Exploring the Macroscopic Properties of Humic Substances Using Modeling and Molecular Simulations
Abstract
:1. Introduction
2. Materials and Methods
3. Results
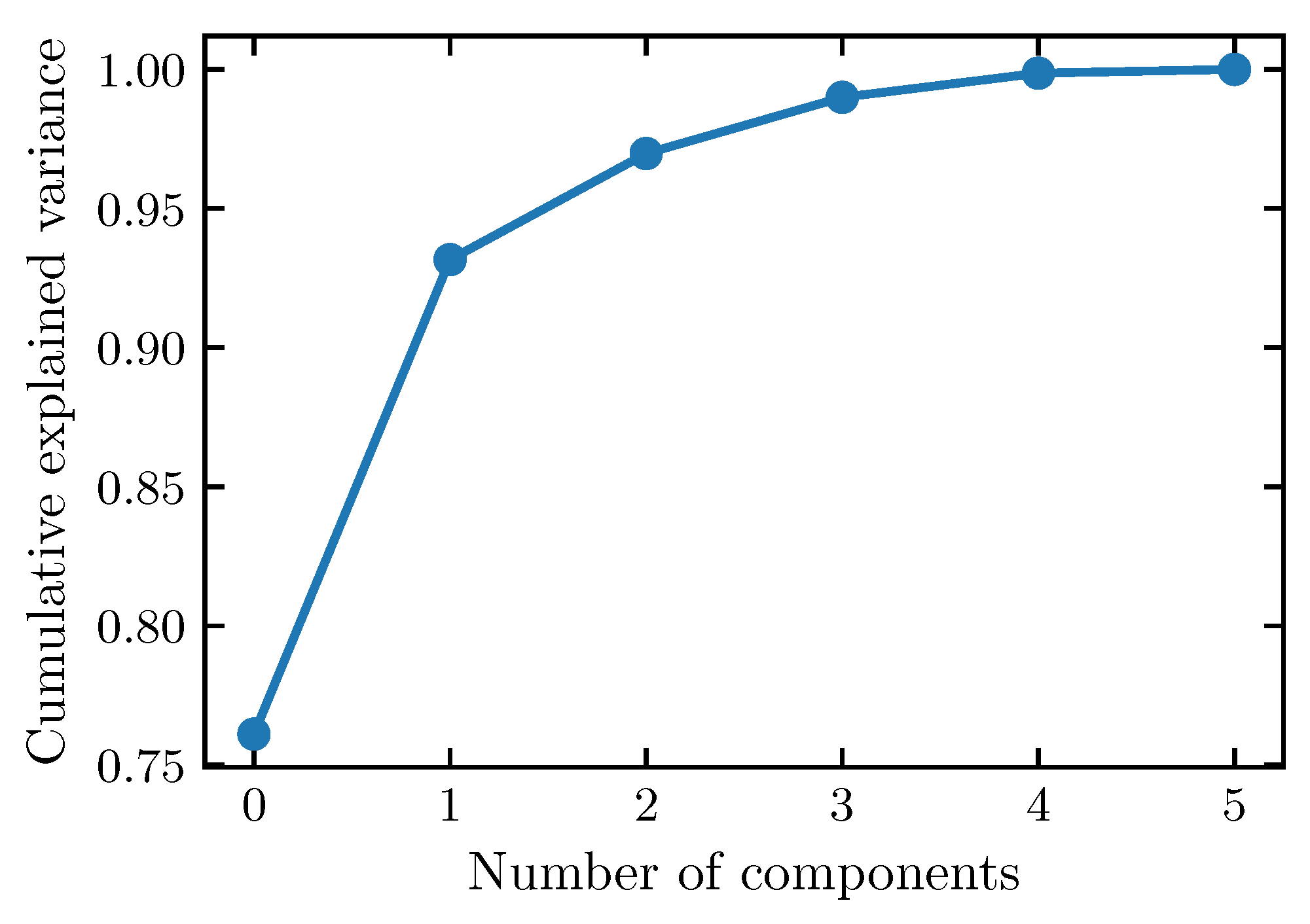
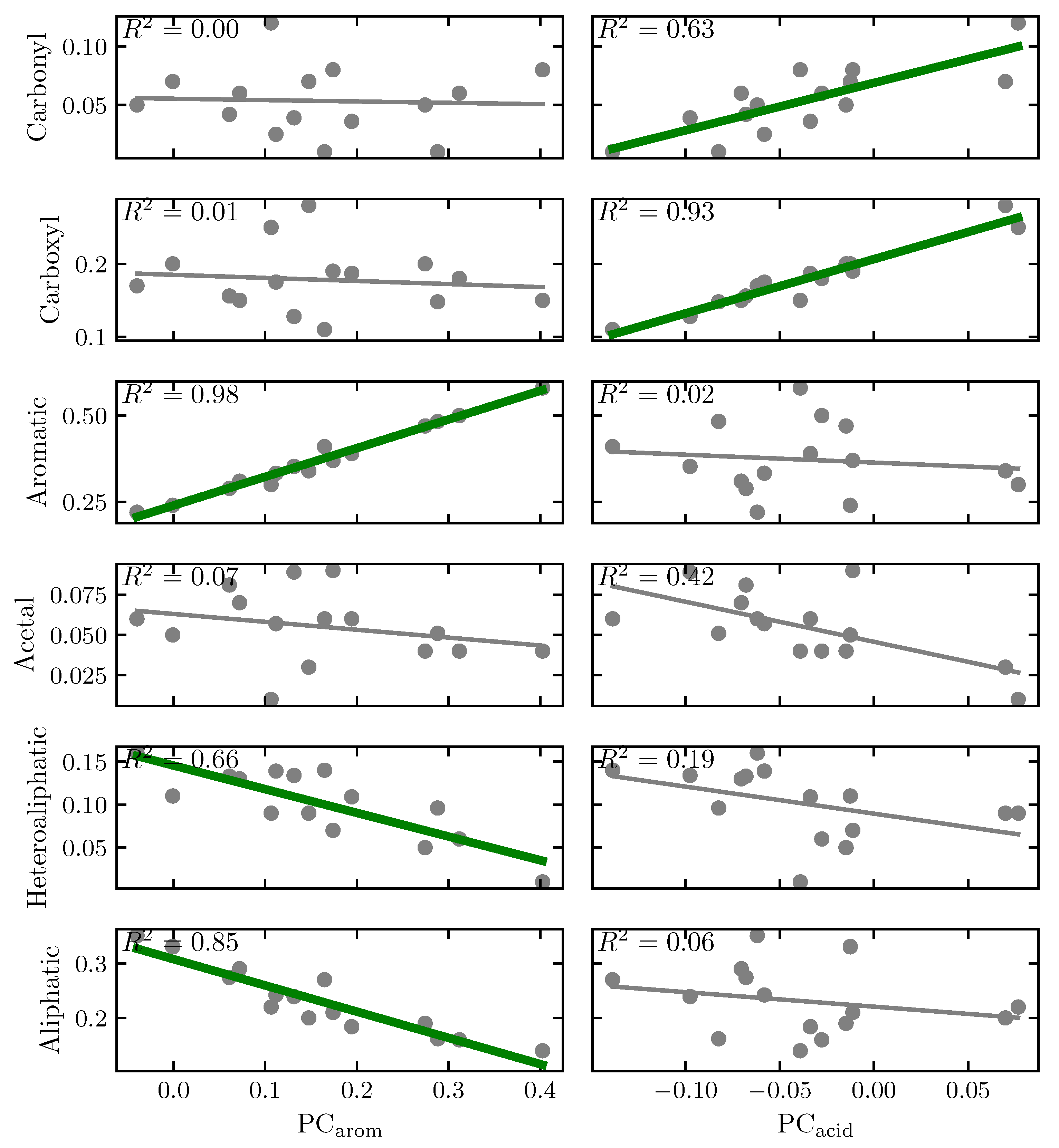
3.1. Principal Component Analysis of the Organic Composition
3.2. Equilibration of Humic Substances
3.3. Physicochemical Properties of Humic Substances
3.3.1. Density
3.3.2. Total Potential Energy
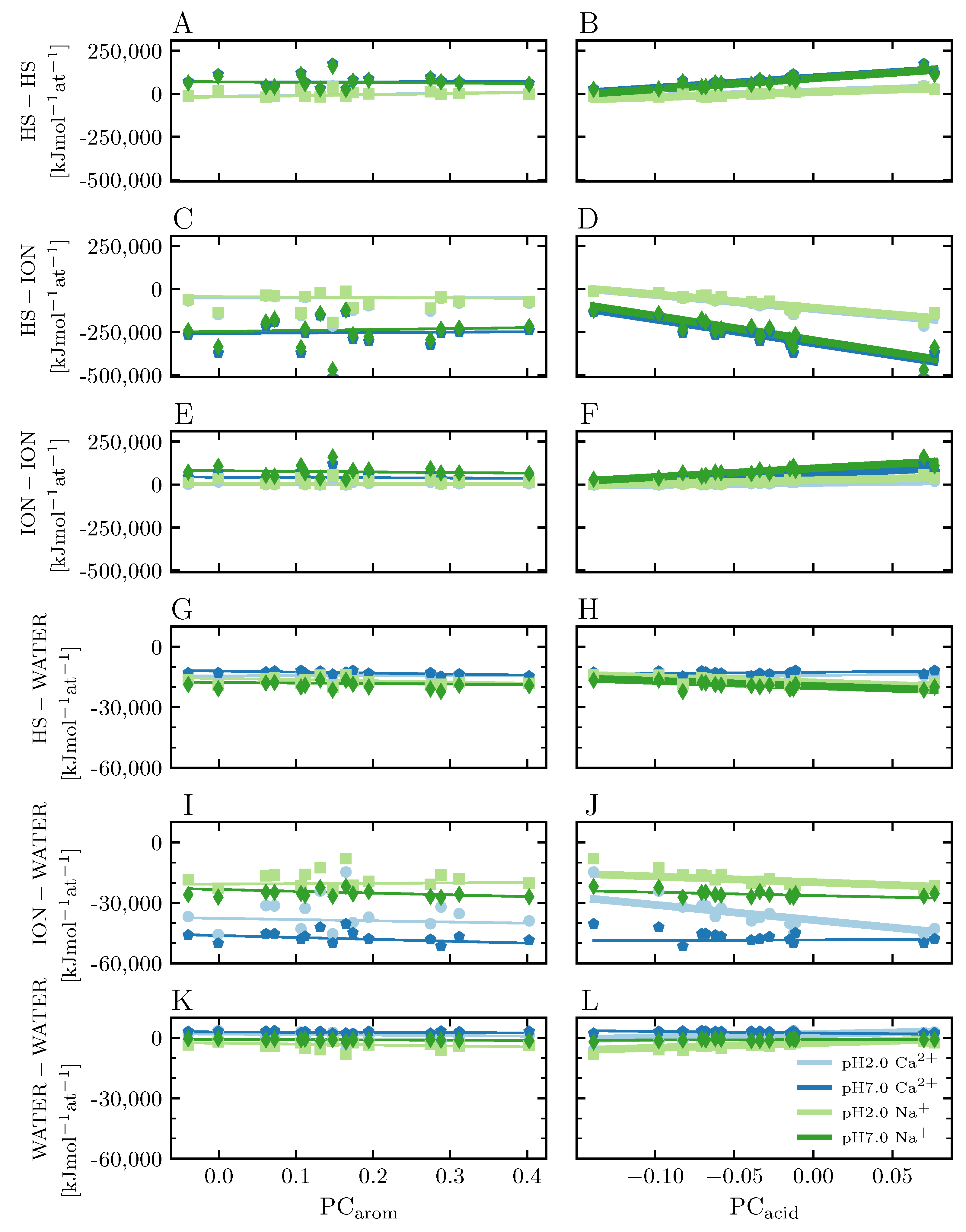
3.3.3. Nonbonded Interaction between Molecules
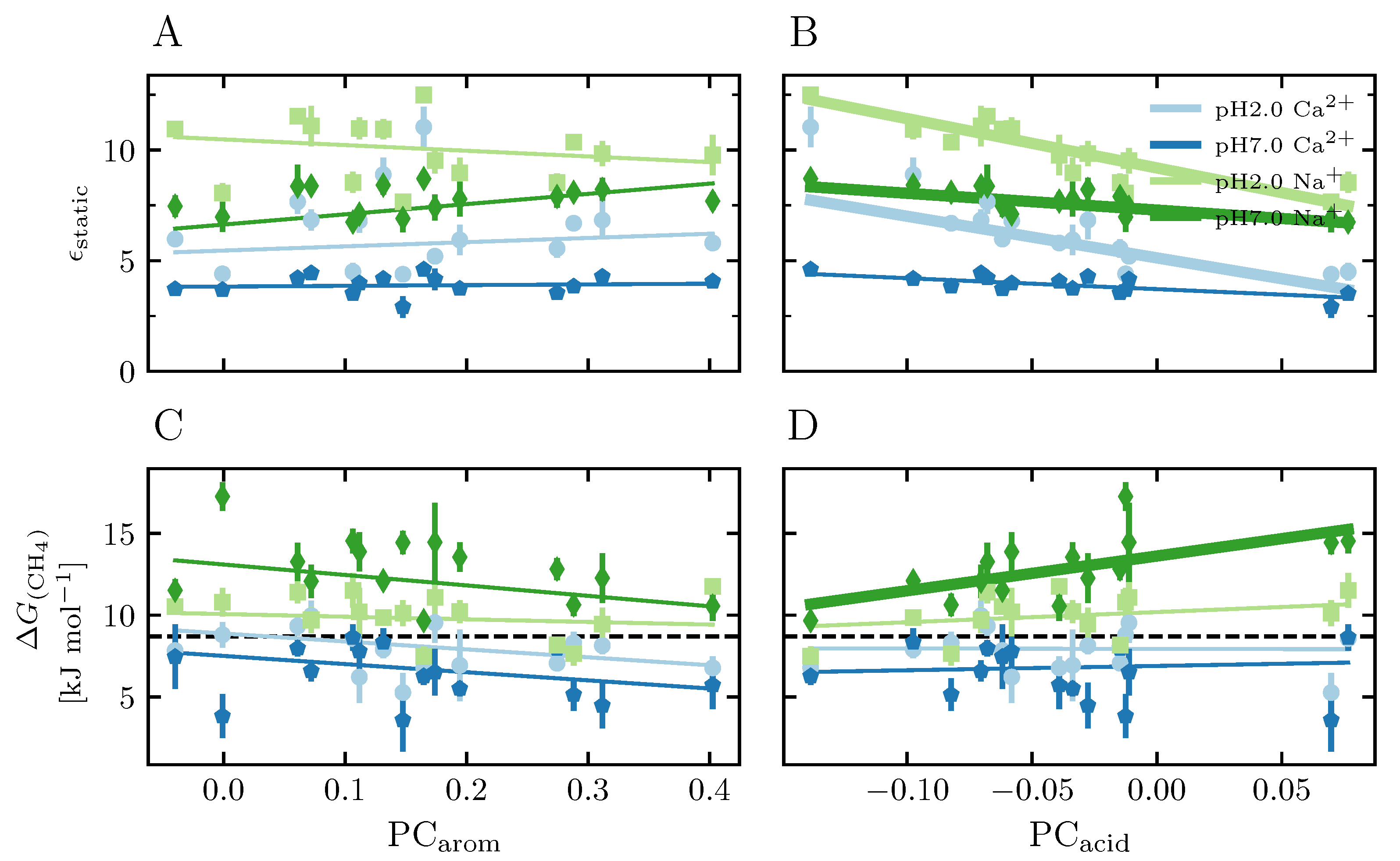
3.3.4. Static Relative Dielectric Constant
3.3.5. Free Energy of Inserting a Methane Molecule
3.3.6. Diffusion
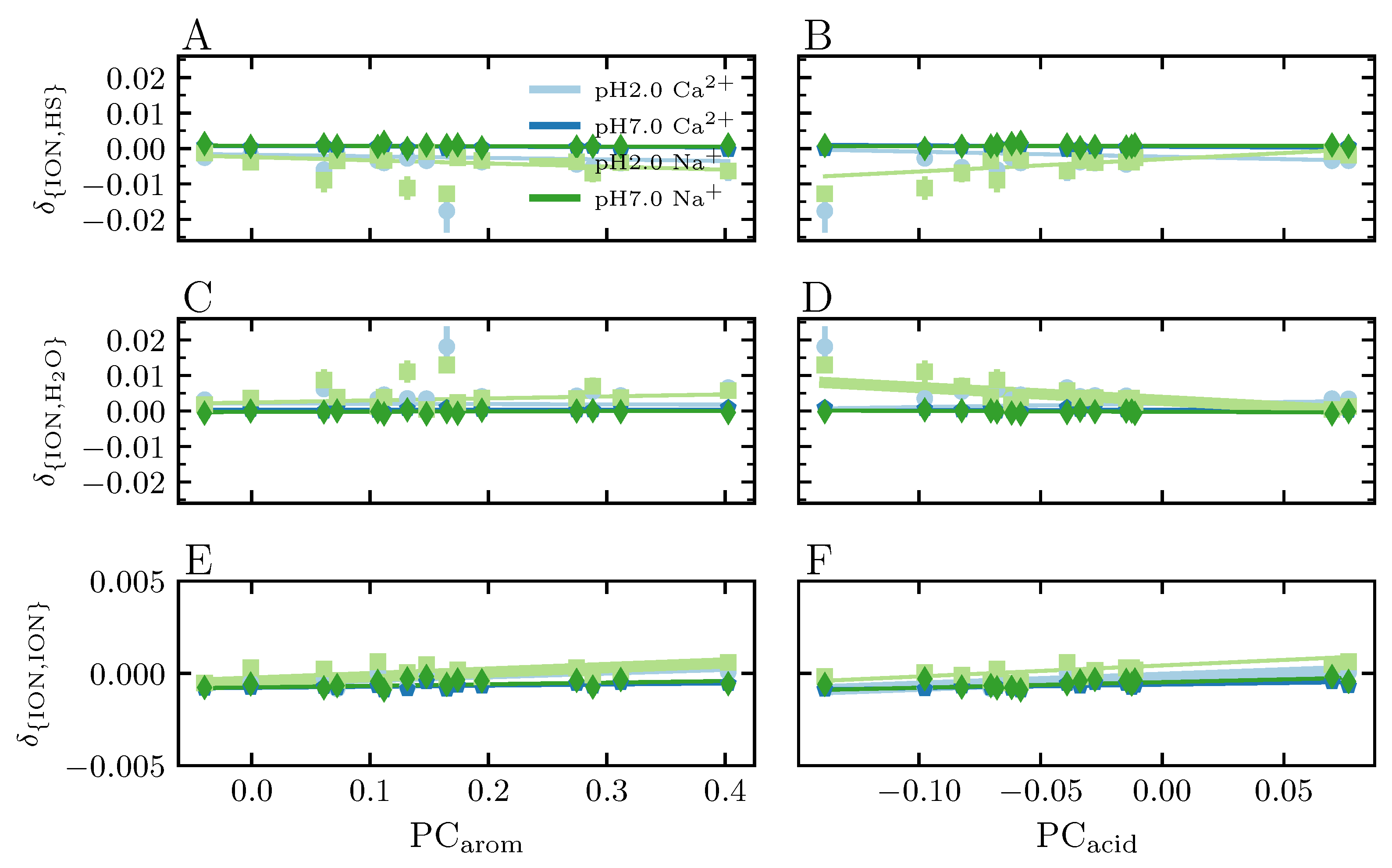
3.3.7. Preferential Solvation
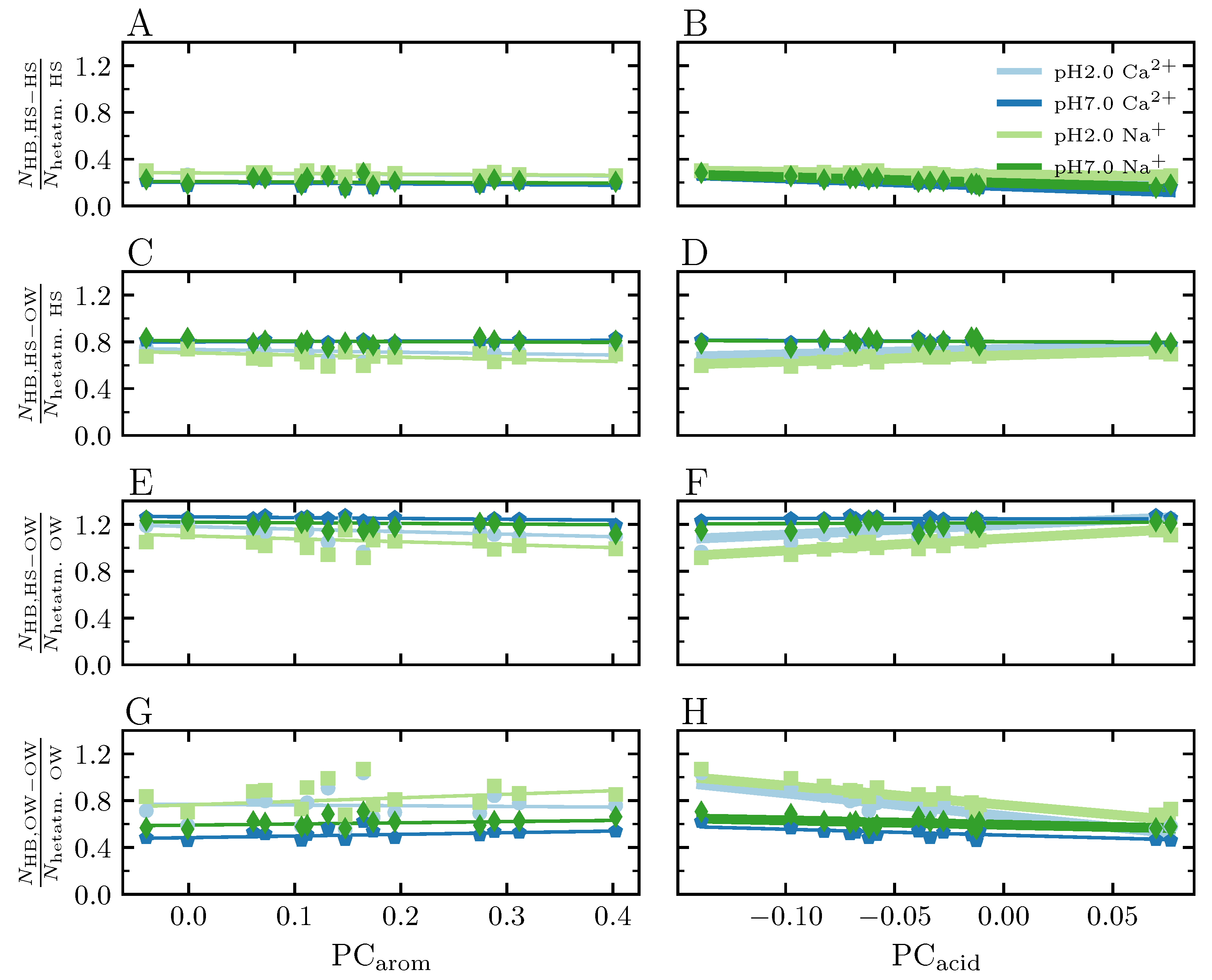
3.3.8. Hydrogen Bonds
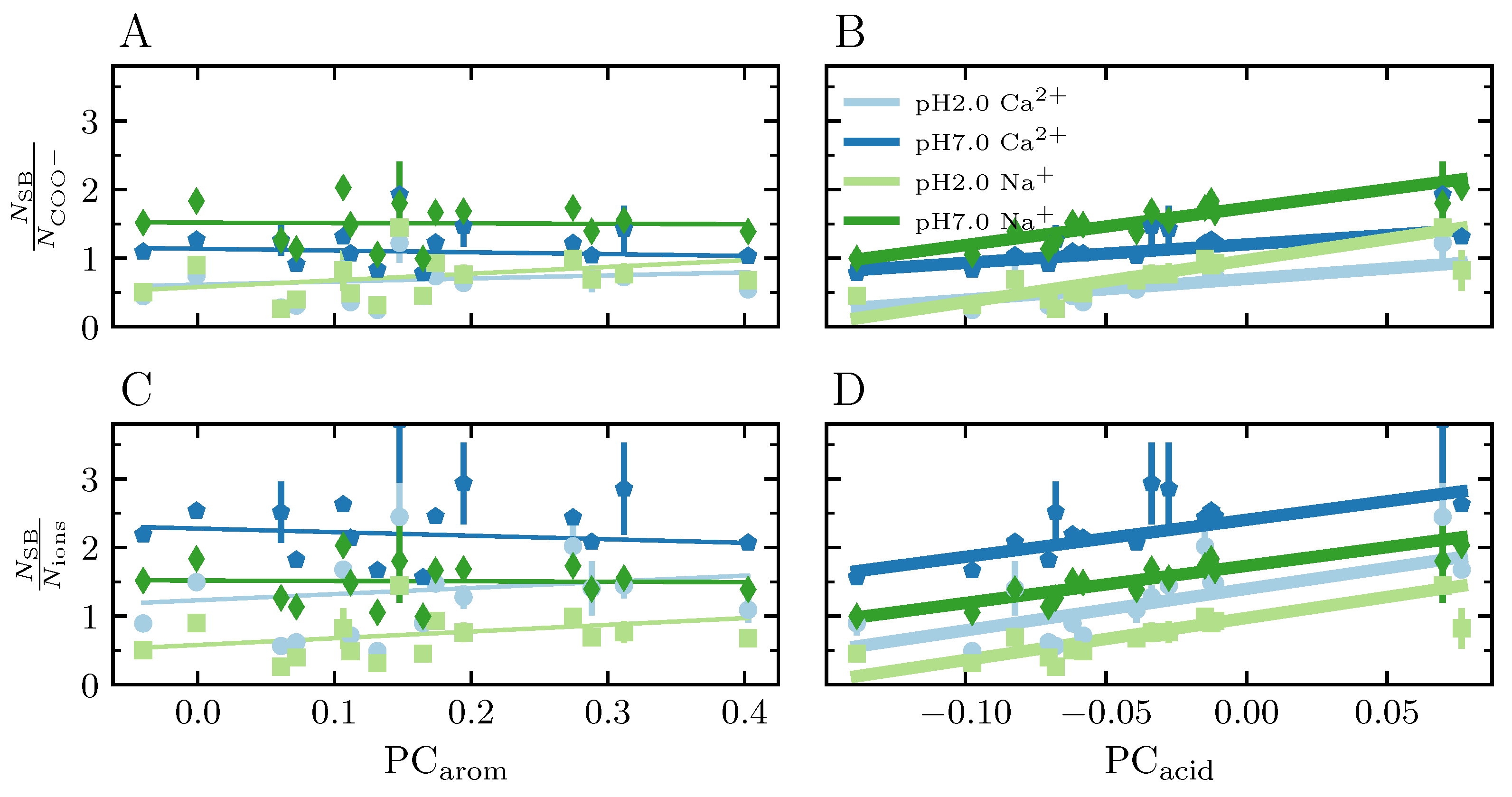
3.3.9. Salt Bridges
4. Discussion
4.1. Humic Substances modeling
4.2. Organic Composition Analysis
4.3. Simulation Setup and Equilibration
4.4. Physical Properties of Modeled Humic Substances
4.4.1. Main Interactions in the System
4.4.2. Effect of Protonated Carboxyl Groups
4.4.3. Salt Bridges Network
4.4.4. The Effect of the Aromaticity in the Studied Systems
