Droughts and Thermo-Priming Enhance Acclimation to Later Drought and Heat Stress in Maize Seedlings by Improving Leaf Physiological Activity
Abstract
1. Introduction
2. Materials and Methods
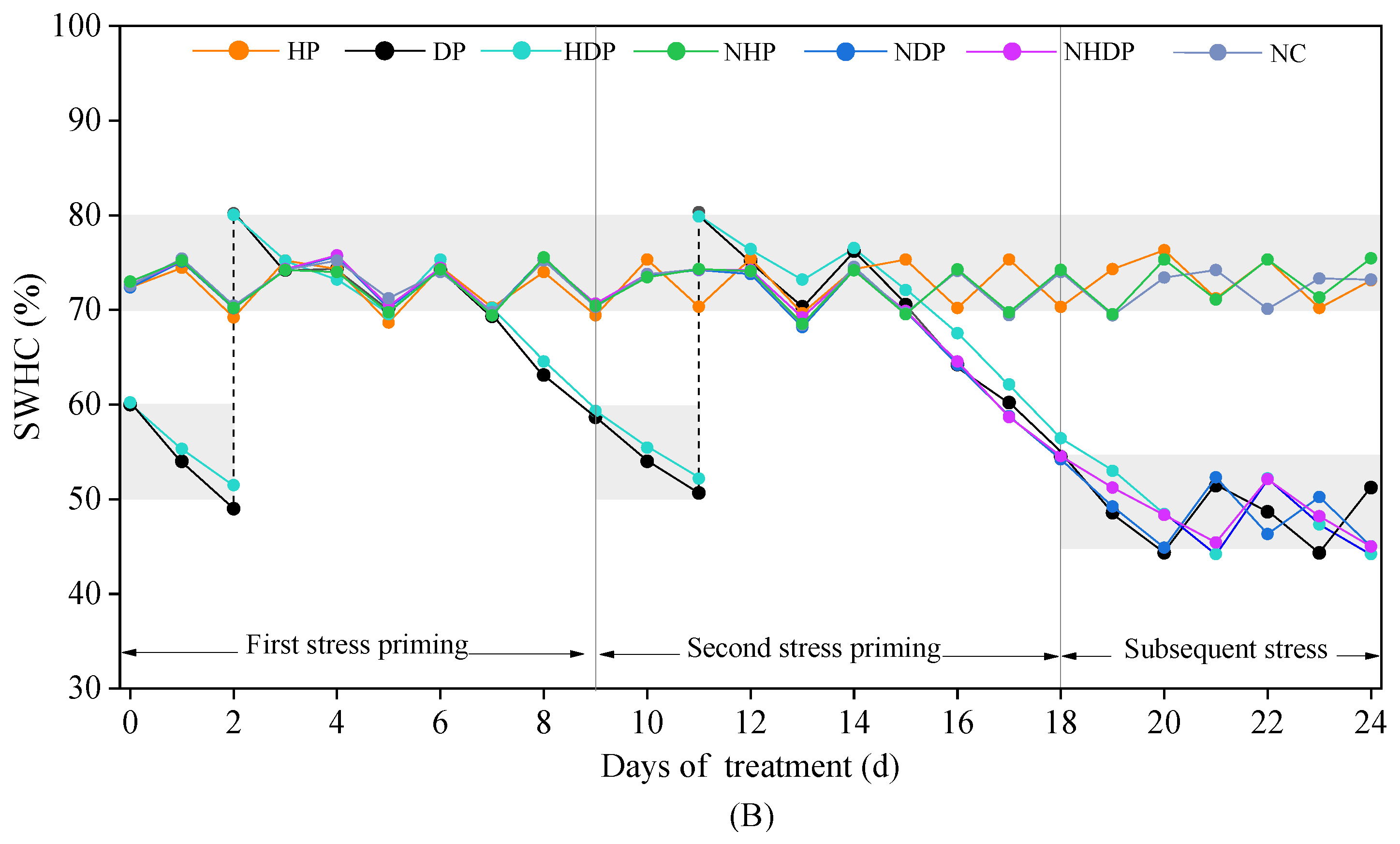
2.1. Plant Culture and Treatments
2.2. Sampling and Measurement
2.2.1. Sampling Method
2.2.2. Growth Traits
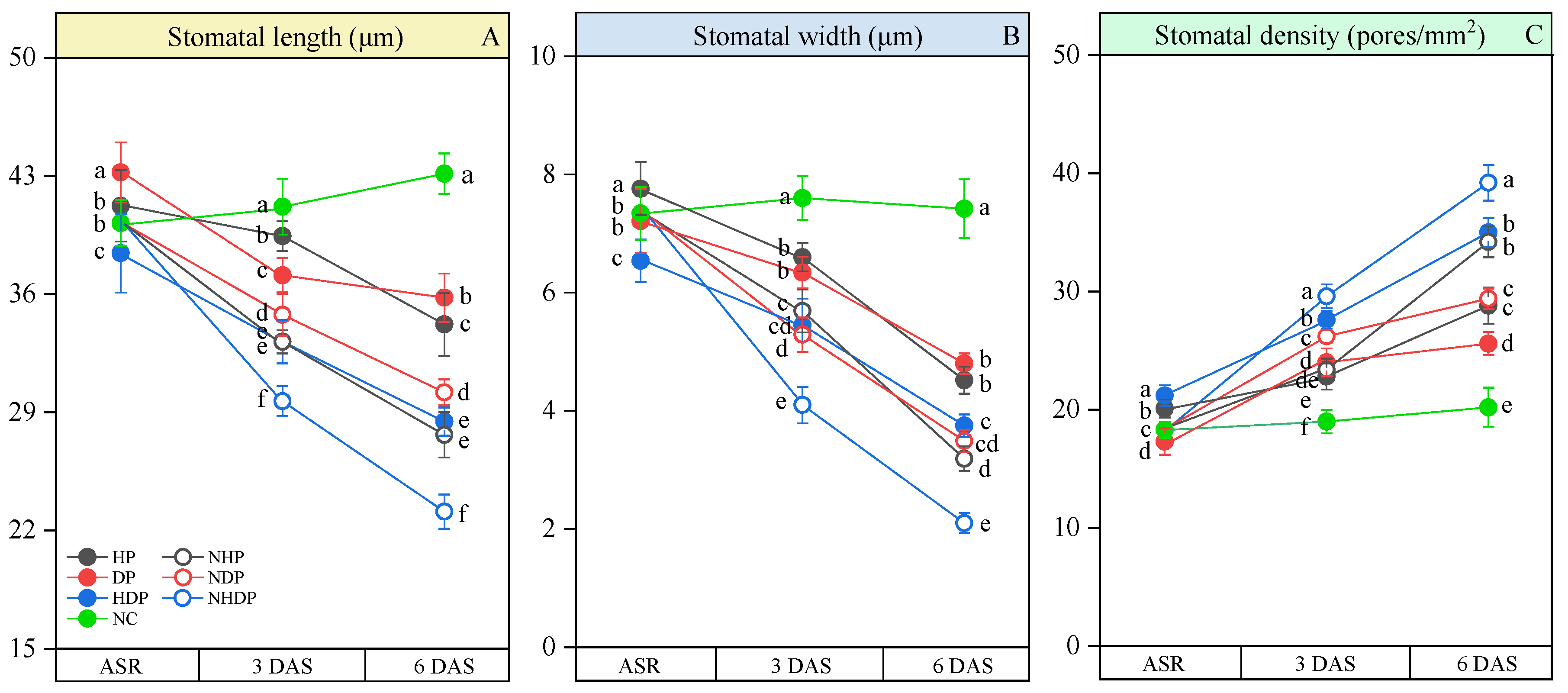
2.2.3. Imprinted Sampling and Microscopic Observation
2.2.4. Leaf Pigment Content and Gas Exchange
2.2.5. The Membrane Injury Index and MDA and O2− Contents
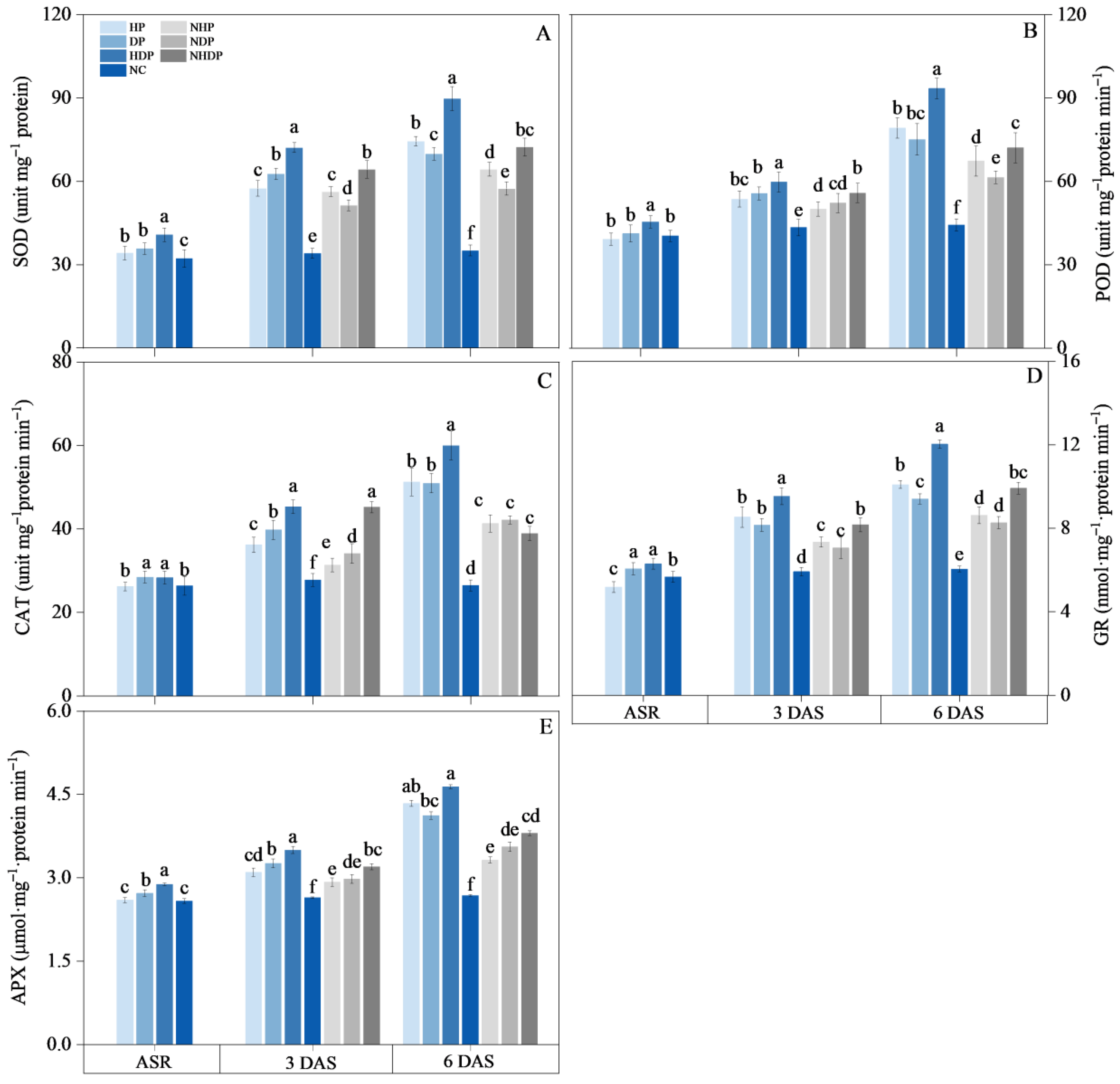
2.2.6. Antioxidant Enzyme Activity
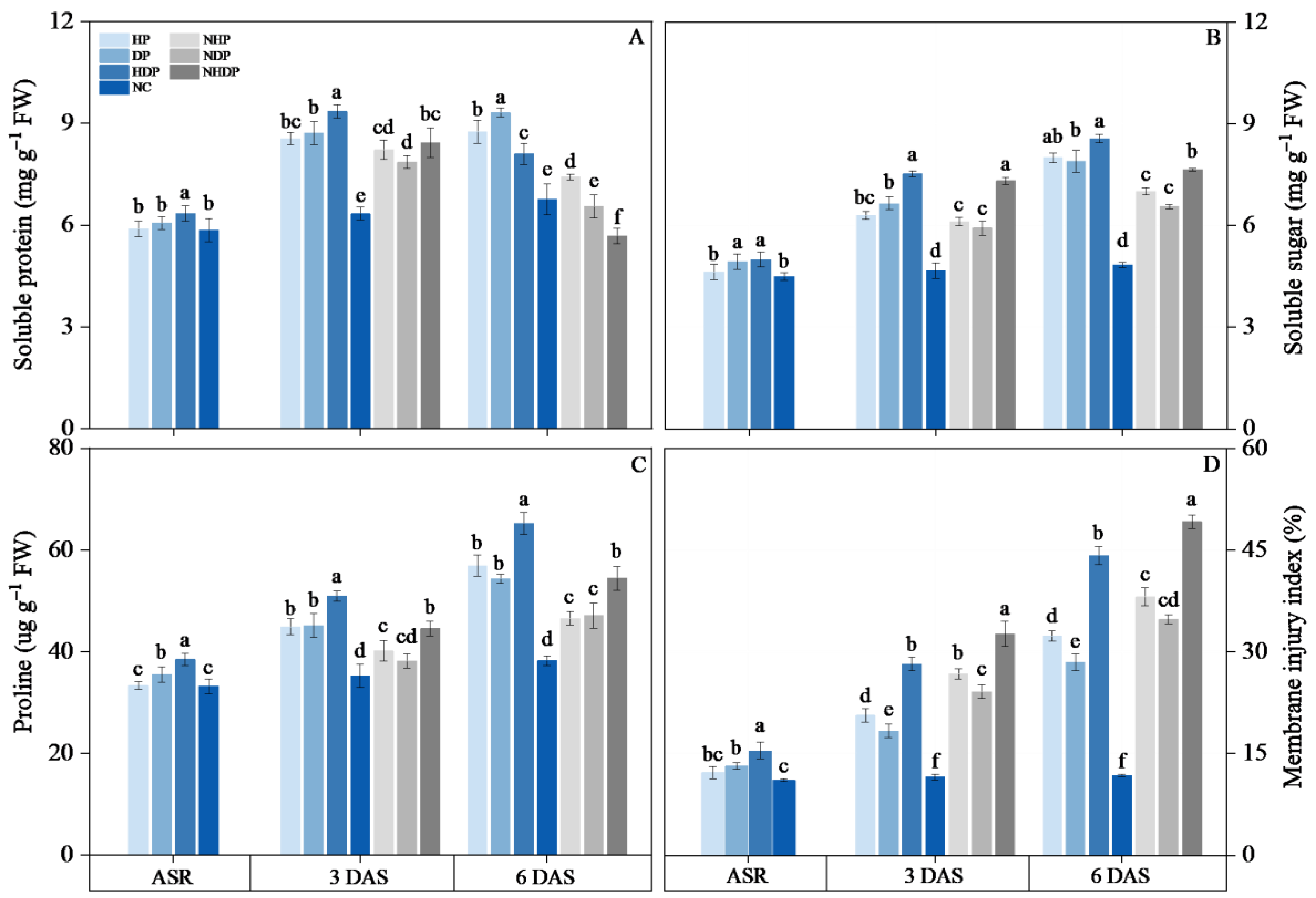
2.2.7. Total Protein and Soluble Protein Content
2.2.8. Soluble Sugar and Proline Contents
2.3. Data Analysis
3. Results
3.1. Injury Index and Aboveground Growth
3.2. Leaf Pigment Contents
3.3. Stomatal Morphology and Gas Exchange
3.4. Superoxide Anion and Malondialdehyde Contents
3.5. Antioxidant Enzyme Activity
3.6. The Contents of Osmoregulatory Substance and Membrane Injury Index
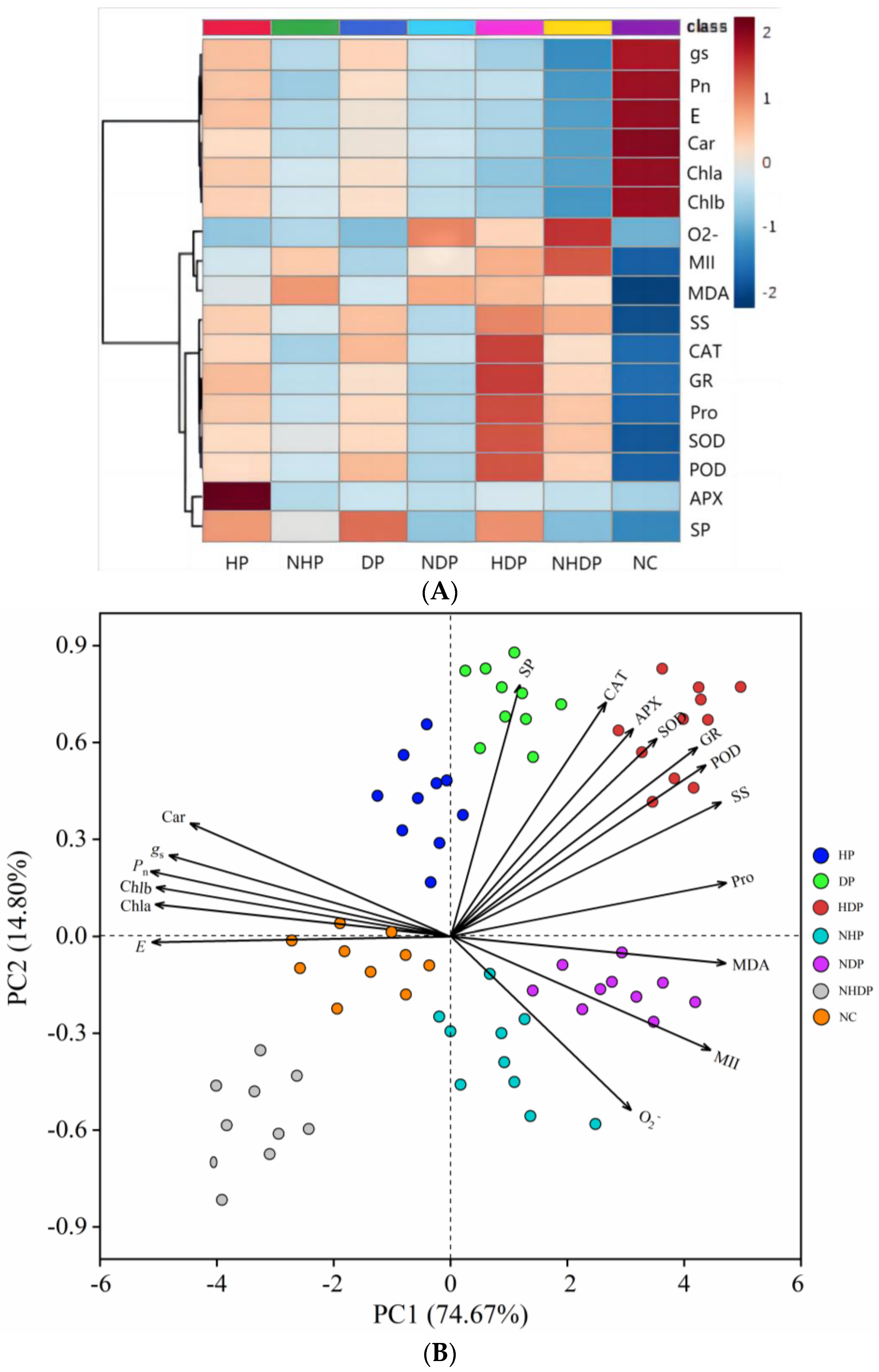
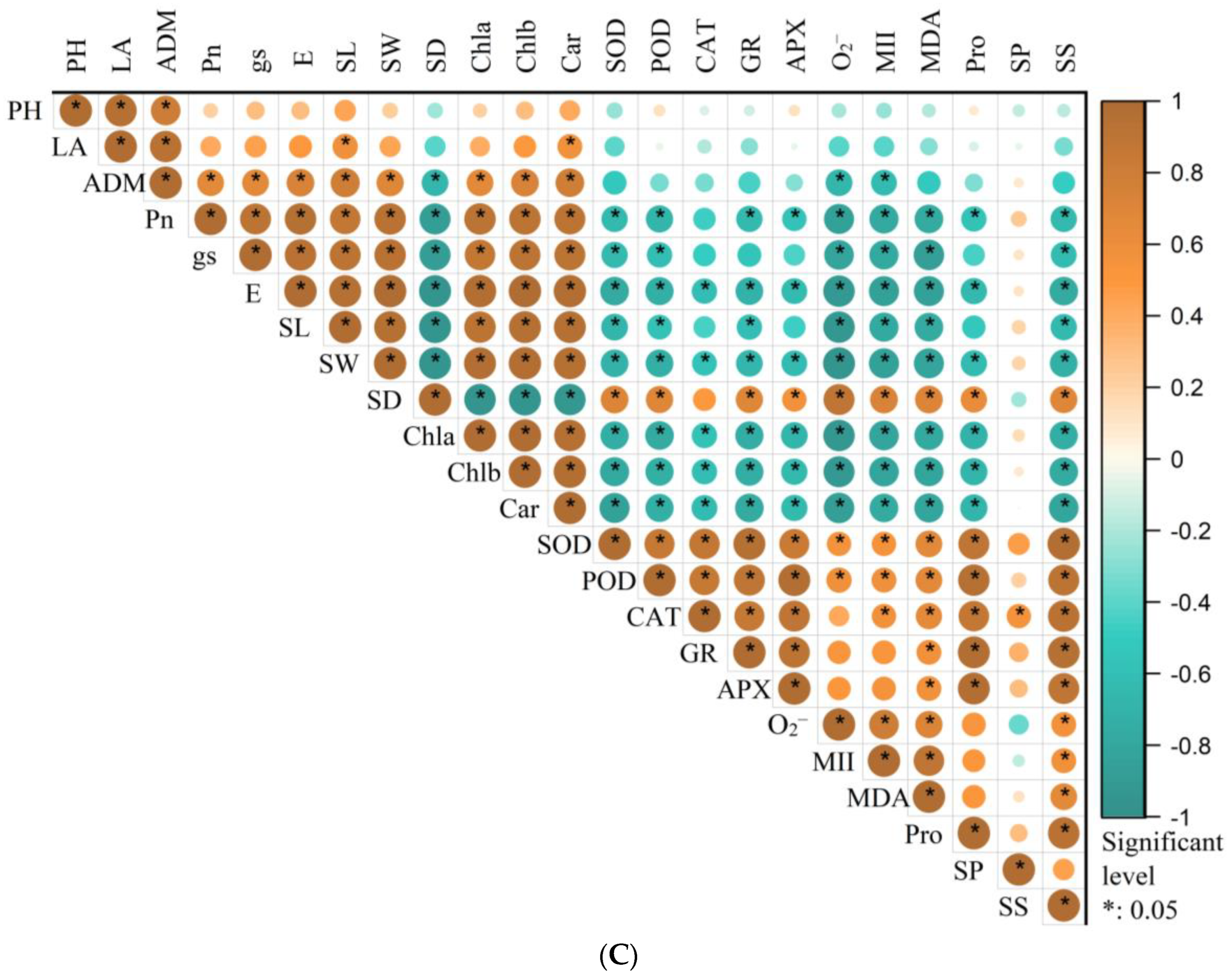
3.7. Responses of Physiological Traits to Stress Priming
3.8. ROC Analysis of Physiological Traits
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lamaoui, M.; Jemo, M.; Datla, R.; Bekkaoui, F. Heat and drought stresses in crops and approaches for their mitigation. Front. Chem. 2018, 6, 26. [Google Scholar] [CrossRef] [PubMed]
- Trnka, M.; Olesen, J.E.; Kersebaum, K.C.; Skjelvag, A.O.; Eitzinger, J.; Seguin, B.; Peltonen-Sainio, P.; Rötter, R.; Iglesias, A.; Orlandini, S.; et al. Agroclimatic conditions in Europe under climate change. Glob. Chang. Biol. 2011, 17, 2298–2318. [Google Scholar] [CrossRef]
- Cairns, J.E.; Hellin, J.; Sonder, K.; Araus, J.L.; MacRobert, J.F.; Thierfelder, C.; Prasanna, B.M. Adapting maize production to climate change in sub-Saharan Africa. Food Secur. 2013, 5, 345–360. [Google Scholar] [CrossRef]
- Zhao, F.Y.; Zhang, D.Y.; Zhao, Y.L.; Wang, W.; Yang, H.; Tai, F.J.; Li, C.H.; Hu, X.L. The difference of physiological and proteomic changes in maize leaves adaptation to drought, heat, and combined both stresses. Front. Plant Sci. 2016, 7, 1471. [Google Scholar] [CrossRef] [PubMed]
