Source–Sink Balance Optimization Depends on Soil Nitrogen Condition So as to Increase Rice Yield and N Use Efficiency
Abstract
:1. Introduction
2. Materials and Methods
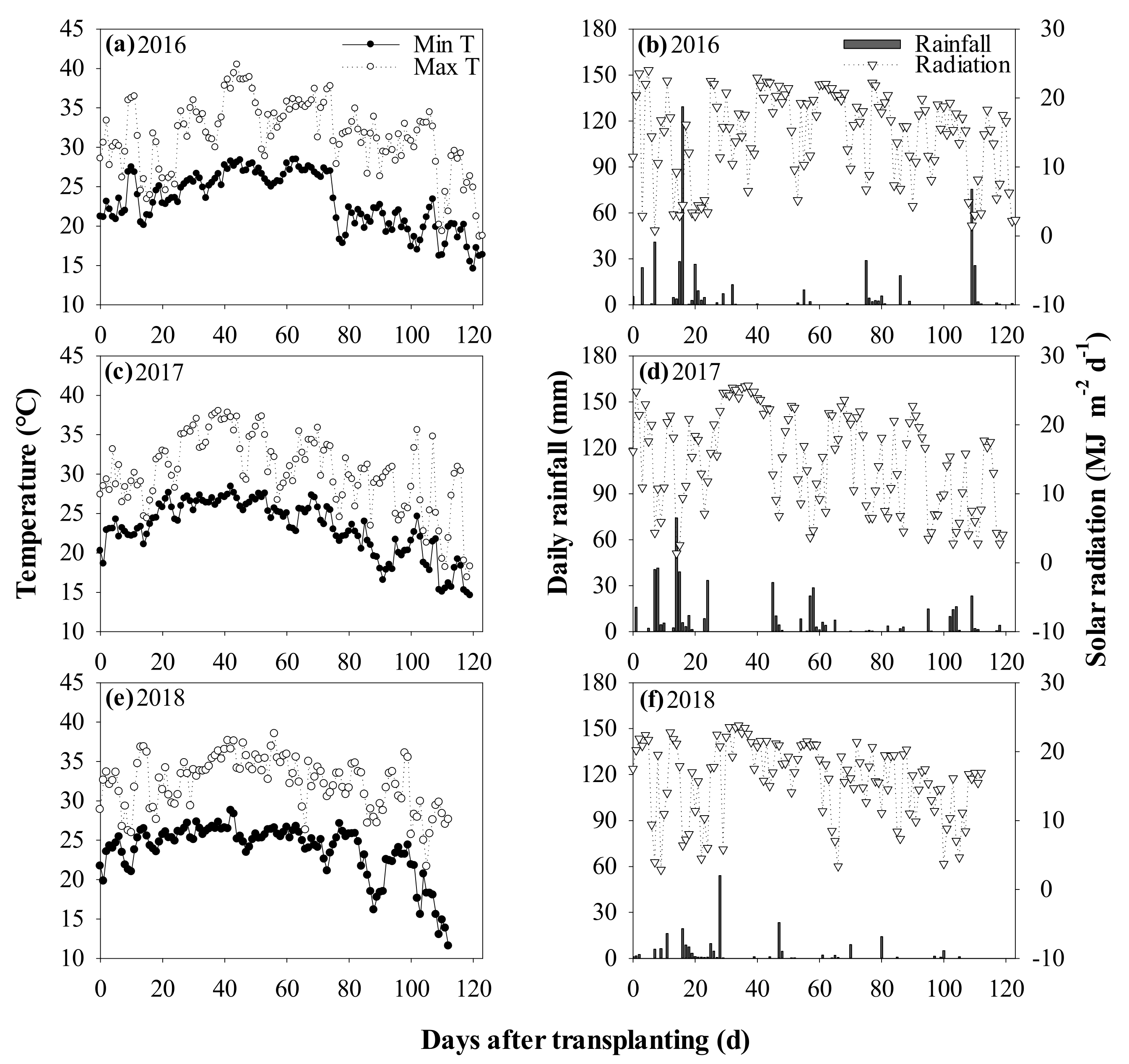
2.1. Site
2.2. Experimental Design and Crop Management
2.3. Sampling and Measurements
2.4. Statistical Analysis
3. Results
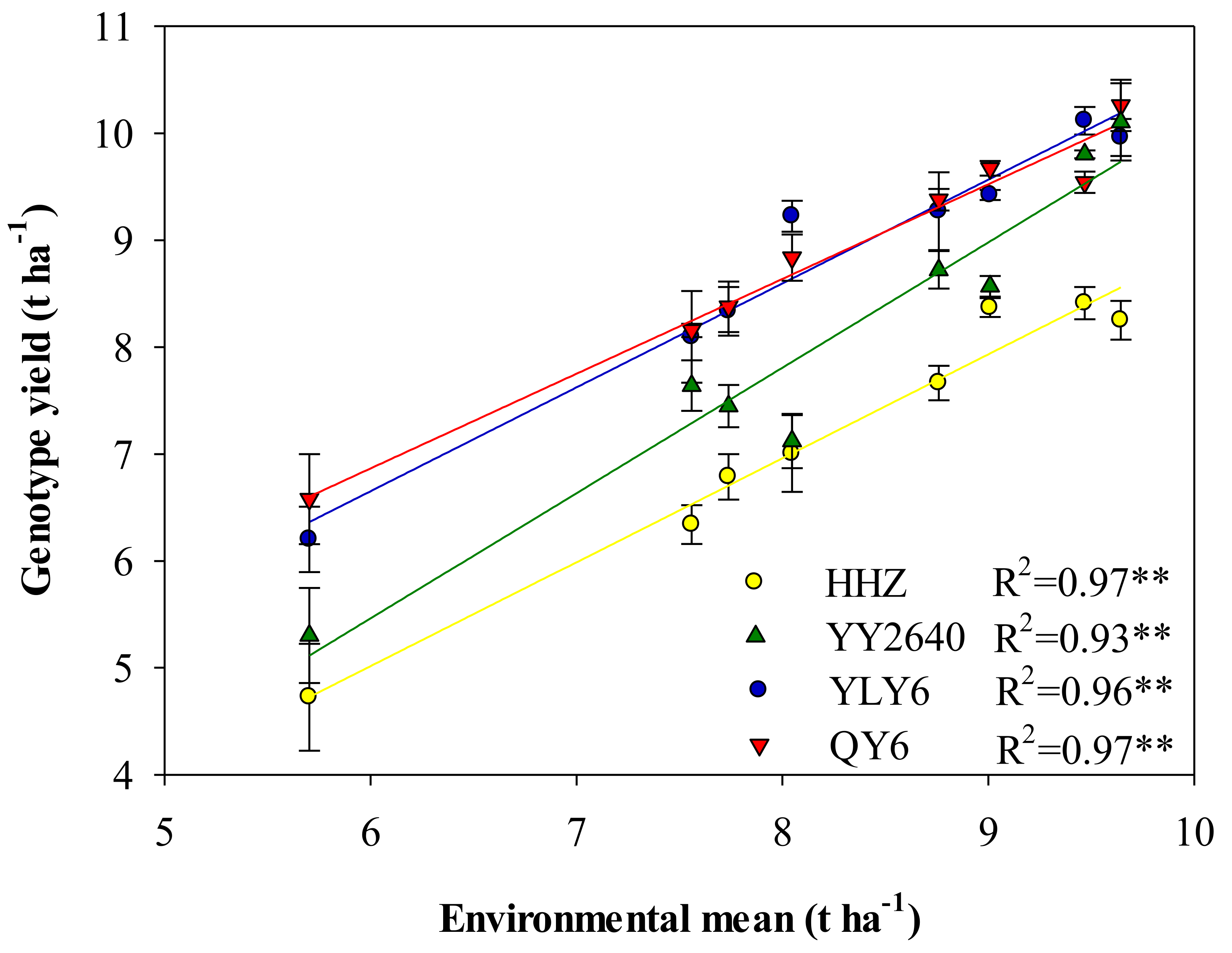
3.1. Growth Duration, Grain Yield, and NUEg
3.2. Sink–Source Relationship
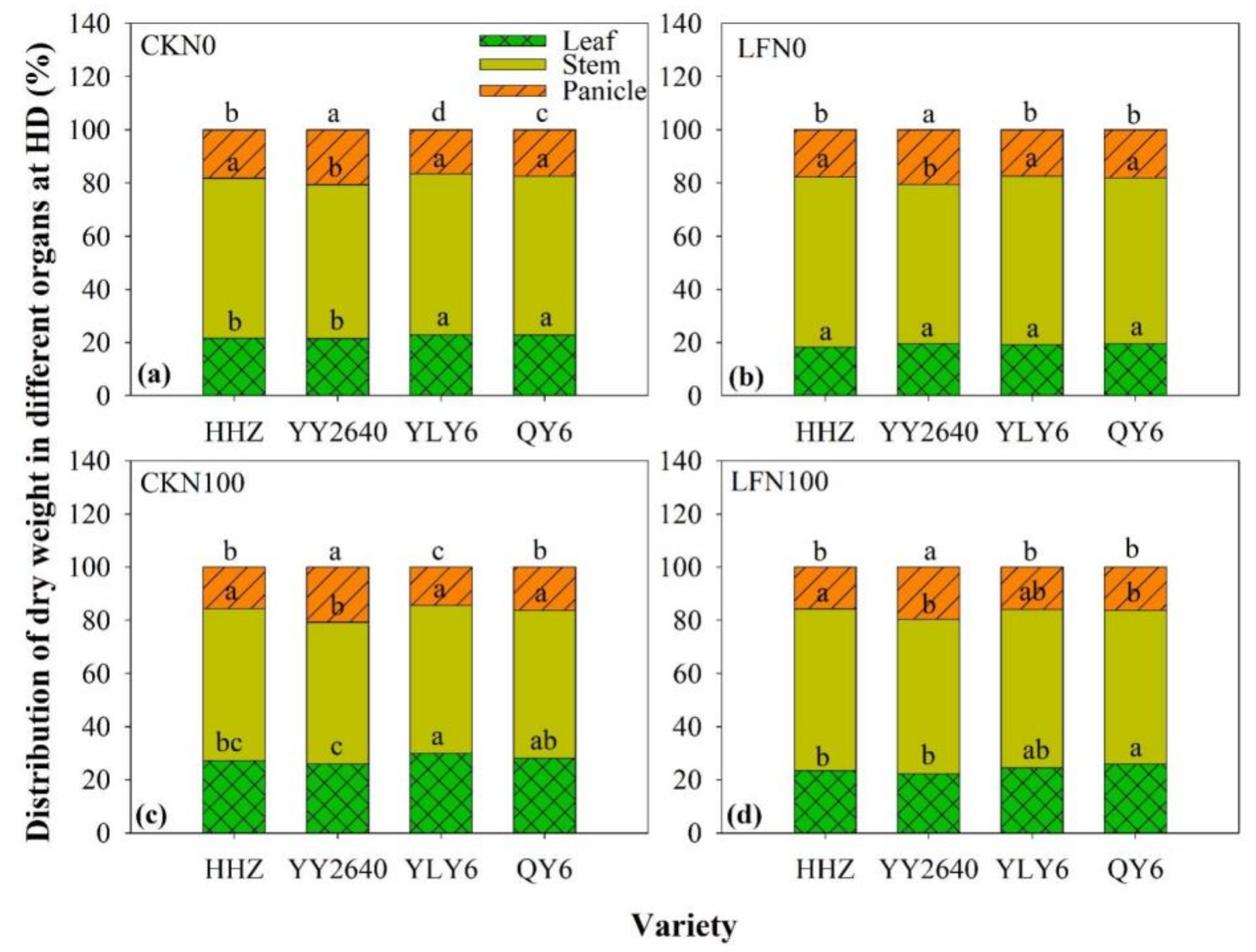
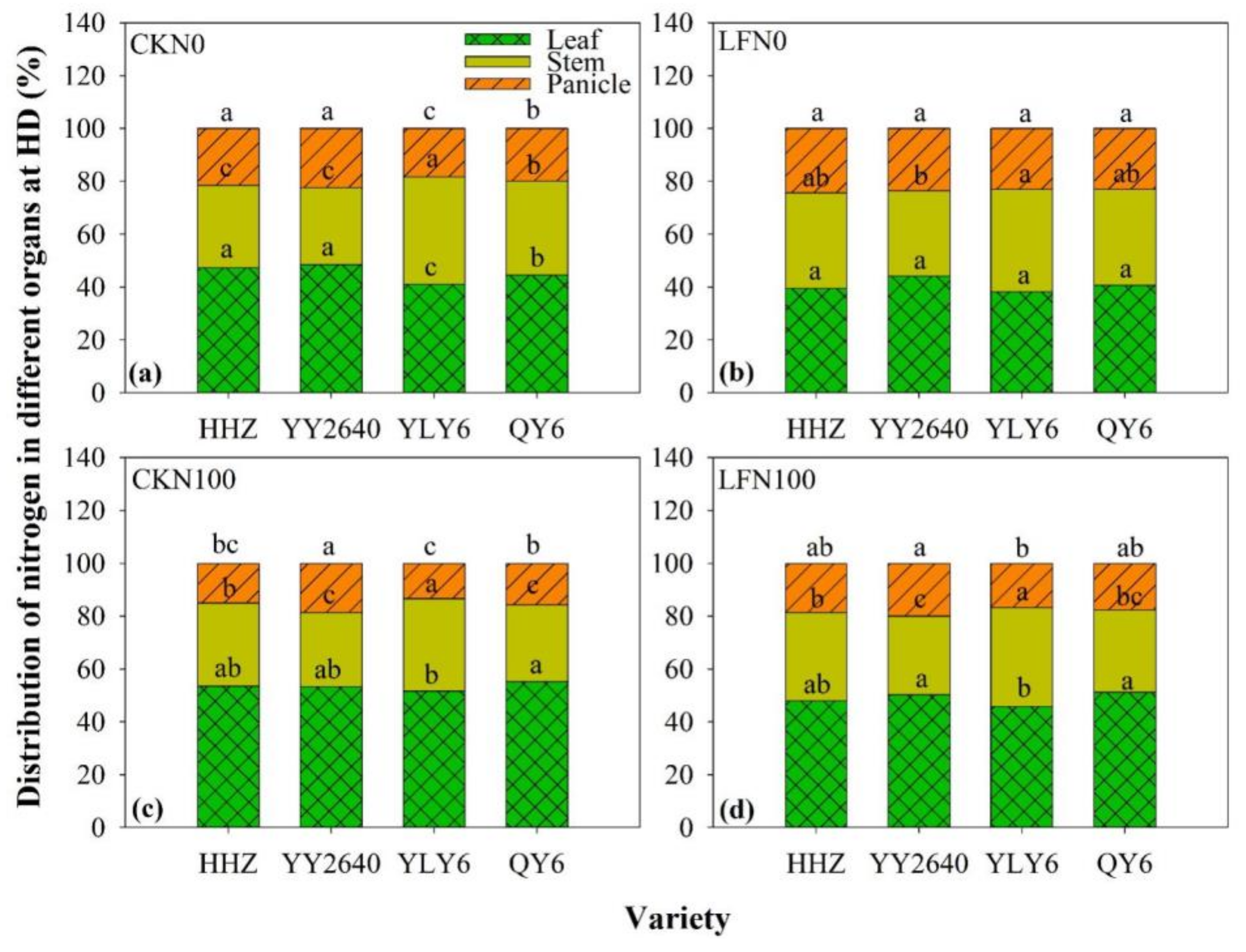
3.3. Dry matter, Nitrogen Accumulation, and Translocation Characteristics
3.4. Dry Matter and Nitrogen Accumulation in Leaf, Stem, and Panicle at HD
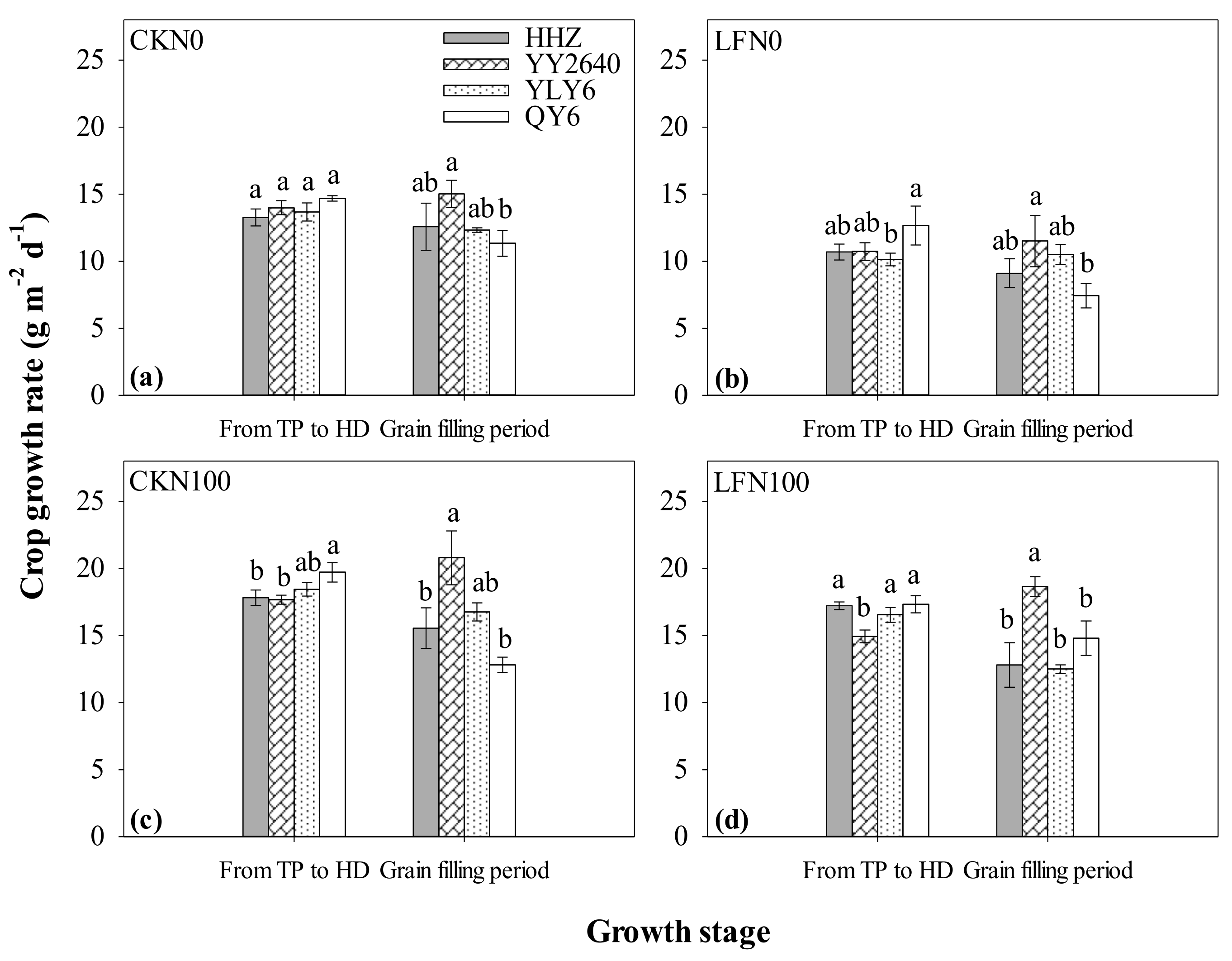
3.5. SPAD and Crop Growth Rate
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, H.P. Rice consumption in China: Can China change rice consumption from quantity to quality? In Rice Is Life: Scientific Perspectives for the 21st Century; Toriyama, K., Heong, K.L., Hardy, B., Eds.; International Rice Research Institute: Los Banos, Philippines, 2005; pp. 497–499. [Google Scholar]
- Wang, G.H.; Zhang, Q.C.; Witt, C.; Buresh, R.J. Opportunities for yield increases and environmental benefits through site-specific nutrient management in rice systems of Zhejiang province, China. Agric. Syst. 2007, 94, 801–806. [Google Scholar] [CrossRef]
- Peng, X.L.; Liu, Y.Y.; Luo, S.; Fan, L.C.; Song, T.X.; Guo, Y.W. Effects of site-specific nitrogen management on yield and dry matter accumulation of rice from cold areas of Northeastern China. Agric. Sci. China 2007, 6, 715–723. [Google Scholar] [CrossRef]
- Yang, X.G.; Chen, F.; Lin, X.M.; Liu, Z.J.; Zhang, H.L.; Zhao, J.; Li, K.N.; Ye, Q.; Li, Y.; Lv, S.; et al. Potential benefits of climate change for crop productivity in china. Agric. Forest Meteorol. 2015, 208, 76–84. [Google Scholar] [CrossRef]
- Zhang, Q.F. Strategies for developing green super rice. Proc. Natl. Acad. Sci. USA 2007, 104, 16402–16409. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.G.; Liu, Z.H.; Anderson, W.; Yang, P.; Wu, W.B.; Tang, H.J.; You, L.Z. Chinese rice production area adaptations to climate changes, 1949–2010. Environ. Sci. Technol. 2015, 49, 2032–2037. [Google Scholar] [CrossRef]
- Grassini, P.; Eskridge, K.M.; Cassman, K.G. Distinguishing between yield advances and yield plateaus in historical crop production trends. Nat. Commun. 2013, 4, 2918. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Q.G.; Goto, S.; Takamura, H. LUCC and accompanied soil degradation in China from 1960s to 1990s. J. Geosci. Res. Northeast Asia 2002, 5, 62–71. [Google Scholar]
