy 2019, 9, 127. [Google Scholar] [CrossRef]
- Fyfe, W.S.; Leonardos, O.H.; Theodoro, S.H. Sustainable farming with native rocks: The transition without revolution. An. Acad. Bras. Ciências 2006, 78, 715–720. [Google Scholar] [CrossRef]
- Theodoro, S.H.; Leonardos, O.H.; Rocha, E.; Macedo, I.; Rego, K.G. Stonemeal of amazon soils with sediments from reservoirs: A case study of remineralization of the Tucuruí degraded land for agroforest reclamation. An. Acad. Bras. Ciências 2013, 85, 23–34. [Google Scholar] [CrossRef]
- Basak, B.; Sarkar, B.; Biswas, D.; Sarkar, S.; Sanderson, P.; Naidu, R. Biointervention of naturally occurring silicate minerals for alternative source of potassium: Challenges and opportunities. Adv. Agron. 2017, 141, 115–145. [Google Scholar]
- Gasparotto, J.; Martinello, K.D.B. Coal as an energy source and its impacts on human health. Energy Geosci. 2021, 2, 113–120. [Google Scholar] [CrossRef]
- Brader, G.; Compant, S.; Vescio, K.; Mitter, B.; Trognitz, F.; Ma, L.-J.; Sessitsch, A. Ecology and genomic insights into plant-pathogenic and plant-nonpathogenic endophytes. Annu. Rev. Phytopathol. 2017, 55, 61–83. [Google Scholar] [CrossRef]
- Souza, R.d.; Ambrosini, A.; Passaglia, L.M. Plant growth-promoting bacteria as inoculants in agricultural soils. Genet. Mol. Biol. 2015, 38, 401–419. [Google Scholar] [CrossRef]
- Sivasakthi, S.; Usharani, G.; Saranraj, P. Biocontrol potentiality of plant growth promoting bacteria (PGPR)-Pseudomonas fluorescens and Bacillus subtilis: A review. Afr. J. Agric. Res. 2014, 9, 1265–1277. [Google Scholar]
- Prashar, P.; Kapoor, N.; Sachdeva, S. Rhizosphere: Its structure, bacterial diversity and significance. Rev. Environ. Sci. Bio/Technol. 2014, 13, 63–77. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, A.; Devi, S.; Patil, S.; Payal, C.; Negi, S. Isolation, screening and characterization of bacteria from Rhizospheric soils for different plant growth promotion (PGP) activities: An in vitro study. Recent Res. Sci. Technol. 2012, 4, 1–5. [Google Scholar]
- Chowdhury, S.P.; Dietel, K.; Rändler, M.; Schmid, M.; Junge, H.; Borriss, R.; Hartmann, A.; Grosch, R. Effects of Bacillus amyloliquefaciens FZB42 on lettuce growth and health under pathogen pressure and its impact on the rhizosphere bacterial community. PLoS ONE 2013, 8, e68818. [Google Scholar] [CrossRef]
- Compant, S.; Duffy, B.; Nowak, J.; Clément, C.; Barka, E.A. Use of plant growth-promoting bacteria for biocontrol of plant diseases: Principles, mechanisms of action, and future prospects. Appl. Environ. Microbiol. 2005, 71, 4951–4959. [Google Scholar] [CrossRef]
- Nelson, L.M. Plant growth promoting rhizobacteria (PGPR): Prospects for new inoculants. Crop Manag. 2004, 3, 1–7. [Google Scholar] [CrossRef]
- Zaki, S.A.; Ouf, S.A.; Albarakaty, F.M.; Habeb, M.M.; Aly, A.A.; Abd-Elsalam, K.A. Trichoderma harzianum-mediated ZnO nanoparticles: A green tool for controlling soil-borne pathogens in cotton. J. Fungi 2021, 7, 952. [Google Scholar] [CrossRef]
- Sala, A.; Vittone, S.; Barrena, R.; Sanchez, A.; Artola, A. Scanning agro-industrial wastes as substrates for fungal biopesticide production: Use of Beauveria bassiana and Trichoderma harzianum in solid-state fermentation. J. Environ. Manag. 2021, 295, 113113. [Google Scholar] [CrossRef]
