Estimation of Drought Tolerance Indices in Upland Cotton under Water Deficit Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Collection of Germplasm
2.2. Experimental Design and Drought Treatment
Turgid weight − Dry weight
Dry weight
2.3. Analysis of Biochemical Parameters
2.3.1. Hydrogen Peroxide
2.3.2. Proline
2.3.3. Peroxidase Activity
2.3.4. Catalase
2.4. Statistical Analysis
3. Results
3.1. Effect of Drought on Morphological Traits
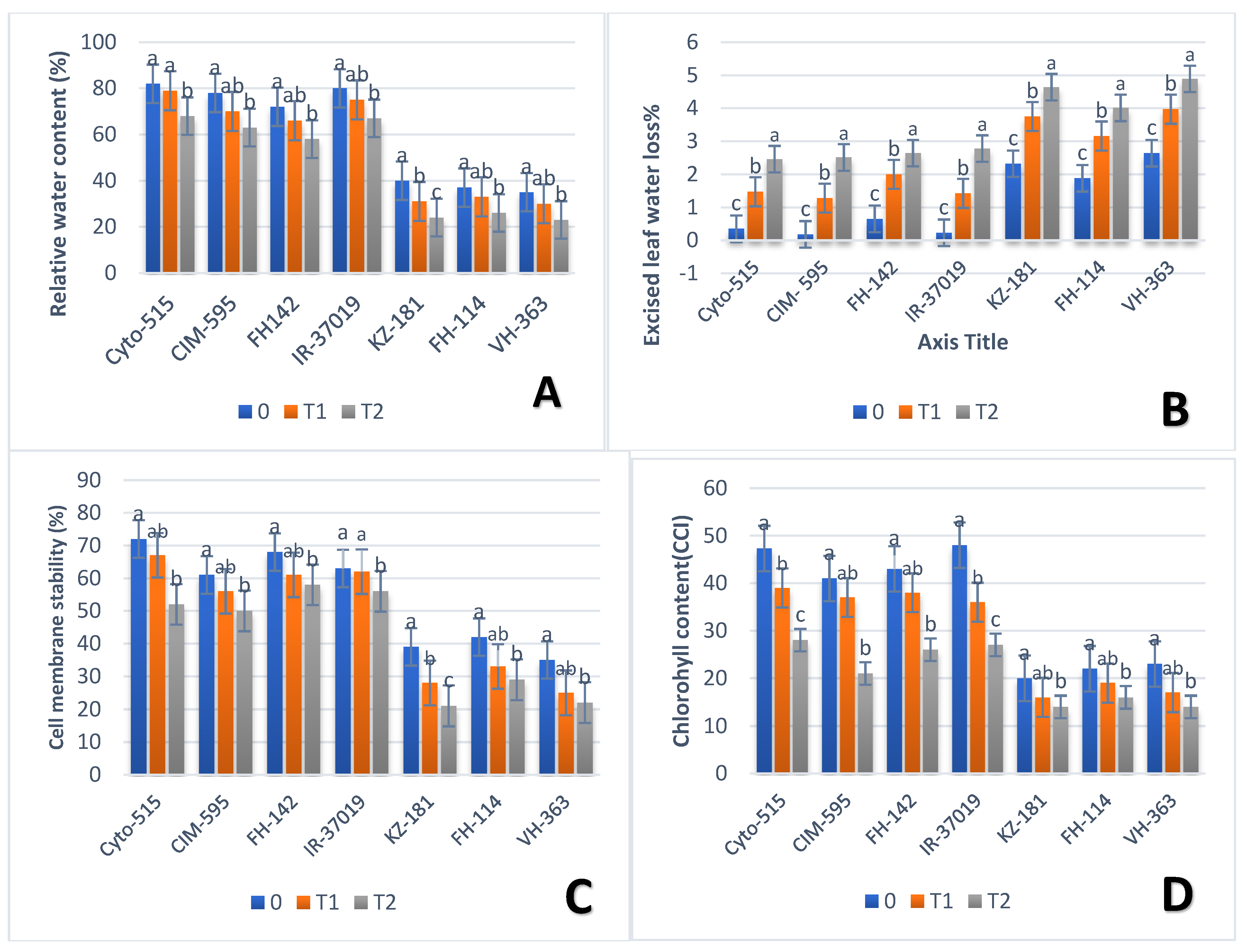