- Li, G.H.; Xue, L.H.; Gu, W.; Yang, C.D.; Wang, S.H.; Ling, Q.H.; Qin, X.; Ding, Y.F. Comparison of yield components and plant type characteristics of high-yield rice between Taoyuan, a ‘special eco-site’ and Nanjing, China. Field Crops Res. 2009, 112, 214–221. [Google Scholar] [CrossRef]
- Long, S.P.; Marshall-Colon, A.; Zhu, X.G. Meeting the global food demand of the future by engineering crop photosynthesis and yield potential. Cell 2015, 161, 56–66. [Google Scholar] [CrossRef] [Green Version]
- Jiang, P.; Xie, X.B.; Huang, M.; Zhou, X.F.; Zhang, R.C.; Chen, J.N.; Wu, D.D.; Xia, B.; Xiong, H.; Xu, F.X.; et al. Characterizing N uptake and use efficiency in rice as influenced by environments. Plant Prod. Sci. 2016, 19, 96–104. [Google Scholar] [CrossRef] [Green Version]
- Zeng, X.B.; Zhang, J.B.; Wei, C.F.; Yu, W.T.; Huang, D.Y.; Xu, M.G.; Xu, J.M. The status and reclamation strategy of low-yield fields in china. Acta Pedolo. Sin. 2014, 51, 675–682. [Google Scholar]
- Ying, J.F.; Peng, S.B.; He, Q.R.; Yang, H.; Yang, C.D.; Visperas, R.M.; Cassman, K.G. Comparison of high-yield rice in tropical and subtropical environments. I. Determinants of grain and dry matter yields. Field Crops Res. 1998, 57, 71–84. [Google Scholar] [CrossRef]
- Yuan, P.R.; Sun, C.Q.; Yang, C.D.; Zhou, N.; Ying, J.F.; Peng, S.B.; Wang, X.Y. Analysis on grain yield and yield components of the 15 t hm−2 high yielding Indica rice (Oryza sativa L.) in Yunnan. Acta Agron. Sin. 2000, 26, 756–762. (In Chinese) [Google Scholar]
- Zhang, H.; Chen, T.T.; Liu, L.J.; Wang, Z.Q.; Yang, J.C.; Zhang, J.H. Performance in grain yield and physiological traits of rice in the Yangtze River basin of china during the last 60 yr. J. Integr. Agric. 2013, 12, 57–66. [Google Scholar] [CrossRef]
- Li, H.W.; Liu, L.J.; Wang, Z.Q.; Yang, J.C.; Zhang, J.H. Agronomic and physiological performance of high-yielding wheat and rice in the lower reaches of Yangtze River of China. Field Crops Res. 2012, 133, 119–129. [Google Scholar] [CrossRef]
- Zhu, G.L.; Peng, S.B.; Huang, J.L.; Cui, K.H.; Nie, L.X.; Wang, F. Genetic improvements in rice yield and concomitant increases in radiation- and nitrogen- use efficiency in Middle Reaches of Yangtze River. Sci. Rep. 2016, 6, 21049. [Google Scholar] [CrossRef] [Green Version]
- Jiang, P. Characteristics, and high-yielding cultivation techniques for good quality rice yongyou 15. Fujian Sci. Technol. Rice Wheat 2013, 31, 47–48. (In Chinese) [Google Scholar]
- Hu, Y.J.; Xing, Z.P.; Gong, J.L.; Liu, G.T.; Zhang, H.C.; Dai, Q.G.; Huo, Z.Y.; Xu, K.; Wei, H.Y.; Guo, B.W.; et al. Study on Population Characteristics and Formation Mechanisms for High Yield of Pot-Seedling Mechanical Transplanting Rice. Sci. Agric. Sin. 2014, 47, 865–879. (In Chinese) [Google Scholar]
