Compost Improving Morphophysiological and Biochemical Traits, Seed Yield, and Oil Quality of Nigella sativa under Drought Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Climate Conditions during the Experiments
2.2. Experimental Site and Soil Characteristics
2.3. Plant Material and Growth Conditions
2.4. Experimental Design and Treatment Distribution
2.4.1. Irrigation Treatments and Irrigation Water Applied
2.4.2. Compost Treatments
2.5. Methodologies and Observations Recorded
2.5.1. Morphological Characteristics
2.5.2. Yield and Its Attributes
2.5.3. Determinations of Relative Water Content (RWC%) and Membrane Stability Index (MSI%)
2.5.4. Estimation of Chlorophyll Content and Total Carbohydrates
2.5.5. Determination of Total Soluble Sugar Concentrations, Total Free Amino Acids, and Free Proline
2.5.6. Determinations of Leaf Nutrients Content (N, P, and K+)
2.5.7. Seed Fixed Oil and Essential Oil Content
2.5.8. Fatty Acid Methyl Ester (FAMEs) Preparation and Fatty-Acid Profile by GC
2.6. Statistical Analysis
3. Results
3.1. Soil Physicochemical Properties
3.2. Growth Attributes
3.3. Relative Water Content (RWC) and Membrane Stability Index (MSI)
3.4. Photosynthetic Pigments
3.5. Yield Components, Seed Yield, and Oil Yield
3.6. NPK Content
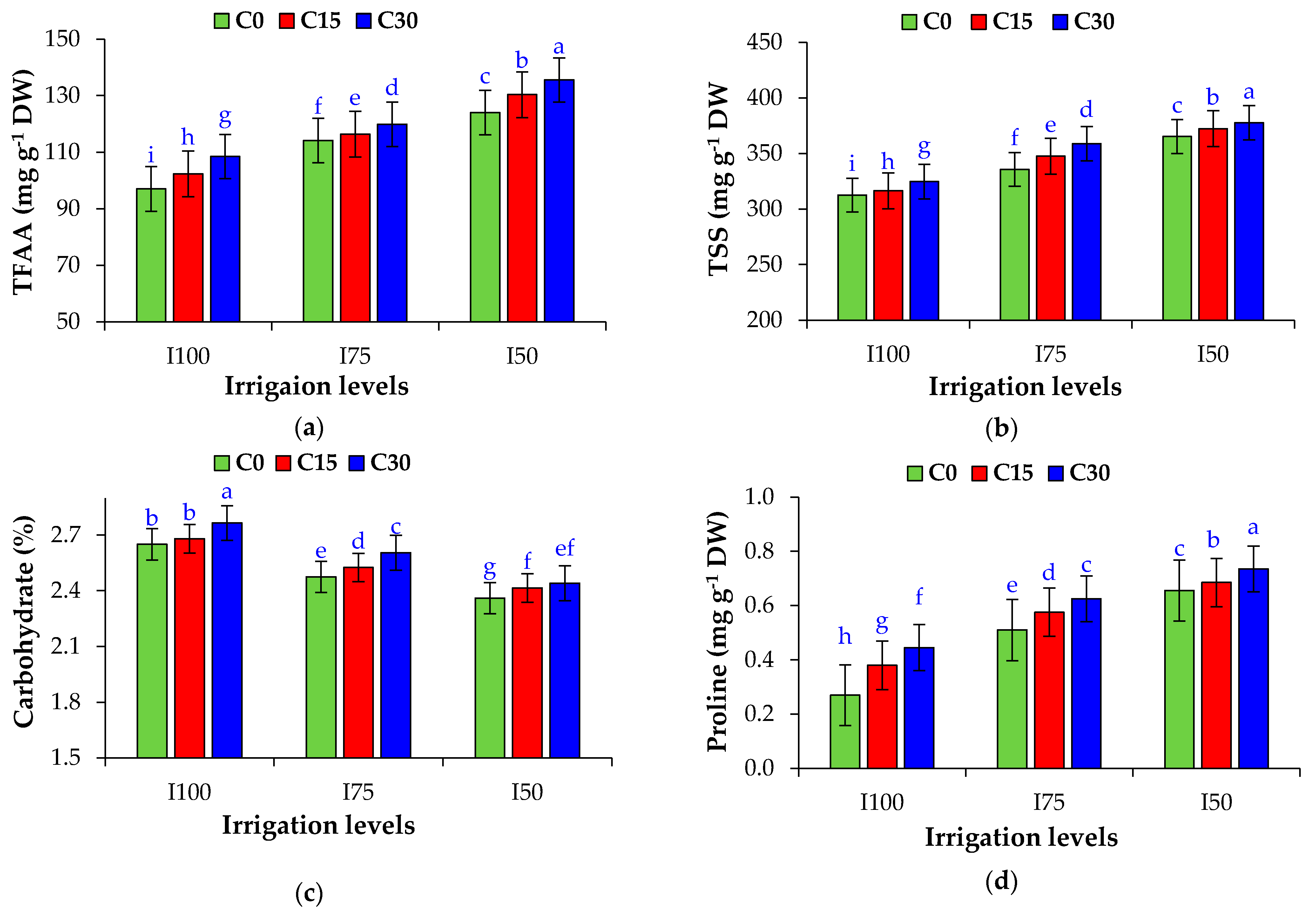
3.7. Total Free Amino Acids (TFAA), Total Soluble Sugar (TSS), Carbohydrate Percentage, and Proline
3.8. Fixed Oil and Essential Oil Percentages
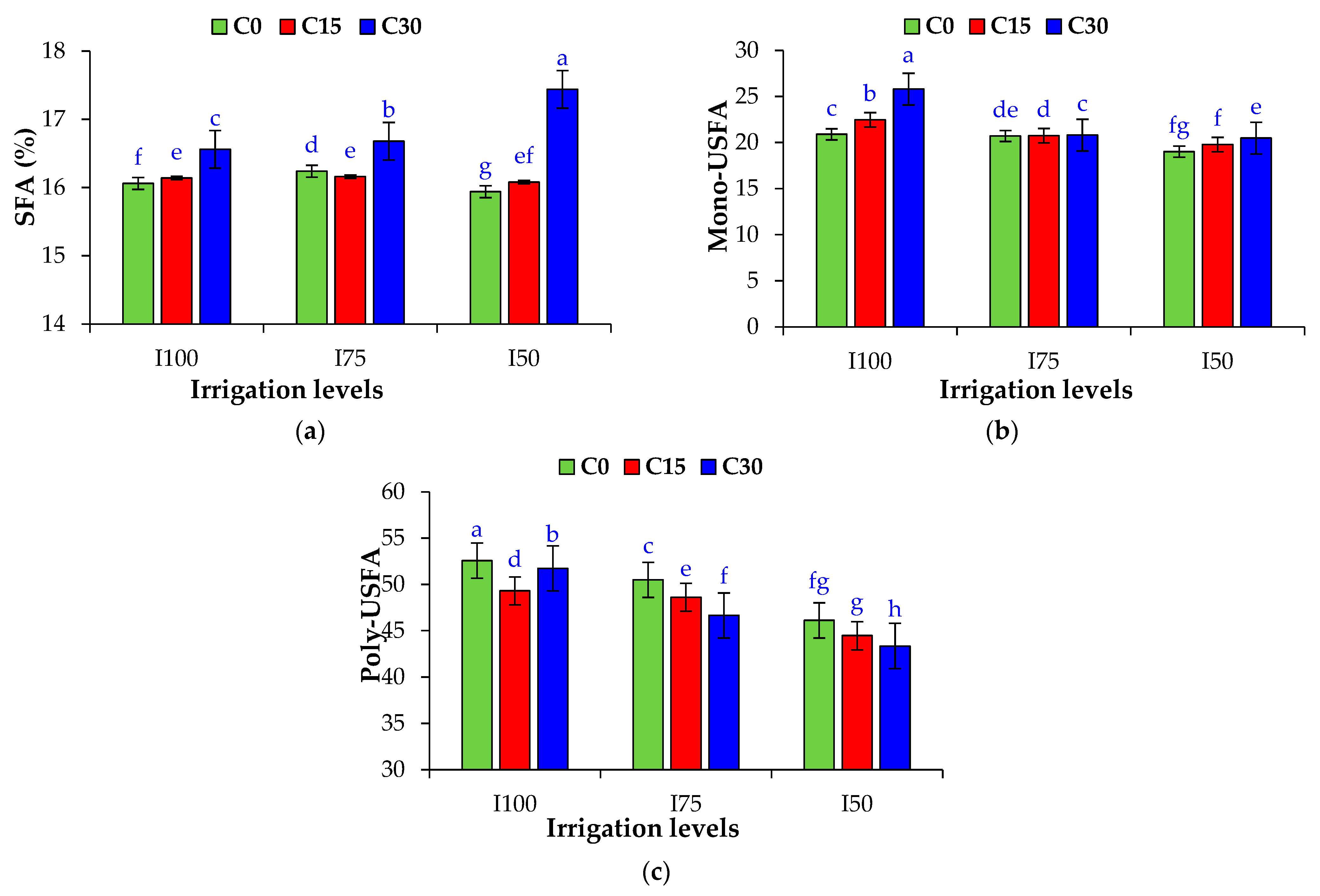
3.9. Fatty-Acid Composition of Fixed Oil
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Taghouti, I.; Cristobal, R.; Brenko, A.; Stara, K.; Markos, N.; Chapelet, B.; Hamrouni, L.; Burši’c, D.; Bonet, J.A. The market evolution of medicinal and aromatic plants: A global supply chain analysis and an application of the delphi method in the Mediterranean area. Forests 2022, 13, 808. [Google Scholar] [CrossRef]
- Sharma, P.; Longvah, T. Nigella (Nigella sativa) seed. In Oilseeds: Health Attributes and Food Applications; Tanwar, B., Goyal, A., Eds.; Springer: Singapore, 2021; pp. 331–350. [Google Scholar] [CrossRef]
- POWO. Plants of the World Online: Nigella sativa L. Facilitated by the Royal Botanic Gardens, Kew. Available online: https://powo.science.kew.org/taxon/urn:lsid:ipni.org:names:711687-1 (accessed on 10 March 2023).
- Amin, B.; Hosseinzadeh, H. Black cumin (Nigella sativa) and its active constituent, thymoquinone: An overview on the analgesic and anti-inflammatory effects. Planta Med. 2016, 82, 8–16. [Google Scholar] [CrossRef]
- Karimi, Z.; Alizadeh, A.M.; Dolatabadi, J.E.N.; Dehghan, P. Nigella sativa and its derivatives as food toxicity protectant agents. Adv. Pharm. Bull. 2019, 9, 22–37. [Google Scholar] [CrossRef]
- Seleiman, M.F.; Al-Suhaibani, N.; Ali, N.; Akmal, M.; Alotaibi, M.; Refay, Y.; Dindaroglu, T.; Abdul-Wajid, H.H.; Battaglia, M.L. Drought stress impact on plants and different approaches to alleviate its adverse effects. Plants 2021, 10, 259. [Google Scholar] [CrossRef]
- Abd El-Gawad, H.G.; Mukherjee, S.; Farag, R.; Abd Elbar, O.H.; Hikal, M.; Abou El-Yazied, A.; Abd Elhady, S.A.; Helal, N.; ElKelish, A.; El Nahhas, N.; et al. Exogenous γ-Aminobutyric Acid (GABA)-Induced Signaling Events and Field Performance Associated with Mitigation of Drought Stress in Phaseolus vulgaris L. Plant Signal. Behav. 2021, 16, 1853384. [Google Scholar] [CrossRef]
- El Nahhas, N.; AlKahtani, M.D.F.; Abdelaal, K.A.A.; Al Husnain, L.; AlGwaiz, H.I.M.; Hafez, Y.M.; Attia, K.A.; El-Esawi, M.A.; Ibrahim, M.F.M.; Elkelish, A. Biochar and Jasmonic Acid Application Attenuates Antioxidative Systems and Improves Growth, Physiology, Nutrient Uptake and Productivity of Faba Bean (Vicia Faba L.) Irrigated with Saline Water. Plant Physiol. Biochem. 2021, 166, 807–817. [Google Scholar] [CrossRef] [PubMed]
