Effects of Inoculating the Diazotrophic Endophyte Bradyrhizobium sp. AT1 on Different Cultivars of Sweet Potato (Ipomoea batatas [L.] Lam.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strain and Culture Conditions
2.2. Colonization by Bradyrhizobium AT1-SR in Three Cultivars of Sweet Potato
2.3. Growth Experiment in Pots and Containers
2.4. Growth Experiments in the Field
2.5. Inoculation of Micropropagated Sweet Potato with AT1 for Evaluation of N2 Fixation
2.6. Statistical Analysis
3. Results
3.1. Colonization of the Apical Stem Cuttings of Three Cultivars of Sweet Potato by AT1-SR
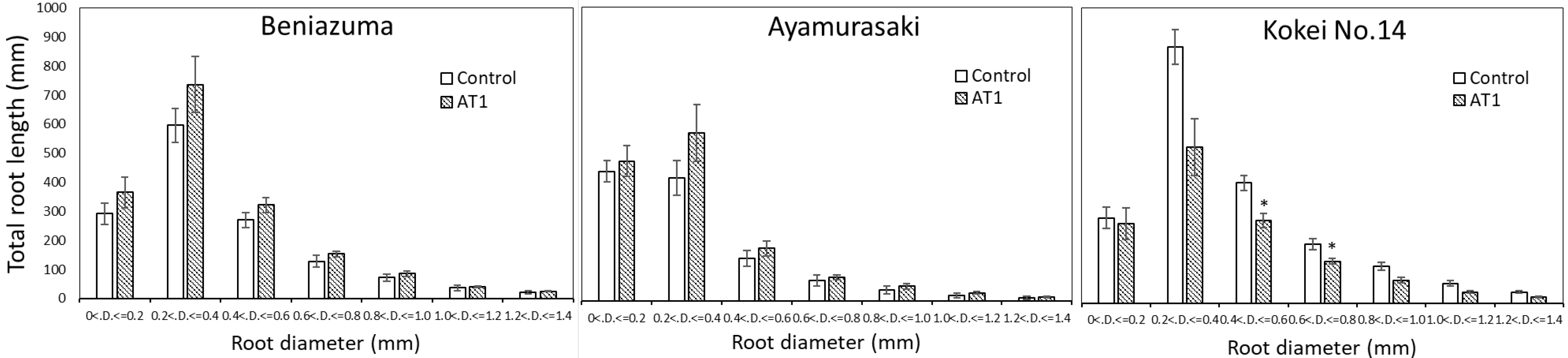
3.2. Growth Experiments in Pots and Containers
3.3. Growth Experiments in the Field
3.4. Growth, ARA, and 15N Dilution Analysis of Micropropagated Sweet Potato
4. Discussion
4.1. Growth Stimulatory Effects of AT1 on Different Sweet Potato Cultivars
4.2. Stimulation of Tuber Growth by AT1 Inoculation
4.3. Biological N2 Fixation Inside Sweet Potato Roots upon AT1 Inoculation
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hill, W.A.; Hortense, D.; Hahn, S.K.; Mulongoy, K.; Adeyeye, S.O. Sweet potato root and biomass production with and without nitrogen fertilization. Agron. J. 1990, 82, 1120–1122. [Google Scholar] [CrossRef]
- Yoneyama, T.; Terakado, J.; Masuda, T. Natural abundance of 15N in sweet potato, pumpkin, sorghum, and castor bean: Possible input of N2-derived nitrogen in sweet potato. Biol Fertil. Soils 1998, 26, 152–154. [Google Scholar] [CrossRef]
- Hill, W.A.; Bacon-Hill, P.; Crossman, S.M.; Stevens, C. Characterization of N2-fixing bacteria associated with sweet potato roots. Can. J. Microbiol. 1983, 29, 860–862. [Google Scholar] [CrossRef]
- Adachi, K.; Nakatani, M.; Mochida, H. Isolation of an endophytic diazotroph, Klebsiella oxytoca, from sweet potato stems in Japan. Soil Sci. Plant Nutr. 2002, 48, 889–895. [Google Scholar] [CrossRef]
- Khan, Z.; Doty, S.L. Characterization of bacterial endophytes of sweet potato plants. Plant Soil 2009, 322, 197–207. [Google Scholar] [CrossRef]
- Marques, J.M.; da Silva, T.F.; Vollú, R.E.; Blank, A.F.; Ding, G.C.; Seldin, L.; Smalla, K. Plant age and genotype affect the bacterial community composition in the tuber rhizosphere of field-grown sweet potato plants. FEMS. Microbiol. Ecol. 2014, 88, 424–435. [Google Scholar] [CrossRef]
- Marques, J.M.; da Silva, T.F.; Vollú, R.E.; de Lacerda, J.R.M.; Blank, A.F.; Smalla, K.; Seldin, L. Bacterial endophytes of sweet potato tuberous roots affected by the plant genotype and growth stage. Appl. Soil Ecol. 2015, 96, 273–281. [Google Scholar] [CrossRef]
- Terakado-Tonooka, J.; Fujihara, S.; Ohwaki, Y. Possible contribution of Bradyrhizobium on nitrogen fixation in sweet potatoes. Plant Soil. 2013, 367, 639–650. [Google Scholar] [CrossRef]