- Wei, H.Y.; Zhang, H.C.; Blumwald, E.; Li, H.L.; Cheng, J.Q.; Dai, Q.G.; Huo, Z.Y.; Xu, K.; Guo, B.W. Different characteristics of high yield formation between inbred japonica super rice and inter-sub-specific hybrid super rice. Field Crops Res. 2016, 198, 179–187. [Google Scholar] [CrossRef]
- Wang, X.Y.; Wei, H.H.; Zhang, H.C.; Sun, J.; Zhang, J.M.; Li, C.; Lu, H.B.; Yang, J.W.; Ma, R.R.; Xu, J.F.; et al. Population characteristics for super-high yielding hybrid rice Yongyou 12 (>13.5 t ha−1). Acta Agron. Sin. 2014, 40, 2149–2159. (In Chinese) [Google Scholar] [CrossRef]
- Venkateswarlu, B. Source-sink interrelationships in lowland rice. Plant Soil 1976, 44, 575–586. [Google Scholar] [CrossRef]
- Shi, W.J.; Xiao, G.; Struik, P.C.; Jagadish, K.S.V.; Yin, X.Y. Quantifying source-sink relationships of rice under high night-time temperature combined with two nitrogen levels. Field Crops Res. 2017, 202, 36–46. [Google Scholar] [CrossRef]
- Adachi, S.; Ohkubo, S.; San, N.S.; Yamamoto, T. Genetic determination for source capacity to support breeding of high-yielding rice (Oryza sativa). Mol. Breed. 2020, 40, 20. [Google Scholar] [CrossRef]
- Cao, X.Z.; Zhu, Q.S. Study on characteristics of the relationship between source and sink rice varieties and their classification. Acta Agron. Sin. 1987, 13, 265–272. (In Chinese) [Google Scholar]
- Zhou, W.X.; Lei, C.; Tu, N.M. Research trends on source-sink relationship of rice. J. Hunan Agric. Univ. 2004, 30, 389–393. (In Chinese) [Google Scholar]
- Huang, L.Y.; Yang, D.S.; Li, X.X.; Peng, S.B.; Wang, F. Coordination of high grain yield and high nitrogen use efficiency through large sink size and high post-heading source capacity in rice. Field Crops Res. 2019, 233, 49–58. [Google Scholar] [CrossRef]
- Ma, G.H.; Zhang, Y.Z. Relationship between source-sink in rice and its progress in research. J. Hubei Agric. Coll. 1992, 12, 61–67. (In Chinese) [Google Scholar]
- He, A.B.; Wang, W.Q.; Jiang, G.L.; Sun, H.J.; Jiang, M.; Man, J.G.; Cui, K.H.; Huang, J.L.; Peng, S.B.; Nie, L.X. Source-sink regulation and its effects on the regeneration ability of ratoon rice. Field Crops Res. 2019, 236, 155–164. [Google Scholar] [CrossRef]
- Liu, L.J.; Zhang, H.; Ju, C.X.; Xiong, Y.W.; Bian, J.L.; Zhao, B.H.; Yang, J.C. Changes in grain yield and root morphology and physiology of mid-season rice in the Yangtze River basin of China during the last 60 years. J. Agric. Sci. 2014, 6, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Ju, C.X.; Tao, J.; Qian, X.Y.; Gu, J.F.; Zhang, H.; Zhao, B.H.; Liu, L.J.; Wang, Z.Q.; Yang, J.C. Leaf photosynthetic characteristics of mid-season indica rice varieties applied at different decades. Acta Agron. Sin. 2016, 42, 415–426. (In Chinese) [Google Scholar] [CrossRef]