- Arafa, S.A.; Attia, K.A.; Niedbała, G.; Piekutowska, M.; Alamery, S.; Abdelaal, K.; Alateeq, T.K.; Ali, M.A.M.; Elkelish, A.; Attallah, S.Y. Seed Priming Boost Adaptation in Pea Plants under Drought Stress. Plants 2021, 10, 2201. [Google Scholar] [CrossRef] [PubMed]
- Elkelish, A.; El-Mogy, M.M.; Niedbała, G.; Piekutowska, M.; Atia, M.A.M.; Hamada, M.M.A.; Shahin, M.; Mukherjee, S.; El-Yazied, A.A.; Shebl, M.; et al. Roles of Exogenous α-Lipoic Acid and Cysteine in Mitigation of Drought Stress and Restoration of Grain Quality in Wheat. Plants 2021, 10, 2318. [Google Scholar] [CrossRef] [PubMed]
- Barłóg, P.; Grzebisz, W.; Łukowiak, R. Fertilizers and fertilization strategies mitigating soil factors constraining efficiency of nitrogen in plant production. Plants 2022, 11, 1855. [Google Scholar] [CrossRef] [PubMed]
- Fallah, S.; Rostaei, M.; Lorigooini, Z.; Surki, A.A. Chemical compositions of essential oil and antioxidant activity of dragonhead (Dracocephalum moldavica) in sole crop and dragonhead- soybean (Glycine max) intercropping system under organic manure and chemical fertilizers. Ind. Crops Prod. 2018, 115, 158–165. [Google Scholar] [CrossRef]
- Lin, W.; Lin, M.; Zhou, H.; Wu, H.; Li, Z.; Lin, W. The effects of chemical and organic fertilizer usage on rhizosphere soil in tea orchards. PLoS ONE. 2019, 14, e0217018. [Google Scholar] [CrossRef] [PubMed]
- Abo-Baker, A.A.; Mostafa, G.G. Effect of Bio-and chemical fertilizers on growth, sepals yield and chemical composition of Hibiscus sabdariffa at new reclaimed soil of south valley area. Asian J. Crop Sci. 2011, 3, 16–25. [Google Scholar] [CrossRef]
- Licata, M.; Maggio, A.M.; La Bella, S.; Tuttolomondo, T. Medicinal and aromatic plants in agricultural research, when considering criteria of multifunctionality and sustainability. Agriculture 2022, 12, 529. [Google Scholar] [CrossRef]
- Yu, H.; Xie, B.; Khan, R.; Shen, G. The changes in carbon, nitrogen components and humic substances during organic-inorganic aerobic co-composting. Bioresour. Technol. 2019, 271, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Ho, T.T.K.; Tra, V.T.; Le, T.H.; Nguyen, N.K.Q.; Tran, C.S.; Nguyen, P.T.; Vo, T.D.H.; Thai, V.N.; Bui, X.T. Compost to improve sustainable soil cultivation and crop productivity. Case Stud. Chem. Environ. Eng. 2022, 6, 100211. [Google Scholar] [CrossRef]
- Kranz, C.N.; McLaughlin, R.A.; Johnson, A.; Miller, G.; Heitman, J.L. The effects of compost incorporation on soil physical properties in urban soils—A concise review. J. Environ. Manag. 2020, 261, 110209. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Wu, Y.; Liang, P.; Song, Q.; Zhang, H.; Wu, J.; Wu, J.; Wu, W.; Liu, X.; Dong, C. Alkaline amendments improve the health of soils degraded by metal contamination and acidification: Crop performance and soil bacterial community responses. Chemosphere 2020, 257, 127309. [Google Scholar] [CrossRef]
- Dai, P.; Cong, P.; Wang, P.; Dong, J.; Dong, Z.; Song, W. Alleviating soil acidification and increasing the organic carbon pool by long-term organic fertilizer on tobacco planting soil. Agronomy 2021, 11, 2135. [Google Scholar] [CrossRef]
- Chen, D.; Ye, X.; Jiang, Y.; Xiao, W.; Zhang, Q.; Zhao, S.; Shao, S.; Gao, N.; Huang, M.; Hu, J. Continuously applying compost for three years alleviated soil acidity and heavy metal bioavailability in a soil-asparagus lettuce system. Front. Plant Sci. 2022, 13, 972789. [Google Scholar] [CrossRef]
- Rostaei, M.; Fallah, S.; Lorigooini, Z.; Surki, A.A. Crop productivity and chemical compositions of black cumin essential oil in sole crop and intercropped with soybean under contrasting fertilization. Ind. Crops Prod. 2018, 125, 622–629. [Google Scholar] [CrossRef]
- Klute, A.; Dirksen, C. Hydraulic Conductivity and Diffusivity: Laboratory Methods. In Methods of Soil Analysis: Part 1 Physical and Mineralogical Methods, 2nd ed.; Soil Science Society of America Amer Soc Agron: Madison, WI, USA, 1986; pp. 687–734. [Google Scholar] [CrossRef]
