Residual Carbon Derived from Different Maize Parts Differed in Soil Organic Carbon Fractions as Affected by Soil Fertility
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site and Tested Materials
2.2. In Situ Experiment Design and Sampling
2.3. Determination of Soil Organic Carbon Fractions
2.3.1. Total Soil Organic Carbon
2.3.2. Soil Dissolved Organic Carbon
2.3.3. Soil Particulate Organic Carbon
2.4. Calculations
2.5. Statistical Analysis
3. Results
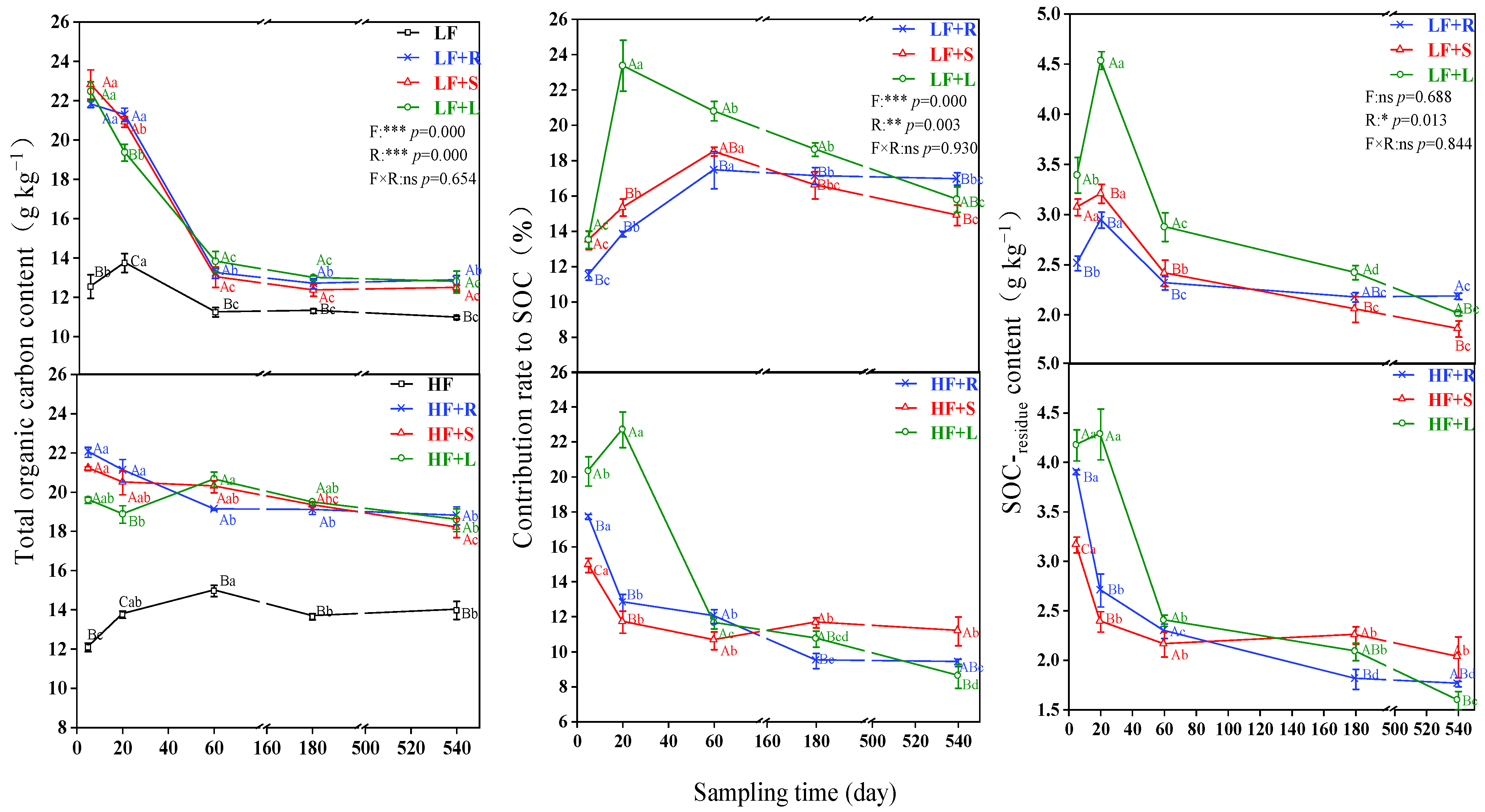
3.1. Distribution of Different Straw Carbon in SOC
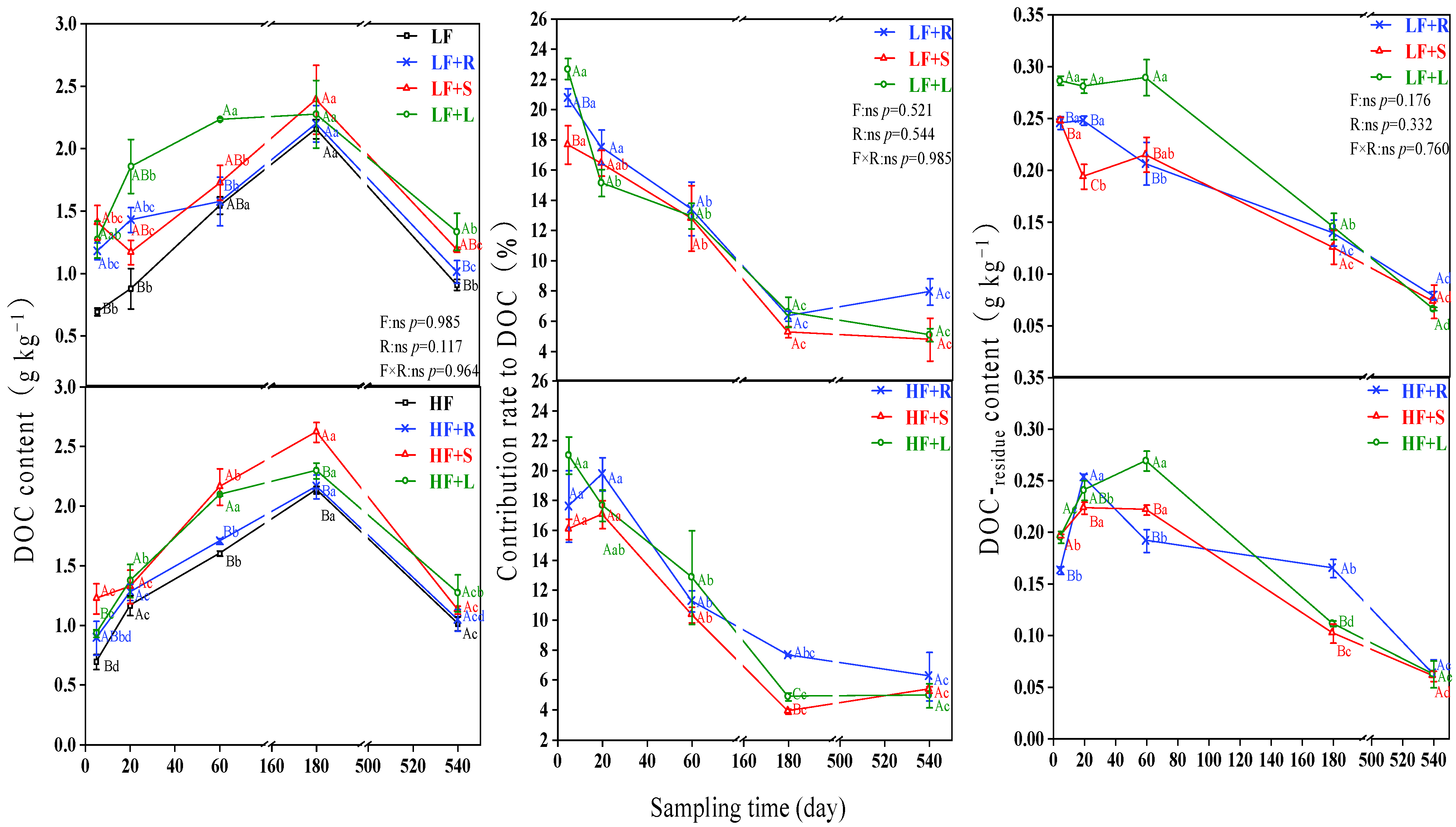
3.2. Distribution of Different Straw in DOC in Soils with Different Fertility
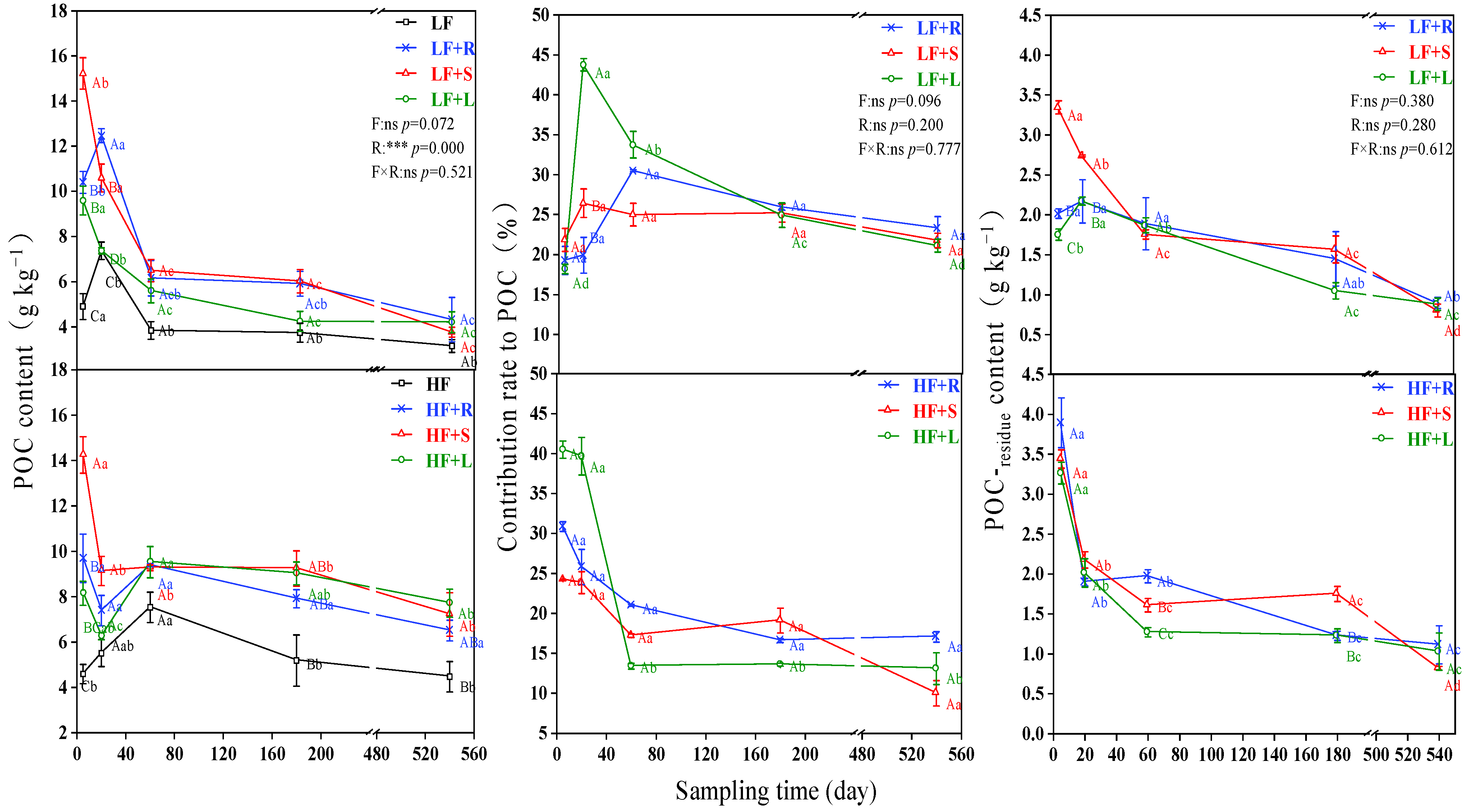
3.3. Distribution of Different Straw in POC in Soils with Different Fertility
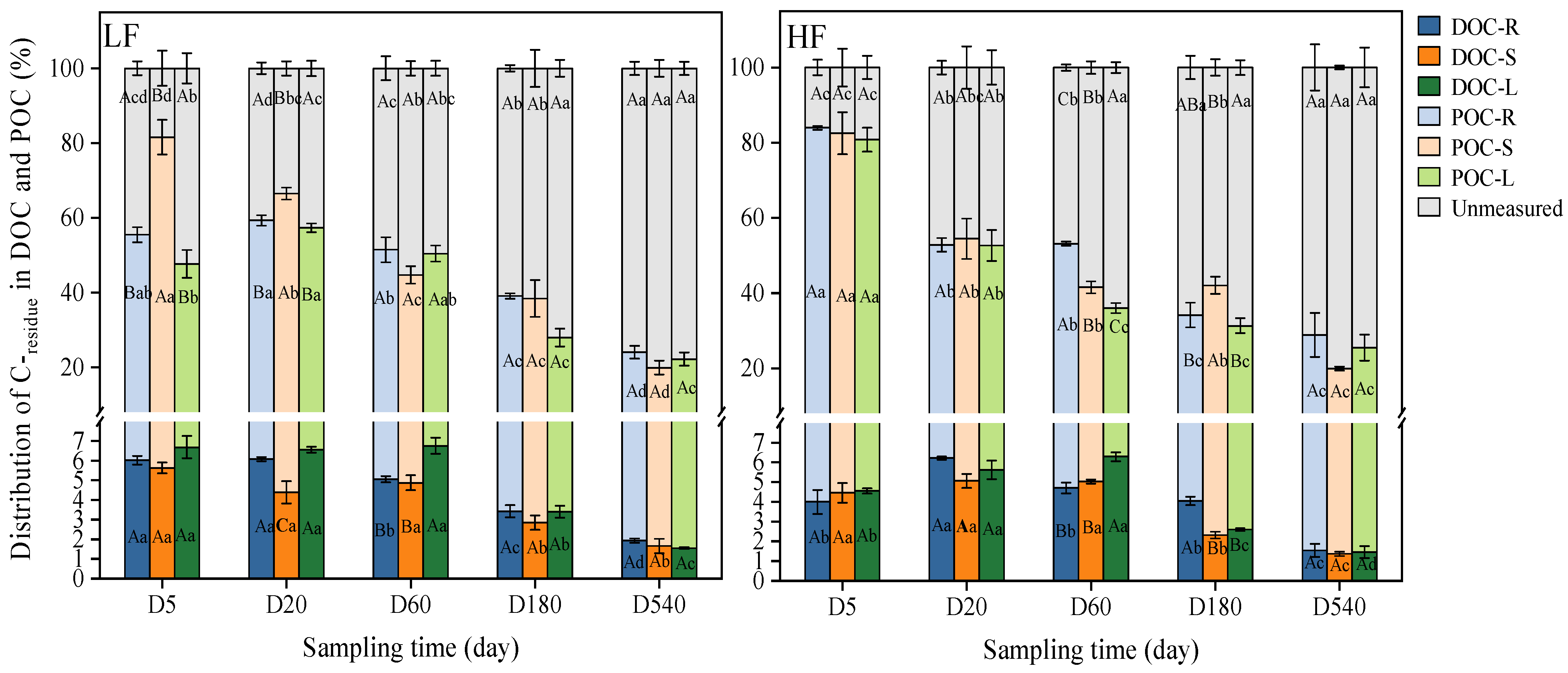
3.4. Distribution of C-residue among SOC Fractions
4. Discussion
4.1. Impact of Residue Types on DOC in the Soil
4.2. Impact of Residue Types on POC in the Soil
4.3. Impact of Residue Types and Fertility on Different SOC Fractions
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Batjes, N.H. Total carbon and nitrogen in the soils of the world. Eur. J. Soil Sci. 2014, 65, 10–21. [Google Scholar] [CrossRef]
- Li, Y.; Wang, X.; Chen, Y.; Luo, Y.; Lian, J.; Niu, Y.; Gong, X.; Yang, H.; Yu, P. Changes in surface soil organic carbon in semiarid degraded Horqin Grassland of northeastern China between the 1980s and the 2010s. Catena 2018, 174, 217–226. [Google Scholar] [CrossRef]
- Oladele, S.O.; Adetunji, A.T. Agro-residue biochar and N fertilizer addition mitigates CO2-C emission and stabilized soil organic carbon pools in a rain-fed agricultural cropland. Int. Soil Water Conserv. Res. 2021, 9, 76–86. [Google Scholar] [CrossRef]