4.5. Extrapolation to Soil Organic Matter
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HS | Humic Substances |
| SOM | Soil Organic Matter |
| HA | Humic Acid |
| FA | Fulvic Acid |
| VSOMM2 | Vienna Soil Organic Matter Modeler 2 |
Appendix A
Appendix A.1. Principal Components Analysis (PCA) of Humic Substances
Appendix A.2. Humic Substances Models
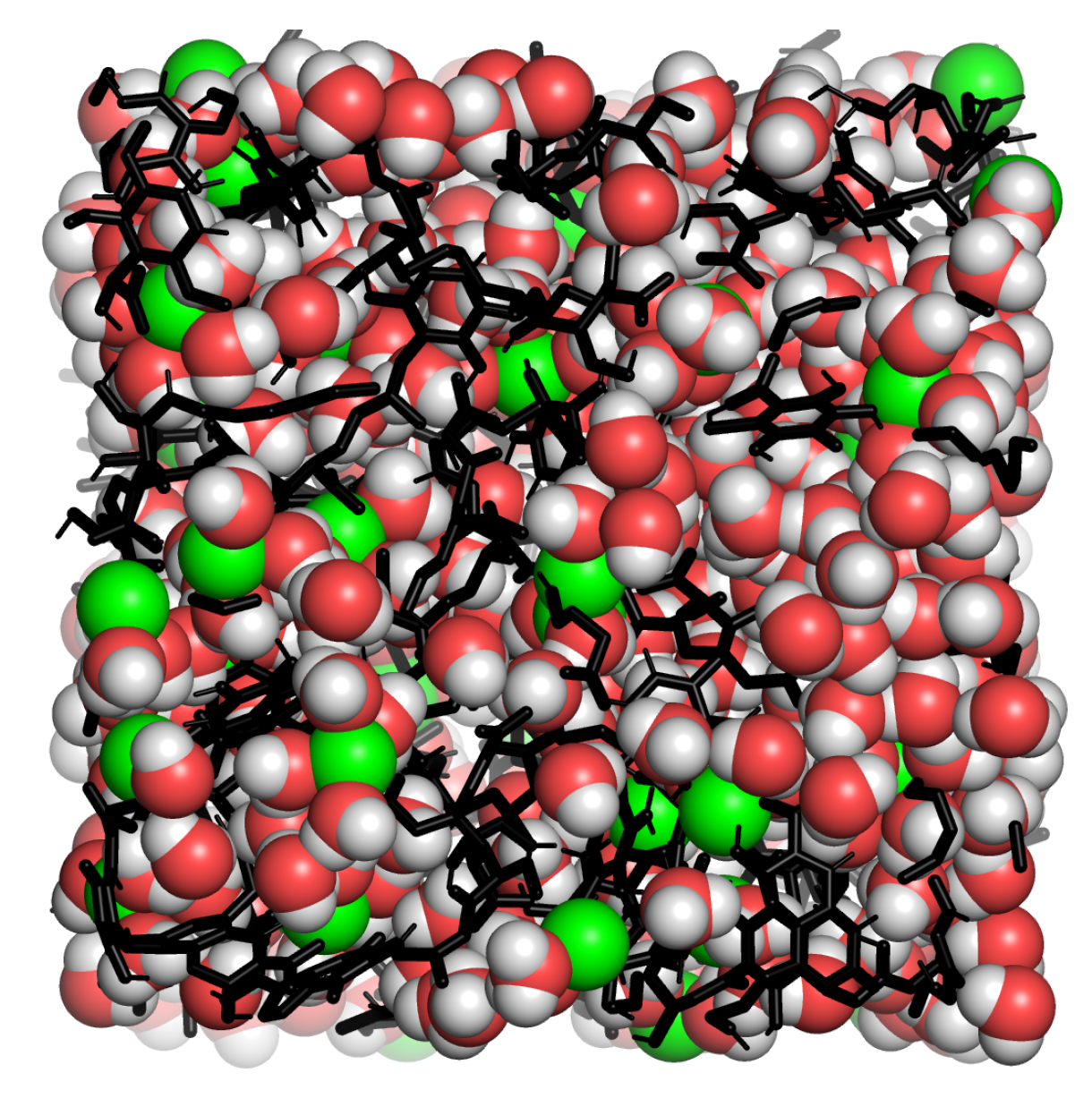
Appendix A.3. Humic Substances Simulations
Appendix A.4. Trajectory Analysis
Appendix A.4.1. Density
Appendix A.4.2. Total Potential Energy
Appendix A.4.3. Nonbonded Interactions Between Molecules
Appendix A.4.4. Static Dielectric Constant
Appendix A.4.5. Free Energy of Inserting a Methane Molecule
Appendix A.4.6. Diffusion Coefficient
Appendix A.4.7. Preferential Solvation
Appendix A.4.8. Number of Hydrogen Bonds
Appendix A.4.9. Number of Salt Bridges
Appendix B. Figures
References
- Masoom, H.; Courtier-Murias, D.; Farooq, H.; Soong, R.; Kelleher, B.P.; Zhang, C.; Maas, W.E.; Fey, M.; Kumar, R.; Monette, M.; et al. Soil Organic Matter in Its Native State: Unravelling the Most Complex Biomaterial on Earth. Environ. Sci. Technol. 2016, 50, 1670–1680. [Google Scholar] [CrossRef] [PubMed]