- Szymańska, R.; Slesak, I.; Orzechowska, A.; Kruk, J. Physiological and biochemical responses to high light and temperature stress in plants. Environ. Exp. Bot. 2017, 139, 165–177. [Google Scholar] [CrossRef]
- Ahluwalia, O.; Singh, P.C.; Bhatia, R. A review on drought stress in plants: Implications, mitigation and the role of plant growth promoting rhizobacteria. Resour. Environ. Sustain. 2021, 5, 100032. [Google Scholar] [CrossRef]
- Crafts-Brandner, S.J. Sensitivity of photosynthesis in a C4 plant, maize, to heat stress. Plant Physiol. 2002, 129, 1773–1780. [Google Scholar] [CrossRef]
- Crafts-Brandner, S.J.; Salvucci, M.E. Rubisco activase constrains the photosynthetic potential of leaves at high temperature and CO2. Proc. Natl. Acad. Sci. USA 2000, 97, 13430–13435. [Google Scholar] [CrossRef]
- Killi, D.; Bussotti, F.; Raschi, A.; Haworth, M. Adaptation to high temperature mitigates the impact of water deficit during combined heat and drought stress in C3 sunflower and C4 maize varieties with contrasting drought tolerance. Physiol. Plant. 2017, 159, 130–147. [Google Scholar] [CrossRef]
- Caemmerer, S.V.; Farquhar, G.D. Some relationships between the biochemistry of photosynthesis and the gas exchange of leaves. Planta 1981, 153, 376–387. [Google Scholar] [CrossRef]
- Lawlor, D.W.; Tezara, W. Causes of decreased photosynthetic rate and metabolic capacity in water-deficient leaf cells: A critical evaluation of mechanisms and integration of processes. Ann. Bot. 2009, 103, 561–579. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, M.B.; Methenni, K.; Nouairi, I.; Zarrouk, M.; Youssef, N.B. Drought priming improves subsequent more severe drought in a drought-sensitive cultivar of olive cv. Chétoui. Sci. Hortic. 2017, 221, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Dumanović, J.; Nepovimova, E.; Natić, M.; Kuča, K.; Jaćević, V. The significance of reactive oxygen species and antioxidant defense system in plants: A Concise Overview. Front. Plant Sci. 2021, 11, 552969. [Google Scholar] [CrossRef] [PubMed]
- Sattar, A.; Sher, A.; Ijaz, M.; Ul-Allah, S.; Rizwan, M.S.; Hussain, M.; Jabran, K.; Cheema, M.A. Terminal drought and heat stress alter physiological and biochemical attributes in flag leaf of bread wheat. PLoS ONE 2020, 15, e0232974. [Google Scholar] [CrossRef] [PubMed]