- Benbi, D.K.; Brar, K.; Toor, A.S.; Sharma, S. Sensitivity of Labile Soil Organic Carbon Pools to Long–Term Fertilizer, Straw and Manure Management in Rice-Wheat System. Pedosphere 2015, 25, 534–545. [Google Scholar] [CrossRef]
- Bongiorno, G.; Bunemann, E.K.; Oguejiofor, C.U.; Meier, J.; Gort, G.; Comans, R.; Mader, P.; Brussaard, L.; de Goede, R. Sen-sitivity of labile carbon fractions to tillage and organic matter management and their potential as comprehensive soil quality indicators across pedoclimatic conditions in Europe. Ecol. Indic. 2019, 99, 38–50. [Google Scholar] [CrossRef]
- Roper, M.M.; Gupta, V.V.S.R.; Murphy, D.V. Tillage practices altered labile soil organic carbon and microbial function without affecting crop yields. Soil Res. 2010, 48, 274–285. [Google Scholar] [CrossRef]
- Tang, H.; Xiao, X.; Li, C.; Pan, X.; Cheng, K.; Shi, L.; Li, W. Short-term responses of soil organic carbon and its labile fractions to different manure Nitrogen input in a double-cropping rice field. J. Agric. Sci. 2020, 158, 119–127. [Google Scholar] [CrossRef]
- Xu, M.; Lou, Y.; Sun, X.; Wang, W.; Baniyamuddin, M.; Zhao, K. Soil organic carbon active fractions as early indicators for total carbon change under straw incorporation. Biol. Fertil. Soils 2011, 47, 745–752. [Google Scholar] [CrossRef]
- Tang, H.; Xiao, X.; Li, C.; Tang, W.; Cheng, K.; Pan, X.; Wang, K.; Li, W. Effects of Different Soil Tillage Systems on Soil Carbon Management Index Under Double-Cropping Rice Field in Southern China. Agron. J. 2019, 111, 440–447. [Google Scholar] [CrossRef]