- Paul, E.A. The nature and dynamics of soil organic matter: Plant inputs, microbial transformations, and organic matter stabilization. Soil Biol. Biochem. 2016, 98, 109–126. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.D.; Dunfield, K.E.; Fraser, T.D.; Wakelin, S.A.; Richardson, A.E.; Condron, L.M. Soil biodiversity and biogeochemical function in managed ecosystems. Soil Res. 2020, 58, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Liu, X.; Zhang, C.; Lu, X. A combined first principles and classical molecular dynamics study of clay-soil organic matters (SOMs) interactions. Geochim. Cosmochim. Acta 2020, 291, 110–125. [Google Scholar] [CrossRef]
- Kibblewhite, M.G.; Ritz, K.; Swift, M.J. Soil health in agricultural systems. Philos. Trans. R. Soc. Biol. Sci. 2008, 363, 685–701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bui, M.; Adjiman, C.S.; Bardow, A.; Anthony, E.J.; Boston, A.; Brown, S.; Fennell, P.S.; Fuss, S.; Galindo, A.; Hackett, L.A.; et al. Carbon capture and storage (CCS): The way forward. Energy Environ. Sci. 2018, 11, 1062–1176. [Google Scholar] [CrossRef] [Green Version]
- Wiesmeier, M.; Urbanski, L.; Hobley, E.; Lang, B.; von Lützow, M.; Marin-Spiotta, E.; van Wesemael, B.; Rabot, E.; Ließ, M.; Garcia-Franco, N.; et al. Soil organic carbon storage as a key function of soils - A review of drivers and indicators at various scales. Geoderma 2019, 333, 149–162. [Google Scholar] [CrossRef]
- Rabot, E.; Wiesmeier, M.; Schlüter, S.; Vogel, H.J. Soil structure as an indicator of soil functions: A review. Geoderma 2018, 314, 122–137. [Google Scholar] [CrossRef]
- Hayes, M.H.; Swift, R.S. Vindication of Humic Substances as a Key Component of Organic Matter in Soil and Water, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2020; Volume 163, pp. 1–37. [Google Scholar] [CrossRef]
- Kögel-Knabner, I.; Rumpel, C. Advances in Molecular Approaches for Understanding Soil Organic Matter Composition, Origin, and Turnover: A Historical Overview, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2018; Volume 149, pp. 1–48. [Google Scholar] [CrossRef]
- Kleber, M.; Johnson, M.G. Advances in Understanding the Molecular Structure of Soil Organic Matter, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2010; Volume 106, pp. 77–142. [Google Scholar] [CrossRef]
- IHSS. 2020. Available online: http://humic-substances.org/ (accessed on 26 March 2023).