- Sun, X.; Tiffany, D.G.; Urriola, P.E.; Shurson, G.G.; Hu, B. Nutrition upgrading of corn-ethanol coproduct by fungal fermentation: Amino acids enrichment and anti-nutritional factors degradation. Food Bioprod. Process. 2021, 130, 1–13. [Google Scholar] [CrossRef]
- De Andrade Barbosa, M.; de Sousa Ferraz, R.L.; Coutinho, E.L.M.; Neto, A.M.C.; da Silva, M.S.; Fernandes, C.; Rigobelo, E.C. Multivariate analysis and modeling of soil quality indicators in long-term management systems. Sci. Total Environ. 2019, 657, 457–465. [Google Scholar] [CrossRef]
- Sanjeev, K.; Manibhushan, T.; Archana, R. Trichoderma: Mass production, formulation, quality control, delivery and its scope in commercialization in India for the management of plant diseases. Afr. J. Agric. Res. 2014, 9, 3838–3852. [Google Scholar]
- Ramos, C.G.; Querol, X.; Dalmora, A.C.; de Jesus Pires, K.C.; Schneider, I.A.H.; Oliveira, L.F.S.; Kautzmann, R.M. Evaluation of the potential of volcanic rock waste from southern Brazil as a natural soil fertilizer. J. Clean. Prod. 2017, 142, 2700–2706. [Google Scholar] [CrossRef]
- Franco, H.C.J.; Otto, R.; Faroni, C.E.; Vitti, A.C.; de Oliveira, E.C.A.; Trivelin, P.C.O. Nitrogen in sugarcane derived from fertilizer under Brazilian field conditions. Field Crops Res. 2011, 121, 29–41. [Google Scholar] [CrossRef]
- Malavolta, E.; Vitti, G.C.; Oliveira, S.A.D. Avaliação do Estado Nutricional das Plantas. Princípios Aplicações. 1989. Available online: https://edisciplinas.usp.br/pluginfile.php/3563546/mod_resource/content/1/Aula%2011_Avalia%C3%A7%C3%A3o%20do%20Estado%20Nutricional_2017.pdf (accessed on 1 February 2023).
- Nakamura, S.; Nitta, Y.; Watanabe, M.; Goto, Y. Analysis of leaflet shape and area for improvement of leaf area estimation method for sago palm (Metroxylon sagu Rottb.). Plant Prod. Sci. 2005, 8, 27–31. [Google Scholar] [CrossRef]
- Kumar, M.; Mondal, P.; Borah, S.; Mahato, K. Physico-chemical evaluation, preliminary phytochemical investigation, fluorescence and TLC analysis of leaves of the plant Lasia spinosa (Lour) Thwaites. Int. J. Pharm. Pharm. Sci. 2013, 5, 306–310. [Google Scholar]
- Ramos, C.G.; Hower, J.C.; Blanco, E.; Oliveira, M.L.S.; Theodoro, S.H. Possibilities of using silicate rock powder: An overview. Geosci. Front. 2022, 13, 101185. [Google Scholar] [CrossRef]
- Cabalar, A.F.; Alosman, S.O. Influence of rock powder on the behaviour of an organic soil. Bull. Eng. Geol. Environ. 2021, 80, 8665–8676. [Google Scholar] [CrossRef]
- Meena, V.S.; Meena, S.K.; Verma, J.P.; Kumar, A.; Aeron, A.; Mishra, P.K.; Bisht, J.K.; Pattanayak, A.; Naveed, M.; Dotaniya, M. Plant beneficial rhizospheric microorganism (PBRM) strategies to improve nutrients use efficiency: A review. Ecol. Eng. 2017, 107, 8–32. [Google Scholar] [CrossRef]
- Hasan, S. Effect of potassium fertilizer, feldspar rock and potassium releasing bacterium (Bacillus circulans) on sweet potato plant under sandy soil conditions. Sci. J. Agric. Sci. 2020, 2, 56–63. [Google Scholar]
- Lobo, L.L.B.; da Silva, M.S.R.D.A.; Carvalho, R.F.; Rigobelo, E.C. The negative effect of coinoculation of plant growth-promoting bacteria is not related to indole-3-acetic acid synthesis. J. Plant Growth Regul. 2022, 11, 1–10. [Google Scholar] [CrossRef]
- Mitter, E.K.; Tosi, M.; Obregón, D.; Dunfield, K.E.; Germida, J.J. Rethinking crop nutrition in times of modern microbiology: Innovative biofertilizer technologies. Front. Sustain. Food Syst. 2021, 5, 606815. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).