- Tang, H.; Cheng, K.; Shi, L.; Li, C.; Wen, L.; Li, W.; Sun, M.; Sun, G.; Long, Z. Effects of long-term organic matter application on soil carbon accumulation and nitrogen use efficiency in a double-cropping rice field. Environ. Res. 2022, 213, 113700. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Shah, T.; Zhang, L.; Liu, H.; Peng, S.; Nie, L. Effect of straw returning on soil organic carbon in rice–wheat rotation system: A review. Food Energy Secur. 2020, 9, e200. [Google Scholar] [CrossRef] [Green Version]
- Ndzelu, B.S.; Dou, S.; Zhang, X. Corn straw return can increase labile soil organic carbon fractions and improve water-stable aggregates in Haplic Cambisol. J. Arid. Land 2020, 12, 1018–1030. [Google Scholar] [CrossRef]
- Li, S.; Gu, X.; Zhuang, J.; An, T.; Pei, J.; Xie, H.; Li, H.; Fu, S.; Wang, J. Distribution and storage of crop residue carbon in aggregates and its contribution to organic carbon of soil with low fertility. Soil Tillage Res. 2016, 155, 199–206. [Google Scholar] [CrossRef]
- Li, Z.; Li, D.; Ma, L.; Yu, Y.; Zhao, B.; Zhang, J. Effects of straw management and nitrogen application rate on soil organic matter fractions and microbial properties in North China Plain. J. Soils Sediments 2018, 19, 618–628. [Google Scholar] [CrossRef]
- Rubino, M.; Lubritto, C.; D’Onofrio, A.; Terrasi, F.; Gleixner, G.; Cotrufo, M.F. An isotopic method for testing the influence of leaf litter quality on carbon fluxes during decomposition. Oecologia 2007, 154, 155–166. [Google Scholar] [CrossRef]
- Clemente, J.S.; Simpson, M.J.; Simpson, A.J.; Yanni, S.F.; Whalen, J.K. Comparison of soil organic matter composition after incubation with maize leaves, roots, and stems. Geoderma 2013, 192, 86–96. [Google Scholar] [CrossRef]