- Anosheh, H.P.; Sadeghi, H.; Emam, Y. Chemical priming with urea and KNO3 enhances maize hybrids (Zea mays L.) seed viability under abiotic stress. J. Crop. Sci. Biotechnol. 2011, 14, 289–295. [Google Scholar] [CrossRef]
- Farman, M.; Nawaz, F.; Majeed, S.; Javeed, H.M.R.; Ahsan, M.; Ahmad, K.S.; Aurangzaib, M.; Bukhari, M.A.; Shehzad, M.A.; Hussain, M.B. Silicon seed priming combined with foliar spray of sulfur regulates photosynthetic and antioxidant systems to confer drought tolerance in maize (Zea mays L.). Silicon 2021, 14, 7901–7917. [Google Scholar] [CrossRef]
- Singhal, R.K.; Pandey, S.; Bose, B. Seed priming with Mg(NO3)2 and ZnSO4 salts triggers physio-biochemical and antioxidant defense to induce water stress adaptation in wheat (Triticum aestivum L.). Plant Stress 2021, 2, 100037. [Google Scholar] [CrossRef]
- Wang, X.; Liu, F.L.; Jiang, D. Priming: A promising strategy for crop production in response to future climate. J. Integr. Agr. 2017, 16, 2709–2716. [Google Scholar] [CrossRef]
- Fan, Y.H.; Ma, C.X.; Huang, Z.L.; Abid, M.; Jiang, S.Y.; Dai, T.B.; Zhang, W.J.; Ma, S.Y.; Jiang, D.G.; Han, X. Heat priming during early reproductive stages enhances thermo- tolerance to post-anthesis heat stress via improving photosynthesis and plant productivity in winter wheat (Triticum aestivum L.). Front. Plant Sci. 2018, 9, 805. [Google Scholar] [CrossRef]
- Zhou, R.; Yu, X.Q.; Li, X.N.; Dos Santos, T.M.; Rosenqvist, E.; Ottosen, C.O. Combined high light and heat stress induced complex response in tomato with better leaf cooling after heat priming—ScienceDirect. Plant Physiol. Biochem. 2020, 151, 1–9. [Google Scholar] [CrossRef]
- Wang, X.; Vignjevic, M.; Liu, F.L.; Jacobsen, S.; Jiang, D.; Wollenweber, B. Drought priming at vegetative growth stages improves tolerance to drought and heat stresses occurring during grain filling in spring wheat. Plant Growth Regul. 2015, 75, 677–687. [Google Scholar] [CrossRef]
- Abid, M.; Tian, Z.; Ata-Ul-Karim, S.T.; Liu, Y.; Cui, Y.; Zahoor, R.; Jiang, D.; Dai, T. Improved tolerance to post-anthesis drought stress by pre-drought priming at vegetative stages in drought-tolerant and-sensitive wheat cultivars. Plant Physiol. Biochem. 2016, 106, 218–227. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Li, J.L.; Zhang, X.Q.; Wei, H.; Cui, L.J. Effects of heat acclimation pretreatment on changes of membrane lipid peroxidation, antioxidant metabolites, and ul-trastructure of chloroplasts in two cool-season turfgrass species under heat stress. Environ. Exp. Bot. 2006, 56, 274–285. [Google Scholar] [CrossRef]