- Page, A.L.; Miller, R.H.; Keeney, D.R. Methods of Soil Analysis Part 2: Chemical and Microbiological Properties; American Society of Agronomy, Inc.: Madison, WI, USA, 1982; pp. 225–246. [Google Scholar] [CrossRef]
- Allen, R.G.; Pereira, L.S.; Raes, D.; Smith, M. Crop evapotranspiration guidelines for computing crop water requirements. In Irrigation and Drainage; FAO, United Nations: Rome, Italy, 1998; pp. 30–42. [Google Scholar]
- Israelsen, O.W.; Hansen, V.E. Irrigation Principles and Practices, 3rd ed.; John Wiley & Sons, Inc.: New York, NY, USA, 1962; p. 448. [Google Scholar] [CrossRef]
- Weatherley, P. Studies in the water relations of the cotton plant. I. The field measurement of water deficits in leaves. New Phytol. 1950, 49, 81–97. [Google Scholar] [CrossRef]
- Sairam, R.K.; Deshmukh, P.S.; Shukla, D.S. Tolerance of drought and temperature stress in relation to increased antioxidant enzyme activity in wheat. J. Agron. Crop Sci. 1997, 178, 171–178. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar] [CrossRef]
- Herbert, D.; Phipps, P.J.; Strange, R.E. Carbohydrate Analysis. Chapter III Chemical Analysis of Microbial Cells. Methods Microbiol. 1971, 5, 265–282. [Google Scholar] [CrossRef]
- Irigoyen, J.J.; Emerich, D.W.; Sanchez-Diaz, M. Water stress induced changes in the concentrations of proline and total soluble sugars in nodulated alfalfa (Medicago sativa) plants. Physiol. Plant. 1992, 8, 55–60. [Google Scholar] [CrossRef]
- Dubey, R.S.; Rani, M. Influence of NaCl salinity on growth and metabolic status of protein and amino acids in rice seedlings. J. Agron. Crop Sci. 1989, 162, 97–106. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldeen, R.P.; Teare, I.D. Rapid determination of free proline for water stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Jackson, M.L. Soil Chemical Analysis, 2nd ed.; Prentice-Hall of India Pvt. Ltd.: New Delhi, India, 1973; Volume 38, p. 336. [Google Scholar]
- AOAC International. Official Methods of Analysis, 18th ed.; The Association of Official Analytical Chemists: Gaithersburg, MD, USA, 2007. [Google Scholar]
- Ackman, R.G. Remarks on official methods employing boron trifluoride in the preparation of methyl esters of the fatty acids of fish oils. J. Amer. Oil Chem. Soc. 1998, 75, 541–545. [Google Scholar] [CrossRef]
- Gomez, K.A.; Gomez, A.A. Statistical Procedures for Agricultural Research, 2nd ed.; John Wiley and Sons: New York, NY, USA, 1984; 680p. [Google Scholar]
- Logsdon, S.D.; Sauer, P.A.; Shipitalo, M.J. Compost improves urban soil and water quality. J. Water Resour. Prot. 2017, 9, 345–357. [Google Scholar] [CrossRef]
- Somerville, P.D.; May, P.B.; Livesley, S.J. Effects of deep tillage and municipal green waste compost amendments on soil properties and tree growth in compacted urban soils. J. Environ. Manag. 2018, 227, 365–374. [Google Scholar] [CrossRef]
- Jagadeesha, N.; Srinivasulu, G.B.; Shet, R.M.; Umesh, M.R.; Kustagi, G.; Ravikumar, B.; Madhu, L.; Reddy, V.C. Effect of organic manures on physical, chemical and biological properties of soil and crop yield in fingermillet-redgram intercropping system. Int. J. Curr. Microbiol. App. Sci. 2019, 8, 1378–1386. [Google Scholar] [CrossRef]
- Abd El-Mageed, T.A.; Abdelkhalik, A.; Abd El-Mageed, S.A.; Semida, W.M. Co-composted poultry litter biochar enhanced soil quality and eggplant productivity under different irrigation regimes. J. Soil Sci. Plant Nutr. 2021, 21, 1917–1933. [Google Scholar] [CrossRef]
- Nigussie, A.; Haile, W.; Agegnehu, G.; Kiflu, A. Growth, nitrogen uptake of maize (Zea mays L.) and soil chemical properties, and responses to compost and nitrogen rates and their mixture on different textured soils: Pot experiment. Appl. Environ. Soil Sci. 2022, 2021, 9931763. [Google Scholar] [CrossRef]