- Terakado-Tonooka, J.; Ohwaki, Y.; Yamakawa, H.; Tanaka, F.; Yoneyama, T.; Fujihara, S. Expressed nifH genes of endophytic bacteria detected in field-grown sweet potatoes (Ipomoea batatas L.). Microbes Environ. 2008, 23, 89–93. [Google Scholar] [CrossRef]
- Villordon, A.; La Bonte, D.; Firon, N.; Carey, E. Variation in nitrogen rate and local availability alter root architecture attributes at the onset of storage root initiation in ‘Beauregard’ sweetpotato. Hort. Sci. 2013, 48, 808–815. [Google Scholar] [CrossRef]
- Okubo, T.; Piromyou, P.; Tittabutr, P.; Teaumroong, N.; Minamisawa, K. Origin and evolution of nitrogen fixation genes on symbiosis islands and plasmid in Bradyrhizobium. Microbes Environ. 2016, 31, 260–267. [Google Scholar] [CrossRef]
- Keele, B.B.; Hamilton, P.B.; Elkan, G.H. Glucose catabolism in Rhizobium japonicum. J. Bacteriol. 1969, 97, 1184–1191. [Google Scholar] [CrossRef]
- Burris, R.H. Nitrogen fixation–assay methods and techniques. Meth. Enzymol. 1972, 24, 415–431. [Google Scholar]
- Chalk, P.M. Estimation of N2 fixation by isotope dilution: An appraisal of techniques involving 15N enrichment and their application. Soil Biol. Biochem. 1985, 17, 389–410. [Google Scholar] [CrossRef]
- Malfanova, N.; Lugtenberg, B.J.J.; Berg, G. Bacterial Eendophytes: Who and Where, and What are they doing there? In Molecular Microbial Ecology of the Rhizosphere; de Bruijn, F.J., Ed.; Wiley Blackwell: Hoboken, NJ, USA, 2013; Volume 1, pp. 393–403. [Google Scholar]
- Yoneyama, T.; Muraoka, T.; Kim, T.H.; Dacanay, E.V.; Nakanishi, Y. The natural 15N abundance of sugarcane and neighboring plants in Brazil, the Philippines and Miyako (Japan). Plant Soil 1997, 189, 239–244. [Google Scholar] [CrossRef]
- Sevilla, M.; Burris, R.H.; Gunapala, N.; Kennedy, C. Comparison of benefit to sugarcane plant growth and 15N2 Incorporation following inoculation of sterile plants with Acetobacter diazotrophicus wild-type and Nif− mutant strains. Mol. Plant-Microbe Interact. 2001, 14, 358–366. [Google Scholar] [CrossRef]
- Oliveira, A.L.M.; Canuto, E.L.; Reis, V.M.; Baldani, J.I. Response of micropropagated sugarcane varieties to inoculation with endophytic diazotrophic bacteria. Braz. J. Microbiol. 2003, 34, 59–61. [Google Scholar] [CrossRef]
- Sasaki, O.; Nishihara, H.; Tsumagari, Y.; Shimotashiro, T. Factors determining the thickening and the shape of the tuberous root in sweet potato (Ipomoea batatas L. Lam). Comparison of two cultivars having different shapes in tuberous root. Jpn. Crop Sci. 2004, 73, 65–70, (In Japanese with English Summary). [Google Scholar] [CrossRef]
- Puri, R.R.; Adachi, F.; Omichi, M.; Saeki, Y.; Yamamoto, A.; Hayashi, S.; Itoh, K. Culture-dependent analysis of endophytic bacterial community of sweet potato (Ipomoea batatas) in different soils and climates. J. Adv. Microbiol. 2018, 13, 1–12. [Google Scholar] [CrossRef]