3.2. Effect of Drought on Physiological Traits
3.3. Effect of Drought on Biochemical Traits
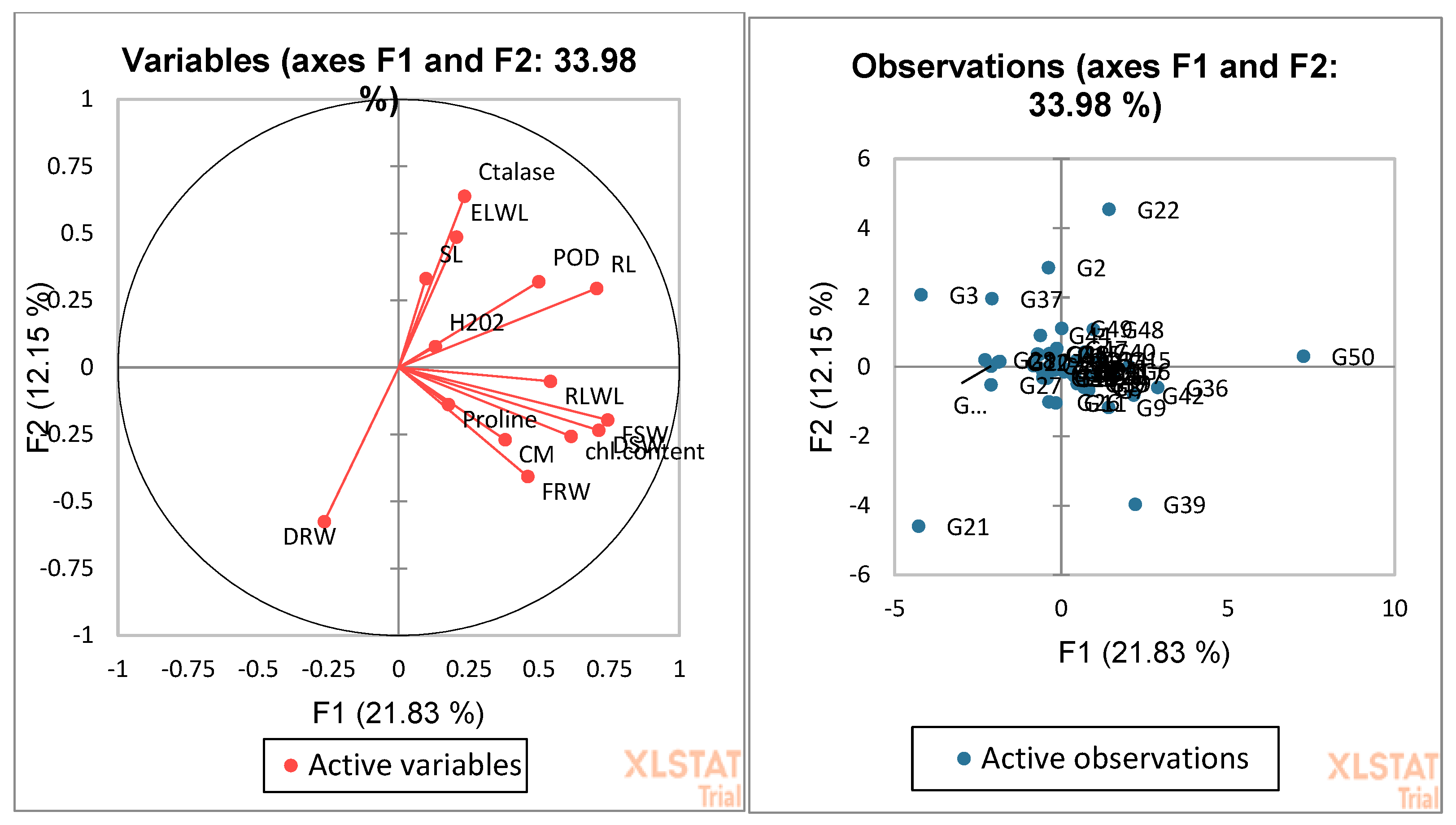
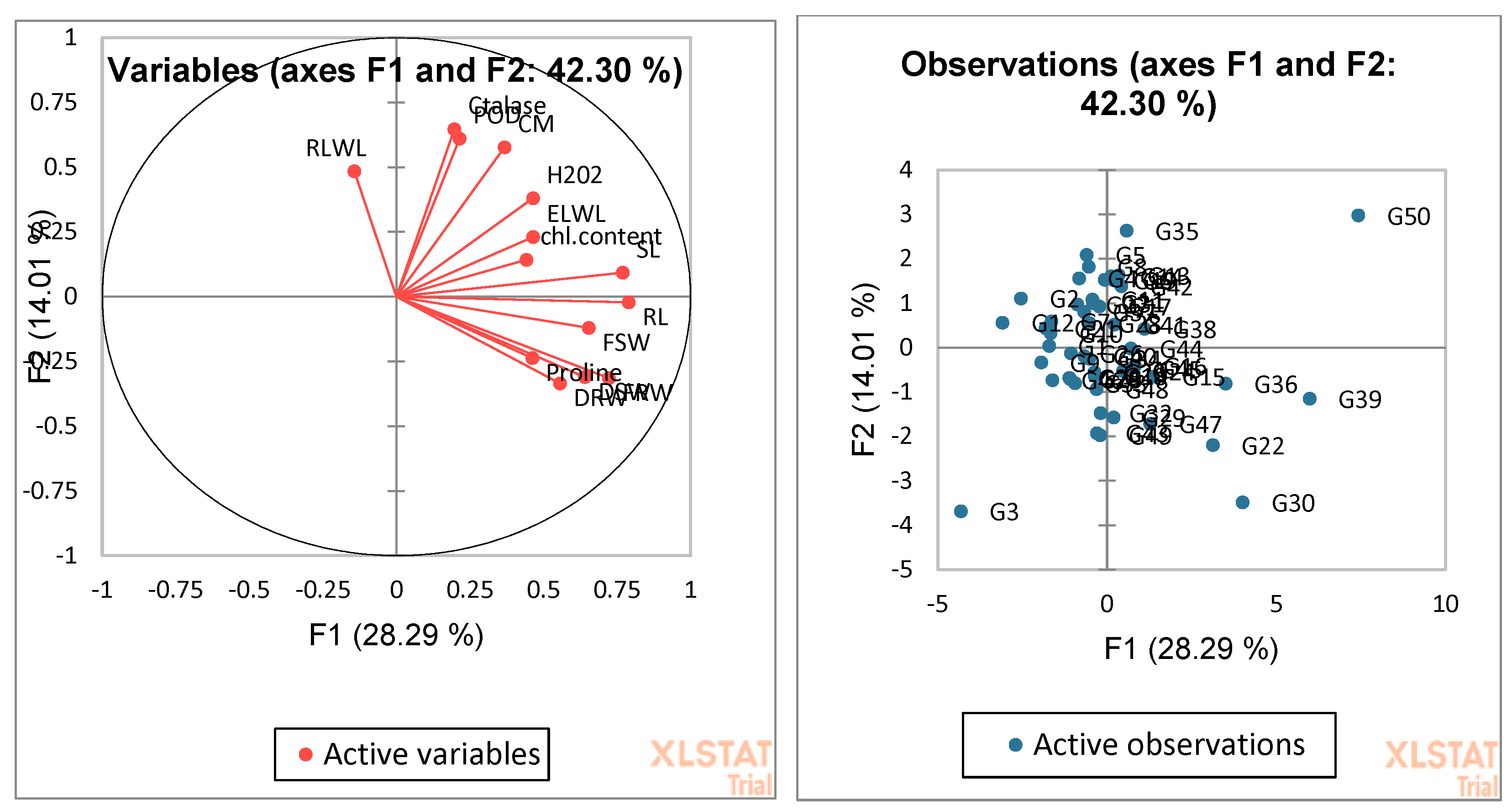
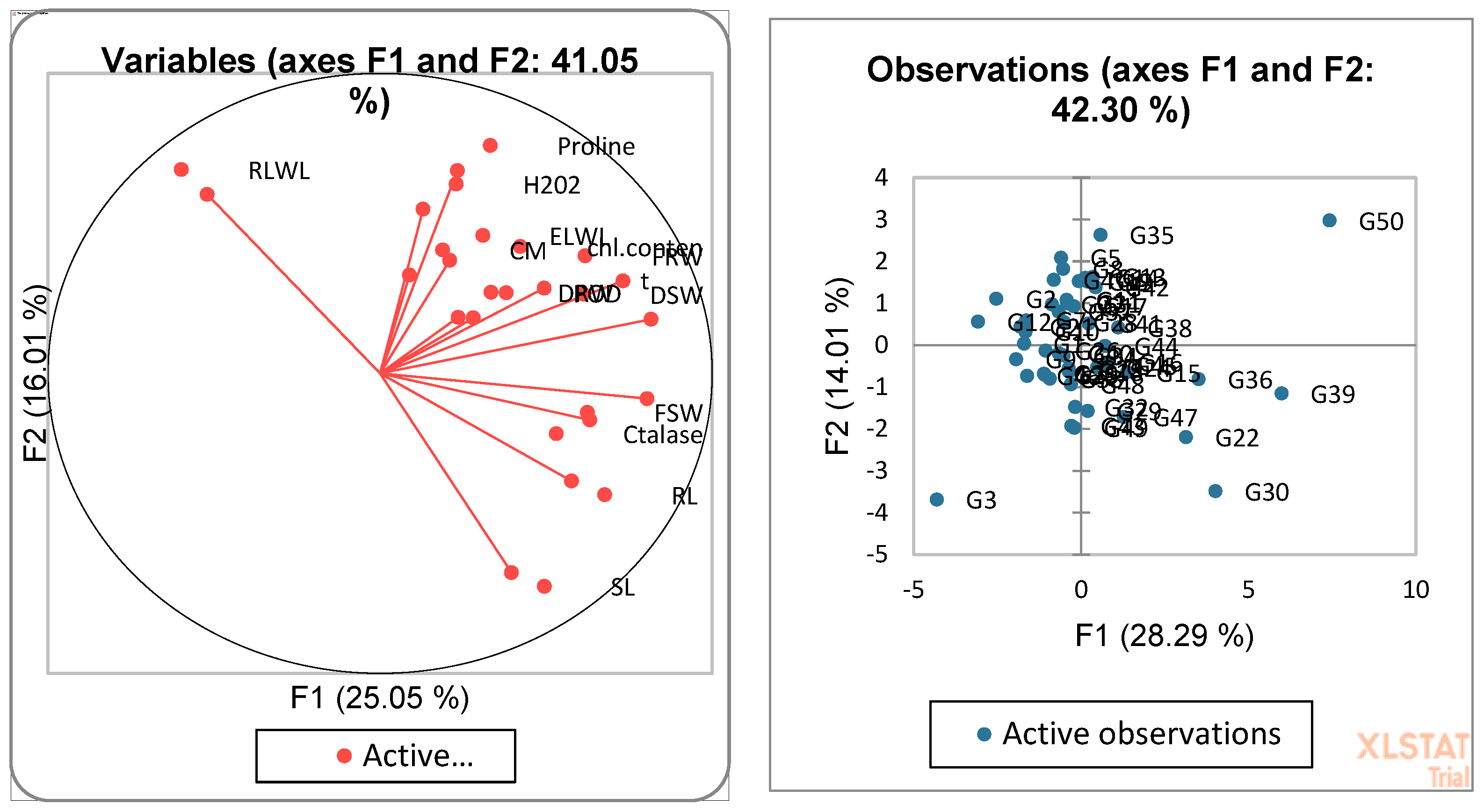
3.4. Biplot Analysis for the Identification of Drought-Tolerant and Susceptible Genotypes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ullah, S.F.; Hashmi, S.M. Financial Development and Economic Growth: Panel Cross—Country Study. Jinnah Bus. Rev. 2016, 4, 9–21. [Google Scholar] [CrossRef]
- Lamaoui, M.; Jemo, M.; Datla, R.; Bekkaoui, F. Heat and Drought Stresses in Crops and Approaches for Their Mitigation. Front. Chem. 2018, 19, 6–26. [Google Scholar] [CrossRef] [PubMed]
- Abdelraheem, A.; Esmaeili, N.; O’Connell, M.; Zhang, J. Progress and Perspective on Drought and Salt Stress Tolerance in Cotton. Ind. Crops Prod. 2019, 130, 118–129. [Google Scholar] [CrossRef]
- Available online: https://www.finance.gov.pk/survey_1617.html (accessed on 17 October 2022).