- Mancy, A.G.; Sheta, M.H. Evaluation of biochar and compost ability to improve soil moisture content and nutrients retention. Al-Azhar J. Agric. Res. 2021, 46, 153–165. [Google Scholar] [CrossRef]
- Hussein, M.; Ali, M.; Abbas, M.H.; Bassouny, M.A. Composting animal and plant residues for improving the characteristics of a clayey soil and enhancing the productivity of wheat plant grown thereon. Egypt. J. Soil Sci. 2022, 62, 195–208. [Google Scholar] [CrossRef]
- Sun, Z.; Hu, Y.; Shi, L.; Li, G.; Pang, Z.; Liu, S.; Chen, Y.; Jia, B. Effects of biochar on soil chemical properties: A global meta-analysis of agricultural soil. Plant Soil Environ. 2022, 68, 272–289. [Google Scholar] [CrossRef]
- Fang, Y.; Xiong, L. General mechanisms of drought response and their application in drought resistance improvement in plants. Cell Mol. Life Sci. 2015, 72, 673–689. [Google Scholar] [CrossRef]
- Abdou, N.M.; El-Saadony, F.M.A.; Roby, M.H.H.; Mahdy, H.A.A.; El-Shehawi, A.M.; Elseehy, M.M.; El-Tahan, M.A.; Abdalla, H.; Saad, M.A.; Abou-Sreea, A.I.B. Foliar spray of potassium silicate, aloe extract composite and their effect on growth and yielding capacity of roselle (Hibiscus sabdariffa L.) under water deficit stress conditions. Saudi J. Biol. Sci. 2022, in press. [Google Scholar] [CrossRef]
- Abou-Sreea, A.I.B.; Roby, M.H.H.; Mahdy, H.A.A.; Abdou, N.M.; Tahan, A.M.; El-Saadony, M.T.; El-Tarabily, K.A.; El-Saadony, F.M.A. Improvement of selected morphological, physiological, and biochemical parameters of roselle (Hibiscus sabdariffa L.) grown under different salinity levels using potassium silicate and Aloe saponaria extract. Plants 2022, 11, 497. [Google Scholar] [CrossRef]
- Abou-Sreea, A.I.B.; Kamal, M.; El Sowfy, D.M.; Rady, M.M.; Mohamed, G.F.; Al-Dhumri, S.A.; AL-Harbi, M.S.; Abdou, N.M. Small-sized nanophosphorus has a positive impact on the performance of fenugreek plants under soil-water deficit stress: A case study under field conditions. Biology 2022, 11, 115. [Google Scholar] [CrossRef]
- Abd El-Mageed, T.A.; Abd El-Mageed, S.A.; El-Saadony, M.T.; Abdelaziz, S.; Abdou, N.M. Plant growth-promoting rhizobacteria improve growth, morph-physiological responses, water productivity, and yield of rice plants under full and deficit drip irrigation. Rice 2022, 15, 16. [Google Scholar] [CrossRef]
- Abdou, N.M.; Abdel-Razek, M.A.; Abd El-Mageed, S.A.; Semida, W.M.; Leilah, A.A.A.; Abd El-Mageed, T.A.; Ali, E.F.; Majrashi, A.; Rady, M.O.A. High nitrogen fertilization modulates morpho-physiological responses, yield, and water productivity of lowland rice under deficit irrigation. Agronomy 2021, 11, 1291. [Google Scholar] [CrossRef]
- Ibrahim, A.; Abd ElMageed, T.; Abohamid, Y.; Abdallah, H.; El-Saadony, M.; AbuQamar, S.; El-Tarabily, K.; Abdou, N.M. Exogenously applied proline enhances morph-physiological responses and yield of drought-stressed maize plants grown under different irrigation systems. Front. Plant Sci. 2022, 13, 897027. [Google Scholar] [CrossRef] [PubMed]
- Bista, D.R.; Heckathorn, S.A.; Jayawardena, D.M.; Mishra, S.; Boldt, J.K. Effects of drought on nutrient uptake and the levels of nutrient-uptake proteins in roots of drought-sensitive and -tolerant grasses. Plants 2018, 7, 28. [Google Scholar] [CrossRef] [PubMed]
- Bista, D.R.; Heckathorn, S.A.; Jayawardena, D.M.; Boldt, J.K. Effect of drought and carbon dioxide on nutrient uptake and levels of nutrient-uptake proteins in roots of barley. Am. J. Bot. 2020, 107, 1401–1409. [Google Scholar] [CrossRef]
- Zhao, Q.; Guo, J.; Shu, M.; Wang, P.; Hu, S. Impacts of drought and nitrogen enrichment on leaf nutrient resorption and root nutrient allocation in four Tibetan plant species. Sci. Total Environ. 2020, 723, 138106. [Google Scholar] [CrossRef]
- Nadal, M.; Flexas, J. Mesophyll conductance to CO2 diffusion: Effects of drought and opportunities for improvement. In Water Scarcity and Sustainable Agriculture in Semiarid Environment; Tejero, I.F.G., Zuazo, V.H.D., Eds.; Elsevier: London, UK, 2018; pp. 404–438. [Google Scholar]