- Itoh, K.; Ohashi, K.; Yakai, N.; Adachi, F.; Hayashi, S. Changes in acetylene reduction activities and nifH genes associated with field-grown sweet potatoes with different nursery farmers and cultivars. Horticulturae 2019, 5, 53. [Google Scholar] [CrossRef]
- Amaral, F.P.; Pankievicz, V.C.S.; Arisi, A.C.M.; Souza, E.M.; Pedrosa, F.; Stacey, G. Differential growth responses of Brachypodium distachyon genotypes to inoculation with plant growth promoting rhizobacteria. Plant Mol. Biol. 2016, 90, 689–697. [Google Scholar] [CrossRef] [PubMed]
- Terakado-Tonooka, J.; Ando, S.; Ohwaki, Y.; Yoneyama, T. NifH gene expression and nitrogen fixation by diazotrophic endophytes in sugarcane and sweet potatoes. In Molecular Microbial Ecology of the Rhizosphere; de Bruijn, F.J., Ed.; Wiley Blackwell: Hoboken, NJ, USA, 2013; Volume 1, pp. 437–444. [Google Scholar]
- Takada, K.; Kimoto, H.; Babil, P.; Shiwachi, H. Analysis of the source of nitrogen during water yam (Dioscuri alate L.) growth using δ15N observations. Trop. Agric. Dev. 2018, 62, 124–131. [Google Scholar]
- Win, K.T.; Okazaki, K.; Ookawa, T.; Yokoyama, T.; Ohwaki, Y. Influence of rice-husk biochar and Bacillus pumilus strain TUAT-1 on yield, biomass production, and nutrient uptake in two forage rice genotypes. PLoS ONE 2019, 14, e0220236. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, M.; Dastogeer, K.M.G.; Sarkodee-Addo, E.; Tokiwa, C.; Isawa, T.; Shinozaki, S.; Okazaki, S. Impact of Azospirillum sp. B510 on the rhizosphere microbiome of rice under field conditions. Agronomy 2022, 12, 1367. [Google Scholar] [CrossRef]
- Minamisawa, K.; Nishioka, K.; Miyaki, T.; Ye, B.; Miyamoto, T.; You, M.; Saito, A.; Saito, M.; Barraquio, W.L.; Teaumroong, N.; et al. Anaerobic nitrogen-fixing consortia consisting of clostridia isolated from gramineous plants. Appl. Environ. Microbiol. 2004, 70, 3096–3102. [Google Scholar] [CrossRef]
- Miaa, S.; Groenigena, J.W.; Voorde, T.F.J.; Orama, N.J.; Bezemer, T.M.; Mommer, L.; Jefferya, S. Biochar application rate affects biological nitrogen fixation in red clover conditional on potassium availability. Agric. Ecosyst. Environ. 2014, 191, 83–91. [Google Scholar] [CrossRef]
- Villordon, A.Q.; La Bonte, D.R.; Firon, N.; Kfir, Y.; Pressman, E.; Schwartz, A. Characterization of adventitious root development in sweetpotato. Hort. Sci. 2009, 44, 651–655. [Google Scholar] [CrossRef]
- Nakatani, M. Factors influencing the rooting of cut-sprouts in sweet potato. Jpn. Agric. Res. Q. 1993, 27, 1–7. [Google Scholar]