- Tuckerman, M.E. Statistical Mechanics: Theory and Molecular Simulation, 1st ed.; Oxford University Press: Oxford, UK, 2010; pp. 95–96. [Google Scholar]
- Ai, Y.; Zhao, C.; Sun, L.; Wang, X.; Liang, L. Coagulation mechanisms of humic acid in metal ions solution under different pH conditions: A molecular dynamics simulation. Sci. Total Environ. 2020, 702, 135072. [Google Scholar] [CrossRef]
- Tan, L.; Yu, Z.; Tan, X.; Fang, M.; Wang, X.; Wang, J.; Xing, J.; Ai, Y.; Wang, X. Systematic studies on the binding of metal ions in aggregates of humic acid: Aggregation kinetics, spectroscopic analyses and MD simulations. Environ. Pollut. 2019, 246, 999–1007. [Google Scholar] [CrossRef]
- Galicia-Andrés, E.; Escalona, Y.; Oostenbrink, C.; Tunega, D.; Gerzabek, M.H. Soil organic matter stabilization at molecular scale: The role of metal cations and hydrogen bonds. Geoderma 2021, 401, 115237. [Google Scholar] [CrossRef]
- Petrov, D.; Tunega, D.; Gerzabek, M.H.; Oostenbrink, C. Molecular Dynamics Simulations of the Standard Leonardite Humic Acid: Microscopic Analysis of the Structure and Dynamics. Environ. Sci. Technol. 2017, 51, 5414–5424. [Google Scholar] [CrossRef]