- Nelissen, H.; Sun, X.H.; Rymen, B.; Jikumaru, Y.; Kojima, M.; Takebayashi, Y.; Abbeloos, R.; Demuynck, K.; Storme, V.; Vuylsteke, M.; et al. The reduction in maize leaf growth under mild drought affects the transition between cell division and cell expansion and cannot be restored by elevated gibberellic acid levels. Plant Biotechnol. J. 2018, 16, 615–627. [Google Scholar] [CrossRef]
- Salehi-Lisar, S.Y.; Bakhshayeshan-Agdam, H. Agronomic Crop Responses and Tolerance to Drought Stress. In Agronomic Crops; Hasanuzzaman, M., Ed.; Springer: Berlin/Heidelberg, Germany, 2020; pp. 63–91. [Google Scholar] [CrossRef]
- You, J.; Chan, Z. ROS regulation during abiotic stress responses in crop plants. Front. Plant Sci. 2015, 6, 1092. [Google Scholar] [CrossRef]
- Bayati, P.; Karimmojeni, H.; Razmjoo, J.; Pucci, M.; Abate, G.; Baldwin, T.C.; Mastinu, A. Physiological, biochemical, and agronomic trait responses of Nigella sativa genotypes to water stress. Horticulturae 2022, 8, 193. [Google Scholar] [CrossRef]
- Forouzandeh, M.; Karimian, M.A.; Mohkami, Z. Effect of drought stress and different types of organic fertilizers on yield of cumin components in Sistan region. Eur. J. Med. Plants 2015, 5, 95–100. [Google Scholar] [CrossRef]
- Joshan, Y.; Sani, B.; Jabbari, H.; Mozafari, H.; Moaveni, P. Effect of drought stress on oil content and fatty acids composition of some safflower genotypes. Plant Soil Environ. 2019, 65, 563–567. [Google Scholar] [CrossRef]
- Bayati, P.; Karimmojeni, H.; Razmjoo, J. Changes in essential oil yield and fatty acid contents in black cumin (Nigella sativa L.) genotypes in response to drought stress. Ind. Crops Prod. 2020, 155, 112764. [Google Scholar] [CrossRef]
- Forouzandeh, M.; Karimian, M.A.; Mohkami, Z. Effect of water stress and different types of organic fertilizers on essential oil content and yield components of Cuminum cyminum. Indian J. Fundam. Appl. Life Sci. 2014, 4, 533–536. [Google Scholar]
- Abdel-Kader, A.A.S.; Saleh, F.E.M.; Ragab, A.A. Effect of organic manure and bio-fertilizers on productivity and quality of cumin (Cuminum cyminum, L.) plant grown in calcareous sandy soil. Assiut J. Agric. Sci. 2016, 47, 473–483. [Google Scholar] [CrossRef]
- Mahmudov, A.V.; Abduraimov, O.S.; Erdonov, S.B.; Gayibov, U.G.; Izotova, L.Y. Bioecological features of Nigella sativa L. in different conditions of Uzbekistan. Plant Sci. Today 2022, 9, 421–426. [Google Scholar] [CrossRef]
- Roussis, I.; Kakabouki, I.; Beslemes, D.; Tigka, E.; Kosma, C.; Triantafyllidis, V.; Mavroeidis, A.; Zotos, A.; Bilalis, D. Nitrogen uptake, use efficiency, and productivity of Nigella sativa L. in response to fertilization and plant density. Sustainability 2022, 14, 3842. [Google Scholar] [CrossRef]
- Omara, A.E.D.; Hafez, E.M.; Osman, H.S.; Rashwan, E.; El-Said, M.A.A.; Alharbi, K.; Abd El-Moneim, D.; Gowayed, S.M. Collaborative impact of compost and beneficial rhizobacteria on soil properties, physiological attributes, and productivity of wheat subjected to deficit irrigation in salt affected soil. Plants 2022, 11, 877. [Google Scholar] [CrossRef]
- Darakeh, S.A.S.S.; Weisany, W.; Diyanat, M.; Ebrahimi, R. Bio-organic fertilizers induce biochemical changes and affect seed oil fatty acids composition in black cumin (Nigella sativa Linn). Ind. Crops Prod. 2021, 164, 113383. [Google Scholar] [CrossRef]
- Wako, F.L.; Aga, M.C.; Negeri, G.T. Response of black cumin to vermicompost and nitrogen fertilizer. Agric. Environ. Lett. 2022, 7, 20066. [Google Scholar] [CrossRef]
- Alaghemand, A.; Khaghani, S.; Bihamta, M.R.; Gomarian, M.; Ghorbanpour, M. Physiological responses of Nigella Sativa ecotypes to drought stress condition. Iran. J. Plant Physiol. 2019, 9, 2695–2702. [Google Scholar]