- Chetanov, N.A.; Bakiev, A.G.; Litvinov, N.A.; Cherlin, V.F. Reptiles: Temperature and Ecology. Saarbrücken: LAP LAMBERT Academic Publishing, 2014. 452 p. Princ. Ecol. 2014, 11, 57–64. [Google Scholar] [CrossRef] [Green Version]
- Joshi, R.; Wani, S.H.; Singh, B.; Bohra, A.; Dar, Z.A.; Lone, A.A.; Pareek, A.; Singla-Pareek, S.L. Transcription Factors and Plants Response to Drought Stress: Current Understanding and Future Directions. Front. Plant Sci. 2016, 7. [Google Scholar] [CrossRef] [Green Version]
- Pettigrew, W.T. Moisture Deficit Effects on Cotton Lint Yield, Yield Components, and Boll Distribution. Agron. J. 2004, 96, 377–383. [Google Scholar] [CrossRef]
- Mohammadkhani, N.; Heidari, R. Effects of Water Stress on Respiration, Photosynthetic Pigments and Water Content in Two Maize Cultivars. Pak. J. Biol. Sci. 2007, 10, 4022–4028. [Google Scholar] [CrossRef] [Green Version]
- Sekmen, A.H.; Ozgur, R.; Uzilday, B.; Turkan, I. Reactive Oxygen Species Scavenging Capacities of Cotton (Gossypium Hirsutum) Cultivars under Combined Drought and Heat Induced Oxidative Stress. Environ. Exp. Bot. 2014, 99, 141–149. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, J.; Feng, K.; Wang, N.; Zhang, S.; Ma, H.; Ge, C.; Shen, Q.; Liu, R.; Zhao, X.; et al. Widely Targeted Metabolomics Reveals the Different Metabolic Changes in Leaves and Roots of Two Cotton Varieties under Drought Stress. J. Agron. Crop Sci. 2021, 207, 1041–1049. [Google Scholar] [CrossRef]
- Lynch, J.P. Harnessing Root Architecture to Address Global Challenges. Plant J. 2021, 109, 415–431. [Google Scholar] [CrossRef]
- Basal, H.; Smith, C.W.; Thaxton, P.S.; Hemphill, J.K. Seedling Drought Tolerance in Upland Cotton. Crop Sci. 2005, 45, 766–771. [Google Scholar] [CrossRef]
- Singh, B.; Norvell, E.; Wijewardana, C.; Wallace, T.; Chastain, D.; Reddy, K.R. Assessing Morphological Characteristics of Elite Cotton Lines from Different Breeding Programmes for Low Temperature and Drought Tolerance. J. Agron. Crop Sci. 2018, 204, 467–476. [Google Scholar] [CrossRef]
- Chaves, M.M.; Flexas, J.; Pinheiro, C. Photosynthesis under Drought and Salt Stress: Regulation Mechanisms from Whole Plant to Cell. Ann. Bot. 2008, 103, 551–560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asif Saleem, M.; Ahmad Malik, T.; Shakeel, A.; Waqas Amjid, M.; Qayyum, A. Genetics of Physiological and Agronomic Traitsinuplandcotton under Drought Stress. Pakjas 2015, 52, 317–324. [Google Scholar]
- Almeselmani, M. Physiological Parameters for Evaluating Drought Tolerance in Durum Wheat Varieties Grown in the Fields in Syria. J. Biol. Today’s World 2012, 1, 53–63. [Google Scholar] [CrossRef]
- Conde, A.; Chaves, M.M.; Geros, H. Membrane Transport, Sensing and Signaling in Plant Adaptation to Environmental Stress. Plant Cell Physiol. 2011, 52, 1583–1602. [Google Scholar] [CrossRef]
- Karim, M.R.; Rahman, M.A. Drought Risk Management for Increased Cereal Production in Asian Least Developed Countries. In Weather and Climate Extremes; Elsevier: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Győri, Z. Evaluation of the Mineral Content of Winter Wheat. MOJ Food Process Technol. 2017, 4, 122–123. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Isoda, A.; LI, M.; Wang, D. Growth and Eco-Physiological Performance of Cotton Under Water Stress Conditions. Agric. Sci. China 2007, 6, 949–955. [Google Scholar] [CrossRef]
- Golabadi, M.; Arzani, A.; Maibody, M. Assessment of Drought Tolerance in Segregating Populations in Durum Wheat. Afr. J. Agric. Res. 2007, 1, 162–171. Available online: https://www.researchgate.net/publication/228787570_Assessment_of_drought_tolerance_in_segregating_Populations_in_durum_wheat (accessed on 18 February 2023).