- Zhu, K.Y.; Yan, J.Q.; Shen, Y.; Zhang, W.Y.; Xu, Y.J.; Wang, Z.Q.; Yang, J.C. Deciphering the morpho–physiological traits for high yield potential in nitrogen efficient varieties (NEVs): A japonica rice case study. J. Integr. Agric. 2022, 21, 947–963. [Google Scholar] [CrossRef]
- Yang, Z.F.; Gao, S.; Xiao, F.; Li, G.H.; Ding, Y.F.; Guo, Q.H.; Paul, M.J.; Liu, Z.H. Leaf to panicle ratio (LPR): A new physiological trait indicative of source and sink relation in japonica rice based on deep learning. Plant Methods 2020, 16, 117. [Google Scholar] [CrossRef]
- Zhong, X.H.; Peng, S.B.; Sanico, A.L.; Liu, H.X. Quantifying the interactive effect of leaf nitrogen and leaf area on tillering on rice. J. Plant Nutr. 2003, 26, 1203–1222. [Google Scholar] [CrossRef]
- Kropff, M.J.; Cassman, K.G.; van Laar, H.H.; Peng, S.B. Nitrogen, and yield potential of irrigated rice. Plant Soil 1993, 155/156, 391–394. [Google Scholar] [CrossRef] [Green Version]
- Yao, Y.L.; Yamamoto, Y.; Wang, Y.L.; Yoshida, T.; Miyazaki, A.; Nitta, Y.; Cai, J.L. Role of nitrogen regulation in sink and source formation of high-yielding rice cultivars. Soil Sci. Plant Nutr. 2000, 46, 825–834. [Google Scholar] [CrossRef]
- Wei, H.H.; Meng, T.Y.; Li, X.Y.; Dai, Q.G.; Zhang, H.C.; Yin, X.Y. Sink-source relationship during rice grain filling is associated with grain nitrogen concentration. Field Crops Res. 2018, 215, 23–38. [Google Scholar] [CrossRef]
- Fu, P.H.; Wang, J.; Zhang, T.; Huang, J.L.; Peng, S.B. High nitrogen input causes poor grain filling of spikelets at the panicle base of super hybrid rice. Field Crop Res. 2019, 244, 107635. [Google Scholar] [CrossRef]
- Li, X.X.; Cao, J.; Huang, J.L.; Xing, D.Y.; Peng, S.B. Effects of topsoil removal on nitrogen uptake, biomass accumulation, and yield formation in puddled-transplanted rice. Field Crops Res. 2021, 265, 10813. [Google Scholar] [CrossRef]
- Finlay, K.; Wilkinson, G. The analysis of adaptation in a plant-breeding programme. Aust. J. Agric. Res. 1963, 14, 742–754. [Google Scholar] [CrossRef] [Green Version]
- Shuai, P. Agronomic Performance of Super and Ordinary Hybrid Rice Varieties Grown under Different Level of Nitrogen Input. Master’s Thesis, Huazhong Agricultural University, Wuhan, China, 2019. (In Chinese). [Google Scholar]
- Yuan, S.; Nie, L.X.; Wang, F.; Huang, J.L.; Peng, S.B. Agronomic performance of inbred and hybrid rice cultivars under simplified and reduced-input practices. Field Crops Res. 2017, 210, 129–135. [Google Scholar] [CrossRef]
- Wu, L.L.; de Boer, H.J.; Zhang, Z.X.; Chen, X.L.; Shi, Y.Y.; Peng, S.B.; Wang, F. The coordinated increase in stomatal density and vein dimensions during genetic improvement in rice. Agron. J. 2020, 112, 2791–2804. [Google Scholar] [CrossRef]