- Wang, X.; Cai, J.; Jiang, D.; Liu, F.L.; Dai, T.B.; Cao, W.X. Pre-anthesis high-temperature acclimation alleviates damage to the flag leaf caused by post-anthesis heat stress in wheat. J. Plant Physiol. 2011, 168, 585–593. [Google Scholar] [CrossRef]
- He, B.; Guo, T.; Huang, H.; Xi, W.; Chen, X. Physiological responses of scaevola aemula seedlings under high temperature stress. S. Afr. J. Bot. 2017, 112, 203–209. [Google Scholar] [CrossRef]
- Karimi, M.; Siddique, K. Crop growth and relative growth rates of old and modern wheat cultivars. Aust. J. Agric. Res. 1991, 42, 13–20. [Google Scholar] [CrossRef]
- Gao, C.; Lu, S.; Wang, Y.Z.; Xu, H.; Gao, X.X.; Gu, Y.W.; Xuan, H.Y.; Wang, B.H.; Yuan, H.H.; Cao, Y.Y. Bismuth vanadium oxide can promote growth and activity in arabidopsis thaliana. Front. Chem. 2021, 9, 766078. [Google Scholar] [CrossRef]
- Lichtenthaler, H.; Wellburn, A. Determinations of total carotenoids and chlorophylls b of leaf extracts in different solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef]
- Deshmukh, P.; Sairam, R.; Shukla, D. Measurement of ion leakage as a screening technique for drought resistance in wheat genotypes. Indian J. Plant Physiol. 1991, 34, 89–91. [Google Scholar]
- Du, Z.; Bramlage, W.J. Modifified thiobarbituric acid assay for measuring lipid oxidation in sugar-rich plant tissue extracts. J. Agric. Food Chem. 1992, 40, 1566–1570. [Google Scholar] [CrossRef]
- Wu, G.L.; Cui, J.; Tao, L.; Yang, H. Fluroxypyr triggers oxidative damage by producing superoxide and hydrogen peroxide in rice (Oryza sativa). Ecotoxicology 2009, 19, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Beauchamp, C.; Fridovich, I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef] [PubMed]
- Kochba, J.; Lavee, S.; Spiegel-Roy, P. Differences in peroxidase activity and isoenzymes in embryogenic and non-embryogenic ‘Shamouti’ orange ovular callus lines. Plant Cell Physiol. 1977, 18, 463–467. [Google Scholar] [CrossRef]