- Rana, V.; Singh, D.; Dhiman, R.; Chaudhary, H.K. Evaluation of Drought Tolerance among Elite Indian Bread Wheat Cultivars. Cereal Res. Commun. 2014, 42, 91–101. [Google Scholar] [CrossRef]
- Hasheminasab, H. Application of Physiological Traits Related to Plant Water Status for Predicting Yield Stability in Wheat under Drought Stress Condition. Annu. Res. Rev. Biol. 2014, 4, 778–789. [Google Scholar] [CrossRef]
- Munjal, R.; Dhanda, S.S. Physiological Evaluation of Wheat (Triticum Aestivum L) Genotypes for Drought Resistance. Indian J. Genet. Plant Breed. 2005, 65, 307–308. [Google Scholar]
- Parveen, A.; Rai, G.K.; Bagati, S.; Rai, P.K.; Singh, P. Morphological, Physiological, Biochemical and Molecular Responses of Plants to Drought Stress. In Abiotic Stress Tolerance Mechanisms in Plants; CRC Press: Boca Raton, FL, USA, 2021; pp. 321–339. [Google Scholar]
- Szabados, L.; Savouré, A. Proline: A Multifunctional Amino Acid. Trends Plant Sci. 2010, 15, 89–97. [Google Scholar] [CrossRef]
- Horváth, E.; Szalai, G.; Janda, T. Induction of Abiotic Stress Tolerance by Salicylic Acid Signaling. J. Plant Growth Regul. 2007, 26, 290–300. [Google Scholar] [CrossRef]
- Anwar Hossain, M.; Hoque, M.A.; Burritt, D.J.; Fujita, M. Proline Protects Plants Against Abiotic Oxidative Stress. In Oxidative Damage to Plants; Elsevier: Amsterdam, The Netherlands, 2014; pp. 477–522. [Google Scholar]
- Mahmood, T.; Khalid, S.; Abdullah, M.; Ahmed, Z.; Shah, M.K.N.; Ghafoor, A.; Du, X. Insights into Drought Stress Signaling in Plants and the Molecular Genetic Basis of Cotton Drought Tolerance. Cells 2019, 9, 105. [Google Scholar] [CrossRef] [Green Version]
- Munne-Bosch, S. Photo- and Antioxidative Protection During Summer Leaf Senescence in Pistacia Lentiscus L. Grown under Mediterranean Field Conditions. Ann. Bot. 2003, 92, 385–391. [Google Scholar] [CrossRef] [Green Version]
- Reddy, A.R.; Chaitanya, K.V.; Vivekanandan, M. Drought-Induced Responses of Photosynthesis and Antioxidant Metabolism in Higher Plants. J. Plant Physiol. 2004, 161, 1189–1202. [Google Scholar] [CrossRef]
- Hasan, M.; Ma, F.; Prodhan, Z.; Li, F.; Shen, H.; Chen, Y.; Wang, X. Molecular and Physio-Biochemical Characterization of Cotton Species for Assessing Drought Stress Tolerance. Int. J. Mol. Sci. 2018, 19, 2636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, C.K.; Rajkumar, B.K.; Kumar, V. Differential Responses of Antioxidants and Osmolytes in Upland Cotton (Gossypium Hirsutum) Cultivars Contrasting in Drought Tolerance. Plant Stress 2021, 2, 100031. [Google Scholar] [CrossRef]
- Xiong, L.; Zhu, J.-K. Molecular and Genetic Aspects of Plant Responses to Osmotic Stress. Plant Cell Environ. 2002, 25, 131–139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fumis, T.D.F.; Pedras, J.F. Variação Nos Níveis de Prolina, Diamina e Poliaminas Em Cultivares de Trigo Submetidas a Déficits Hídricos. Pesqui. Agropecuária Bras. 2002, 37, 449–453. [Google Scholar] [CrossRef]
- Mafakheri, A.; Siosemardeh, A.; Bahramnejad, B.; Struik, P.C.; Sohrabi, Y. Effect of Drought Stress on Yield, Proline and Chlorophyll Contents in Three Chickpea Cultivars. Aust. J. Crop Sci. 2010, 4, 580–585. [Google Scholar]
- Nikolaeva, M.K.; Maevskaya, S.N.; Shugaev, A.G.; Bukhov, N.G. Effect of Drought on Chlorophyll Content and Antioxidant Enzyme Activities in Leaves of Three Wheat Cultivars Varying in Productivity. Russ. J. Plant Physiol. 2010, 57, 87–95. [Google Scholar] [CrossRef]