- Ozer, H.; Coban, F.; Sahin, U.; Ors, S. Response of black cumin (Nigella sativa L.) to deficit irrigation in a semi-arid region: Growth, yield, quality, and water productivity. Ind. Crops Prod. 2020, 144, 112048. [Google Scholar] [CrossRef]
- Shahbazi, E.; Safipor, B.; Saeidi, K.; Golkar, P. Responses of Nigella damascena L. and Nigella sativa L. to drought stress: Yield, fatty acid composition and antioxidant activity. J. Agric. Sci. Tech. 2022, 24, 693–705. [Google Scholar]
- Bibiano, C.S.; Carvalho, A.A.; Bertolucci, S.K.V.; Torres, S.S.; Corrêa, R.M.; Pinto, J.E.B.P. Organic manure sources play fundamental roles in growth and quali-quantitative production of essential oil from Dysphania ambrosioides L. Ind Crops Prod. 2019, 139, 111512. [Google Scholar] [CrossRef]
- Chiyaneh, S.F.; Chiyaneh, E.R.; Amirnia, R.; Afshar, R.K.; Siddique, K.H.M. Changes in the essential oil, fixed oil constituents, and phenolic compounds of ajowan and fenugreek in intercropping with pea affected by fertilizer sources. Ind. Crops Prod. 2022, 178, 114587. [Google Scholar] [CrossRef]
- Mohamed, H.I.; Latif, H.H. Improvement of drought tolerance of soybean plants by using methyl jasmonate. Physiol. Mol. Biol. Plants 2017, 23, 545–556. [Google Scholar] [CrossRef]
- Borzoo, S.; Mohsenzadeh, S.; Kahrizi, D. Water-deficit stress and genotype variation induced alteration in seed characteristics of Camelina sativa. Rhizosphere 2020, 20, 100427. [Google Scholar] [CrossRef]
- Aslam, M.N.; Nelson, M.N.; Kailis, S.G.; Bayliss, K.L.; Speijers, J.; Cowling, W.A. Canola oil increases in polyunsaturated fatty acids and decreases in oleic acid in drought-stressed Mediterranean-type environments. Plant Breed. 2009, 128, 348–355. [Google Scholar] [CrossRef]
- Silva, L.R.; Pereira, M.J.; Azevedo, J.; Mulas, R.; Velazquez, E.; González-Andrés, F.; Valentão, P.; Andrade, P.B. Inoculation with Bradyrhizobium japonicum enhances the organic and fatty acids content of soybean (Glycine max (L.) Merrill) seeds. Food chem. 2013, 141, 3636–3648. [Google Scholar] [CrossRef]
- Sharifi, P.; Shorafa, M.; Mohammadi, M.H. Comparison of the effect of cow manure, vermicompost, and azolla on safflower growth in a saline-sodic soil. Commun. Soil Sci. Plant Anal. 2019, 50, 1417–1424. [Google Scholar] [CrossRef]
- Shaaban, A.; Al-Elwany, O.A.A.I.; Abdou, N.M.; Hemida, K.A.; El-Sherif, A.M.A.; Abdel-Razek, M.A.; Semida, W.M.; Mohamed, G.F.; Abd El-Mageed, T.A. Filter Mud Enhanced Yield and Soil Properties of Water-Stressed Lupinus termis L. in Saline Calcareous Soil. J. Soil Sci. Plant Nutr. 2022, 22, 1572–1588. [Google Scholar] [CrossRef]


| Depth (cm) | Particle Size Distribution | ρb (g cm−3) | Porosity (%) | Ksat (cm h−1) | θ Fc. (%) | θ W.P. (%) | A.W. (%) | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Sand (%) | Silt (%) | Clay (%) | Texture | |||||||
| 0–25 | 9.16 | 19.66 | 71.18 | Clay | 1.23 | 53.58 | 0.91 | 43.12 | 22.67 | 20.45 |
| 25–50 | 8.11 | 20.84 | 71.05 | Clay | 1.24 | 53.21 | 0.92 | 41.30 | 22.58 | 18.72 |
| Mean | 8.64 | 20.25 | 71.12 | Clay | 1.24 | 53.40 | 0.92 | 42.21 | 22.62 | 19.585 |
| Soil | Compost | ||
|---|---|---|---|
| Properties | Value | Properties | Value |
| pH [at a soil-to-water(w/v) ratio of 1:2.5] | 7.62 | pH [at a soil-to-water(w/v) ratio of 1:2.5] | 7.31 |
| ECe (dS·m−1; soil paste extract) | 2.89 | Ece (dS·m−1; soil paste extract) | 2.97 |
| CEC (cmol·kg−1) | 33.20 | ||
| CaCO3 (g·kg−1) | 3.54 | CaCO3 (g·kg−1) | 1.60 |
| O.M (%) | 1.44 | O.M (%) | 57.46 |
| O.C (%) | 0.84 | O.C (%) | 33.00 |
| Available nutrients | Total nutrients | ||
| N (%) | 0.07 | N (%) | 1.30 |
| P (mg·kg−1 soil) | 6.02 | P (g·kg−1) | 6.30 |
| K (mg·kg−1 soil) | 55.70 | K (g·kg−1) | 8.50 |
| Soil Physical Properties (Average of Two Seasons) $ | ||||||||
|---|---|---|---|---|---|---|---|---|
| Compost Application | ρb | Ksat | T.P | Q.D.P >30 µ | S.D.P 30–9 µ | W.H.P 9.02 µ | θFc | A.W. (%) |