- Nakatani, M.; Oyanagi, A.; Watanabe, Y.; Komeichi, M. Effects of soil temperatures on the rooting of cut-sprouts of sweet potato (Ipomoea batatas Lam.): II. Varietal differences in the optimum soil temperature for rooting and the rooting ability under the low soil temperature. Jpn. J. Crop Sci. 1989, 58, 35–41, (In Japanese with English Summary). [Google Scholar] [CrossRef]
- Loretan, P.A.; Bonsi, C.K.; Mortley, D.G.; Wheeler, R.M.; Mackowiak, C.L.; Hill, W.A.; Morris, C.E.; Trotman, A.A.; David, P.P. Effects of several environmental factors on sweetpotato growth. Adv. Space Res. 1994, 14, 277–280. [Google Scholar] [CrossRef]
- Hill, J.; Douglas, D.; David, P.; Mortley, D.; Trotman, A.; Bonsi, C. Biomass accumulation in hydroponically grown sweetpotato in a controlled environment: A preliminary study. Acta Hortic. 1996, 440, 25–30. [Google Scholar] [CrossRef]
- Mortley, D.; Hill, J.; Loretan, P.; Bonsi, C.; Hill, W.; Hileman, D.; Terse, A. Elevated carbon dioxide influences yield and photosynthetic responses of hydroponically-grown sweetpotato. Acta Hortic. 1996, 440, 31–36. [Google Scholar] [CrossRef]
- Kano, Y.; Ming, Z.J. Effects of soil temperature on the thickening growth and the quality of sweetpotato during the latter part of their growth. Environ. Control Biol. 2000, 38, 113–120. [Google Scholar] [CrossRef]
- Pardales, J.R.; Yamauchi, A. Regulation of root development in sweet potato and cassava by soil moisture during their establishment period. Plant Soil 2003, 255, 201–208. [Google Scholar] [CrossRef]
- Heerden, P.D.R.; Laurie, R. Effects of prolonged restriction in water supply on photosynthesis, shoot development and storage root yield in sweet potato. Physiol. Plant. 2008, 134, 99–109. [Google Scholar] [CrossRef]
- Zhang, X.; Tong, J.; Dong, M.; Akhtar, K.; He, B. Isolation, identification and characterization of nitrogen fixing endophytic bacteria and their effects on cassava production. Peer J. 2002, 10, e12677. [Google Scholar] [CrossRef]
- McDavid, C.R.; Alamu, S. The effect of growth regulators on tuber initiation and growth in rooted leaves of two sweet potato cultivars. Ann. Bot. 1980, 45, 363–364. [Google Scholar] [CrossRef]
- Matsuo, T.; Yoneda, T.; Ito, S. Identification of free cytokinins and the changes in endogenous levels during tuber development of sweet potato (Ipomoea batatas Lam.). Plant Cell Physiol. 1983, 24, 1305–1312. [Google Scholar]
- Nakatani, M.; Komeichi, M. Changes in the endogenous level of zeatin riboside, abscisic acid and indole acetic acid during formation and thickening of tuberous roots in sweet potato. Jpn. J. Crop Sci. 1991, 60, 91–100, (In Japanese with English Summary). [Google Scholar] [CrossRef]
- Ku, A.T.; Huang, Y.S.; Wang, Y.S.; Ma, D.; Yeh, K.W. IbMADS1 (Ipomoea batatas MADS-box 1 gene) is involved in tuberous root initiation in sweet potato (Ipomoea batatas). Ann. Bot. 2008, 102, 57–67. [Google Scholar] [CrossRef]