- Mishra, V.; Cherkauer, K.A.; Shukla, S. Assessment of Drought Due to Historic Climate Variability and Projected Future Climate Change in the Midwestern United States. J. Hydrometeorol. 2010, 11, 46–68. [Google Scholar] [CrossRef] [Green Version]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants: Protective role of exogenous polyamines. J. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid Determination of Free Proline for Water-Stress Studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Fielding, J.L.; Hall, J.L. A Biolchemical and Cytochemical Study of Peroxidase Activity in Roots OfPisum Sativum. J. Exp. Bot. 1978, 29, 969–981. [Google Scholar] [CrossRef]
- Chance, B.; Maehly, A.C. Assay of Catalases and Peroxidases. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1955; pp. 764–775. [Google Scholar]
- Steel, R.G.D.; Torrie, J.H. Principles and Procedures of Statistics, a Biometrical Approach, 2nd ed.; McGraw-Hill Book Company: New York, NY, USA, 1980. [Google Scholar]
- Ruzdik, N.M.; Karov, I.; Mitrev, S.; Gjorgjieva, B.; Kovacevik, B.; Kostadinovska, E. Evaluation of Sunflower (Helianthus annuus L.) Hybrids Using Multivariate Statistical Analysis. Helia 2015, 38, 175–187. [Google Scholar] [CrossRef]
- Ahmad, N.; Munir, I.; Khan, I.A.; Ali, W.; Muhammad, W.; Habib, R.; Khan, R.S.; Swati, Z.A. PCR-Based Genetic Diversity of Rapeseed Germplasm Using RAPD Markers. Biotechnology 2007, 6, 334–338. [Google Scholar] [CrossRef] [Green Version]
- Eissa, A.M.; Jenkins, J.N.; Vaughan, C.E. Inheritance of Seedling Root Length and Relative Root Weight in Cotton. Crop Sci. 1983, 23, 1107–1111. [Google Scholar] [CrossRef]
- Lidon, Z. An Overview on Drought Induced Changes in Plant Growth, Water Relationsand Photosynthesis. Emir. J. Food Agric. 2012, 24, 57. [Google Scholar] [CrossRef] [Green Version]
- Azhar, N.; Hussain, B.; Abbasi, K.Y.; Ashraf, M.Y. Water Stress Mediated Changes in Growth, Physiology and Secon. Pak. J. Bot. 2011, 43, 15–19. [Google Scholar]
- Azhar, F.M.; Ali, Z.; Akhtar, M.M.; Khan, A.A.; Trethowan, R. Genetic Variability of Heat Tolerance, and Its Effect on Yield and Fibre Quality Traits in Upland Cotton (Gossypium Hirsutum L.). Plant Breed. 2009, 128, 356–362. [Google Scholar] [CrossRef]
- Ahmad, R.T.; Malik, T.A.; Khan, I.A.; Jaskani, M. Genetic Analysis of Some Morpho-Physiological Traits Related to Drought Stress in Cotton (Gossypium Hirsutum). Int. J. Agric. Biol. 2009, 11, 235–240. Available online: https://www.researchgate.net/publication/228547792_Genetic_Analysis_of_Some_Morpho-Physiological_Traits_Related_to_Drought_Stress_in_Cotton_Gossypium_hirsutum.1 (accessed on 22 February 2023).
- Hasanuzzaman, M.; Shabala, L.; Brodribb, T.J.; Zhou, M.; Shabala, S. Understanding Physiological and Morphological Traits Contributing to Drought Tolerance in Barley. J. Agron. Crop Sci. 2018, 205, 129–140. [Google Scholar] [CrossRef]
- Hussain, H.A.; Men, S.; Hussain, S.; Chen, Y.; Ali, S.; Zhang, S.; Zhang, K.; Li, Y.; Xu, Q.; Liao, C.; et al. Interactive Effects of Drought and Heat Stresses on Morpho-Physiological Attributes, Yield, Nutrient Uptake and Oxidative Status in Maize Hybrids. Sci. Rep. 2019, 9, 3890. [Google Scholar] [CrossRef] [Green Version]
- Zou, J.; Hu, W.; LI, Y.; He, J.; Zhu, H.; Zhou, Z. Screening of Drought Resistance Indices and Evaluation of Drought Resistance in Cotton (Gossypium hirsutum L.). J. Integr. Agric. 2020, 19, 495–508. [Google Scholar] [CrossRef]
- Ullah, A.; Sun, H.; Yang, X.; Zhang, X. Drought Coping Strategies in Cotton: Increased Crop per Drop. Plant Biotechnol. J. 2017, 15, 271–284. [Google Scholar] [CrossRef] [Green Version]




| Code | Genotypes | Code | Genotypes | Code | Genotypes | Code | Genotypes | Code | Genotypes |