- Srinivasan, V.; Kumar, P.; Long, S.P. Decreasing, not increasing, leaf area will raise crop yields under global atmospheric change. Global Change Biol. 2017, 23, 1626–1635. [Google Scholar] [CrossRef]
- Wang, F.; Peng, S.B. Yield potential and nitrogen use efficiency of China’s super rice. J. Integr. Agric. 2017, 16, 1000–1008. [Google Scholar] [CrossRef] [Green Version]
- Zhu, K.Y.; Zhou, Q.; Shen, Y.; Yan, J.Q.; Xu, Y.J.; Wang, Z.Q.; Yang, J.C. Agronomic and physiological performance of an indica–japonica rice variety with a high yield and high nitrogen use efficiency. Crop Sci. 2020, 60, 1556–1568. [Google Scholar] [CrossRef]
- Liu, K.; Li, T.T.; Chen, Y.; Huang, J.; Qiu, Y.Y.; Li, S.Y.; Wang, H.; Zhu, A.; Zhuo, X.X.; Yu, F.; et al. Effects of root morphology and physiology on the formation and regulation of large panicles in rice. Field Crops Res. 2020, 258, 107946. [Google Scholar] [CrossRef]
- Byrd, G.T.; Sage, R.F.; Brown, R.H. A comparison of dark respiration between C3 and C4 plants. Plant Physiol. 1992, 100, 191–198. [Google Scholar] [CrossRef] [Green Version]
- Loomis, R.S.; Connor, D.J. Crop Ecology: Productivity and Management in Agricultural Systems; Cambridge University Press: Cambridge, UK, 1992; p. 538. [Google Scholar]
- Mitchell, P.L.; Sheehy, J.E. Supercharging rice photosynthesis to increase yield. New Phytol. 2006, 171, 688–693. [Google Scholar] [CrossRef]
- Gong, J.L.; Xing, Z.P.; Hu, Y.J.; Zhang, H.C.; Dai, Q.G.; Huo, Z.Y.; Xu, K.; Wei, H.Y.; Gao, H. Difference of characteristics of photosynthesis, matter production and translocation between indica and japonica super rice. Acta Agron. Sin. 2014, 40, 497–510. (In Chinese) [Google Scholar] [CrossRef]
- Fu, J.; Huang, Z.H.; Wang, Z.Q.; Yang, J.C.; Zhang, J.H. Pre-anthesis non-structural carbohydrate reserve in the stem enhances the sink strength of inferior spikelets during grain filling of rice. Field Crops Res. 2011, 123, 170–182. [Google Scholar] [CrossRef]
- Sharma, N.; Sinha, V.B.; Gupta, N.; Rajpal, S.; Kuchi, S.; Sitaramam, V.; Parsad, R.; Raghuram, N. Phenotyping for nitrogen use efficiency: Rice genotypes differ in N-responsive germination, oxygen consumption, seed urease activities, root growth, crop duration, and yield at low N. Front. Plant Sci. 2018, 9, 1452. [Google Scholar] [CrossRef]
- Suriyagoda, L.; Sirisena, D.; Kekulandara, D.; Bandaranayake, P.; Samarasinghe, G.; Wissuwa, M. Biomass and nutrient accumulation rates of rice cultivars differing in their growth duration when grown in fertile and low-fertile soils. J. Plant Nutr. 2020, 43, 251–269. [Google Scholar] [CrossRef]


| Year | Treatment | pH | OM (g kg−1) | TN (g kg−1) | AN (mg kg−1) | OP (mg kg−1) | AK (mg kg−1) |
|---|---|---|---|---|---|---|---|
| 2016 | CK | 4.96 a | 30.64 a | 2.37 a | / | 15.38 a | 152.38 a |