- Dhindsa, R.S.; Plumb-dhindsa, P.; Thorp, T.A. Leaf senescence: Correlated with increased levels of membrane permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. J. Exp. Bot. 1981, 32, 93–101. [Google Scholar] [CrossRef]
- Schaedle, M.; Bassham, J.A. Chloroplast glutathione reductase. Plant Physiol. 1977, 59, 1011–1012. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar]
- Lowry, H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Li, L.; He, G.Z. Experimental Guide for Plant Physiology; Higher Education Press: Beijing, China, 2021. [Google Scholar]
- Bates, L.S.; Waldeen, R.P.; Teare, I.D. Rapid determination of free proline for water stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Mu, Q.; Cai, H.J.; Sun, S.K.; Wen, S.S.; Xu, J.T.; Dong, M.Q.; Saddique, Q. The physiological response of winter wheat under short-term drought conditions and the sensitivity of different indices to soil water changes. Agric. Water Manag. 2021, 243, 106475. [Google Scholar] [CrossRef]
- Worley, B.; Powers, R. Multivariate analysis in metabolomics. Curr. Metab. 2013, 1, 92–107. [Google Scholar]
- Hussain, H.A.; Men, S.N.; Hussain, S.; Chen, Y.L.; Ali, S.; Zhang, S.; Zhang, K.P.; Li, Y.; Xu, Q.W.; Liao, C.Q. Interactive effects of drought and heat stresses on morpho-physiological attributes, yield, nutrient uptake and oxidative status in maize hybrids. Sci. Rep. 2019, 9, 3890. [Google Scholar] [CrossRef] [PubMed]
- Saeidi, M.; Zabihe-Mahmoodabad, R. Evaluation of drought stress on relative water content and chlorophyll content of sesame (Sesamum indicum L.) genotypes at early flowering stage. Res. J. Environ. Sci. 2009, 3, 345–350. [Google Scholar]
- Cornic, G. Drought stress inhibits photosynthesis by decreasing stomatal aperture–not by affecting ATP synthesis. Trends Plant Sci. 2000, 5, 187–188. [Google Scholar] [CrossRef]
- Kinoshita, T.; Toh, S.; Torii, K.U. Chemical control of stomatal function and development. Curr. Opin. Plant Biol. 2021, 60, 102010. [Google Scholar] [CrossRef]
- Loreto, F.; Tricoli, D.; Marco, G. On the relationship between electron transport rate and photosynthesis in leaves of the C4 plantSorghum bicolorexposed to water stress, temperature changes and carbon metabolism inhibition. Funct. Plant Biol. 1995, 22, 885–892. [Google Scholar] [CrossRef]