- Petrov, D.; Tunega, D.; Gerzabek, M.H.; Oostenbrink, C. Molecular modelling of sorption processes of a range of diverse small organic molecules in Leonardite humic acid. Eur. J. Soil Sci. 2020, 71, 831–844. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Escalona, Y.; Petrov, D.; Oostenbrink, C. Modeling soil organic matter: Changes in macroscopic properties due to microscopic changes. Geochim. Cosmochim. Acta 2021, 307, 228–241. [Google Scholar] [CrossRef]
- Thorn, K.A.; Folan, D.W.; MacCarthy, P. Characterization of the International Humic Substances Society Standard and Reference Fulvic and Humic Acids by Solution State Carbon-13 (13C) and Hydrogen-1 (1H) Nuclear Magnetic Resonance Spectrometry; Water-Resources Investigations Report 89-4196 (USGS); U.S. Geological Survey: Reston, VA, USA, 1989; Volume 13, p. 99.
- Niederer, C.; Goss, K.U.; Schwarzenbach, R.P. Sorption equilibrium of a wide spectrum of organic vapors in leonardite humic acid: Modeling of experimental data. Environ. Sci. Technol. 2006, 40, 5374–5379. [Google Scholar] [CrossRef]
- Escalona Balboa, Y.I. Towards The Understanding of Soil Organic Matter via Molecular Modeling and Simulations. 2021. Available online: https://permalink.obvsg.at/bok/AC16438986 (accessed on 26 March 2023).