|---|---|---|---|---|---|---|---|---|---|
| G1 | SB-149 | G11 | IUB-13 | G21 | VH-363 | G31 | Kehkshan | G41 | KZ-189 |
| G2 | FH-452 | G12 | FH-114 | G22 | IR-3701 | G32 | Mubarak | G42 | FH-172 |
| G3 | KZ-181 | G13 | Cyto-178 | G23 | FH-170 | G33 | Bahar-2017 | G43 | BS-80 |
| G4 | KZ-191 | G14 | CRS-2007 | G24 | Cyto-124 | G34 | FH-215 | G44 | NS-121 |
| G5 | VH-341 | G15 | S-9 | G25 | AGC-2 | G35 | VH-228 | G45 | FH-118 |
| G6 | AA-703 | G16 | VH-259 | G26 | Ghouri | G36 | FH-142 | G46 | FH-169 |
| G7 | Tipo-1 | G17 | Debal | G27 | VH-339 | G37 | NIAB-777 | G47 | AGC-501 |
| G8 | MNH-992 | G18 | FH-154 | G28 | MNH-888 | G38 | VH-330 | G48 | NIAB-820 |
| G9 | Tarzan | G19 | Cyto-179 | G29 | FH-458 | G39 | CIM-595 | G49 | FH-490 |
| G10 | CRS-2 | G20 | VH-377 | G30 | AA-802 | G40 | Cyto-608 | G50 | Cyto-515 |
| Normal | 75% (FC) Water Stress | 50% (FC) Water Stress | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Traits | Genotypes | Coding | Max. Value Min. Value | Genotypes | Coding | Max. Value Min. Value | Genotypes | Coding | Max. Value Min. Value |
| SL | Cyto-515 | G50 | 25 | Cyto-515 | G50 | 20 | CIM-595 | G39 | 10 |
| VH-363 | G27 | 9 | VH-363 | G21 | 4 | VH-363 | G21 | 1 | |
| RL | Cyto-515 | G50 | 18 | FH-142 | G36 | 17 | Cyto-515 | G50 | 13 |
| FH-114 | G12 | 5 | VH-363 | G21 | 6.67 | VH-363 | G21 | 0.96 | |
| FSW | Cyto-515 | G50 | 5 | Cyto-515 | G50 | 3.45 | Cyto-515 | G50 | 3.76 |
| KZ-181 | G3 | 2 | KZ-181 | G3 | 1.12 | FH-114 | G13 | 0.9 | |
| FRW | Cyto-515 | G50 | 3 | CIM-595 | G39 | 1.38 | IR-3701 | G22 | 0.9 |
| FH-114 | G32 | 1 | KZ-181 | G3 | 1.01 | AA-802 | G30 | 0.03 | |
| DSW | CIM-595 | G39 | 3 | Cyto-515 | G50 | 2.42 | FH-142 | G36 | 1.2 |
| VH-363 | G27 | 0.75 | FH-114 | G12 | 0.88 | FH-114 | G12 | 0.62 | |
| DRW | Cyto-515 | G38 | 1.18 | AA-802 | G30 | 0.12 | Kehkshan | G31 | 0.15 |
| FH-114 | G12 | 0.02 | FH-172 | G42 | 34 | VH-363 | G21 | 0.06 | |
| Chlr | R-3701 | G22 | 48 | FH-142 | G36 | 43 | Cyto-178 | G13 | 35.12 |
| KZ-181 | G3 | 20 | CRS-2 | G10 | 17.03 | FH-118 | G45 | 14.23 | |
| ELWL | AA-802 | G30 | 0.7 | FH-114 | G12 | 3.16 | CIM-595 | G39 | 2.51 |
| CIM-595 | G39 | 0.35 | VH-363 | G21 | 3.97 | FH-114 | G50 | 4.01 | |
| RLWL | Cyto-608 | G-49 | 83 | IR-3701 | G22 | 75 | IR-3701 | G22 | 67 |
| FH-490 | G35 | 10.67 | FH-172 | G42 | 25 | KZ-181 | G3 | 24 | |
| CMS | Cyto-178 | G13 | 69.35 | Kehkshan | G31 | 58.67 | FH-142 | G12 | 58 |
| KZ-181 | G3 | 21 | KZ-181 | G21 | 21 | FH-114 | G36 | 29 | |
| H2O2 | Debal | G17 | 1.40 | CIM-595 | G39 | 0.17 | Cyto-515 | G39 | 0.27 |
| AA-802 | G30 | 0.22 | VH-363 | G21 | 0.18 | FH-114 | G12 | 0.054 | |
| Proln | FH-458 | G29 | 0.59 | FH-142 | G36 | 0.39 | IR-3701 | G22 | 0.72 |
| FH-114 | G32 | 0.07 | FH-114 | G12 | 0.087 | VH-363 | G21 | 0.016 | |
| POD | Cyto-515 | G50 | 15 | Cyto-515 | G50 | 18.67 | CIM-595 | G39 | 60 |
| FH-114 | G12 | 6 | CRS-2 | G10 | 3.11 | KZ-181 | G3 | 18 | |
| CAT | IR-3701 | G22 | 16 | CIM-595 | G39 | 31 | CIM-595 | G39 | 45 |
| VH-363 | G21 | 8 | KZ-181 | G3 | 11 | VH-363 | G21 | 12 | |
| Source of Variation | DF | RL | SL | FRW | FSW | DSW | DRW | Chlr | CMS | ELWL | RWC | H2O2 | POD | CAT | Proline |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Drought | 2 | 313.06 ** | 713.14 ** | 2.88 ** | 10.36 ** | 2.66 ** | 0.06 ** | 895.84 ** | 12426.02 ** | 10.35 ** | 81703.5 ** | 16.68 ** | 5656.29 ** | 2139.93 ** | 10.93 ** |
| Genotypes | 49 | 37.3 ** | 48.61 ** | 2.85 ** | 1.32 ** | 0.64 ** | 0.64 ** | 148.41 ** | 104.64 ** | 6.01 ** | 1091.7 ** | 3.63 ** | 25.59 ** | 341.15 ** | 24.67 ** |