| C0 | 1.23 a | 1.11 c | 53.58 c | 8.66 c | 9.34 c | 20.53 c | 42.68 c | 20.53 c |
| C15 | 1.19 b | 1.35 b | 55.09 b | 9.51 b | 10.84 b | 22.73 b | 44.87 b | 22.73 b |
| C30 | 1.14 c | 1.65 a | 56.98 a | 11.13 a | 12.64 a | 23.88 a | 46.21 a | 23.88 a |
| Soil Chemical Properties (Average of Two Seasons) | ||||||||
| Compost Application | pH | ECe (dS·m−1) | CEC (cmol·kg−1) | O.M (%) | N (%) | P (mg·kg−1) | K (mg·kg−1) | |
| C0 | 7.71 a | 2.55 c | 34.18 c | 1.42 c | 0.06 c | 7.22 c | 48.65 c | |
| C15 | 7.65 b | 2.87 b | 36.98 b | 1.66 b | 0.08 b | 9.18 b | 86.37 b | |
| C30 | 7.64 b | 3.12 a | 39.64 a | 1.74 a | 0.09 a | 11.34 a | 102.17 a | |
| Treatments | Plant Height (cm) | Stem Diameter (cm) | Fresh Weight (g) | Dry Weight (g) | |
|---|---|---|---|---|---|
| Irrigation Levels | ** | ** | ** | ** | |
| I100 | 55.87 a | 0.90 a | 72.05 a | 33.26 a | |
| I75 | 47.48 b | 0.66 b | 50.85 b | 23.44 b | |
| I50 | 38.03 c | 0.51 c | 34.88 c | 16.24 c | |
| Compost (t·ha1) | ** | ** | ** | ** | |
| C0 | 43.09 c | 0.60 c | 44.07 c | 20.46 c | |
| C15 | 47.20 b | 0.67 b | 53.15 b | 24.63 b | |
| C30 | 51.11 a | 0.80 a | 60.58 a | 27.85 a | |
| Interaction: I × C | ** | ** | ** | ** | |
| I100 | C0 | 52.67 c | 0.73 c | 60.26 c | 27.77 c |
| C15 | 54.76 b | 0.85 b | 73.22 b | 33.90 b | |
| C30 | 60.19 a | 1.11 a | 82.67 a | 38.10 a | |
| I75 | C0 | 43.79 e | 0.59 e | 43.02 e | 20.11 f |
| C15 | 46.50 d | 0.66 d | 50.37 d | 23.32 e | |
| C30 | 52.17 c | 0.72 c | 59.18 c | 26.90 d | |
| I50 | C0 | 32.81 g | 0.46 g | 28.91 h | 13.51 i |
| C15 | 40.33 f | 0.52 f | 35.85 g | 16.67 h | |
| C30 | 40.95 f | 0.56 ef | 39.88 f | 18.55 g | |
| Treatments | Ch a (mg·g−1) | Ch b (mg·g−1) | Carotenoids (mg·g−1) | |
|---|---|---|---|---|
| Irrigation Levels | ** | ** | ** | |
| I100 | 1.45 a | 0.75 a | 0.70 a | |
| I75 | 1.31 b | 0.66 b | 0.61 b | |
| I50 | 1.21 c | 0.54 c | 0.48 c | |
| Compost (t ha1) | ** | ** | ** | |
| C0 | 1.29 c | 0.62 c | 0.57 c | |
| C15 | 1.32 b | 0.64 b | 0.59 b | |
| C30 | 1.36 a | 0.68 a | 0.63 a | |
| Interaction: I × C | ** | ** | ** | |
| I100 | C0 | 1.41 b | 0.72 b | 0.67 b |
| C15 | 1.45 b | 0.73 b | 0.68 b | |
| C30 | 1.50 a | 0.79 a | 0.74 a | |
| I75 | C0 | 1.27 d | 0.63 d | 0.58 d |
| C15 | 1.31 c | 0.66 cd | 0.59 d | |
| C30 | 1.35 c | 0.67 c | 0.64 c | |
| I50 | C0 | 1.18 f | 0.51 g | 0.45 g |
| C15 | 1.21 ef | 0.54 f | 0.49 f | |
| C30 | 1.23 de | 0.57 e | 0.51 e | |
| Treatments | Capsules Weight (g plant−1) | Capsule Weight (g) | Seeds Weight (g·capsule−1) | Seed Yield (kg·ha−1) | Fixed Oil (kg·ha−1) | Essential Oil (kg·ha−1) | |
|---|---|---|---|---|---|---|---|
| Irrigation Levels | ** | ** | ** | ** | ** | ** | |
| I100 | 18.49 a | 0.38 a | 0.23 a | 749.00 a | 219.45 a | 1.60 a | |
| I75 | 12.80 b | 0.30 b | 0.18 b | 502.22 b | 129.54 b | 0.85 b | |
| I50 | 7.58 c | 0.22 c | 0.13 c | 296.52 c | 68.74 c | 0.39 c | |
| Compost (t ha1) | ** | ** | ** | ** | ** | ** | |
| C0 | 10.91 c | 0.27 c | 0.17 c | 439.08 c | 113.39 c | 0.74 c | |
| C15 | 12.90 b | 0.30 b | 0.18 b | 517.13 b | 138.17 b | 0.94 b | |
| C30 | 15.04 a | 0.33 a | 0.20 a | 591.53 a | 166.17 a | 1.17 a | |
| Interaction: I × C | ** | ** | ** | ** | ** | ** | |
| I100 | C0 | 16.18 c | 0.36 c | 0.21 c | 651.82 c | 179.34 c | 1.28 c |
| C15 | 18.00 b | 0.38 b | 0.23 b | 731.22 b | 213.77 b | 1.54 b | |
| C30 | 21.29 a | 0.40 a | 0.24 a | 863.97 a | 265.24 a | 1.99 a | |
| I75 | C0 | 10.22 f | 0.26 f | 0.15 f | 410.04 f | 104.36 f | 0.64 e |
| C15 | 13.18 e | 0.31 e | 0.18 e | 521.44 e | 131.87 e | 0.89 d | |
| C30 | 15.01 d | 0.34 d | 0.20 d | 575.19 d | 152.40 d | 1.04 d | |
| I50 | C0 | 6.33 i | 0.19 i | 0.12 h | 255.40 i | 56.47 i | 0.30 g |
| C15 | 7.53 h | 0.22 h | 0.13 g | 298.73 h | 68.88 h | 0.39 fg | |
| C30 | 8.88 g | 0.24 g | 0.14 g | 335.43 g | 80.87 g | 0.47 f | |
| Treatments | N (mg·g−1 DW) | P (mg·g−1 DW) | K (mg·g−1 DW) | |