- Shahbaz, M.; Kuzyakov, Y.; Sanaullah, M.; Heitkamp, F.; Zelenev, V.; Kumar, A.; Blagodatskaya, E. Microbial decomposition of soil organic matter is mediated by quality and quantity of crop residues: Mechanisms and thresholds. Biol. Fertil. Soils 2017, 53, 287–301. [Google Scholar] [CrossRef]
- An, T.; Schaeffer, S.; Li, S.; Fu, S.; Pei, J.; Li, H.; Zhuang, J.; Radosevich, M.; Wang, J. Carbon fluxes from plants to soil and dynamics of microbial immobilization under plastic film mulching and fertilizer application using 13C pulse-labeling. Soil Biol. Biochem. 2015, 80, 53–61. [Google Scholar] [CrossRef]
- De Troyer, I.; Amery, F.; Van Moorleghem, C.; Smolders, E.; Merckx, R. Tracing the source and fate of dissolved organic matter in soil after incorporation of a 13C labelled residue: A batch incubation study. Soil Biol. Biochem. 2011, 43, 513–519. [Google Scholar] [CrossRef]
- Sun, L.; Sun, Z.; Hu, J.; Yaa, O.-K.; Wu, J. Decomposition Characteristics, Nutrient Release, and Structural Changes of Maize Straw in Dryland Farming under Combined Application of Animal Manure. Sustainability 2021, 13, 7609. [Google Scholar] [CrossRef]
- Meng, F.; Dungait, J.A.; Xu, X.; Bol, R.; Zhang, X.; Wu, W. Coupled incorporation of maize (Zea mays L.) straw with nitrogen fertilizer increased soil organic carbon in Fluvic Cambisol. Geoderma 2017, 304, 19–27. [Google Scholar] [CrossRef]
- Kelleher, B.P.; Simpson, A.J. Humic substances in soils, are they really chemically distinct. Environ. Sci. Technol. 2006, 70, 4080–4094. [Google Scholar] [CrossRef] [PubMed]