| LF | 4.84 b | 28.64 a | 2.19 a | / | 13.46 b | 107.95 b | |
| 2017 | CK | 4.77 b | 28.50 a | 1.86 a | 172.18 a | 27.86 a | 107.48 a |
| LF | 5.02 a | 24.36 b | 1.60 b | 140.80 b | 21.18 b | 67.77 b | |
| 2018 | CK | 5.04 b | 29.02 a | 2.33 a | 139.48 a | 24.30 a | 112.15 a |
| LF | 5.12 a | 29.53 a | 2.20 a | 129.47 b | 21.70 b | 94.83 b |
| Year | N Rate (kg ha−1) | Variety | Total Growth Period (d) | Vegetative Growth Period (d) | Grain Filling Period (d) | |||
|---|---|---|---|---|---|---|---|---|
| CK | LF | CK | LF | CK | LF | |||
| 2016 | 100 | HHZ | 130 | 130 | 61 | 61 | 39 | 39 |
| YY2640 | 132 | 117 | 58 | 58 | 47 | 32 | ||
| YLY6 | 152 | 145 | 71 | 69 | 51 | 46 | ||
| QY6 | 144 | 143 | 66 | 64 | 50 | 51 | ||
| Mean | 140 | 134 | 64 | 63 | 47 | 42 | ||
| 2017 | 100 | HHZ | 132 | 130 | 62 | 62 | 40 | 41 |
| YY2640 | 132 | 130 | 60 | 60 | 41 | 41 | ||
| YLY6 | 152 | 146 | 70 | 70 | 50 | 47 | ||
| QY6 | 146 | 138 | 65 | 65 | 49 | 43 | ||
| Mean | 141 | 136 | 64 | 64 | 45 | 43 | ||
| 2018 | 0 | HHZ | 123 | 120 | 56 | 56 | 36 | 33 |
| YY2640 | 125 | 122 | 54 | 54 | 41 | 38 | ||
| YLY6 | 135 | 133 | 65 | 65 | 42 | 40 | ||
| QY6 | 136 | 135 | 59 | 62 | 46 | 45 | ||
| Mean | 130 | 128 | 59 | 59 | 41 | 39 | ||
| 2018 | 100 | HHZ | 124 | 120 | 57 | 56 | 40 | 37 |
| YY2640 | 127 | 122 | 54 | 54 | 43 | 38 | ||
| YLY6 | 141 | 139 | 65 | 65 | 44 | 46 | ||
| QY6 | 138 | 137 | 62 | 59 | 48 | 47 | ||
| Mean | 133 | 130 | 60 | 59 | 44 | 42 | ||
| Year | N Rate (kg ha−1) | Variety | NUEg (kg kg−1) | |
|---|---|---|---|---|
| CK | LF | |||
| 2016 | 100 | HHZ | 40.5 b | 43.7 c |
| YY2640 | 47.0 a | 51.8 b | ||
| YLY6 | 45.3 a | 55.6 a | ||
| QY6 | 45.9 a | 51.8 b | ||
| Mean | 44.7 B | 50.7 A | ||
| 2017 | 100 | HHZ | 37.8 b | 46.8 c |
| YY2640 | 41.1 a | 51.4 b | ||
| YLY6 | 42.7 a | 57.0 a | ||
| QY6 | 43.5 a | 58.9 a | ||
| Mean | 41.3 B | 53.5 A | ||
| 2018 | 0 | HHZ | 49.5 c | 48.9 c |
| YY2640 | 54.6 b | 55.0 bc | ||
| YLY6 | 60.0 a | 60.9 ab | ||
| QY6 | 59.7 a | 65.9 a | ||
| Mean | 56.0 A | 57.7 A | ||
| 2018 | 100 | HHZ | 41.2 b | 49.6 b |
| YY2640 | 47.4 a | 56.4 a | ||
| YLY6 | 49.5 a | 57.4 a | ||
| QY6 | 48.7 a | 54.5 a | ||
| Mean | 46.7 B | 54.4 A | ||
| Year | N Rate (kg ha−1) | Variety | Spikelets m−2 (×103) at MA | Leaf Area Index at HD (m2 m−2) | Spikelets to Leaf-Area Ratio (No. cm−2) | |||
|---|---|---|---|---|---|---|---|---|
| CK | LF | CK | LF | CK | LF | |||
| 2016 | 100 | HHZ | 54.5 b | 41.0 ab | 5.53 c | 3.95 b | 1.00 b | 1.04 b |
| YY2640 | 58.0 a | 46.6 a | 4.89 c | 3.77 b | 1.19 a | 1.25 a | ||
| YLY6 | 42.0 c | 40.7 b | 8.20 a | 5.42 a | 0.52 c | 0.76 c | ||
| QY6 | 41.1 c | 34.5 c | 7.28 b | 5.22 a | 0.57 c | 0.67 c | ||
| Mean | 48.9 A | 40.7 B | 6.48 A | 4.59 B | 0.82 B | 0.93 A | ||