- Tanaka, M.; Kato, N.; Nakayama, H.; Nakatani, M.; Takahata, Y. Expression of class 1 Knotted1-like homeobox genes in the storage roots of sweetpotato (Ipomoea batatas). J. Plant Physiol. 2008, 165, 1726–1735. [Google Scholar] [CrossRef]
- Ravi, V.; Naskar, S.K.; Makeshkumar, T.; Babu, B.; Krishnan, B.S.P. Molecular physiology of storage root formation and development in sweet potato (Ipomoea batatas (L.) lam.). J. Root Crops 2009, 35, 1–27. [Google Scholar]
- Dong, T.; Zhu, M.; Yu, J.; Han, R.; Tang, C.; Xu, T.; Liu, J.; Li, Z. RNA-Seq and iTRAQ reveal multiple pathways involved in storage root formation and development in sweet potato (Ipomoea batatas L.). BMC Plant Biol. 2019, 19, 136. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Cai, Z.; Huang, J.; Wang, A.; Ntambiyukuri, A.; Chen, B.; Zheng, G.; Li, H.; Huang, Y.; Zhan, J.; et al. Transcriptomic analysis of tuberous root in two sweet potato varieties reveals the important genes and regulatory pathways in tuberous root development. BMC Genom. 2022, 23, 473. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Yang, R.; Bartels, D.; Dong, T.; Duan, H. Roles of Abscisic Acid and Gibberellins in Stem/Root Tuber Development. Int. J. Mol. Sci. 2022, 23, 4955. [Google Scholar] [CrossRef]
- Noh, S.A.; Lee, H.S.; Huh, E.J.; Huh, G.H.; Peak, K.H.; Shin, J.S.; Bae, J.M. SRD1 is involved in the auxin-mediated initial thickening growth of storage root by enhancing proliferation of metaxylem and cambium cells in sweet potato (Ipomoea batatas). J. Exp. Bot. 2010, 61, 1337–1349. [Google Scholar] [CrossRef]
- Kondhare, K.R.; Patil, A.B.; Giri, A.P. Auxin: An emerging regulator of tuber and storage root development. Plant Sci. 2021, 306, 110854. [Google Scholar] [CrossRef]
- Singh, V.; Sergeeva, L.; Lighterink, W.; Aloni, R.; Zemach, H.; Doron-Faigenboim, A.; Yang, J.; Zhang, P.; Shabtai, S.; Firoin, N. Gibberellin promotes sweetpotato root vascular lignification and reduces storage-root formation. Front. Plant Sci. 2019, 10, 1320. [Google Scholar] [CrossRef]
- Lugtenberg, B.; Malfanova, N.; kamilova, F.; Berg, G. Plant growth promotion by microbes. In Molecular Microbial Ecology of the Rhizosphere; de Bruijn, F.J., Ed.; Wiley Blackwell: Hoboken, NJ, USA, 2013; Volume 2, pp. 561–573. [Google Scholar]
- Tatsukuni, Y.; Ueda, M. Rhizobial gibberellin negatively regulates host nodule number. Sci. Rep. 2016, 6, 27998. [Google Scholar] [CrossRef]
- Bloemberg, G.V.; Lugtenberg, B.J. Molecular basis of plant growth promotion and biocontrol by rhizobacteria. Curr. Opin. Plant Biol. 2001, 4, 343–350. [Google Scholar] [CrossRef]
- Persello-Cartieaux, F.; Nussaume, L.; Robaglia, C. Tales from the underground: Molecular plant-rhizobacteria interactions. Plant Cell Environ. 2003, 26, 189–199. [Google Scholar] [CrossRef]