- Machinet, G.E.; Bertrand, I.; Chabbert, B.; Recous, S. Decomposition in soil and chemical changes of maize roots with genetic variations affecting cell wall quality. Eur. J. Soil Sci. 2009, 60, 176–185. [Google Scholar] [CrossRef]
- Kögel-Knabner, I. The macromolecular organic composition of plant and microbial residues as inputs to soil organic matter: Fourteen years on. Soil Biol. Biochem. 2017, 105, A3–A8. [Google Scholar] [CrossRef]
- Guggenberger, G.; Christensen, B.; Zech, W. Land-use effects on the composition of organic matter in particle-size separates of soil: I. Lignin and carbohydrate signature. Eur. J. Soil Sci. 1994, 45, 449–458. [Google Scholar] [CrossRef]
- Guillaumie, S.; San-Clemente, H.; Deswarte, C.; Martinez, Y.; Lapierre, C.; Murigneux, A.; Barrière, Y.; Pichon, M.; Goffner, D. MAIZEWALL. Database and Developmental Gene Expression Profiling of Cell Wall Biosynthesis and Assembly in Maize. Plant Physiol. 2006, 143, 339–363. [Google Scholar] [CrossRef] [Green Version]
- Hatfield, R.D.; Chaptman, A.K. Comparing Corn Types for Differences in Cell Wall Characteristics and p-Coumaroylation of Lignin. J. Agric. Food Chem. 2009, 57, 4243–4249. [Google Scholar] [CrossRef]
- Shahbaz, M.; Kätterer, T.; Thornton, B.; Börjesson, G. Dynamics of fungal and bacterial groups and their carbon sources during the growing season of maize in a long-term experiment. Biol. Fertil. Soils 2020, 56, 759–770. [Google Scholar] [CrossRef] [Green Version]
- Wei, L.; Ge, T.; Zhu, Z.; Ye, R.; Peñuelas, J.; Li, Y.; Lynn, T.M.; Jones, D.L.; Wu, J.; Kuzyakov, Y. Paddy soils have a much higher microbial biomass content than upland soils: A review of the origin, mechanisms, and drivers. Agric. Ecosyst. Environ. 2021, 326, 107798. [Google Scholar] [CrossRef]