| 2017 | 100 | HHZ | 51.0 b | 43.3 a | 6.42 c | 4.79 b | 0.80 b | 0.91 b |
| YY2640 | 58.4 a | 43.5 a | 6.00 c | 4.29 b | 0.98 a | 1.03 a | ||
| YLY6 | 41.4 c | 38.1 b | 9.45 a | 5.64 a | 0.44 c | 0.68 c | ||
| QY6 | 41.4 c | 36.3 b | 8.05 b | 5.94 a | 0.52 c | 0.61 c | ||
| Mean | 48.0 A | 40.3 B | 7.48 A | 5.16 B | 0.68 A | 0.81 A | ||
| 2018 | 0 | HHZ | 45.3 a | 36.9 a | 3.29 b | 2.14 b | 1.39 a | 1.75 a |
| YY2640 | 44.2 a | 32.7 b | 3.07 b | 2.11 b | 1.45 a | 1.56 a | ||
| YLY6 | 31.4 c | 25.8 c | 4.00 a | 2.32 ab | 0.79 b | 1.13 b | ||
| QY6 | 37.2 b | 31.3 b | 4.33 a | 3.13 a | 0.86 b | 1.06 b | ||
| Mean | 39.5 A | 31.7 B | 3.67 A | 2.42 B | 1.12 B | 1.37 A | ||
| 2018 | 100 | HHZ | 51.5 b | 45.3 ab | 5.88 b | 4.71 b | 0.88 b | 0.97 b |
| YY2640 | 59.8 a | 46.4 a | 4.88 c | 3.68 c | 1.23 a | 1.26 a | ||
| YLY6 | 43.1 c | 38.0 c | 7.73 a | 5.48 ab | 0.56 c | 0.70 c | ||
| QY6 | 45.4 c | 42.0 b | 7.83 a | 6.27 a | 0.58 c | 0.68 c | ||
| Mean | 50.0 A | 42.9 B | 6.58 A | 5.03 B | 0.81 B | 0.90 A | ||
| Year | N Rate (kg ha−1) | Variety | Pre-Flowering Dry Matter Accumulation (g m−2) | Post-Flowering Dry Matter Accumulation (g m−2) | Pre-Flowering Dry Matter Translocation (g m−2) | |||
|---|---|---|---|---|---|---|---|---|
| CK | LF | CK | LF | CK | LF | |||
| 2016 | N100 | HHZ | 1026.2 b | 872.6 c | 672.0 bc | 471.4 b | 128.3 b | 119.4 b |
| YY2640 | 851.3 c | 811.2 c | 1027.5 a | 650.3 a | −19.2 c | 125.0 b | ||
| YLY6 | 1278.4 a | 1064.4 a | 559.3 c | 550.1 ab | 316.4 a | 253.1 a | ||
| QY6 | 1106.2 b | 961.0 b | 770.1 b | 629.6 a | 180.4 b | 165.2 ab | ||
| Mean | 1065.5 A | 927.3 B | 757.2 A | 575.3 B | 151.5 A | 165.7 A | ||
| 2017 | N100 | HHZ | 915.8 c | 872.7 b | 695.2 a | 447.1 b | 50.0 b | 163.6 b |
| YY2640 | 1049.5 b | 897.8 b | 786.9 a | 547.5 a | 72.0 b | 141.1 b | ||
| YLY6 | 1223.5 a | 1052.4 a | 650.5 a | 560.4 a | 252.2 a | 277.3 a | ||
| QY6 | 1109.1 b | 1014.3 a | 738.1 a | 567.8 a | 175.2 ab | 273.2 a | ||
| Mean | 1074.5 A | 959.3 B | 717.7 A | 530.7 A | 137.3 A | 213.8 A | ||
| 2018 | N0 | HHZ | 790.2 b | 640.8 b | 452.9 b | 300.6 b | 143.0 ab | 120.3 ab |
| YY2640 | 805.6 b | 626.2 b | 616.1 a | 437.4 a | 95.9 b | 71.4 b | ||
| YLY6 | 894.0 ab | 666.9 ab | 518.1 ab | 420.6 ab | 214.8 a | 125.2 ab | ||
| QY6 | 917.6 a | 795.1 a | 521.8 ab | 334.5 ab | 206.4 ab | 242.9 a | ||
| Mean | 851.8 A | 682.3 B | 527.2 A | 373.2 B | 165.0 A | 139.9 A | ||
| 2018 | N100 | HHZ | 1000.8 b | 950.6 b | 622.2 b | 473.9 b | 119.8 b | 213.7 a |
| YY2640 | 1007.4 b | 857.4 c | 894.4 a | 708.9 a | 81.8 b | 112.2 b | ||
| YLY6 | 1273.2 a | 1077.0 a | 737.5 ab | 575.0 ab | 252.2 a | 249.0 a | ||
| QY6 | 1225.2 a | 1079.9 a | 615.5 b | 695.8 a | 280.7 a | 185.4 ab | ||