- Boiero, L.; Perrig, D.; Masciarelli, O.; Penna, C.; Cassán, F.; Luna, V. Phytohormone production by three strains of Bradyrhizobium japonicum and possible physiological and technological implications. Appl. Microbiol. Biotechnol. 2007, 74, 874–880. [Google Scholar] [CrossRef]
- Bashan, Y.; de-Bashan, L.E. How the plant growth-promoting bacterium Azospirillum promotes plant growth—A critical assessment. Adv. Agron. 2010, 108, 77–136. [Google Scholar]
- Yoneyama, T.; Terakado-Tonooka, J.; Minamisawa, K. Exploration of bacterial N2-fixation systems in association with soil-grown sugarcane, sweet potato, and paddy rice: A review and synthesis. Soil Sci. Plant Nutr. 2017, 63, 578–590. [Google Scholar] [CrossRef]
- Yoneyama, T.; Terakado-Tonooka, J.; Bao, Z.; Minamisawa, K. Molecular analyses of the distribution and function of diazotrophic rhizobia and methanotrophs in the tissues and rhizosphere of non-leguminous plants. Plants 2019, 8, 408. [Google Scholar] [CrossRef]
- Okamoto, T.; Shinjo, R.; Nishihara, A.; Uesaka, K.; Tanaka, A.; Sugiura, D.; Kondo, M. Genotypic variation of endophytic nitrogen-fixing activity and bacterial flora in rice stem based on sugar content. Front. Plant Sci. 2021, 12, 719259. [Google Scholar] [CrossRef]
- You, M.; Nishiguchi, T.; Saito, A.; Isawa, T.; Mitsui, H.; Minamisawa, K. Expression of the nifH gene of a Herbaspirillum endophyte in wild rice species: Daily rhythm during the light-dark cycle. Appl. Environ. Microbiol. 2005, 71, 8183–8190. [Google Scholar] [CrossRef]
| Site | Number of Cells (1 × 103 CFU g fw−1) | ||
|---|---|---|---|
| Beniazuma | Ayamurasaki | Kokei No. 14 | |
| Shoots | N.D. | N.D. | N.D. |
| Roots | 1.6 ± 0.2 | 3.3 ± 0.7 | 4.1 ± 0.7 |
| Treatment | Sweet Potato Cultivar | |||
|---|---|---|---|---|
| Beniazuma | Ayamurasaki | Kokei No. 14 | ||
| Root length (cm) | Control | 1466 ± 117 | 1376 ± 83 | 2039 ± 199 |
| AT1 | 1781 ± 156 ** | 1413 ± 181 | 1379 ± 263 ** | |
| Root tips (number) | Control | 4388 ± 348 | 2545 ± 175 | 3319 ± 714 |
| AT1 | 4825 ± 381 | 2600 ± 289 | 3129 ± 369 | |
| Root forks (number) | Control | 9322 ± 1195 | 9169 ± 1038 | 13,776 ± 2149 |
| AT1 | 11,470 ± 1457 | 9808 ± 2010 | 8169 ± 1702 ** | |
| Shoots (g dw) | Control | 4.69 ± 0.42 | 4.62 ± 0.28 | 4.45 ± 0.76 |
| AT1 | 4.20 ± 0.84 | 3.80 ± 0.44 | 2.12 ± 0.24 ** | |
| Roots (g dw) | Control | 0.40 ± 0.06 | 0.26 ± 005 | 0.37 ± 0.18 |
| AT1 | 0.43 ± 0.08 | 0.24 ± 0.03 | 0.24 ± 0.05 ** | |
| Treatment | Dry Weight (g Plant−1) | |||
|---|---|---|---|---|
| Beniazuma | Ayamurasaki | Kokei No. 14 | ||
| Shoots | Control | 4.26 ± 0.85 | 8.46 ± 0.87 | 7.78 ± 1.44 |
| AT1 | 12.11 ± 1.41 ** | 9.23 ± 1.30 | 6.35 ± 0.72 | |
| Roots + Tubers | Control | 2.08 ± 0.68 | 2.66 ± 1.18 | 5.64 ± 1.19 |
| AT1 | 2.52 ± 1.15 | 5.37 ± 1.23 ** | 5.29 ± 1.29 | |