- Wang, B.; An, S.; Liang, C.; Liu, Y.; Kuzyakov, Y. Microbial necromass as the source of soil organic carbon in global ecosystems. Soil Biol. Biochem. 2021, 162, 108422. [Google Scholar] [CrossRef]
- Xu, Y.; Gao, X.; Liu, Y.; Li, S.; Liang, C.; Lal, R.; Wang, J. Differential accumulation patterns of microbial necromass induced by maize root vs. shoot residue addition in agricultural Alfisols. Soil Biol. Biochem. 2021, 164, 108474. [Google Scholar] [CrossRef]
- Zhu, Z.; Ge, T.; Hu, Y.; Zhou, P.; Wang, T.; Shibistova, O.; Guggenberger, G.; Su, Y.; Wu, J. Fate of rice shoot and root residues, rhizodeposits, and microbial assimilated carbon in paddy soil–Part 2: Turnover and microbial utilization. Plant Soil 2017, 416, 243–257. [Google Scholar] [CrossRef]
- Zheng, X.D.; Liang, C.M.; Chen, X.B.; Hu, Y.J.; Qiu, H.S.; Xia, Y.H.; Liu, Z.Z.; Wei, L.; Ge, T.D.; Gunina, A.; et al. Plant res-idue-derived hydrophilic and hydrophobic fractions contribute to the formation of soil organic matter. Biol. Fertil. Soils 2021, 57, 1021–1028. [Google Scholar] [CrossRef]
- Liang, C.; Amelung, W.; Lehmann, J.; Kästner, M. Quantitative assessment of microbial necromass contribution to soil organic matter. Glob. Chang. Biol. 2019, 25, 3578–3590. [Google Scholar] [CrossRef]
- Blaud, A.; Lerch, T.; Chevallier, T.; Nunan, N.; Chenu, C.; Brauman, A. Dynamics of bacterial communities in relation to soil aggregate formation during the decomposition of 13C-labelled rice straw. Appl. Soil Ecol. 2012, 53, 1–9. [Google Scholar] [CrossRef]