| Drought Genotypes | 98 | 15.84 ** | 15.34 ** | 1.01 ** | 0.62 ** | 0.16 ** | 0.27 ** | 39.33 ** | 88.56 ** | 3.46 ** | 1482.53 ** | 4.05 ** | 19.82 ** | 347.41 ** | 23.15 ** |
| Error | 300 | 5.95 | 2.98 | 0.47 | 0.21 | 0.03 | 0.02 | 18.61 | 16.18 | 2.92 | 1240.4 | 3.71 | 16.23 | 324.7 | 23.43 |
| Total | 449 |
| Cluster 1 | Cluster 2 | Cluster 3 | Cluster 4 | Cluster 5 | |
|---|---|---|---|---|---|
| SL | 12.70 | 15.46 | 12.20 | 15.28 | 15.64 |
| RL | 8.41 | 8.81 | 7.10 | 10.10 | 10.14 |
| FSW | 0.98 | 1.19 | 0.87 | 1.23 | 1.31 |
| FRW | 0.45 | 0.52 | 0.31 | 0.82 | 0.85 |
| DSW | 0.26 | 0.25 | 0.21 | 0.4 | 0.51 |
| DRW | 0.14 | 0.12 | 0.09 | 0.36 | 0.37 |
| Chlr | 34.96 | 36.03 | 34 | 36.43 | 38.29 |
| ELWL | 1.43 | 1.48 | 1.68 | 1.66 | 1.23 |
| RLWL | 73.33 | 75.23 | 63.33 | 79.13 | 81.38 |
| CMS | 44.7 | 41.30 | 32.5 | 56.25 | 56.85 |
| H2O2 | 1.34 | 1.36 | 1.24 | 1.37 | 1.41 |
| Proline | 0.19 | 0.13 | 0.11 | 0.20 | 0.34 |
| POD | 7.63 | 7.50 | 7.09 | 7.52 | 7.85 |
| CAT | 24.88 | 26.82 | 17.06 | 23.77 | 29.83 |
| Cluster 1 | Cluster 2 | Cluster 3 | Cluster 4 | Cluster 5 | |
|---|---|---|---|---|---|
| SL | 7.81 | 12 | 13.21 | 10.63 | 11.47 |
| RL | 4.46 | 6.70 | 7.64 | 6.58 | 6.20 |
| FSW | 0.30 | 0.56 | 0.84 | 0.52 | 0.76 |
| FRW | 0.11 | 0.34 | 0.99 | 0.48 | 0.40 |
| DSW | 0.05 | 0.48 | 0.69 | 0.68 | 0.48 |
| DRW | 0.20 | 0.22 | 0.44 | 0.43 | 0.41 |
| Chlr | 11.34 | 37 | 37.67 | 37.47 | 31.79 |
| ELWL | 2.38 | 2.34 | 2.23 | 2.25 | 2.37 |
| RLWL | 41.44 | 54.33 | 56.59 | 49.09 | 51.45 |
| CMS | 24.23 | 37.04 | 37.22 | 36.69 | 29.66 |
| H2O2 | 5.25 | 5.43 | 5.53 | 5.42 | 5.33 |
| Proline | 0.40 | 0.41 | 0.58 | 0.50 | 0.57 |
| POD | 16.06 | 17.75 | 17.78 | 15.70 | 16.22 |
| CAT | 52.86 | 58.2 | 59.82 | 59.57 | 58.43 |
| Cluster 1 | Cluster 2 | Cluster 3 | Cluster 4 | Cluster 5 | |
|---|---|---|---|---|---|
| SL | 13.27 | 7.54 | 13.53 | 19.66 | 14.40 |
| RL | 8.37 | 4.93 | 7.77 | 7.69 | 11.28 |
| FSW | 0.53 | 0.47 | 0.98 | 1.20 | 1.25 |
| FRW | 0.24 | 0.24 | 0.43 | 0.69 | 1.30 |
| DSW | 0.12 | 0.09 | 0.23 | 0.56 | 0.78 |
| DRW | 0.21 | 0.19 | 0.20 | 0.22 | 0.24 |
| Chlr | 34.85 | 24.85 | 30.63 | 38.6 | 39.7 |
| ELWL | 3.77 | 3.88 | 3.75 | 3.63 | 3.34 |
| RLWL | 34.59 | 34.15 | 36.28 | 35.07 | 35.67 |
| CMS | 31.03 | 30.80 | 33.08 | 22.35 | 31.52 |
| H2O2 | 0.53 | 0.26 | 0.30 | 0.44 | 0.74 |
| Proline | 0.32 | 0.25 | 0.35 | 0.28 | 0.37 |
| POD | 11.93 | 10.81 | 11.19 | 11.54 | 12.65 |
| CAT | 32.53 | 30.56 | 39.03 | 30.84 | 39.41 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aslam, S.; Hussain, S.B.; Baber, M.; Shaheen, S.; Aslam, S.; Waheed, R.; Seo, H.; Azhar, M.T. Estimation of Drought Tolerance Indices in Upland Cotton under Water Deficit Conditions. Agronomy 2023, 13, 984. https://doi.org/10.3390/agronomy13040984
Aslam S, Hussain SB, Baber M, Shaheen S, Aslam S, Waheed R, Seo H, Azhar MT. Estimation of Drought Tolerance Indices in Upland Cotton under Water Deficit Conditions. Agronomy. 2023; 13(4):984. https://doi.org/10.3390/agronomy13040984
Chicago/Turabian StyleAslam, Sidra, Syed Bilal Hussain, Muhammad Baber, Sabahat Shaheen, Seema Aslam, Raheela Waheed, Hyojin Seo, and Muhammad Tehseen Azhar. 2023. "Estimation of Drought Tolerance Indices in Upland Cotton under Water Deficit Conditions" Agronomy 13, no. 4: 984. https://doi.org/10.3390/agronomy13040984
APA StyleAslam, S., Hussain, S. B., Baber, M., Shaheen, S., Aslam, S., Waheed, R., Seo, H., & Azhar, M. T. (2023). Estimation of Drought Tolerance Indices in Upland Cotton under Water Deficit Conditions. Agronomy, 13(4), 984. https://doi.org/10.3390/agronomy13040984