|---|---|---|---|---|
| Irrigation Levels | ** | ** | ** | |
| I100 | 1.53 a | 0.42 a | 1.60 a | |
| I75 | 1.39 b | 0.34 b | 1.37 b | |
| I50 | 1.26 c | 0.27 c | 1.18 c | |
| Compost (t·ha−1) | ** | ** | ** | |
| C0 | 1.35 c | 0.31 c | 1.32 c | |
| C15 | 1.39 b | 0.35 b | 1.39 b | |
| C30 | 1.44 a | 0.38 a | 1.44 a | |
| Interaction: I × C | ** | ** | ** | |
| I100 | C0 | 1.50 c | 0.38 bc | 1.53 c |
| C15 | 1.53 b | 0.41 b | 1.60 b | |
| C30 | 1.58 a | 0.47 a | 1.66 a | |
| I75 | C0 | 1.34 f | 0.31 de | 1.31 f |
| C15 | 1.37 e | 0.35 cd | 1.37 e | |
| C30 | 1.44 d | 0.36 c | 1.43 d | |
| I50 | C0 | 1.21 i | 0.25 f | 1.12 i |
| C15 | 1.27 h | 0.28 ef | 1.19 h | |
| C30 | 1.31 g | 0.30 e | 1.23 g | |
| Treatments | SFA | USFA | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| M-USFA | P-USFA | ||||||||||
| Myristic | Palmitic | Stearic | Arachidic | Palmitoleic | Oleic | Eicosenoic | Linoleic | Linolenic | Eicosadienoic | ||
| (14:0) | (16:0) | (18:0) | (20:0) | (16:1) | (18:1) | (20:1) | (18:2) | (18:3) | (20:2) | ||
| Irrigation Levels | ** | ** | ** | ** | ** | ** | ** | ** | ** | ** | |
| I100 | 0.17 c | 12.29 c | 3.64 a | 0.16 b | 0.18 a | 21.82 a | 0.38 a | 48.47 a | 0.57 a | 3.25 a | |
| I75 | 0.19 b | 12.74 b | 3.27 b | 0.17 a | 0.16 b | 20.24 b | 0.35 b | 45.30 b | 0.51 b | 2.77 b | |
| I50 | 0.21 a | 12.93 a | 2.83 c | 0.17 a | 0.16 b | 19.27 c | 0.32 c | 41.66 c | 0.47 c | 2.51 c | |
| Compost (t ha1) | ** | ** | ** | ** | ** | ** | ** | ** | ** | ** | |
| C0 | 0.18 c | 12.63 b | 3.10 c | 0.16 b | 0.16 b | 19.70 c | 0.34 c | 46.63 a | 0.51 b | 2.58 c | |
| C15 | 0.19 b | 12.56 b | 3.21 b | 0.17 a | 0.17 a | 20.48 b | 0.35 b | 45.17 b | 0.51 b | 2.66 b | |
| C30 | 0.20 a | 12.76 a | 3.43 a | 0.17 a | 0.17 a | 21.16 a | 0.37 a | 43.62 c | 0.54 a | 3.08 a | |
| Interaction: I × C | ** | ** | ** | ** | ** | ** | ** | ** | ** | ** | |
| I100 | C0 | 0.16 f | 12.24 d | 3.50 b | 0.16 c | 0.17 b | 20.35 c | 0.37 b | 49.34 a | 0.56 c | 2.67 c |
| C15 | 0.17 e | 12.27 d | 3.54 b | 0.16 c | 0.18 a | 21.91 b | 0.37 b | 48.73 a | 0.57 b | 2. 78 c | |
| C30 | 0.17 e | 12.35 cd | 3.88 a | 0.16 c | 0.18 a | 23.21 a | 0.41 a | 47.33 b | 0.58 a | 3.82 a | |
| I75 | C0 | 0.18 d | 12.78 c | 3.12 e | 0.16 c | 0.16 c | 20.22 c | 0.33 c | 47.32 b | 0.52 e | 2.65 d |
| C15 | 0.18 d | 12.56 b | 3.25 d | 0.17 b | 0.16 c | 20.23 c | 0.36 c | 45.34 c | 0.49 g | 2.78 c | |
| C30 | 0.20 c | 12.87 b | 3.44 c | 0.17 b | 0.17 b | 20.27 c | 0.37 b | 43.24 d | 0.53 d | 2.87 b | |
| I50 | C0 | 0.21 b | 12.87 b | 2.69 h | 0.17 b | 0.16 c | 18.53 e | 0.32 e | 43.24 d | 0.44 i | 2.43 f |
| C15 | 0.21 b | 12.86 b | 2.84 g | 0.17 b | 0.16 c | 19.30 d | 0.32 e | 41.45 e | 0.47 h | 2.54 e | |
| C30 | 0.22 a | 13.07 a | 2.97 f | 0.18 a | 0.16 c | 19.99 c | 0.33 c | 40.30 f | 0.51 f | 2.55 e | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdou, N.M.; Roby, M.H.H.; AL-Huqail, A.A.; Elkelish, A.; Sayed, A.A.S.; Alharbi, B.M.; Mahdy, H.A.A.; Abou-Sreea, A.I.B. Compost Improving Morphophysiological and Biochemical Traits, Seed Yield, and Oil Quality of Nigella sativa under Drought Stress. Agronomy 2023, 13, 1147. https://doi.org/10.3390/agronomy13041147
Abdou NM, Roby MHH, AL-Huqail AA, Elkelish A, Sayed AAS, Alharbi BM, Mahdy HAA, Abou-Sreea AIB. Compost Improving Morphophysiological and Biochemical Traits, Seed Yield, and Oil Quality of Nigella sativa under Drought Stress. Agronomy. 2023; 13(4):1147. https://doi.org/10.3390/agronomy13041147
Chicago/Turabian StyleAbdou, Nasr M., Mohamed H. H. Roby, Arwa Abdulkreem AL-Huqail, Amr Elkelish, Ali A. S. Sayed, Basmah M. Alharbi, Hayam A. A. Mahdy, and Alaa Idris Badawy Abou-Sreea. 2023. "Compost Improving Morphophysiological and Biochemical Traits, Seed Yield, and Oil Quality of Nigella sativa under Drought Stress" Agronomy 13, no. 4: 1147. https://doi.org/10.3390/agronomy13041147