- Cui, S.; Liang, S.; Zhang, X.; Li, Y.; Liang, W.; Sun, L.; Wang, J.; Bezemer, T.M.; Li, Q. Long-term fertilization management affects the C utilization from crop residues by the soil micro-food web. Plant Soil 2018, 429, 335–348. [Google Scholar] [CrossRef]
- Bell, J.M.; Smith, J.L.; Bailey, V.L.; Bolton, H. Priming effect and C storage in semi-arid no-till spring crop rotations. Biol. Fertil. Soils 2003, 37, 237–244. [Google Scholar] [CrossRef]
- Xu, Y.; Ding, F.; Gao, X.; Wang, Y.; Li, M.; Wang, J. Mineralization of plant residues and native soil carbon as affected by soil fertility and residue type. J. Soils Sediments 2018, 19, 1407–1415. [Google Scholar] [CrossRef]
- Wang, R.-J.; Song, J.-S.; Feng, Y.-T.; Zhou, J.-X.; Xie, J.-Y.; Khan, A.; Che, Z.-X.; Zhang, S.-L.; Yang, X.-Y. Changes in soil organic carbon pools following long-term fertilization under a rain-fed cropping system in the Loess Plateau, China. J. Integr. Agric. 2021, 20, 2512–2525. [Google Scholar] [CrossRef]
- Stockmann, U.; Adams, M.A.; Crawford, J.W.; Field, D.J.; Henakaarchchi, N.; Jenkins, M.; Minasny, B.; McBratney, A.B.; de Courcelles, V.d.R.; Singh, K.; et al. The knowns, known unknowns and unknowns of sequestration of soil organic carbon. Agric. Ecosyst. Environ. 2013, 164, 80–99. [Google Scholar] [CrossRef]
- Sun, L.; Sun, Z.; Hu, J.; Opoku-Kwanowaa, Y.; Wu, J. Combined application of animal manure and stover enhance labile organic carbon and benefit the microbial community in dark brown soil. Arch. Agron. Soil Sci. 2021, 69, 519–532. [Google Scholar] [CrossRef]
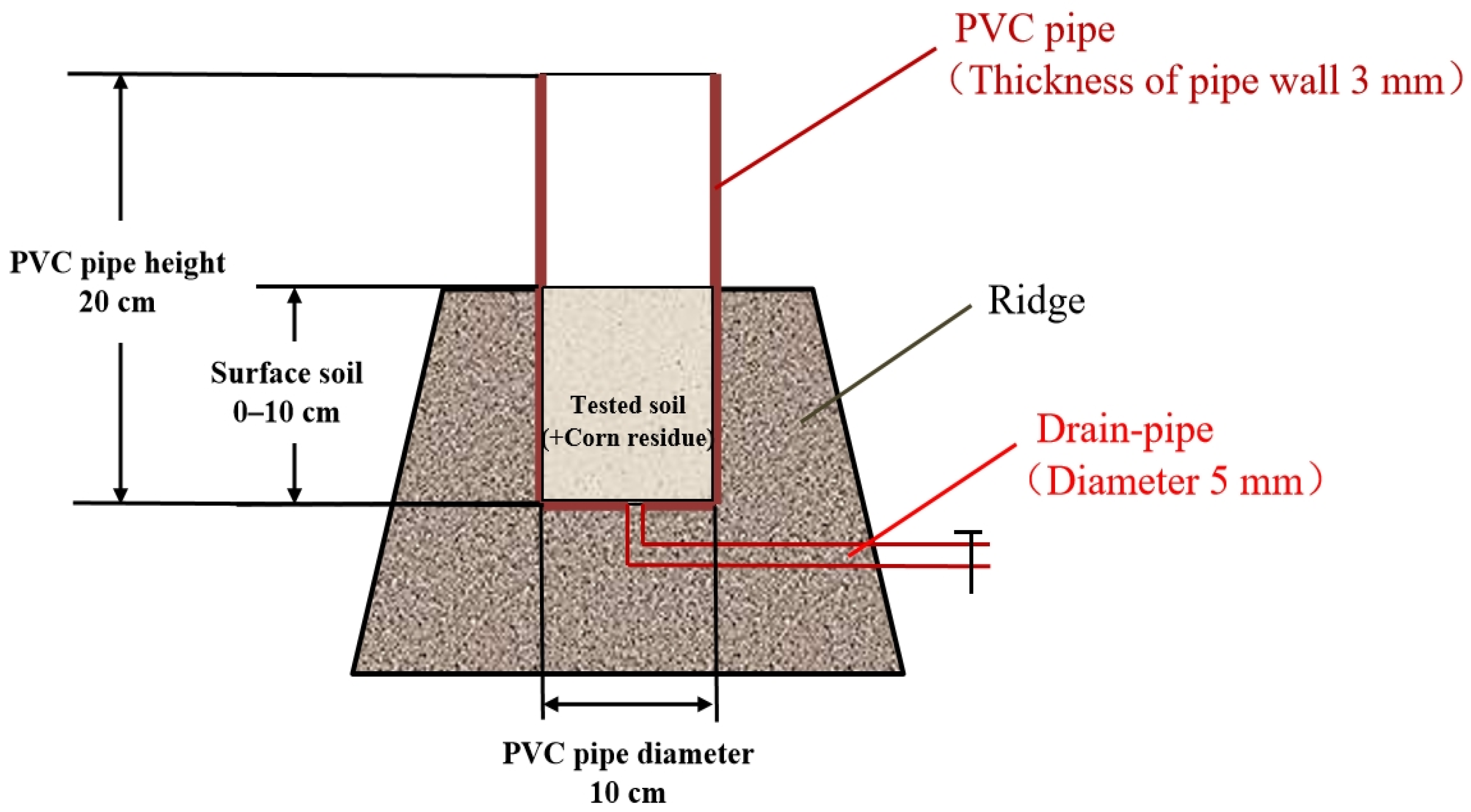

| Soil Fertility | Fertilizer | Soil Organic Carbon (g kg−1) | Total Nitrogen (g kg−1) | C/N | δ13C (‰) |