- Orzechowska, A.; Trtílek, M.; Tokarz, K.M.; Szymańska, R.; Niewiadomska, E.; Rozpądek, P.; Wątor, K. Thermal analysis of stomatal response under salinity and high light. Int. J. Mol. Sci. 2021, 22, 4663. [Google Scholar] [CrossRef] [PubMed]
- Deva, C.R.; Urban, M.O.; Challinor, A.J.; Falloon, P.; Svitákova, L. Enhanced leaf cooling is a pathway to heat tolerance in common bean. Front Plant Sci. 2020, 11, 19. [Google Scholar] [CrossRef]
- Wang, X.; Cai, J.; Liu, F.L.; Dai, T.B.; Cao, W.X.; Wollenweber, B.; Jiang, D. Multiple heat priming enhances thermo-tolerance to a later high temperature stress via improving subcellular antioxidant activities in wheat seedlings. Plant Physiol. Biochem. 2014, 74, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Engels, C.; Marschne, H. Plant uptake and utilization of nitrogen. In Nitrogen Fertilization and the Environment; Bacon, P.E., Ed.; Marcel Dekker: New York, NY, USA, 1995; pp. 41–83. [Google Scholar]
- Ru, C.; Wang, K.F.; Hu, X.T.; Chen, D.Y.; Wang, W.E.; Yang, H.S. Nitrogen modulates the efects of heat, drought, and combined stresses on photosynthesis, antioxidant capacity, cell osmoregulation, and grain yield in winter wheat. J. Plant Growth Regul. 2023, 42, 1681–1703. [Google Scholar] [CrossRef]
- Mansoor, S.; Ali Wani, O.; Lone, J.K.; Manhas, S.; Kour, N.; Alam, P.; Ahmad, A.; Ahmad, P. Reactive oxygen species in plants: From source to sink. Antioxidants 2022, 11, 225. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Gupta, D.; Nayyar, H. Comparative response of maize and rice genotypes to heat stress: Status of oxidative stress and antioxidants. Acta Physiol. Plant. 2012, 34, 75–86. [Google Scholar] [CrossRef]
- Wang, X.; Wollenweber, B.; Liu, F.L.; Jiang, D. Heat priming induces intra- and trans-generational thermo-tolerance in crop plants. In Priming-Mediated Stress and Cross-Stress Tolerance in Crop Plants; Academic Press: Cambridge, MA, USA, 2020; pp. 175–181. [Google Scholar]
- Zhang, X.X.; Huang, B.R. Drought priming-induced heat tolerance: Metabolic pathways and molecular mechanisms. In Priming-Mediated Stress and Cross-Stress Tolerance in Crop Plants; Academic Press: Cambridge, MA, USA, 2020; pp. 149–160. [Google Scholar]