| Mean | 1126.7 A | 991.2 B | 717.4 A | 613.4 A | 183.6 A | 190.1 A | ||
| Year | N Rate (kg ha−1) | Variety | Pre-Flowering N (kg ha−1) | Post-Flowering N (kg ha−1) | Pre-Flowering N Translocation (kg ha−1) | |||
|---|---|---|---|---|---|---|---|---|
| CK | LF | CK | LF | CK | LF | |||
| 2016 | 100 | HHZ | 145.1 b | 104.9 a | 52.7 a | 30.6 ab | 58.0 b | 43.5 b |
| YY2640 | 154.0 ab | 114.5 a | 60.5 a | 36.3 ab | 81.2 ab | 60.0 a | ||
| YLY6 | 172.2 a | 118.4 a | 21.1 b | 26.3 b | 99.5 a | 70.4 a | ||
| QY6 | 154.1 ab | 115.7 a | 53.2 a | 38.0 a | 78.9 ab | 58.6 a | ||
| Mean | 156.3 A | 113.4 B | 46.9 A | 32.8 A | 79.4 A | 58.1 B | ||
| 2017 | 100 | HHZ | 158.8 a | 110.6 a | 38.0 a | 20.4 a | 66.2 b | 52.3 b |
| YY2640 | 179.6 a | 113.0 a | 29.9 a | 21.1 a | 85.9 ab | 62.4 ab | ||
| YLY6 | 184.5 a | 122.8 a | 27.7 a | 24.4 a | 98.0 a | 73.9 a | ||
| QY6 | 172.1 a | 122.3 a | 39.0 a | 20.7 a | 86.5 ab | 75.3 a | ||
| Mean | 173.7 A | 117.2 B | 33.6 A | 21.7 A | 84.1 A | 66.0 A | ||
| 2018 | 0 | HHZ | 83.5 b | 58.9 a | 37.0 ab | 26.4 ab | 33.1 b | 20.2 b |
| YY2640 | 89.8 ab | 60.1 a | 40.8 a | 32.4 ab | 44.7 ab | 24.0 ab | ||
| YLY6 | 91.4 ab | 58.9 a | 31.0 ab | 33.1 a | 52.3 a | 26.4 ab | ||
| QY6 | 100.0 a | 71.0 a | 22.0 b | 17.3 b | 57.4 a | 41.4 a | ||
| Mean | 91.2 A | 62.2 B | 32.7 A | 27.3 A | 46.9 A | 28.0 B | ||
| 2018 | 100 | HHZ | 142.9 b | 117.3 a | 37.8 ab | 22.0 b | 57.0 b | 57.1 b |
| YY2640 | 148.9 ab | 98.5 b | 57.6 a | 47.5 a | 68.0 b | 45.8 c | ||
| YLY6 | 166.0 a | 115.8 a | 34.4 b | 29.0 b | 90.1 a | 64.5 ab | ||
| QY6 | 166.9 a | 131.8 a | 17.9 b | 32.3 ab | 96.4 a | 72.3 a | ||
| Mean | 156.2 A | 115.8 B | 36.9 A | 32.7 A | 77.9 A | 59.9 B | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Zhou, Y.; Shuai, P.; Wang, X.; Peng, S.; Wang, F. Source–Sink Balance Optimization Depends on Soil Nitrogen Condition So as to Increase Rice Yield and N Use Efficiency. Agronomy 2023, 13, 907. https://doi.org/10.3390/agronomy13030907
Li X, Zhou Y, Shuai P, Wang X, Peng S, Wang F. Source–Sink Balance Optimization Depends on Soil Nitrogen Condition So as to Increase Rice Yield and N Use Efficiency. Agronomy. 2023; 13(3):907. https://doi.org/10.3390/agronomy13030907
Chicago/Turabian StyleLi, Xiaoxiao, Yongjin Zhou, Peng Shuai, Xinyu Wang, Shaobing Peng, and Fei Wang. 2023. "Source–Sink Balance Optimization Depends on Soil Nitrogen Condition So as to Increase Rice Yield and N Use Efficiency" Agronomy 13, no. 3: 907. https://doi.org/10.3390/agronomy13030907
APA StyleLi, X., Zhou, Y., Shuai, P., Wang, X., Peng, S., & Wang, F. (2023). Source–Sink Balance Optimization Depends on Soil Nitrogen Condition So as to Increase Rice Yield and N Use Efficiency. Agronomy, 13(3), 907. https://doi.org/10.3390/agronomy13030907