- Escalona, Y.; Petrov, D.; Oostenbrink, C. Vienna soil organic matter modeler 2 (VSOMM2). J. Mol. Graph. Model. 2021, 103, 107817. [Google Scholar] [CrossRef]
- Schmid, N.; Eichenberger, A.P.; Choutko, A.; Riniker, S.; Winger, M.; Mark, A.E.; Van Gunsteren, W.F. Definition and testing of the GROMOS force-field versions 54A7 and 54B7. Eur. Biophys. J. 2011, 40, 843–856. [Google Scholar] [CrossRef]
- Schmid, N.; Christ, C.D.; Christen, M.; Eichenberger, A.P.; Van Gunsteren, W.F. Architecture, implementation and parallelisation of the GROMOS software for biomolecular simulation. Comput. Phys. Commun. 2012, 183, 890–903. [Google Scholar] [CrossRef]
- Oostenbrink, C.; Villa, A.; Mark, A.E.; Van Gunsteren, W.F. A biomolecular force field based on the free enthalpy of hydration and solvation: The GROMOS force-field parameter sets 53A5 and 53A6. J. Comput. Chem. 2004, 25, 1656–1676. [Google Scholar] [CrossRef]
- Reif, M.M.; Hünenberger, P.H.; Oostenbrink, C. New interaction parameters for charged amino acid side chains in the GROMOS force field. J. Chem. Theory Comput. 2012, 8, 3705–3723. [Google Scholar] [CrossRef]
- Eichenberger, A.P.; Allison, J.R.; Dolenc, J.; Geerke, D.P.; Horta, B.A.C.; Meier, K.; Oostenbrink, C.; Schmid, N.; Steiner, D.; Wang, D.; et al. GROMOS++ software for the analysis of biomolecular simulation trajectories. J. Chem. Theory Comput. 2011, 7, 3379–3390. [Google Scholar] [CrossRef] [PubMed]
- Demontoux, F.; Le Crom, B.; Ruffié, G.; Wigneron, J.P.; Grant, J.P.; Mironov, V.L.; Lawrence, H. Electromagnetic characterization of soil-litter media: Application to the simulation of the microwave emissivity of the ground surface in forests. Eur. Phys. J. Appl. Phys. 2008, 44, 303–315. [Google Scholar] [CrossRef]
- Adar, R.M.; Markovich, T.; Levy, A.; Orland, H.; Andelman, D. Dielectric constant of ionic solutions: Combined effects of correlations and excluded volume. J. Chem. Phys. 2018, 149, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Widom, B. Some Topics in the Theory of Fluids. J. Chem. Phys. 1963, 39, 2808–2812. [Google Scholar] [CrossRef]
- Lehmann, J.; Solomon, D.; Kinyangi, J.; Dathe, L.; Wirick, S.; Jacobsen, C. Spatial complexity of soil organic matter forms at nanometre scales. Nat. Geosci. 2008, 1, 238–242. [Google Scholar] [CrossRef]
- Piccolo, A.; Spaccini, R.; Savy, D.; Drosos, M. The Soil Humeome: Chemical Structure, Functions and Technological Perspectives. In Sustainable Agrochemistry; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar] [CrossRef]
- Trout, C.C.; Kubicki, J.D. UV Resonance Raman Spectra and Molecular Orbital Calculations of Salicylic and Phthalic Acids Complexed to Al3+ in Solution and on Mineral Surfaces. J. Phys. Chem. A 2004, 108, 11580–11590. [Google Scholar] [CrossRef]
- Watts, H.D.; Mohamed, M.N.A.; Kubicki, J.D. Comparison of Multistandard and TMS-Standard Calculated NMR Shifts for Coniferyl Alcohol and Application of the Multistandard Method to Lignin Dimers. J. Phys. Chem. B 2011, 115, 1958–1970. [Google Scholar] [CrossRef]
- Trout, C.C.; Tambach, T.; Kubicki, J.D. Correlation of observed and model vibrational frequencies for aqueous organic acids: UV resonance Raman spectra and molecular orbital calculations of benzoic, salicylic, and phthalic acids. Spectrochim. Acta Part Mol. Biomol. Spectrosc. 2005, 61, 2622–2633. [Google Scholar] [CrossRef]
- Gotsmy, M.; Escalona, Y.; Oostenbrink, C.; Petrov, D. Exploring the structure and dynamics of proteins in soil organic matter. Proteins Struct. Funct. Bioinform. 2021, 89, 925–936. [Google Scholar] [CrossRef]
- Galicia-Andrés, E.; Oostenbrink, C.; Gerzabek, M.H.; Tunega, D. On the Adsorption Mechanism of Humic Substances on Kaolinite and Their Microscopic Structure. Minerals 2021, 11, 1138. [Google Scholar] [CrossRef]
- Schellekens, J.; Buurman, P.; Kalbitz, K.; Zomeren, A.V.; Vidal-Torrado, P.; Cerli, C.; Comans, R.N. Molecular Features of Humic Acids and Fulvic Acids from Contrasting Environments. Environ. Sci. Technol. 2017, 51, 1330–1339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, K.L.; Smith, B.A.; Ball, W.P.; Fairbrother, D.H. Assessing the colloidal properties of engineered nanoparticles in water: Case studies from fullerene C60 nanoparticles and carbon nanotubes. Environ. Chem. 2010, 7, 10–27. [Google Scholar] [CrossRef] [Green Version]