- Chakraborty, P.; Dwivedi, P. Seed priming and its role in mitigating heat stress responses in crop plants. J. Soil Sci. Plant Nut. 2021, 21, 1718–1734. [Google Scholar] [CrossRef]
- Chen, T.H.H.; Murata, N. Enhancement of tolerance of abiotic stress by metabolic engineering of betaines and other compatible solutes. Curr. Opin. Plant Biol. 2002, 5, 250–257. [Google Scholar] [CrossRef]
- Mu, Q.; Dong, M.Q.; Xu, J.T.; Cao, Y.X.; Ding, Y.B.; Sun, S.K.; Cai, H.J. Photosynthesis of winter wheat effectively reflected multiple physiological responses under short-term drought–rewatering conditions. J. Sci. Food Agric. 2022, 102, 2472–2483. [Google Scholar] [CrossRef]
- Li, D.X.; Li, X.D.; Sun, H.C.; Wang, W.X.; Liu, L.T.; Zhang, Y.J. Effects of drought on soluble protein content and protective enzyme system in cotton leaves. Front. Agric. China 2010, 4, 56–62. [Google Scholar] [CrossRef]
- Koch, K. Carbohydrate-modulated gene expression in plants. Annu. Rev. Plant Biol. 1996, 47, 509–540. [Google Scholar] [CrossRef]
- Lehr, P.P.; Hernández-Montes, E.; Ludwig-Müller, J.; Keller, M.; Zörb, C. Abscisic acid and proline are not equivalent markers for heat, drought and combined stress in grapevines. Aust. J. Grape Wine Res. 2022, 28, 119–130. [Google Scholar] [CrossRef]
- Rizhsky, L.; Liang, H.J.; Shuman, J.L.; Shulaev, V.; Davletova, S.; Mittler, R. When defense pathways collide: The response of Arabidopsis to a combination of drought and heat stress. Plant Physiol. 2004, 134, 1683–1696. [Google Scholar] [CrossRef]




| Periods | Treatments | Injury Index | Plant Height | Leaf Area | Aboveground Dry Mass | Relative Growth Rate |
|---|---|---|---|---|---|---|
| (%) | (cm) | (cm2/Plant) | (g/Plant) | (mg g−1 day−1) | ||
| ASR | HP | 0.0 ± 0.00 a | 64.53 ± 2.16 a | 394.18 ± 9.12 a | 1.22 ± 0.06a | - |
| DP | 0.0 ± 0.00 a | 63.01 ± 1.34 b | 378.67 ± 8.32 b | 1.17 ± 0.11 b | - | |
| HDP | 0.0 ± 0.00 a | 61.15 ± 1.55 c | 374.21 ± 15.22 b | 1.13 ± 0.08 c | - | |
| NC | 0.0 ± 0.00 a | 63.21 ± 0.56 ab | 401.11 ± 12.42 a | 1.25 ± 0.03 a | - | |
| 6 DAS | HP | 26.5 ± 0.50 d | 74.63 ± 2.13 b | 618.19 ± 6.80 b | 1.96 ± 0.13 b | 123.33 ± 4.32 b |
| DP | 25.4 ± 0.34 d | 72.54 ± 1.22 c | 590.76 ± 14.20 c | 1.88 ± 0.08 c | 118.33 ± 3.78 b | |
| HDP | 32.2 ± 1.15 c | 68.89 ± 3.21 f | 553.21 ± 16.21 d | 1.65 ± 0.15 e | 86.67 ± 1.88 c | |