| Region | Site | Treatment | Dry Weight (g Plant−1) | N (g Plant−1) | ||||
|---|---|---|---|---|---|---|---|---|
| Beniazuma | Ayamurasaki | Kokei No. 14 | Beniazuma | Ayamurasaki | Kokei No. 14 | |||
| Tsukuba | Vines | Control | 623 ± 130 | 321 ± 54 | 474 ± 91 | 7.14 ± 1.22 | 2.87 ± 0.47 | 7.87 ± 2.06 |
| AT1 | 688 ± 97 | 526 ± 58 * | 664 ± 181 | 8.77 ± 2.55 | 7.41 ± 1.31 * | 10.76 ± 4.52 | ||
| Tubers | Control | 255 ± 47 | 361 ± 29 | 454 ± 73 | 1.45 ± 0.29 | 2.24 ± 0.29 | 2.46 ± 0.23 | |
| AT1 | 298 ± 40 | 472 ± 47 * | 415 ± 44 | 1.60 ± 0.57 | 2.61 ± 0.42 | 2.07 ± 0.61 | ||
| Saga | Vines | Control | 371 ± 42 | 155 ± 23 | 430 ± 68 | 7.12 ± 0.7 | 3.10 ± 0.61 | 5.74 ± 1.07 |
| AT1 | 558 ± 109 | 187 ± 30 | 386 ± 75 | 7.68 ± 1.59 | 3.21 ± 0.52 | 6.26 ± 0.99 | ||
| Tubers | Control | 201 ± 19 | 312 ± 44 | 664 ± 68 | 0.76 ± 0.07 | 1.94 ± 0.39 | 2.79 ± 0.45 | |
| AT1 | 511 ± 44 * | 414 ± 43 * | 553 ± 84 | 1.97 ± 0.24 * | 2.65 ± 0.33 * | 2.05 ± 0.21 | ||
| Site | Treatment | Dry Weight (g Plant−1) | N (mg Plant−1) | 15N Atom % Excess | % Ndfa ** |
|---|---|---|---|---|---|
| Shoots | Control | 0.31 ± 0.02 | 3.26 ± 0.31 | 0.78 ± 0.02 | - |
| AT1 | 0.38 ± 0.01 * | 3.42 ± 0.17 * | 0.67 ± 0.01 * | 14.2 ± 0.4 | |
| Roots | Control | 0.43 ± 0.01 | 4.05 ± 0.10 | 0.80 ± 0.03 | - |
| AT1 | 0.56 ± 0.06 * | 5.23 ± 0.38 * | 0.69 ± 0.03 * | 13.8 ± 0.5 |
| Inoculation | Acetylene | ARA (nmol C2H4 g fw−1 h−1) | |
|---|---|---|---|
| Shoots | Roots | ||
| − | + | 0.02 ± 0.007 | 0.02 ± 0.007 |
| + | + | 0.03 ± 0.008 | 0.56 ± 0.12 * |
| + | − | N.D. | N.D. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Terakado-Tonooka, J.; Tanaka, F.; Karasawa, T.; Suzuki, A.; Ohwaki, Y. Effects of Inoculating the Diazotrophic Endophyte Bradyrhizobium sp. AT1 on Different Cultivars of Sweet Potato (Ipomoea batatas [L.] Lam.). Agronomy 2023, 13, 963. https://doi.org/10.3390/agronomy13040963
Terakado-Tonooka J, Tanaka F, Karasawa T, Suzuki A, Ohwaki Y. Effects of Inoculating the Diazotrophic Endophyte Bradyrhizobium sp. AT1 on Different Cultivars of Sweet Potato (Ipomoea batatas [L.] Lam.). Agronomy. 2023; 13(4):963. https://doi.org/10.3390/agronomy13040963
Chicago/Turabian StyleTerakado-Tonooka, Junko, Fukuyo Tanaka, Toshihiko Karasawa, Akihiro Suzuki, and Yoshinari Ohwaki. 2023. "Effects of Inoculating the Diazotrophic Endophyte Bradyrhizobium sp. AT1 on Different Cultivars of Sweet Potato (Ipomoea batatas [L.] Lam.)" Agronomy 13, no. 4: 963. https://doi.org/10.3390/agronomy13040963
APA StyleTerakado-Tonooka, J., Tanaka, F., Karasawa, T., Suzuki, A., & Ohwaki, Y. (2023). Effects of Inoculating the Diazotrophic Endophyte Bradyrhizobium sp. AT1 on Different Cultivars of Sweet Potato (Ipomoea batatas [L.] Lam.). Agronomy, 13(4), 963. https://doi.org/10.3390/agronomy13040963