|---|---|---|---|---|---|
| Low fertility (LF) | No fertilizer (0 kg N hm−2) | 11.55 ± 0.09 B | 1.30 ± 0.03 B | 8.87 ± 0.15 A | −17.88 ± 0.41 B |
| High fertility (HF) | Manure fertilizer (270 kg N hm−2) | 20.61 ± 0.25 A | 2.32 ± 0.01 A | 8.89 ± 0.08 A | −19.45 ± 0.04 A |
| Factor | Df | Distribution-DOC | Distribution-POC | Unmeasured | |||
|---|---|---|---|---|---|---|---|
| F | p | F | p | F | p | ||
| S | 4 | 149.627 | <0.001 | 54.432 | <0.001 | 61.848 | <0.001 |
| F | 1 | 13.000 | <0.01 | 14.577 | <0.001 | 13.942 | <0.001 |
| R | 2 | 16.260 | <0.001 | 8.692 | <0.001 | 7.782 | <0.01 |
| S × F | 4 | 52.657 | <0.001 | 101.074 | <0.001 | 111.221 | <0.001 |
| S × R | 8 | 3.639 | <0.01 | 6.355 | <0.001 | 5.636 | <0.001 |
| F × R | 2 | 2.419 | 0.098 | 5.987 | <0.01 | 6.780 | <0.01 |
| S × F × R | 8 | 3.578 | <0.01 | 3.102 | <0.01 | 2.874 | <0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, M.; Pei, J.; Yu, Y.; Wang, S. Residual Carbon Derived from Different Maize Parts Differed in Soil Organic Carbon Fractions as Affected by Soil Fertility. Agronomy 2023, 13, 1121. https://doi.org/10.3390/agronomy13041121
Wang M, Pei J, Yu Y, Wang S. Residual Carbon Derived from Different Maize Parts Differed in Soil Organic Carbon Fractions as Affected by Soil Fertility. Agronomy. 2023; 13(4):1121. https://doi.org/10.3390/agronomy13041121
Chicago/Turabian StyleWang, Mengmeng, Jiubo Pei, Yaxi Yu, and Siyin Wang. 2023. "Residual Carbon Derived from Different Maize Parts Differed in Soil Organic Carbon Fractions as Affected by Soil Fertility" Agronomy 13, no. 4: 1121. https://doi.org/10.3390/agronomy13041121