| NC | 0.0 ± 0.00 e | 78.12 ± 1.28 a | 701.15 ± 5.40 a | 2.35 ± 0.11 a | 183.33 ± 4.21 a | |
| NHP | 31.0 ± 1.00 c | 71.56 ± 1.77 d | 576.17 ± 21.30 c | 1.80 ± 0.09 c | 91.67 ± 1.43 c | |
| NDP | 34.8 ± 1.32 b | 69.77 ± 0.99 e | 555.32 ± 16.30 d | 1.71 ± 0.08 d | 76.67 ± 3.21 d | |
| NHDP | 43.3 ± 2.48 a | 68.67 ± 1.23 f | 512.45 ± 7.80 e | 1.53 ± 0.04 f | 46.67 ± 1.98 e |
| Traits | Heat Stress | Drought Stress | Combined Stress | ||||||
|---|---|---|---|---|---|---|---|---|---|
| AUC | YI | BT | AUC | YI | BT | AUC | YI | BT | |
| SOD | 0.941 ** | 0.829 | 54.88 | 0.751 * | 0.576 | 50.35 | 0.907 ** | 0.845 | 65.17 |
| POD | 0.971 ** | 0.874 | 49.38 | 0.889 ** | 0.74 | 52.50 | 0.830 * | 0.723 | 57.35 |
| CAT | 0.891 ** | 0.825 | 32.32 | 0.827 * | 0.694 | 35.41 | 0.749 * | 0.701 | 39.70 |
| GR | 0.824 * | 0.699 | 7.44 | 0.958 ** | 0.886 | 7.20 | 0.934 ** | 0.815 | 8.31 |
| APX | 0.772 * | 0.633 | 2.91 | 0.69 ns | 0.576 | 3.02 | 0.759 * | 0.631 | 3.25 |
| SP | 0.683 ns | 0.423 | 7.57 | 0.705 ns | 0.667 | 7.71 | 0.702 ns | 0.583 | 8.11 |
| SS | 0.829 * | 0.722 | 5.91 | 0.919 ** | 0.774 | 6.08 | 0.866 ** | 0.729 | 7.28 |
| Pro | 0.838 * | 0.732 | 41.21 | 0.802 * | 0.684 | 41.71 | 0.958 ** | 0.833 | 45.56 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ru, C.; Hu, X.; Chen, D.; Wang, W. Droughts and Thermo-Priming Enhance Acclimation to Later Drought and Heat Stress in Maize Seedlings by Improving Leaf Physiological Activity. Agronomy 2023, 13, 1124. https://doi.org/10.3390/agronomy13041124
Ru C, Hu X, Chen D, Wang W. Droughts and Thermo-Priming Enhance Acclimation to Later Drought and Heat Stress in Maize Seedlings by Improving Leaf Physiological Activity. Agronomy. 2023; 13(4):1124. https://doi.org/10.3390/agronomy13041124
Chicago/Turabian StyleRu, Chen, Xiaotao Hu, Dianyu Chen, and Wene Wang. 2023. "Droughts and Thermo-Priming Enhance Acclimation to Later Drought and Heat Stress in Maize Seedlings by Improving Leaf Physiological Activity" Agronomy 13, no. 4: 1124. https://doi.org/10.3390/agronomy13041124
APA StyleRu, C., Hu, X., Chen, D., & Wang, W. (2023). Droughts and Thermo-Priming Enhance Acclimation to Later Drought and Heat Stress in Maize Seedlings by Improving Leaf Physiological Activity. Agronomy, 13(4), 1124. https://doi.org/10.3390/agronomy13041124