- Tatzber, M.; Stemmer, M.; Spiegel, H.; Katzlberger, C.; Haberhauer, G.; Gerzabek, M.H. Impact of different tillage practices on molecular characteristics of humic acids in a long-term field experiment - An application of three different spectroscopic methods. Sci. Total Environ. 2008, 406, 256–268. [Google Scholar] [CrossRef] [PubMed]
- Kalinichev, A.G.; Iskrenova-Tchoukova, E.; Ahn, W.Y.; Clark, M.M.; Kirkpatrick, R.J. Effects of Ca2+ on supramolecular aggregation of natural organic matter in aqueous solutions: A comparison of molecular modeling approaches. Geoderma 2011, 169, 27–32. [Google Scholar] [CrossRef] [Green Version]
- Iskrenova-Tchoukova, E.; Kalinichev, A.G.; Kirkpatrick, R.J. Metal Cation Complexation with Natural Organic Matter in Aqueous Solutions: Molecular Dynamics Simulations and Potentials of Mean Force. Langmuir 2010, 26, 15909–15919. [Google Scholar] [CrossRef] [Green Version]
- Xu, F.; Wei, C.; Zeng, Q.; Li, X.; Alvarez, P.J.; Li, Q.; Qu, X.; Zhu, D. Aggregation Behavior of Dissolved Black Carbon: Implications for Vertical Mass Flux and Fractionation in Aquatic Systems. Environ. Sci. Technol. 2017, 51, 13723–13732. [Google Scholar] [CrossRef]
- Wei, P.; Xu, F.; Fu, H.; Qu, X. Impact of origin and structure on the aggregation behavior of natural organic matter. Chemosphere 2020, 248, 125990. [Google Scholar] [CrossRef]
- Kalinichev, A.G.; Kirkpatrick, R.J. Molecular dynamics simulation of cationic complexation with natural organic matter. Eur. J. Soil Sci. 2007, 58, 909–917. [Google Scholar] [CrossRef]
- Baalousha, M.; Motelica-Heino, M.; Coustumer, P.L. Conformation and size of humic substances: Effects of major cation concentration and type, pH, salinity, and residence time. Colloids Surfaces Physicochem. Eng. Asp. 2006, 272, 48–55. [Google Scholar] [CrossRef]
- Aquino, A.J.; Tunega, D.; Schaumann, G.E.; Haberhauer, G.; Gerzabek, M.H.; Lischka, H. The functionality of cation bridges for binding polar groups in soil aggregates. Int. J. Quantum Chem. 2011, 111, 1531–1542. [Google Scholar] [CrossRef]
- Ritchie, J.D.; Michael Perdue, E. Proton-binding study of standard and reference fulvic acids, humic acids, and natural organic matter. Geochim. Cosmochim. Acta 2003, 67, 85–96. [Google Scholar] [CrossRef]
- Berendsen, H.J.; Postma, J.P.; Van Gunsteren, W.F.; Dinola, A.; Haak, J.R. Molecular dynamics with coupling to an external bath. J. Chem. Phys. 1984, 81, 3684–3690. [Google Scholar] [CrossRef] [Green Version]
- Tironi, I.G.; Sperb, R.; Smith, P.E.; Van Gunsteren, W.F. A generalized reaction field method for molecular dynamics simulations. J. Chem. Phys. 1995, 102, 5451–5459. [Google Scholar] [CrossRef]
- Heinz, T.N.; Van Gunsteren, W.F.; Hünenberger, P.H. Comparison of four methods to compute the dielectric permittivity of liquids from molecular dynamics simulations. J. Chem. Phys. 2001, 115, 1125–1136. [Google Scholar] [CrossRef]
- Ryckaert, J.P.; Ciccotti, G.; Berendsen, H.J. Numerical integration of the cartesian equations of motion of a system with constraints: Molecular dynamics of n-alkanes. J. Comput. Phys. 1977, 23, 327–341. [Google Scholar] [CrossRef] [Green Version]
- Schröder, C.; Steinhauser, O. Using fit functions in computational dielectric spectroscopy. J. Chem. Phys. 2010, 132, 244109. [Google Scholar] [CrossRef]
- Krüger, P.; Schnell, S.K.; Bedeaux, D.; Kjelstrup, S.; Vlugt, T.J.H.; Simon, J.M. Kirkwood-Buff integrals for finite volumes. J. Phys. Chem. Lett. 2013, 4, 235–238. [Google Scholar] [CrossRef]
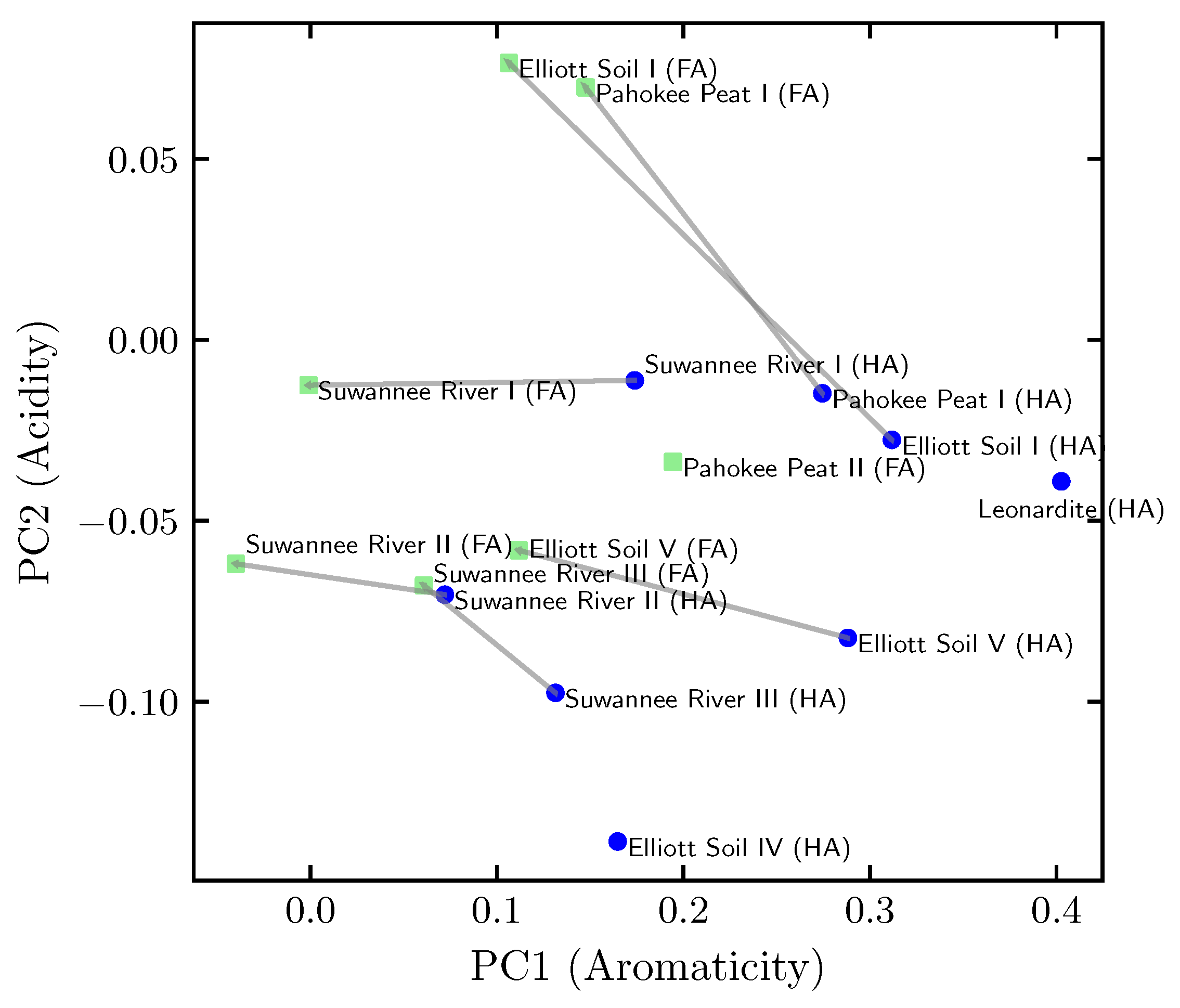





| Negative | Positive | |
|---|---|---|
| PC | high hetero- & aliphatic content | high aromatic content |
| e.g., Suwannee River II (FA) | e.g., Leonardite (HA) | |
| PC | low carboxyl and carbonyl content | high carboxyl and carbonyl content |
| e.g., Elliott Soil IV (HA) | e.g., Elliott Soil I (FA) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Escalona, Y.; Petrov, D.; Galicia-Andrés, E.; Oostenbrink, C. Exploring the Macroscopic Properties of Humic Substances Using Modeling and Molecular Simulations. Agronomy 2023, 13, 1044. https://doi.org/10.3390/agronomy13041044
Escalona Y, Petrov D, Galicia-Andrés E, Oostenbrink C. Exploring the Macroscopic Properties of Humic Substances Using Modeling and Molecular Simulations. Agronomy. 2023; 13(4):1044. https://doi.org/10.3390/agronomy13041044
Chicago/Turabian StyleEscalona, Yerko, Drazen Petrov, Edgar Galicia-Andrés, and Chris Oostenbrink. 2023. "Exploring the Macroscopic Properties of Humic Substances Using Modeling and Molecular Simulations" Agronomy 13, no. 4: 1044. https://doi.org/10.3390/agronomy13041044





